Abstract
Thiazides and thiazide-type diuretics are recommended as first-line agents for the treatment of hypertension, but contemporary information on their use in clinical practice is lacking. We examined patterns and correlates of thiazide prescription in a cross-sectional analysis of baseline data from participants enrolled in the Systolic Blood Pressure Intervention Trial (SPRINT). We examined baseline prescription of thiazides in 7582 participants receiving at least one antihypertensive medication by subgroup, and used log-binomial regression to calculate adjusted prevalence ratios for thiazide prescription (versus no thiazide). Forty-three percent of all participants were prescribed a thiazide at baseline, but among participants prescribed a single agent, the proportion was only 16%. The prevalence of thiazide prescription differed significantly by demographic factors, with younger participants, women, and blacks all having higher adjusted prevalence of thiazide prescription than other corresponding subgroups. Participants in the lowest category of kidney function (estimated glomerular filtration rate <30 mL/min per 1.73m2) were half as likely to be prescribed a thiazide as participants with preserved kidney function. In conclusion, among persons with hypertension and heightened cardiovascular risk, we found that thiazide prescription varied significantly by demographics and kidney disease status, despite limited evidence about relative differences in effectiveness.
Keywords: hypertension, randomized trial, thiazides, antihypertensive medications, chronic kidney disease, race
Introduction
Thiazide-type diuretics are among the first-line agents recommended for use in the treatment of hypertension by multiple clinical practice guidelines published over the past decade1–3. The Seventh Report of the Joint National Committee (JNC 7), published in 2003, gave one of the strongest endorsements for thiazide prescription, recommending that they be given as initial therapy for most patients with hypertension2. These recommendations were based in large part on the lower incidence of several cardiovascular disease outcomes following treatment with chlorthalidone compared with other antihypertensive agents (i.e., the angiotensin-converting enzyme inhibitors (ACEI) lisinopril, the alpha blocker doxazosin, or the calcium channel blocker amlodipine) reported in the landmark Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)4,5.
An increase in thiazide prescription was observed after publication of ALLHAT and JNC7, but the magnitude and duration of this effect was relatively modest and short-lived6,7. Moreover, reported rates of thiazide prescription remained lower than for other antihypertensive medication classes8–10. However, even the most recent of these studies only included patients through 2010, and so may not represent the most up-to-date information on the use of thiazides in clinical practice.
We therefore sought to examine patterns and correlates of thiazide prescription in a contemporary cohort of patients with hypertension. We conducted a cross-sectional analysis of baseline data from the Systolic Blood Pressure Intervention Trial (SPRINT), a randomized clinical trial that enrolled persons with hypertension and other cardiovascular risk factors between November 2010 and March 201311. We hypothesized that a minority of the overall cohort would have evidence of thiazide prescription, and that older patients and patients with chronic kidney disease (CKD) would have lower prevalence of prescription than other subgroups.
Methods
Study Participants
SPRINT is a multicenter clinical trial sponsored by the National Institutes of Health comparing two strategies for control of systolic blood pressure (SBP) and effects on cardiovascular, brain, and renal outcomes11,12. Briefly, between November 2010 and March 2013, participants with a history of hypertension (treated or untreated SBP ≥130 mm Hg) and age> 75 years, or age ≥50 years with at least one of the following cardiovascular risk factors were enrolled: history of cardiovascular disease, Framingham risk score for 10-year cardiovascular disease event ≥15%, or CKD, defined as an estimated glomerular filtration rate (eGFR) of 20 to 59 mL/min per 1.73m2 using the 4-variable Modification of Diet in Renal Disease (MDRD) equation13. Participants with a history of stroke, diabetes mellitus, polycystic kidney disease, dementia, non-adherence, eGFR <20 mL/min per 1.73m2 or ≥1 gram of proteinuria/day (or the equivalent) were not eligible for participation. Participants were randomly assigned to a standard SBP target (<140 mm Hg) or to a lower SBP target (<120 mm Hg).
For the present analysis, we restricted the cohort to participants prescribed at least one antihypertensive medication at baseline, prior to any changes in medications by the SPRINT investigators. All participants provided written informed consent for participation in the trial. The trial was approved by the Institutional Review Board at each site and is registered with ClinicalTrials.gov (NCT01206062).
Baseline characteristics and Antihypertensive Medications
Trained study personnel ascertained information about participant baseline characteristics during the screening or randomization visit. Fasting blood and urine samples were collected at that time. All antihypertensive medications prescribed prior to the time of the screening visit (i.e., baseline antihypertensive medications) were documented and classified into the following categories: thiazides (e.g., hydrochlorothiazide, chlorthalidone, metolazone), ACEIs, angiotensin II receptor blockers (ARBs), beta-blockers, calcium channel blockers, loop diuretics, mineralocorticoid receptor antagonists, direct renin-inhibitors, alpha-blockers, centrally acting agents, and direct vasodilators.
Statistical Analysis
Our main outcome variable was evidence of baseline thiazide prescription. We examined thiazide prescription by subgroups based on sociodemographic variables, history of cardiovascular conditions, and kidney function, and plotted the unadjusted results. We used log-binomial regression to calculate prevalence ratios (95% confidence intervals [CI]) for thiazide prescription versus no thiazide prescription, and included the following variables in the model: age, sex, race, insurance status, cardiovascular comorbid conditions, eGFR, and presence of albuminuria (defined as a spot albumin-to-creatinine ratio ≥30 mg/g). For common outcomes, prevalence ratios are a more consistent approximation of relative risks than odds ratios derived from logistic regression14. We defined statistical significance based on two-sided p-values <0.05. All analyses were conducted using SAS 9.4 (Cary, NC).
Results
Of the 9361 participants enrolled in SPRINT, 8426 (90% of total cohort) were taking at least one antihypertensive medication at baseline. 7582 had complete data needed for this analysis (90% of treated participants), forming the present cohort. The 43% of participants prescribed a thiazide at baseline were generally younger, with a larger proportion of women, non-white race and uninsured status. They also had a lower prevalence of cardiovascular disease than participants not prescribed a thiazide at baseline (Table).
Table.
Baseline characteristics of SPRINT participants prescribed at least 1 antihypertensive medication. All values are % unless otherwise noted
| Variable | Overall N=7582 |
Thiazide N=3275 |
No Thiazide N=4307 |
|---|---|---|---|
| Age y, mean (SD) | 68.2 (9.4) | 67.3 (9.2) | 68.8 (9.5) |
| Age categories (years) | |||
| 50–59 | 20.2 | 22.4 | 18.5 |
| 60–69 | 36.2 | 37.9 | 34.9 |
| 70–79 | 30.7 | 29.1 | 31.9 |
| 80+ | 12.8 | 10.6 | 14.6 |
| Female sex | 36.7 | 40.2 | 34.0 |
| Race / Ethnicity | |||
| White | 57.0 | 53.5 | 59.7 |
| Black | 30.2 | 36.3 | 25.5 |
| Hispanic | 11.1 | 8.9 | 12.8 |
| Other | 1.8 | 1.3 | 2.1 |
| Insurance status | |||
| Private | 42.2 | 42.1 | 42.3 |
| Medicare | 26.3 | 24.7 | 27.5 |
| Medicaid | 7.0 | 6.2 | 7.7 |
| Veterans Affairs | 14.6 | 15.0 | 14.4 |
| None | 9.8 | 12.0 | 8.1 |
| No insurance for drugs | 19.3 | 21.4 | 17.8 |
| History of cardiovascular disease: | |||
| Coronary artery disease | 13.9 | 9.5 | 17.3 |
| Myocardial infarction | 8.5 | 5.6 | 10.7 |
| Heart failure | 3.7 | 2.3 | 4.8 |
| Atrial fibrillation | 8.4 | 5.9 | 10.3 |
| Arrhythmia | 17.6 | 14.8 | 19.7 |
| Body mass index kg/m2, mean (SD) | 30 (5.8) | 30.5 (5.8) | 29.6 (5.8) |
| Systolic blood pressure, mm Hg, mean (SD) | 139.1 (15.5) | 137.8 (15.2) | 140.2 (15.8) |
| Diastolic blood pressure, mm Hg, mean (SD) | 77.5 (11.8) | 77.7 (11.6) | 77.3 (12) |
| Heart rate, beats/min, mean (SD) | 66.0 (12) | 66.7 (11.5) | 65.3 (11.5) |
| Baseline laboratory values | |||
| Estimated glomerular filtration rate category (mL/min per 1.73m2) | |||
| ≥60 | 69.7 | 71.9 | 68.1 |
| 45–59 | 19.8 | 19.9 | 19.8 |
| 30–44 | 8.6 | 7.4 | 9.4 |
| 20–30 | 1.9 | 0.8 | 2.7 |
| Albuminuria mg/g: | |||
| < 30 | 80.1 | 83.8 | 77.3 |
| 30–300 | 16.9 | 14.2 | 19.0 |
| > 300 | 3.0 | 2.0 | 3.7 |
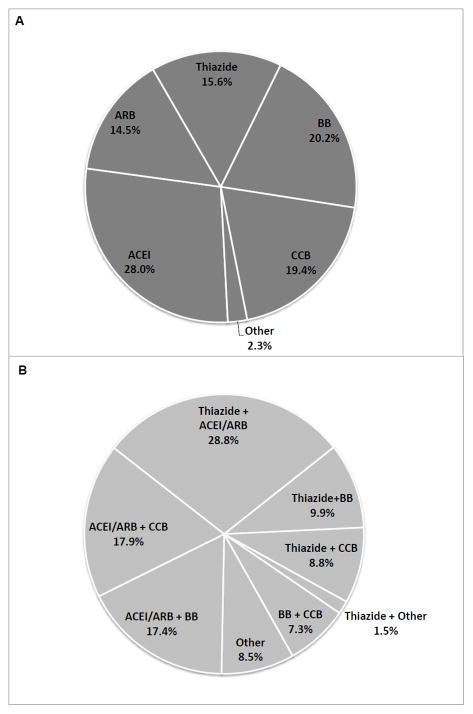
Large proportions of the cohort were prescribed ACEIs or ARBs (65%), beta-blockers (40%) or dihydropyridine calcium channel blockers (33%) at baseline (Table S1). For the 2436 participants (32% of the cohort) prescribed a single antihypertensive agent at baseline, only 16% received a thiazide, while 43% received either an ACEI or an ARB (Figure 1A). Of the 2967 participants prescribed two antihypertensive medications at baseline, fewer than half received a thiazide (Figure 1B). Of the 2179 participants prescribed three or more antihypertensive medications at baseline, 62% received a thiazide.
Figure 1.
Distribution of antihypertensive medication class prescription among SPRINT participants prescribed A) a single agent at baseline (N=2436); and B) two agents at baseline (N=2967). Abbreviations: ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin II receptor blocker; BB = beta-blocker; CCB = calcium channel blocker;
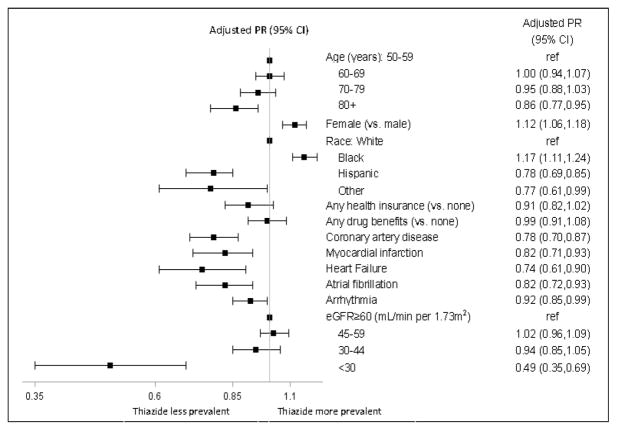
In unadjusted analyses, we saw a stepwise difference in the prevalence of thiazide prescription with older age, from 48% in participants aged 50–59 years down to 36% in participants aged ≥80 years (Figure S1). In multivariable-adjusted models, there was a 14% (CI 5% to 23%) lower adjusted prevalence of thiazide prescription among participants aged ≥80 years compared with participants aged 50–59 years (Figure 2). Women were more likely than men to have thiazides prescribed, and thiazide prescription also varied by race/ethnicity, with blacks having the highest and Hispanics the lowest prevalence of thiazide prescription (Figures S1 & 2). Although participants who lacked health insurance or drug benefits had higher unadjusted prevalence of thiazide prescription, the results were not statistically different after adjusting for other baseline variables (Figures S1 and 2).
Figure 2.
Adjusted prevalence ratios (PR) and 95% confidence intervals (CI) of thiazide prescription in SPRINT participants receiving at least 1 antihypertensive medication at baseline.
Abbreviation: eGFR = estimated glomerular filtration rate
Participants with a history of coronary disease, myocardial infarction, heart failure, atrial fibrillation, or cardiac arrhythmias were less likely to have thiazides prescribed at baseline than participants without these comorbid conditions in unadjusted and adjusted models (Figures S2 and 2). We saw a stepwise difference in the prevalence of thiazide prescription in participants with lower eGFR, from 45% in participants with preserved eGFR to 19% in participants with an eGFR<30 mL/min per 1.73m2 (Figure S2). In adjusted models, there was a 51% (CI 31% to 65%) lower prevalence of thiazide prescription for participants with an eGFR< 30 mL/min per 1.73m2 compared with participants with an eGFR ≥60 mL/min per 1.73m2 (Figure 2).
Discussion
We conducted a cross-sectional analysis of baseline data from SPRINT, a diverse cohort of persons with hypertension and other cardiovascular risk factors enrolled between November 2010 and March 2013. We found that 43% of the overall cohort was prescribed a thiazide at baseline, but that the prevalence of thiazide prescription was only 16% among participants treated with a single agent. Over half of patients prescribed two antihypertensive medications at baseline, and over one-third of patients taking three or more agents did not receive a thiazide. Our results, based on participants enrolled between 2010 and 2013, show that thiazide prescription continues to be suboptimal, and are consistent with previous studies. For example, a study of managed care patients initiating antihypertensive medications in 2004–20058 showed that 37% of all patients had a regimen that included a thiazide, and only 21% of patients receiving a single drug used a thiazide. Similarly, only 18% of Medicare beneficiaries initiating antihypertensive medications in 2010 started treatment with a thiazide9, despite recommendations published as part of broadly endorsed clinical practice guidelines2.
In our study, thiazide prescription differed significantly by certain participant demographics, with participants in older age categories having a lower prevalence of thiazide prescription compared with the younger participants (50–59 years). The Hypertension in the Very Elderly Trial (HYVET)15, which randomized patients ≥80 years old to receive the thiazide-type diuretic indapamide (with or without an ACEI) or placebo, showed fewer adverse events in the active treatment group. However, fears about higher risks of adverse events associated with thiazides may persist in a non-trial, clinical setting. In an observational analysis of older veterans16, initiation with a thiazide was associated with a 2 to 3-fold higher risk of an adverse event, such as hypokalemia, hyponatremia, or acute kidney injury, compared with propensity score-matched non-users (p<0.001). However, caveats of that study included its atypical patient population (hypertension treated for at least 9 months with second-line agents only), and other methodological issues17.
We also found that women had a 12% higher adjusted prevalence of thiazide prescription compared with men, a phenomenon observed in several other studies10,18–20 despite the lack of evidence to indicate differences in thiazide effectiveness by sex21,22. Potential explanations for the difference may relate to concerns of adverse effects of thiazides on sexual function and metabolic syndrome in men. In women, thiazides may be used preferentially to treat more frequent complaints of edema. There may also be heightened concerns for osteoporosis in women, and thiazides inhibit calcium excretion, preserve bone mineral density and may lower the risk of hip fracture23.
Racial and ethnic differences in thiazide prescription were observed in our study: blacks were 17% more likely, but Hispanics 22% less likely than non-Hispanic whites to receive thiazides, consistent with previous studies8, 12. Current guidelines recommend preferential prescription of thiazides (or calcium channel blockers) in blacks, based in part on results from ALLHAT, which, in a pre-specified subgroup analysis, showed a larger SBP reduction and improved cardiovascular outcomes with chlorthalidone versus lisinopril24 (even in those with the metabolic syndrome25). Reasons for the lower prevalence of thiazide prescription in Hispanics are unclear, as Hispanic ALLHAT participants had better blood pressure control than non-Hispanics prescribed chlorthalidone26.
We found a lower prevalence of thiazide prescription with lower eGFR, as participants in the lowest eGFR category had an adjusted 51% lower prevalence of thiazide prescription compared with participants without CKD. The guidelines from the National Kidney Foundation, published in 2002, recommend changing from a thiazide to a loop diuretic when the estimated GFR falls below 30 mL/min per 1.73m2 (with the exception of the thiazide metolazone)27, citing lower effectiveness of thiazides with impaired kidney function However, recent studies provide evidence to the contrary. A study of 60 patients with CKD (mean eGFR 38 mL/min per 1.73m2) versus 60 non-CKD controls (mean eGFR 76 mL/min per 1.73m2) showed a similar decrease of about 20 mm Hg in SBP in both groups after 8 weeks of taking chlorthalidone in addition to other non-diuretic antihypertensive medications28. A pilot study of 14 participants with CKD (eGFR 20–45 mL/min per 1.73m2) and poorly controlled hypertension showed that home SBP fell by 10, 13 and 9 mm Hg after 4, 8 and 12 weeks and 24-hour SBP was reduced by 10.5 mm Hg after 12 weeks of treatment with chlorthalidone added to other antihypertensive medications29. Interestingly, albuminuria was also reduced by 40% in that study. Larger, controlled studies are needed to confirm these findings, but they challenge the conventional wisdom that thiazides are not effective in advanced CKD. Clinical practice guidelines may need updating to incorporate newer evidence.
Although our analysis has several strengths, such as its large sample size, diverse participant population including a significant proportion of participants older than 75 years and/or with CKD, there are also several limitations that should be considered. First, we did not have information on medication prescriptions prior to enrollment in SPRINT. Thus, we are unable to determine whether some participants may have previously been initiated on a thiazide but then discontinued the drug due to adverse side effects, allergies, or other intolerances. Second, we relied on participant reporting to collect information about baseline medication prescriptions, and did not have more objective information (i.e., pharmacy fill data, electronic health records) to verify the participants’ reports. We also could not determine whether lack of baseline thiazide prescription was due to indications for other drugs, physician preference or participant non-adherence. Finally, results from persons recruited into a randomized clinical trial such as SPRINT may not be fully generalizable to the overall population of persons with hypertension. However, a recent study using data from the National Health and Nutrition Examination survey found that a substantial proportion of US adults would meet SPRINT eligibility criteria30.
Perspectives
In a contemporary, diverse cohort with hypertension and other cardiovascular risk factors, we show that thiazides prescription remains suboptimal. Thiazides are prescribed less often than other classes of antihypertensive medications among participants on monotherapy, and thiazides were not prescribed for over half of patients on two antihypertensive medications and for over one-third of patients on three or more antihypertensive medications at baseline. Moreover, thiazide prescription varied significantly by demographics and CKD status, despite limited evidence about differences in effectiveness in any particular subgroup. At the time of SPRINT enrollment (2010–2013), JNC 7 and most other guidelines recommended thiazides as at least one of the first-line agents2, suggesting that contemporary prescribing practices are not consistent with U.S. hypertension guidelines. Our results suggest the need for focused interventions so that clinical practice patterns more closely reflect practice guidelines, which may improve global clinical outcomes.
Supplementary Material
Novelty and Significance.
1) What is New?
We report on patterns and correlates of thiazides and thiazide-type prescriptions in a large, contemporary cohort of participants enrolled between November 2010 and March 2013 in the Systolic Blood Pressure Intervention Trial (SPRINT), a multicenter trial of two different blood pressure targets in the United States.
2) What is Relevant?
Among participants receiving 1, 2 or 3+ blood pressure medication, 16%, 49% and 62% were prescribed a thiazide, respectively.
Certain demographic subgroups, such as older participants, men, and non-blacks, were less likely to have a thiazide prescription, even though there is little evidence that thiazides are more or less effective in any given population.
Participants with more advanced kidney disease had lower prevalence of thiazide prescription, but recent studies suggest thiazides may still work well in this subgroup.
3) Summary
In the 7582 SPRINT participants included in our analysis, thiazide prescription remained suboptimal at baseline. Thiazide prescription varied significantly by demographics and kidney disease status, despite limited evidence about differences in effectiveness in any particular subgroup. Our results suggest the need for focused interventions so that clinical practice patterns more closely reflect guidelines, which may improve global clinical outcomes.
Acknowledgments
Sources of Funding
Dr. Chang is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): 5K23DK095914.
The Systolic Blood Pressure Intervention Trial11 is funded by the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: ClinicalTrials.gov Identifier: NCT01206062.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS.
Footnotes
Disclosures
Dr. Cushman reports serving as an uncompensated consultant to Takeda Pharmaceuticals.
Contributor Information
Tara I. Chang, Stanford University School of Medicine
Gregory Evans, Wake Forest School of Medicine.
Alfred K. Cheung, University of Utah
William C. Cushman, Memphis Veterans Affairs Medical Center
Matthew J Diamond, The Medical College of Georgia at Georgia Regents University.
Jamie P. Dwyer, Vanderbilt University
Yonghong Huan, University of Pennsylvania.
Dalane Kitzman, Wake Forest School of Medicine.
John B. Kostis, Robert Wood Johnson Medical School
Suzanne Oparil, University of Alabama at Birmingham.
Anjay Rastogi, University of California, Los Angeles.
Christianne Roumie, Tennessee Valley Health Care Center & Vanderbilt University.
Rukmani Sahay, Stanford University School of Medicine.
Randall S. Stafford, Stanford University School of Medicine
Addison A. Taylor, Michael E. DeBakey VA Medical Center and Baylor College of Medicine
Jackson T. Wright, Jr., Case Western Reserve University
Glenn M. Chertow, Stanford University School of Medicine
References
- 1.Daskalopoulou SS, Khan NA, Quinn RR, et al. The 2012 Canadian hypertension education program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol. 2012;28:270–287. doi: 10.1016/j.cjca.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037–2114. doi: 10.1016/j.jacc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 4.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 5.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2003;42:239–246. doi: 10.1161/01.HYP.0000086521.95630.5A. [DOI] [PubMed] [Google Scholar]
- 6.Stafford RS, Bartholomew LK, Cushman WC, Cutler JA, Davis BR, Dawson G, Einhorn PT, Furberg CD, Piller LB, Pressel SL, Whelton PK for the ALLHAT Collaborative Research Group. Impact of the ALLHAT/JNC7 Dissemination Project on thiazide-type diuretic use. Arch Intern Med. 2010;170:851–858. doi: 10.1001/archinternmed.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stafford RS, Monti V, Furberg CD, Ma J. Long-term and short-term changes in antihypertensive prescribing by office-based physicians in the United States. Hypertension. 2006;48:213–218. doi: 10.1161/01.HYP.0000229653.73128.b6. [DOI] [PubMed] [Google Scholar]
- 8.Muntner P, Krousel-Wood M, Hyre AD, Stanley E, Cushman WC, Cutler JA, Piller LB, Goforth GA, Whelton PK. Antihypertensive prescriptions for newly treated patients before and after the main Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial results and Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure guidelines. Hypertension. 2009;53:617–623. doi: 10.1161/HYPERTENSIONAHA.108.120154. [DOI] [PubMed] [Google Scholar]
- 9.Kent ST, Shimbo D, Huang L, Diaz KM, Kilgore ML, Oparil S, Muntner P. Antihypertensive medication classes used among medicare beneficiaries initiating treatment in 2007–2010. PLoS One. 2014;9:e105888. doi: 10.1371/journal.pone.0105888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126:2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 11.Wright JT, Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D for the Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 14.Thompson ML, Myers JE, Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: what is to be done? Occup Environ Med. 1998;55:272–277. doi: 10.1136/oem.55.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 16.Makam AN, John Boscardin W, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62:1039–1045. doi: 10.1111/jgs.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Einhorn PT, Whelton PK, Davis BR, Wright JT, Cushman WC, Zieman SJ. Real-world evidence supports optimally dosed thiazide-type diuretics as preferred in treatment regimens of older adults with hypertension. J Am Geriatr Soc. 2015;63:1045–1047. doi: 10.1111/jgs.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klungel OH, de Boer A, Paes AH, Seidell JC, Bakker A. Sex differences in antihypertensive drug use: determinants of the choice of medication for hypertension. J Hypertens. 1998;16:1545–1553. doi: 10.1097/00004872-199816100-00021. [DOI] [PubMed] [Google Scholar]
- 19.Keyhani S, Scobie JV, Hebert PL, McLaughlin MA. Gender disparities in blood pressure control and cardiovascular care in a national sample of ambulatory care visits. Hypertension. 2008;51:1149–1155. doi: 10.1161/HYPERTENSIONAHA.107.107342. [DOI] [PubMed] [Google Scholar]
- 20.Doumas M, Papademetriou V, Faselis C, Kokkinos P. Gender differences in hypertension: myths and reality. Curr Hypertens Rep. 2013;15:321–330. doi: 10.1007/s11906-013-0359-y. [DOI] [PubMed] [Google Scholar]
- 21.Turnbull F, Woodward M, Neal B, Barzi F, Ninomiya T, Chalmers J, Perkovic V, Li N, MacMahon S. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J. 2008;29:2669–2680. doi: 10.1093/eurheartj/ehn427. [DOI] [PubMed] [Google Scholar]
- 22.Oparil S, Davis BR, Cushman WC, Ford CE, Furberg CD, Habib GB, Haywood LJ, Margolis K, Probstfield JL, Whelton PK, Wright JT. Mortality and morbidity during and after ALLHAT: Results by gender. Hypertension. 2013;61:977–986. doi: 10.1161/HYPERTENSIONAHA.111.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aung K, Htay T. Thiazide diuretics and the risk of hip fracture. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD005185.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Wright JT, Dunn J, Cutler JA, Davis BR, Cushman WC, Ford CE, Haywood LJ, Leenen FH, Margolis KL, Papdemetriou V, Probstfield JL, Whelton PK, Habib GB for the ALLHAT Collaborative Research Group. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595–1608. doi: 10.1001/jama.293.13.1595. [DOI] [PubMed] [Google Scholar]
- 25.Wright JT, Jr, Harris-Haywood S, Pressel S, et al. Clinical outcomes by race in hypertensive patients with and without the metabolic syndrome: Antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) Arch Intern Med. 2008;168:207–217. doi: 10.1001/archinternmed.2007.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolis KL, Piller LB, Ford CE, Henriquez MA, Cushman WC, Einhorn PT, Colon PJ, Vidt DG, Christian R, Wong ND, Wright JT, Goff DC for the ALLHAT Collaborative Research Group. Blood Pressure Control in Hispanics in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension. 2007;50:854–861. doi: 10.1161/HYPERTENSIONAHA.107.092650. [DOI] [PubMed] [Google Scholar]
- 27.National Kidney Foundation K/DOQI Guidelines. [Accessed May 25, 2010];KDOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. 2002 http://www.kidney.org/professionals/KDOQI/guidelines_bp/index.htm.
- 28.Cirillo M, Marcarelli F, Mele AA, Romano M, Lombardi C, Bilancio G. Parallel-group 8-week study on chlorthalidone effects in hypertensives with low kidney function. Hypertension. 2014;63:692–697. doi: 10.1161/HYPERTENSIONAHA.113.02793. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal R, Sinha AD, Pappas MK, Ammous F. Chlorthalidone for poorly controlled hypertension in chronic kidney disease: an interventional pilot study. Am J Nephrol. 2014;39:171–182. doi: 10.1159/000358603. [DOI] [PubMed] [Google Scholar]
- 30.Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of results from the Systolic Blood Pressure Intervention Trial (SPRINT) to the US adult population. J Am Coll Cardiol. 2015 doi: 10.1016/j.jacc.2015.10.037. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.