Abstract
Carbon monoxide is an intrinsic signaling molecule with importance on par with that of nitric oxide. During the past decade, pharmacological studies have amply demonstrated the therapeutic potential of carbon monoxide. However, such studies were mostly based on CO inhalation and metal-based CO releasing molecules (CO-RMs). The field is now at the stage that a major effort is needed to develop pharmaceutically acceptable forms of CO for delivery via various routes such as oral, injection, infusion, or topical applications. This review examines the state of the art, discusses existing hurdles to overcome, and proposes developmental strategies necessary to address remaining drug delivery issues.
Keywords: Carbon monoxide, CO-RMs, targeted delivery, metal-free, photo-sensitive, enzyme-activated
1. CO pharmacology and drug delivery issues
Carbon monoxide is most widely known as a poisonous gas that originates from combustion of fossil fuels. This is largely due to the fact that each year many die from CO poisoning, which gives CO high visibility in that regard. However, research in recent years has convincingly demonstrated the role of CO as a gasotransmitter having critical physiological functions in mammals1-7 with importance on par with that of nitric oxide (NO), which was the subject of the 1998 Nobel Prize. Along with its physiological roles, CO is now accepted as a potential therapeutic agent and has entered multiple clinical trials (www.clinicaltrials.gov).
There have been numerous research publications demonstrating the tremendous potential of using CO as a therapeutic agent and several very high quality reviews for readers who are interested in a comprehensive understanding of CO-related research.1, 3-8 Therefore, there is no need to extensively review the literature. Instead, this article will focus on examining critical issues remaining in developing CO-based therapeutics. Briefly, CO is produced in all cells by one of two known heme oxygenases (Hmox1, HO-1 and Hmox2, HO-2),9, 10 and each possesses strong cytoprotective functions to the cell evidenced by the fact that absence of either, particularly the stress response isoform HO-1, is incredibly detrimental to the cell and organism.7, 11-13 On a daily basis, an average person produces about 500 μmol of CO largely related to erythrocyte turnover in the spleen, leading to about 2% of the hemoglobin being CO-bound,14, 15 which is being used as a surrogate indicator of CO exposure levels. However, elevations in exhaled CO measured in samples of exhaled breath and indicative of elevated HO-1 have been observed in patients with a variety of illnesses including asthma, diabetes and shock. Cellular and animal pharmacological experiments suggest numerous therapeutic indications where induction of HO-1 or administration of CO imparts benefits in treating conditions such as sepsis, bacterial infection, cancer, inflammation, and circadian clock regulation, stroke, erectile dysfunction, and heart attack.1, 3-7, 13, 16-17 A very prominent example of CO's therapeutic effect is its ability to protect the cardiomyocyte from cell death, and help maintain overall cardiovascular health.18-21 Such protection is effective in alleviating the cardiotoxicity of chemotherapeutic agents such as doxorubicin.19, 20, 22 It is also interesting to note that CO sensitizes cancer cells, but not normal cells, to the genotoxin doxorubicin by 1000-fold in part through an anti-Warburg effect,23 leading to metabolic exhaustion. Furthermore, in contrast to cancer cells, CO spares normal cells in the cancer-laden tissue.20, 23-25 Collectively these findings support CO being used in cancer chemotherapy with a two-fold advantage. First, CO will allow using a lower dose of chemotherapy and thus chemosparing, which will reduce cardiotoxicity. Second, CO offers protection of normal cells in the surrounding areas. Given the large amount of information available on CO pharmacology, the time has come for intense medicinal chemistry effort, which has been lagging way behind the biological assessment work.
Contrary to popular belief, inhaled CO is quite safe to use, possessing safety margins wider than many clinically used drugs and even some nutrients. For example, the normal glucose level is 5.8 mM and yet 14 mM of glucose can be life threatening.26 With insulin, there is no consistent level that is considered “normal” because of the daily fluctuation. However, it is commonly believed that staying below 10 μIU/mL is healthy,27 and yet 50 μIU/mL would completely suppress hepatic glucose output, which would be life-threatening without therapeutic intervention by administering glucose to the patient.28 With many metal ions, the safety window is even narrower. For example, potassium significantly increases incidents of sudden cardiac death at a concentration slightly above the normal range (5.5 mEq).29 Considering the reversible nature of CO binding to heme, employing CO for therapeutic applications does not present unusual safety challenges when compared to the development of other small molecule drugs.
Physiologically speaking, the amount of CO-bound hemoglobin (COHb) in healthy humans averages < 1% (range 1-6%) while smokers can have up to 14% COHb,30 which is generally considered tolerable.31 It is commonly believed that 10% COHb would have therapeutic effect for inhaled CO and the FDA has set 12-14% as the upper limit for the clinical trials. 6, 32 In some instances CO-releasing molecules (CO-RMs), e.g. CO-RM-3 and CO-RM-A1, pharmacologic effects were observed without altering the serum COHb levels.33-35 The lethal dose of CO is reported to be over 50% COHb,36, 37 but some animal studies suggest that 50% COHb is far from lethal.38 As a result, the safety margin is well over 10-fold in many applications. At this point, it is important to make the distinction between 50% COHb in the blood and an inhalation process that would produce 50% COHb. Simply having 50% COHb in erythrocytes may only have a significant effect on the oxygen-carrying ability of the blood. This by itself may not be a major issue as indicated by the fact that those with anemia possessing hemoglobin levels that are 50% of the normal level can still perform relatively normal daily activities without life-threatening consequences. However, an inhalation process that leads to 50% COHb may have many other effects such as inhibition of cytochrome oxidase (Complex IV in the electron transport chain of the oxidative phosphorylation process) and other enzymes including p450. This distinction also brings out the significance of controlled delivery of CO, especially with the ability to target certain tissue sites. This can be accomplished using systems that allow for tethering targeting molecules to CO-prodrugs, and will be discussed in detail in various sections below.
With the demonstrated therapeutic effect and safety profiles of CO, the development of CO-based therapeutics has only the hurdle of developing pharmaceutically acceptable forms of CO-based therapeutics that can be used for different indications. The focus of this review examinees various available forms of delivery in the context of suitability and limitations in clinical applications.
2. Existing CO-delivery methods
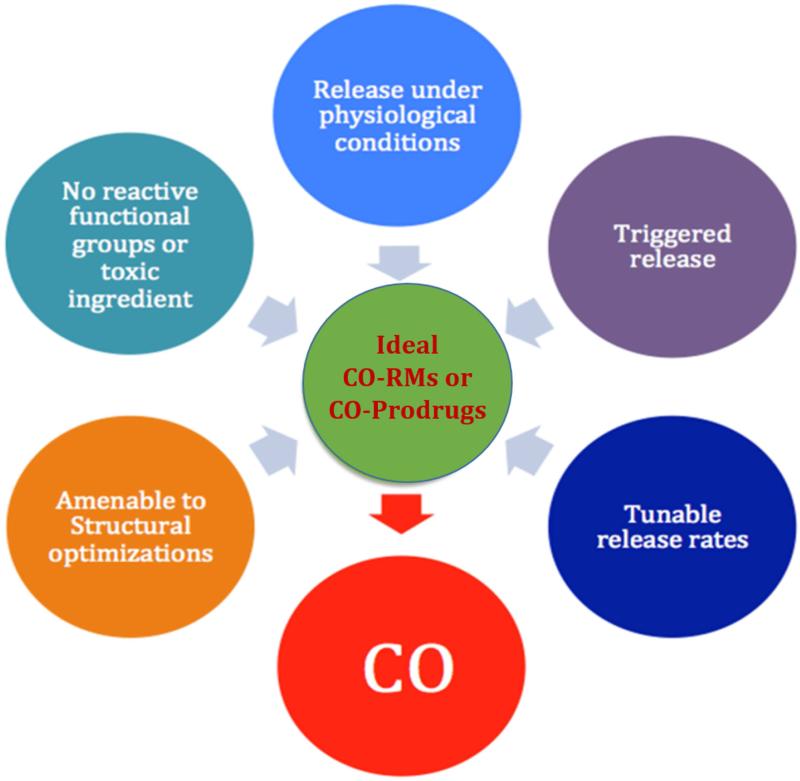
When one considers the therapeutic applications of CO, the form of delivery is a primary concern. Because CO is a gas, inhalation is the natural consideration for administration. Gas inhalation, however, has many issues including the limitation that it can only be used in primarily hospital settings. Consequently, it would be desirable to develop formulations that allow for delivery via oral, intravenous, intraperitoneal, sub-cutaneous, or other routes. This would require the development of “caged” CO for release at the appropriate time and rate. There have been recent efforts in developing metal-based CO-RMs (or CO-prodrugs),17, 39-42 encapsulated CO-RMs,43-46 photo-sensitive organic CO-RMs,47-49 and organic CO-prodrugs that spontaneously release CO under physiological conditions.50 In addition to normal delivery route challenges, there are some special factors related to CO-based therapeutics, all of which would affect the development of pharmaceutically acceptable forms of a CO-prodrug. First, as is true with all gasotransmitters, the dosage question is not only a concentration issue, but also involves release and therefore exposure rates. The same dosage at different release rates may mean very different effective concentrations. At this time, there have not been studies that examine the effect of sustained release of CO versus a single bolus administration. Much of this is due to the difficulty in tuning the release rates of currently available CO-RMs. Therefore, there is a need to develop CO-RMs with release rates that can be controlled over specific kinetics to allow systematic pharmacodynamic and tissue distribution studies to be executed. Second, much of earlier studies using inhaled CO were based on daily administrations of CO over a 1-2 h exposure period.51-54 Such studies suggest that continuous administration is not necessary. There could be two or more reasons that contribute to this. On one hand, inhaled CO can be “stored” in the form of COHb, which serves as a “reservoir” for sustained release. On the other hand, it is entirely possible that CO triggers a cascade reaction/signaling pathway. Once the cascade has been initiated, there would be no need for a sustained level of CO to achieve the effect. Based on the known mechanisms of action, both scenarios are possible and could influence CO-RM design. The de-convolution of all these factors will be much more feasible by having CO-RMs with tunable release rates. Third, CO has various therapeutic indications. For each individual indication, one can envision the need for different CO release rates and different pharmaceutical properties such as permeability and solubility, which would require the ability to tune the structures of CO-RMs for optimal physicochemical properties and ultimately efficacy. Fourth, currently available CO-delivery methods lack targetability. CO as a gas molecule has high diffusivity and high affinity to hemoproteins. It is expected to potentially have multiple targets depending on the binding affinity and heme targets present, which can change as the cell expresses different proteins. For minimized side effect, it would be desirable to be able to target CO to a specific location or type of tissue. This would require conjugation chemistry to tether targeting molecules for various applications. For all these reasons, we have developed criteria listed in Figure 1 to guide our CO-prodrug design efforts. In the next section, we will examine the various classes of CO-RMs in detail in the context of desirable pharmaceutical and medicinal properties.
Figure 1.
Desirable features for effective CO-RMs or CO-prodrugs
i. Inhalation
The easiest form of delivery of CO is by inhalation as a gas. This is also the form widely used in successful animal studies for the past decade. For example, Otterbein et al. demonstrated that CO in the concentration range of 100-500 ppm has protective effect against lethal hyperoxia conditions.55 In this study, rats were exposed to 98% O2 or 98% O2 with different concentrations of CO for 72 h. CO-treatment group showed a remarkable increase in survival rate. Rats that were exposed to 100 ppm CO had a survival rate of 50%, and 250 or 500 ppm CO groups had a survival rate of 100%. However, those without CO treatment had a survival rate of 0%. In a rat inflammation model,56 exposure to 250 ppm CO for 1 h before treatment with a lethal dosage of lipopolysaccharides (LPS) led to a remarkably improved survival rate (80%) compared with controls without CO treatment (14%). In a recent report, CO was able to enhance bacterial killing by augmenting the host's innate immune response.57 The ability for CO to prevent liver injury caused by LPS induced inflammation was verified by the observation of significant down-regulation of a liver injury marker, serum alanine aminotransferase. CO is also known to promote liver regeneration and protection against fulminant hepatitis.58, 59 Another exciting function of CO is its ability to stimulate mitochondrial biogenesis. In a mouse model, Piantadosi et al.20 showed that CO can prevent doxorubicin induced myocardial damage by stimulating mitochondrial biogenesis. One group of mice was given doxorubicin (15 mg/kg) alone, and the other was exposed to 1 h of CO treatment (500 ppm) 24 h prior to doxorubicin treatment and 1 h of CO treatment (500 ppm) 7 days after doxorubicin administration. The group that was treated with CO showed a significant increase of mtDNA copies, mitochondrial transcription factor A level, and DNA polymerase-γ expression. Such results suggest that CO was effective in countering doxorubicin's inhibitory effect on mitochondrial activities.
With all the success with inhaled CO in preclinical animal studies, five clinical trials have been initiated to examine safety and efficacy in human.54, 60-63 Brigham and Women's Hospital initiated clinical trials to test the efficiency of low concentrations of CO in treating idiopathic pulmonary fibrosis in July 2011 and the studies are ongoing at this time. Clinical trials aimed at examining the antihypertensive effective in the pulmonary artery started at the University of Illinois at Chicago in July 2012 and are ongoing. Aimed at evaluating the ability for CO to decrease lung inflammation, the National Institutes of Health Clinical Center initiated a clinical trial in October 2004, and this study has been completed though no result has been posted yet. In August 2007, INO Therapeutics initiated clinical trials to test the safety and tolerability of CO in kidney transplant patients. Meanwhile, Weill Medical College of Cornell University initiated clinical trials to exam the safety of inhaled CO in treating acute respiratory distress syndrome in April 2015. This study is still recruiting participants. Table 1 summarizes some key clinical trials involving inhaled CO.
Table 1.
| Sponsor | Start Date | Subject | Phase | Status |
|---|---|---|---|---|
| Brigham and Women's Hospital | July 2011 | Effect of inhaled carbon monoxide in treating idiopathic pulmonary fibrosis | 2 | Ongoing |
| University of Illinois at Chicago | July 2012 | Carbon monoxide for the treatment of severe pulmonary arterial hypertension | 1 2 |
Ongoing |
| NIH Clinical Center | October 2004 | Prevention effect of carbon monoxide in lung inflammation | 1 | Completed |
| INO Therapeutics | August 2007 | Safety and tolerability study of inhaled carbon monoxide in kidney transplant patients | 2 | Withdrawn |
| Weill Medical College of Cornell University | April 2015 | Safety study of inhaled carbon monoxide to treat acute respiratory distress syndrome | 1 | Recruiting |
The above examples clearly demonstrate the growing interests in CO as a therapeutic agent. Even with all the success and promises of inhaled CO in animal studies, direct translation into human is difficult. In human applications, there is much less control of the specific environment, compliance, and dosage of administration. Therefore inhaled CO, even with clear efficacy in treating numerous conditions, is likely only suitable for administration in hospitals, clinics, and other carefully controlled settings. In addition, administration of inhaled CO has other issues to consider, particularly in spontaneously breathing individuals. First, the appropriate dose and therefore the effectiveness of inhaled CO will be highly dependent on respiratory function of the patient. Different respiratory rates, depth of breath and masks or nasal cannulas will all impact the dose delivered. Because lung capacity and physical conditions of patients can vary significantly, the difference in both efficacy and toxicity among individuals with various conditions is something that has to be considered in administering inhaled CO. Second, patient may have very different hematological conditions depending on other illness. A substantial change of hemoglobin level could make dramatic differences in terms of effective doses of CO delivered within a fixed period of time. Third, inhaled CO will impact the lung first regardless of what the desired site of action is. Therefore treating lung disease versus kidney or liver failure will likely be very different in terms of the amount required to inhale to observe any biologic or medical effects. Fourth, the inhalation form lacks targetability, and the availability of CO at the site of action is entirely dependent on tissue perfusion and diffusion of the gas from the blood into the tissue. This is imprecise and subject to perturbations by many factors. For all these reasons, there is a strong need for “CO in a pill” or “CO in an ampule” much the same way that nitroglycerin was designed to be a “caged” form of NO. The following sections discuss these aspects.
ii. Metal-based CO-RMs
In developing solid or liquid dosage of CO, metal complexes can be considered the pioneering work in this field first described by Motterlini and Foresti (Table 2). The first example of metal-based CO-RMs, which can release carbon monoxide (CO) in aqueous solution, was reported in 2002.17 They reported dimanganese decacarbonyl64 (CO-RM-1) and tricarbonyldichlororuthenium (II) dimer (CO-RM-2), which release CO in a concentration dependent manner. CO release from CO-RM-1 is induced by light whereas in the case of CO-RM-2, CO is released in DMSO solution.
Table 2.
CO-RMs structures and CO release properties
| Compounds | Chemical structures | Solubility | Half-life (min) |
|---|---|---|---|
| CO-RM-1 [Mn2(CO)10]17, 64 |
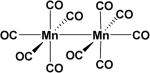
|
DMSO Ethanol | t½ < 1 min |
| CO-RM-2 [Ru(CO)3Cl2]217 |
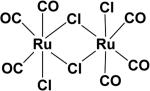
|
DMSO Ethanol | t½ ≈ 1 min |
| CO-RM-3 [Ru(CO)3Cl-glycinate]39 |
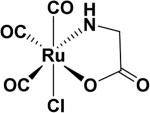
|
H2O | t½ ≈ 1 min (37 °C, pH = 7.4) |
| CO-RM-A140 |
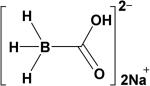
|
H2O | t½ ≈ 21 min (37 °C, pH = 7.4) |
| CO-RM-401 Mn(CO)4(S2CR)]42 |
|
H2O | t½ < 4 min |
| Mn (CO)3RR141 |
|
Ethanol | t½ = 5000 min for R = 2, 2’-bipy t½ = 2600 min for R = 2, 2’-bipy, R1 = Br |
In 2003, the same group reported on a water soluble CO-RM, tricarbonylchloro(glycinato) ruthenium (II) (CO-RM-3),39 which liberates CO under physiological conditions and showed protection of myocardial cells and tissues against ischemia-reperfusion injury.
Motterlini and co-workers also created a water soluble sodium boranocarbonate Na2[H3BCO2] (CO-RM-A1),40 which releases CO in aqueous solutions. Unlike the original CO-RMs, CO-RM-A1 does not contain a transition metal and releases CO with a slower rate compared to CO-RM-3 with a half-life of ~21 min at 37 °C.
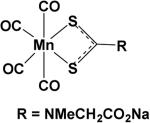
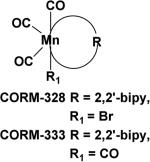
CO-RMs based on a new Mn complex structure [Mn(CO)3RR1]41 (R = 2, 2’-bipyridine (bipy), R1 = CO, Br) are known to generate CO upon irradiation in the UV region. Aimed at addressing the issue of water solubility, a class of manganese based CO-RMs with water soluble groups such as –CH2COOH in [Mn(CO)4(S2CNMeCH2CO2H)] (CO-RM-401) was introduced.42 These compounds (CO-RM-401) have improved water solubility. A 25% decrease in cell viability was observed after 24 h treatment with 100 μM of CO-RM-401 in RAW264.7 macrophages, indicating mild cytotoxicity at a relatively high concentration. CO-RM-401 successfully inhibited nitrite production, which is an indicator of inflammation, in response to treatment with 1μg.ml−1 of LPS.
The metal-based CO-RMs described above have played very important roles so far in helping the understanding of CO's role in physiological, pathological, and pharmacological processes. Unfortunately, their further development as clinically useful therapeutics has faced many hurdles. The initial issue of solubility has been overcome by newer CO-RMs. However, it has been hard to overcome the stigma of metal toxicity, which is probably warranted for some applications, while others may be perception issues. At this time, it needs to be noted that metal complexes have been developed as therapeutics.65 For example, cisplatin is a platinum complex widely used in cancer chemotherapy.66 There are other similar metal complexes that are in use or being developed. Even arsenic trioxide, a compound considered very toxic and often used historically as a poison, has been proven to be quite effective in treating selected forms of leukemia and is approved for clinical use.67 All such examples demonstrate one point, i.e., just because a compound contains a transition metal does not and should not automatically disqualify the compound from clinical development. However, there is no doubt that the use of transition metals adds a layer of complexity in pharmaceutical development compared with traditional small molecule and protein/peptide-based drugs. To date, the use of transition metal complexes has been largely limited to short-term use or otherwise life-threatening situations such as cancer. In the body, metal concentrations are tightly regulated. Perturbation of metal concentrations can lead to serious or even life-threatening situations. With trace elements such as cobalt, selenium, copper, and molybdenum, the allowable concentration range is even smaller. As a result, the long-term health effect of most transition metals is of concern, and has not been adequately studied. This issue poses a hurdle for the development of metal-based CO-RMs for application with non-life-threatening or chronic conditions. With CO-RM-A1, which contains a boron atom, the situation is similar in one way that boron is considered an ultra-trace element with no known physiological functions in mammals. In addition, the release of CO from CO-RM-A1 is accompanied by the formation of one net hydroxide. Therefore, the effect of CO-RM-A1 on the pH in certain locations cannot be overlooked. Once CO has been discharged, all metal-based CO-RMs form new compounds termed iCO-RMs and it is nearly impossible to create the inactive iCO-RM for testing as an appropriate control for the original compound. For all these reasons, the further development of metal-based CO-RMs faces many hurdles, which are not routinely encountered by the traditional pharmaceutical industry. This by itself will present a serious developability issue.68, 69 Furthermore, because of the extraordinarily high cost of pharmaceutical development, it is unfortunate that sometimes perceived issues and uncertainties can be a major hurdle as well. As a result, there is a strong desire to develop other CO-RMs where metal-related issues, whether real or perceived, can be controlled and/or tested for many future clinical applications. This would include the development of metal-free organic CO-prodrugs.
iii. Encapsulated metal-based CO-RMs
Given the issues described above with transition metal-containing CO-RMs, some clever approaches have been developed to prevent metal leakage through encapsulation. Basically, the system contains two components: macromolecular carriers and metal-based CO-RMs. The CO-RMs are bound to the macromolecular carriers by either covalent bond or non-covalent adhesion. The macromolecules could be polymeric micelles, nanoparticles, copolymer, proteins, etc. Recently, there have been many papers published in this field,8, 32, 44, 48, 70-78 and this section only has a few selected examples (Table 3) for discussion of various issues. This selection is not based on “importance” of these papers, but rather whether there are appropriate issues to discuss. Readers are referred to the literature for a comprehensive review of this area.73
Table 3.
Encapsulated CO-RMs and CO release properties
| Refs | Macromolecular carriers | CO-RMs | Trigger release | Release rate |
|---|---|---|---|---|
| Hubbell43 | Micelles | CO-RM-3 | Thiol containing compounds | Thiol concentration dependent. About 10% CO release after 60 min at 10 mM of cysteine. |
| Schatzschneider44 | SiO2 nanoparticles | Photo-CO-RM | Light | Similar to the parent CO-RMs. |
| Schiller45 | Nanoporous fibrous non-wovens | CO-RM-1 | Light | Wavelength dependent |
| Bernardes46 | Proteins (BSA) | CO-RM-3 | Proteins | In 1mg/ml BSA with 50 eq. CO-RM-3, CO release finished in 4 h. |
In 2010, Hubbell et al.43 reported CO-releasing micelles, which showed slowed diffusion in tissues and improved ability to target distal tissue drainage sites. Basically, the micelles were formed by triblock copolymers: a hydrophilic poly(ethylene glycol) block, a poly(ornithine acrylamide) block bearing [Ru(CO)3Cl-(ornithinate)]moieties and a hydrophobic poly(n-butylacrylamide) block. The copolymeric micelles were quite stable under physiological conditions and in serum, but released CO when treated with thiol-containing compounds such as cysteine and glutathione. To study the anti-inflammatory effects of the micelles, THP-1 Blue cells, which are derived from human monocyte THP-1 cells, were used. In these cells, activation of NF-κB, a transcription factor that induces a pro-inflammatory response, leads to the expression of a secreted embryonic alkaline phosphatase (SEAP); thus SEAP levels correlate with NF-κB activation. In particular, upon treating THP-1 Blue cells with 200 μM of Ru(CO)3Cl(ornithinate) encapsulated in micelles, a substantial suppression of SEAP production induced by LPS was observed. On the other hand, treating with CO-RM-3 alone did not show the same beneficial effects, which might be due to nonspecific effects caused by the Ru compound. It was shown that CO release rates from these micelles were slower than from CO-RM-3. It was also found that the stealth feature of poly(ethylene glycol) could significantly reduce the toxicity of the parent CO drug. All of these properties showed that CO-releasing micelles might be a potential delivery system in the therapeutic applications of CO. However, this method may only be applicable in selected local delivery applications, such as in the gastrointestinal system, which allows the materials to be excreted without entering the systemic circulation.
Schatzschneider et al.44 developed a method of using silicon dioxide nanoparticles containing azido groups at the surface as a macromolecular carrier. By applying copper-catalyzed azide-alkyne 1, 3-dipolar cycloaddition (CuAAC “click” reaction), they successfully loaded a photoactivatable CO-releasing molecule (Photo-CO-RM) based on [Mn(CO)3(tpm)]+(tpm= tris(pyrazolyl)methane) containing an alkyne-functionalized TPM ligand on the carrier. These Photo-CO-RM based nanoparticles displayed similar photoinducible CO-release properties as the parent Photo-CO-RM. Silicon dioxide nanoparticles are seeing increased usage in drug delivery in recent years and this kind of functionalized nanoparticle carriers may provide a useful platform as delivery agents for CO-RMs in solid tumors.
In 2014, Schiller et al.45 embedded water-insoluble, photoactive CO-RM-1 into nanoporous fibrous non-wovens of polylactic acid. Effective CO release into the surrounding medium is initiated by light stimulation of the high surface area materials. The metal complexes were non-covalently embedded into the polymer matrices via electrospinning. It was found that CO release rate was wavelength dependent as measured by CO binding to myoglobin. Irradiation at 365 nm resulted in faster release than at 480 nm by four fold, and one milligram of the non-woven materials could release 3.4 μmol of CO. This CO delivery platform was also tested by light-induced eradication of mouse fibroblast 3T3 cells grown on the non-wovens, and the hybrid material showed no toxicity in dark and became strongly cytotoxic when light was applied. These nanoporous fibrous non-wovens also provide a possible CO delivery platform.
In 2015, Bernardes et al.46 demonstrated that RuII(CO)2–protein complexes, generated by the reaction of decomposition products of CO-RM-3 with the His residues exposed on proteins in aqueous solution, spontaneously release CO in aqueous solution. Based on such findings, a bovine serum albumin (BSA)–RuII(CO)2 complex was synthesized. Treating cancer cells with this complex led to inhibition of inflammatory responses. To further show successful CO delivery in vivo, administration of (BSA)–RuII(CO)2 in mice bearing colon carcinoma tumors resulted in enhanced CO accumulation at the tumor site. This study suggested the use of RuII(CO)2–protein complexes as an alternative for the efficient and tissue specific delivery of therapeutic CO in vivo.
There are several advantages for using the large and complex “encapsulation” forms of CO-RMs. First, until now, most well studied CO-RMs are metal containing compounds, with various concerns described earlier. The outside “capsule” could dramatically reduce the toxicity issue by trapping the metal fragment in the insoluble matrices. Secondly, the “capsule” could also contribute to site-specific drug uptake by using macromolecular carriers with different targeting molecules tethered to the surface. Finally, “encapsulated CO-RMs” could also increase the bioavailability of the parent CO-RMs. One potential issue with these encapsulation methods is the eventual fate of both the encapsulating materials and the metal, if the application is systemic. One specific site of application for such encapsulated materials without this concern is the gastrointestinal system, where direct excretion is possible without entering into the general systemic circulation. This would address the issue of the eventual fate of the metal ions and insoluble matrix materials.
iii. Enzyme-trigger CO-RMs
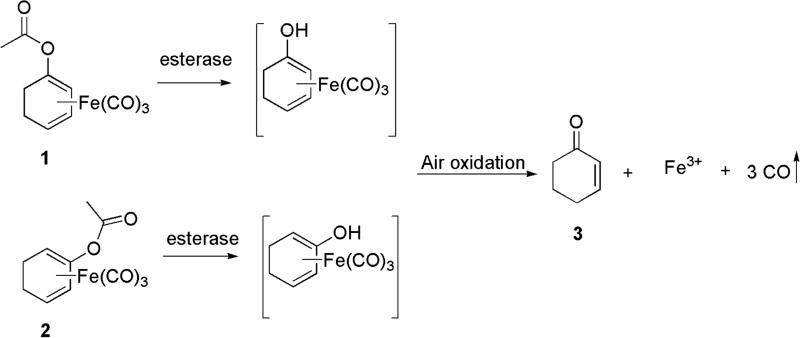
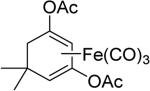
One issue in the development of an effective CO-RM is the controlled release of CO. Current CO-RMs generally rely on reactions with water or pH changes or the presence of oxygen for CO release, which lacks precise control. Controlled CO release could enhance tissue targeting and minimize toxicity, and could greatly improve the therapeutic potential of CO-RMs. Using an enzyme to trigger CO release from CO-RMs is emerging as a promising strategy to address this issue. In 2011, Schmalz et al.79 developed acyloxybutadiene iron tricarbonyl complexes. This design takes advantage of the tight complexation of a diene with iron, which can bind and carry CO. Enzymatic cleavage of the ester group would lead to enol-ketone tautomerization and the conversion of the diene to an α, β-unsaturated ketone, which would lose affinity for Fe(II) and result in its oxidation to Fe(III) and the subsequent release of CO (Figure 2).80 These enzyme-triggered CO-RMs (ET-CO-RMs) (1 and 2) are stable under physiological conditions. The biological effects of these ET-CO-RMs are related to both the organic components and the rate of CO release.81 Table 4 lists several such examples.
Figure 2.
The general concept of ET-CO-RMs
Table 4.
ET-CO-RMs structures and CO release properties
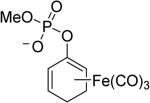
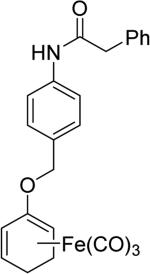
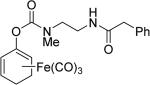
In one study, ET-CO-RMs successfully inhibited NO production in LPS-stimulated RAW267.4 cells.79 Specifically, treatment of cells with compound 4 at 15 μM resulted in up to 68% suppression of LPS-induced NO formation relative to control cells, which were only treated with LPS. To improve the water solubility of ET-CO-RMs, cyclohexadienyl methyl phosphate iron tricarbonyl complex-based compound 5 was synthesized.82 Besides improved water solubility, it showed less toxicity than other esterase sensitive ET-CO-RMs, and also a moderate level of anti-inflammatory effect. The IC20 of compound 5 in RAW 246.7 cells was determined to be 252 μM, and 100 μM of 5 resulted in 31% inhibition of NO production in RAW264.7 cells. In 2015, to further address the site-specific targeting issue, protease-activated ET-CO-RMs (6 and 7) were synthesized.83 This type of ET-CO-RMs comprise of a protease-specific peptide, which is used as a targeting moiety, a self-immolative linker, which was used to tune the rate of CO release, and an oxycyclohexadiene–Fe(CO)3 moiety, which would release CO after dissociation of the iron complex from the diene moiety. By using different self-immolative linkers, various release rates were achieved. This enzyme-activated strategy represents a new and interesting direction for the development of tissue-specific CO-RMs.
Enzyme trigger is an excellent option in controllable drug delivery, and has been successfully used in other areas.84 The designs described above are very innovative, reflecting a beautiful combinatorial use of chelation chemistry, enzymatic reactions, and organic chemistry. However, at this stage all the designs and studies only focused on metal-based CO-RMs. Because iron is released from these ET-CO-RMs, the issue of iron accumulation must be considered when such prodrugs are used on a daily basis for an extended period of time.
v. Photo-sensitive organic CO-RMs
Although a wide range of metal-based CO-RMs have been developed, and showed promising CO-associated pharmacological effects both in vitro and in vivo, the metal containing inactive byproducts after CO release are of safety concerns as discussed earlier. Ideally, one would want to have small organic molecules capable of releasing CO under physiological conditions. There are well-defined paths and criteria for addressing general developability issues in developing small molecule-based pharmaceuticals.68, 79, 84, 85 One approach is to focus on small molecule CO-prodrugs. The chemistry of CO-release from a small organic molecule is not without precedents. However, most examples rely on either light or high temperature to achieve CO release. This section focuses on photo-sensitive organic CO-prodrugs, which have the advantage of triggered release with spatio-temporal control, and minimized off-site effects. However, photo-sensitive CO-prodrugs also have the limitations of only delivering the prodrug and consequently CO to a site accessible by light either by direct irradiation or an optical fiber. The discussions below focus on the examples of available photo-activatable systems (Table 5).
Table 5.
Summary of the properties of photo-sensitive CO-RMs
| CO-RMs | Wavelength (nm) | Release Rate |
|---|---|---|
|
500 | t½ = 270 min |
|
470 | Complete in 10 min. |
|
419 or >546 | Complete in 10 min. |
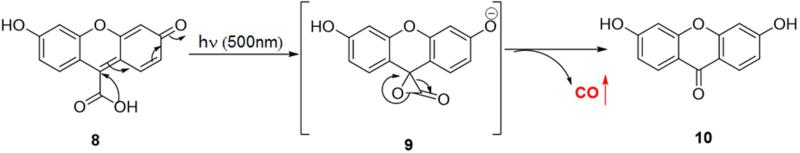
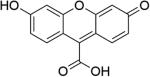
The chemistry of UV or near UV light induced decarbonylation has been well studied.86-89 Fluorescein analogue 8 (Figure 3) was the first reported transition metal free and water soluble photo-sensitive CO-RM.47 After irradiation with visible light (λ = 503 ± 15 nm) in phosphate buffer saline (PBS) buffer (pH = 7.4), compound 8 could release CO with a half-life of around 4.5 h. This release rate is much slower compared to most metal based CO-RMs, which tend to have half-lives in the range of minutes. Release of CO was demonstrated by elucidating the chemical structure of the by-product 10 and using a standard hemoglobin binding assay. The photo-release mechanism was believed to involve an α-lactone intermediate 9, which is known to undergo ready decarbonylation.90 There was no CO-associated biology data reported for compound 8.
Figure 3.
The photo-release mechanism for fluorescein analogue 8
As one can see from the structure of compound 8, the hydroxyl group could serve as a handle for tethering different targeting moieties for precise spatio-control of CO release. However, compound 8 still has much to be desired for application in biology and medicine due to several limitations, including the difficulty in tuning the CO release rate. Furthermore, the short wavelength needed for CO release and the low penetrating power of light at this wavelength mean that this may only be applicable to topical application. However for research applications, such photo-control may provide advantages because of the precision with which one can exert spatio-temporal control of the CO release process.
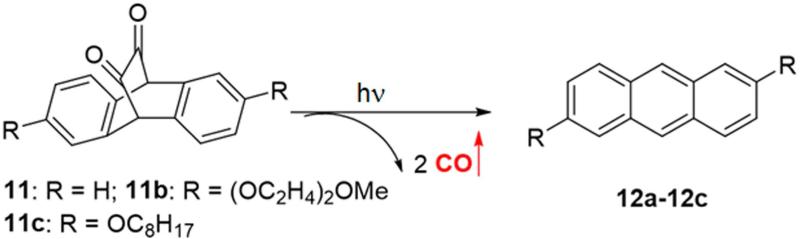
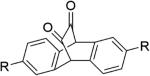
Another example is the photoreaction of cyclic unsaturated diketones, which release two molecules of CO after irradiation.91-94 In a clever use of diketone photochemistry, Liao et al. designed and synthesized three unsaturated cyclic α-diketones (11a-11c, Figure 4), and studied their CO release properties upon visible light irradiation.48 The results showed that all three compounds (11a-11c) could release CO in organic solvents upon irradiation at 470 nm, and CO release was finished in 10 min. However, when irradiating the aqueous solution of compounds 11a-11c, no CO release was observed. This was attributed to the tendency for the diketone groups to exist in the hydrated form, which would not be photo-active, at least not in the same way. To overcome this hydration problem, compounds 11a-11c were encapsulated in Pluronic F127 micelles, which have a hydrophobic inner environment, and protect the carbonyl from hydration. As expected, the encapsulated compounds 11a-11c could release CO in aqueous solution upon irradiation at 470 nm, and the CO release yield from encapsulated 11a, 11b, and 11c was 78%, 71% and 90% respectively. One unique feature of this type of CO-RMs is the formation of a fluorescent byproduct, which greatly facilitates “real time” monitoring of CO release. After incubation of encapsulated 11c with acute myeloid leukemia (AML) KG-1 cells for 24 h, irradiation at 470 nm for six 30-second pulses afforded a bright blue fluorescence in the cells. These results confirmed that 11c was taken up by the cells and photo-induced CO release happened intracellularly. Additionally, cell proliferation and viability assays showed no cytotoxicity for the byproduct or photo-damage under experimental conditions.
Figure 4.
Photoreaction of the diketone compounds
It is clear that unsaturated diketones 11a-11c could serve as excellent research tools for the investigation of CO biology in vitro. However, in vivo applications will unfortunately have the same limitations as other photo-sensitive CO-RMs. Similar to fluorescein analogue 8, tuning the CO release rate will be hard. In addition, encapsulation of diketones 11a-11c into micelles may decrease light penetration efficiency.
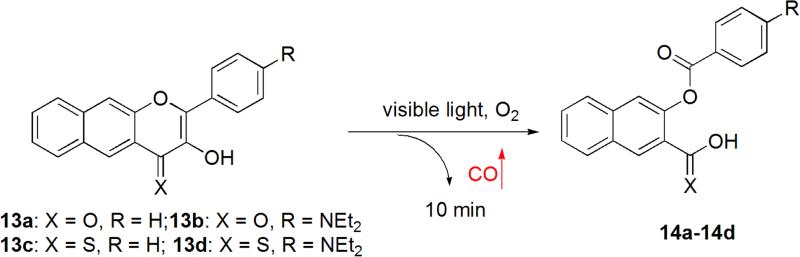
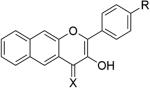
Very recently, Berreau et al. designed and synthesized 3-hydroxyflavone-based CO-RMs, which can be activated by visible light in the presence of oxygen. This is a very innovative use of flavone chemistry. By modifying the substituent on the 3-hydroxyflavone scaffold (Figure 5), the activation wavelength can be tuned from 419 to 546 nm.49 For example, exposure of 13a to light (419 nm) in an aerobic organic or aqueous solution resulted in quantitative CO release (0.96 equiv) in 10 min. Further structural modifications afforded three new analogues 13b, 13c and 13d. The activation wavelength for compounds 13b and 13c remained the same as that of 13a. However, the activation wavelength for compound 13d red-shifted to >546 nm, which is very close to the photodynamic therapy window (>600 nm). Therefore, with this class of CO-prodrugs, it is possible to tune the activation wavelength to a range that is acceptable in human therapy. This can be achieved by using different substituents on the 3-hydroxyflavone scaffold. Another advantage for this type of the prodrugs is its fluorescent change after CO release, allowing for tracking of 13a-13d in cells prior to CO release and monitoring of CO release in real time based on fluorescence emission changes. However, issues still remain for this type of CO-prodrugs. For example, CO release is dependent on the presence of oxygen for 13a, 13b and 13c. Although 13d can release CO in the absence of oxygen, the CO quantity released decreased by 70% compared to the one released in the presence of oxygen. Such properties could limit the applicability of these CO-RMs under hypoxia conditions (e.g. cancer). Compounds with the 3-hydroxyflavone scaffold are known to possess various bioactivities including anti-oxidation, anti-inflammation, and anti-cancer activity.95-98 This aspect will need to be considered in optimizing such structures.
Figure 5.
The photoreaction of 3-hydroxyflavone analogues
In summary, photo-sensitive organic CO-RMs have great potential in applications with precise spatio-temporal control. One possible direction in the field is the development of CO-RMs activated by infrared or near infrared light, which possess improved tissue penetration compared to visible light. It will also be desirable to see more effort in tuning CO release rates, which will be important for future applications.
vi. Organic CO-prodrugs capable of releasing CO under physiological conditions
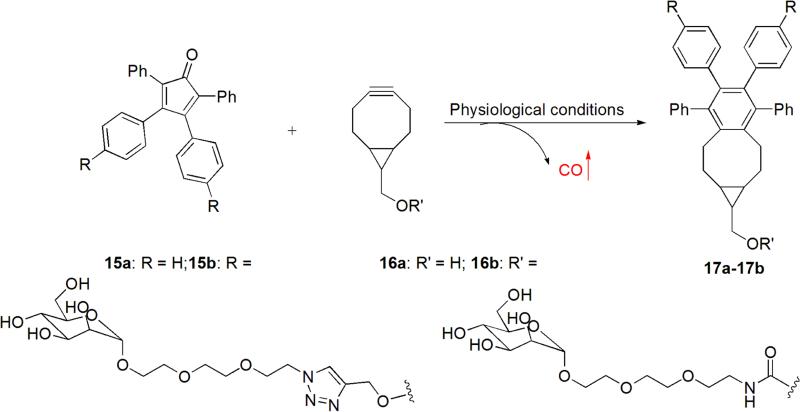
In order to side-step metal-related issues and achieve targeted delivery, novel CO-RMs with the following characteristics are highly desirable: metal free, tunable CO release rate, the ability to release CO with an endogenous (e.g. enzyme) or exogenous (e.g. chemical) trigger under physiological conditions, and the option to allow conjugation of targeting molecules or other functional groups for physicochemical property tuning. Wang and colleagues recently reported a prototype of an organic reaction that leads to CO release under physiological conditions, which allows for the “caging” of CO in the form of a ketone and then its “click and release.”50
The design originated from an inverse-electron-demand Diels Alder reaction (DAinv) between tetraphenylcyclopentadienone (TPCPD) and an alkyne, which results in the formation of a strained intermediate with the ability to undergo a chelatropic reaction to release CO. In terms of the chemistry concept for these reactions, this is not new. However, most literature reactions between TPCPD and an ordinary alkyne require harsh conditions (e.g. reflux in toluene), and cannot be used as CO-RMs under physiological conditions. Since the reaction rate of DAinv is a matter of the energy gap between the LUMO of the diene and HOMO of the dienophile in a DAinv, it was reasoned that a strained alkyne (e.g. bicyclo-[6.1.0]nonyne (BCN)), which is known to possess a high HOMO energy level due to its highly strained nature,99 should be able to “click” with TPCPD and release CO under physiological conditions. Specifically, the reaction between TPCPD-1 (15a) and strained alkyne 16a was examined (Figure 6). It was found that the reaction went smoothly in methanol at room temperature with a second order rate constant of 0.61 M−1s−1. CO generation was further confirmed by the CO-myoglobin assay. In order to improve the water solubility and attenuate the potential toxicity of the reactants, mannose was conjugated to the core structures of 15a and 16a by a hydrophilic triethyleneglycol linker, yielding compounds 15b and 16b. In cell viability studies, 15b and 16b did not show any cytotoxicity to RAW 264.7 cells at 1 mM after 24 h of incubation. Compounds 15b and 16b were evaluated for their anti-inflammatory effect in RAW 264.7 cells. The results showed that co-treatment with 15b and 16b attenuated LPS-induced TNF-α secretion, and neither 15b nor 16b alone showed any anti-inflammatory effect. Taken together, the observed anti-inflammatory effect was associated with the CO generation by a “click and release” process between 15b and 16b.
Figure 6.
The click reaction between BCNs and TPCPDs
The “click and release” strategy demonstrated both CO formation and CO-associated biological effects under physiological conditions. Since the CO generation rate is related to the reaction rate between TPCPD and a strained alkyne, it is easy to tune the CO release rate by modifying the electron density of TPCPD or using different strained alkynes with various HOMO energy levels (e.g. cyclooctyne, fluorocyclooctyne, etc).99, 100 Additionally, both components are easy to be functionalized for different purposes including targeting and improving pharmacokinetics profiles among others. However, the “prototype” reaction has its own issues where improvements are needed. For example, this is a bimolecular process, and cannot be readily used as a CO-prodrug, except in selected situations, for two reasons. First, prodrug concentration and reaction rates cannot be adjusted independent of each other because bimolecular reaction rates are concentration-dependent. Second, to “synchronize” the pharmacokinetic properties of two reaction components will be very challenging in a drug delivery system. In order to overcome these limitations, unimolecular systems, which combine the alkyne and the cyclopentadienone moieties into one molecule, is much more desirable. Such an approach can be used for the preparation of CO prodrugs that are stable in vitro, and can undergo intramolecular click reactions to release CO under physiological conditions. The Wang lab has research underway along this direction.101
3. Conclusions
The CO field has provided all the necessary background supporting the clinical utility in patients suffering from numerous pathologies; however, development has been slowed by a series of unfortunate events. On the one hand, ample studies have demonstrated the therapeutic benefits of inhaled CO; and yet the adequacy of the delivery systems available so far remains a significant hurdle. Metal based CO-RMs as well as the family of photo-sensitive CORMs, which essentially mimic that observed with inhaled gas, have not been able to identify a lead compound with acceptable pharmacologic characteristics primarily because medicinal chemistry efforts are lagging behind significantly. However, this is an area with tremendous potential and conditions are ripe for intense medicinal chemistry efforts. The pioneering work of metal-based CO-RMs and inhaled CO has made monumental contributions to the understanding of CO biology as well as to the identification of pharmaceutical issues that need to be addressed. The work on encapsulated CO-RMs, photo-sensitive CO-RMs, and enzyme-triggered CO-RMs has brought several drug delivery issues to the forefront. At this time, the development of entirely organic CO-prodrugs is becoming a reality with the “click and release” chemistry reported.50 Considering the vast experience of the pharmaceutical industry with small organic molecules, these organic pro-drugs hold promise for further successful clinical development. The encapsulated forms of CO-RMs also have realistic potential in localized delivery of CO for treating colitis and skin conditions, among others. The photo-sensitive prodrugs may allow for precise control of delivery, which will be very useful in research and topical applications. The traditional metal-based CO-RMs may find applications in situations where long-term application is not needed. Taken together, we expect to see an increased level of interest in developing CO-based therapeutics as well as continued advances in understanding CO biology. Collectively, all of these will lead to an accelerated pace marching toward clinical testing of CO-based therapeutics.
Acknowledgement
Work in the authors’ lab is partially supported by the National Institutes of Health (CA180519, AI104168, and CA180805).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dedicated to Professor Ronald T. Borchardt on the occasion of his retirement.
References
- 1.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 2.Otterbein LE, Bach FH, Alam J, Soares M, Tao LH, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 3.Barbagallo I, Marrazzo G, Frigiola A, Zappala A, Li VG. Role of carbon monoxide in vascular diseases. Curr Pharm Biotechnol. 2012;13:787–796. doi: 10.2174/138920112800399086. [DOI] [PubMed] [Google Scholar]
- 4.Chau LY. Heme oxygenase-1: emerging target of cancer therapy. J Biomed Sci. 2015;22:22–28. doi: 10.1186/s12929-015-0128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Was H, Dulak J, Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets. 2010;11:1551–1570. doi: 10.2174/1389450111009011551. [DOI] [PubMed] [Google Scholar]
- 6.Wegiel Hanto DW, Otterbein LE. The social network of carbon monoxide in medicine. Trends Mol Med. 2013;19:3–11. doi: 10.1016/j.molmed.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wegiel B, Nemeth Z, Correa-Costa M, Bulmer AC, Otterbein LE. Heme oxygenase-1: a metabolic nike. Antioxid Redox Signal. 2014;20:1709–1722. doi: 10.1089/ars.2013.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinemann SH, Hoshi T, Westerhausen M, Schiller A. Carbon monoxide-physiology, detection and controlled release. Chem Commun. 2014;50:3644–3660. doi: 10.1039/c3cc49196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenhunen R, Marver HS, Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970;75:410–421. [PubMed] [Google Scholar]
- 10.McCoubrey WKJ, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 11.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 12.Otterbein LE, Soares MP, Bach FH. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 13.Scott JR, Chin BY, Bilban MH, Otterbein LE. Restoring HOmeostasis: is heme oxygenase-1 ready for the clinic? Trends Pharmacol Sci. 2007;28:200–205. doi: 10.1016/j.tips.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Hartsfield CL. Cross talk between carbon monoxide and nitric oxide. Antioxid Redox Signal. 2002;4:301–307. doi: 10.1089/152308602753666352. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 16.Motterlini R, Mann BE, Johnson TR, Clark JE, Foresti R, Green CJ. Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr Pharmacol Design. 2003;9:2525–2539. doi: 10.2174/1381612033453785. [DOI] [PubMed] [Google Scholar]
- 17.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 18.Zhao S, Lin Q, Li H, He Y, Fang X, Chen F, Chen C, Huang Z. Carbon monoxide releasing molecule-2 attenuated ischemia/reperfusion-induced apoptosis in cardiomyocytes via a mitochondrial pathway. Mol Med Rep. 2014;9:754–762. doi: 10.3892/mmr.2013.1861. [DOI] [PubMed] [Google Scholar]
- 19.Kim DS, Chae SW, Kim HR, Chae HJ. CO and bilirubin inhibit doxorubicin-induced cardiac cell death. Immunopharmacol Immunotoxicol. 2009;31:64–70. doi: 10.1080/08923970802354762. [DOI] [PubMed] [Google Scholar]
- 20.Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavitrano M, Smolenski RT, Musumeci A, Maccherini M, Slominska E, Di Florio E, Bracco A, Mancini A, Stassi G, Patti M, Giovannoni R, Froio A, Simeone F, Forni M, Bacci ML, D'Alise G, Cozzi E, Otterbein LE, Yacoub MH, Bach FH, Calise F. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004;18:1093–1095. doi: 10.1096/fj.03-0996fje. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho PS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira1 PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014;34:106–135. doi: 10.1002/med.21280. [DOI] [PubMed] [Google Scholar]
- 23.Wegiel B, Gallo D, Csizmadia E, Harris C, Belcher J, Vercellotti GM, Penacho N, Seth P, Sukhatme V, Ahmed A, Pandolfi PP, Helczynski L, Bjartell A, Persson JL, Otterbein LE. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013;73:7009–7021. doi: 10.1158/0008-5472.CAN-13-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soni H, Pandya G, Patel P, Acharya A, Jain M, Mehta AA. Beneficial effects of carbon monoxide-releasing molecule-2 (CORM-2) on acute doxorubicin cardiotoxicity in mice: Role of oxidative stress and apoptosis. Toxicol Appl Pharmacol. 2011;253:70–80. doi: 10.1016/j.taap.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardern RD, Quinn ND. Emergency management of diabetic ketoacidosis in adults. Emerg Med J. 2003;20:210–213. doi: 10.1136/emj.20.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:2694–2698. doi: 10.1210/jcem.83.8.5054. [DOI] [PubMed] [Google Scholar]
- 28.Gundgurthi A, Kharb S, Dutta MK, Pakhetra R, Garg MK. Insulin poisoning with suicidal intent. Indian J Endocr Metab. 2012;16:S120–S122. doi: 10.4103/2230-8210.94254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCullough PA, Beaver TM, Bennett-Guerrero E, Emmett M, Fonarow GC, Goyal A, Herzog CA, Kosiborod M, Palmer BF. Acute and chronic cardiovascular effects of hyperkalemia: new insights into prevention and clinical management. Rev Cardiovasc Med. 2014;15:11–23. [PubMed] [Google Scholar]
- 30.Light A, Grass CPD, Krause J. Carboxyhemoglobin levels in smokers vs. non-smokers in a smoking environment. Respir Care. 2007;52:1576. [Google Scholar]
- 31.Knauert M, Vangala S, Haslip M, Lee PJ. Therapeutic applications of carbon monoxide. Oxid Med Cell Longev. 2013;2013:360815. doi: 10.1155/2013/360815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romao CC, Blattler WA, Seixas JD, Bernardes GJ. Developing drug molecules for therapy with carbon monoxide. Chem Soc Rev. 2012;41:3571–3583. doi: 10.1039/c2cs15317c. [DOI] [PubMed] [Google Scholar]
- 33.Vera T, Henegar JR, Drummond HA, Rimoldi JM, Stec DE. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J Am Soc Nephrol. 2005;16:950–958. doi: 10.1681/ASN.2004090736. [DOI] [PubMed] [Google Scholar]
- 34.Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, Dawn B, Motterlini R, Bolli R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1649–1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fagone P, Mangano K, Coco M, Perciavalle V, Garotta G, Romao CC, Nicoletti F. Therapeutic potential of carbon monoxide in multiple sclerosis. Clin Exp Immunol. 2012;167:179–187. doi: 10.1111/j.1365-2249.2011.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Research Council (US) Committee on Acute Exposure Guideline Levels . Acute exposure guideline levels for selected airborne chemicals: Volume 8. Carbon monoxide acute exposure guideline levels. National Academies Press (US); Washington (DC): 2010. Available from: http://www.ncbi.nlm.nih.gov/books/NBK220007/ [PubMed] [Google Scholar]
- 37.Von Burg R. Carbon monoxide. J Appl Toxicol. 1999;19:379–386. doi: 10.1002/(sici)1099-1263(199909/10)19:5<379::aid-jat563>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Goldbaum LR, Orellano T, Dergal E. Mechanism of the toxic action of carbon monoxide. Ann Clin Lab Sci. 1976;6:372–376. [PubMed] [Google Scholar]
- 39.Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide–releasing molecule. Circ Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 40.Motterlini R, Sawle P, Hammad J, Bains S, Alberto R, Foresti R, Green CJ. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005;19:284–286. doi: 10.1096/fj.04-2169fje. [DOI] [PubMed] [Google Scholar]
- 41.Zuckerbraun BS. Therapeutic delivery of carbon monoxide: WO2008/003953. Expert Opin Ther Pat. 2008;18:1321–1325. [Google Scholar]
- 42.Crook SH, Mann BE, Meijer AJHM, Adams H, Sawle P, Scapens D, Motterlini R. [Mn(CO)4{S2CNMe(CH2CO2H)}], a new water-soluble CO-releasing molecule. Dalton Trans. 2011;40:4230–4235. doi: 10.1039/c1dt10125k. [DOI] [PubMed] [Google Scholar]
- 43.Hasegawa U, van der Vlies AJ, Simeoni E, Wandrey C, Hubbell JA. Carbon monoxide-releasing micelles for immunotherapy. J Am Chem Soc. 2010;132:18273–18280. doi: 10.1021/ja1075025. [DOI] [PubMed] [Google Scholar]
- 44.Dordelmann G, Pfeiffer H, Birkner A, Schatzschneider U. Silicium dioxide nanoparticles as carriers for photoactivatable CO-releasing molecules (PhotoCORMs). Inorg Chem. 2011;50:4362–4367. doi: 10.1021/ic1024197. [DOI] [PubMed] [Google Scholar]
- 45.Bohlender C, Glaser S, Klein M, Weisser J, Thein S, Neugebauer U, Popp J, Wyrwa R, Schiller A. Light-triggered CO release from nanoporous non-wovens. J Mater Chem B. 2014;2:1454–1463. doi: 10.1039/c3tb21649g. [DOI] [PubMed] [Google Scholar]
- 46.Chaves-Ferreira M, Albuquerque IS, Matak-Vinkovic D, Coelho AC, Carvalho SM, Saraiva LM, Romão CC, Bernardes GJL. Spontaneous CO release from RuII(CO)2–protein complexes in aqueous solution, cells, and mice. Angew Chem Int Ed. 2015;54:1172–1175. doi: 10.1002/anie.201409344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antony LAP, Slanina T, Šebej P, Šolomek T, Klán P. Fluorescein analogue xanthene-9-carboxylic acid: A transition-metal-free CO releasing molecule activated by green light. Org Lett. 2013;15:4552–4555. doi: 10.1021/ol4021089. [DOI] [PubMed] [Google Scholar]
- 48.Peng P, Wang C, Shi Z, Johns VK, Ma L, Oyer J, Copik A, Igarashi R, Liao Y. Visible-light activatable organic CO-releasing molecules (PhotoCORMs) that simultaneously generate fluorophores. Org Biomol Chem. 2013;11:6671–6674. doi: 10.1039/c3ob41385c. [DOI] [PubMed] [Google Scholar]
- 49.Anderson SN, Richards JM, Esquer HJ, Benninghoff AD, Arif AM, Berreau LM. A structurally-tunable 3-hydroxyflavone motif for visible light-induced carbon monoxide-releasing molecules (CORMs). ChemistryOpen. 2015 doi: 10.1002/open.201500167. aticle in press, doi: 10.1002/open.201500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D, Viennois E, Ji K, Damera K, Draganov A, Zheng Y, Dai C, Merlin D, Wang B. A click-and-release approach to CO prodrugs. Chem Commun. 2014;50:15890–15893. doi: 10.1039/c4cc07748b. [DOI] [PubMed] [Google Scholar]
- 51.Kohmoto J, Nakao A, Kaizu T, Tsung A, Ikeda A, Tomiyama K, Billiar TR, Choi AM, Murase N, McCurry KR. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery. 2006;140:179–185. doi: 10.1016/j.surg.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Raman KG, Barbato JE, Ifedigbo E, Ozanich BA, Zenati MS, Otterbein LE, Tzeng E. Inhaled carbon monoxide inhibits intimal hyperplasia and provides added benefit with nitric oxide. J Vasc Surg. 2006;44:151–158. doi: 10.1016/j.jvs.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Bathoorn E, Slebos DJ, Postma DS, Koeter GH, van Oosterhout AJ, van der Toorn M, Boezen HM, Kerstjens HA. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J. 2007;30:1131–1137. doi: 10.1183/09031936.00163206. [DOI] [PubMed] [Google Scholar]
- 54. ClinicalTrials.gov. ClinicalTrials.gov Record as of May 25, 2015. Study of inhaled carbon monoxide to treat idiopathic pulmonary fibrosis. Identifier: NCT01214187 First received: September 30, 2010; Last updated: May 11, 2015; Last verified: May 2015.
- 55.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688–694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 56.Sarady JK, Zuckerbraun BS, Bilban M, Wagner O, Usheva A, Liu F, Ifedigbo E, Zamora R, Choi AM, Otterbein LE. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. FASEB J. 2004;18:854–856. doi: 10.1096/fj.03-0643fje. [DOI] [PubMed] [Google Scholar]
- 57.Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, Kaczmarek E, Lee PJ, Zuckerbraun BS, Flavell R, Soares MP, Otterbein LE. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest. 2014;124:4926–4940. doi: 10.1172/JCI72853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuckerbraun BS, Billiar TR, Otterbein SL, Kim PK, Liu F, Choi AM, Bach FH, Otterbein LE. Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. J Exp Med. 2003;198:1707–1716. doi: 10.1084/jem.20031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuramitsu K, Gallo D, Yoon M, Chin BY, Csizmadia E, Hanto DW, Otterbein LE. Carbon monoxide enhances early liver regeneration in mice after hepatectomy. Hepatology. 2011;53:2016–2026. doi: 10.1002/hep.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. ClinicalTrials.gov. ClinicalTrials.gov Record as of May 25, 2015. Carbon monoxide therapy for severe pulmonary arterial hypertension (CO in PAH). Identifier: NCT01523548 First received: January 27, 2012; Last updated: April 1, 2015; Last verified: April 2015.
- 61. ClinicalTrials.gov. ClinicalTrials.gov Record as of May 25, 2015. Carbon monoxide to prevent lung inflammation. Identifier: NCT00094406 First received: October 16, 2004; Last updated: May 20, 2015; Last verified: May 2015.
- 62. ClinicalTrials.gov. ClinicalTrials.gov Record as of May 25, 2015. Safety and tolerability study of inhaled carbon monoxide in kidney transplant patients. Identifier: NCT00531856 First received: September 18, 2007; Last updated: April 3, 2015; Last verified: April 2015.
- 63. ClinicalTrials.gov. Safety study of inhaled carbon monoxide to treat acute respiratory distress syndrome (ARDS) ClinicalTrials.gov Identifier: NCT02425579 record as of May 25, 2015, First received: March 17, 2015; Last updated: August 21, 2015; Last verified: August 2015. [Google Scholar]
- 64.Dahl LF, Rundle RE. The crystal structure of dimanganese decacarbonyl Mn2(CO)10. Acta Crystallogr. 1963;16:419–426. [Google Scholar]
- 65.Gaynora D, Griffith DM. The prevalence of metal-based drugs as therapeutic or diagnostic agents: beyond platinum. Dalton Trans. 2012;41:13239–13257. doi: 10.1039/c2dt31601c. [DOI] [PubMed] [Google Scholar]
- 66.Apps MG, Choi EHY, Wheate NJ. The state-of-play and future of platinum drugs. Endocr Relat Cancer. 2015;22:R219–R233. doi: 10.1530/ERC-15-0237. [DOI] [PubMed] [Google Scholar]
- 67.Lengfelder E, Lo-Coco F, Ades L, Montesinos P, Grimwade D, Kishore B, Ramadan SM, Pagoni M, Breccia M, Huerta AJG, Nloga AM, González-Sanmiguel JD, Schmidt A, Lambert J-F, Lehmann S, Di Bona E, Cassinat B, Hofmann W-K, Görlich D, Sauerland M-C, Fenaux P, Sanz M. Arsenic trioxide-based therapy of relapsed acute promyelocytic leukemia: registry results from the European LeukemiaNet. Leukemia. 2015 doi: 10.1038/leu.2015.12. in press, doi:10.1038/leu.2015.1012. [DOI] [PubMed] [Google Scholar]
- 68.Han C, Wang B. Factors that impact the developability of drug candidates-An overview. In: Wang B, Siahaan T, Soltero R, editors. Drug delivery: principles and applications. John Wiley and Sons; New York: 2005. pp. 1–15. [Google Scholar]
- 69.Han C, David C, Wang B. Evaluation of drug candidates for preclinical development: Pharmacokinetics, metabolism, pharmaceutics, and toxicology. John Wiley and Sons; New York: 2010. [Google Scholar]
- 70.Pfeiffer H, Rojas A, Niesel J, Schatzschneider U. Sonogashira and “Click” reactions for the N-terminal and side-chain functionalization of peptides with [Mn(CO)3(tpm)]+-based CO releasing molecules (tpm = tris(pyrazolyl)methane). Dalton Trans. 2009;14:4292–4298. doi: 10.1039/b819091g. [DOI] [PubMed] [Google Scholar]
- 71.Nagel C, McLean S, Poole RK, Braunschweig H, Kramer T, Schatzschneider U. Introducing [Mn(CO)3(tpa-κ3N)]+ as a novel photoactivatable CO-releasing molecule with well-defined iCORM intermediates-synthesis, spectroscopy, and antibacterial activity. Dalton Trans. 2014;43:9986–9997. doi: 10.1039/c3dt51848e. [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Gallego S, Bernardes GJ. Carbon-monoxide-releasing molecules for the delivery of therapeutic CO in vivo. Angew Chem Int Ed. 2014;53:9712–9721. doi: 10.1002/anie.201311225. [DOI] [PubMed] [Google Scholar]
- 73.Inaba H, Fujita K, Ueno T. Design of biomaterials for intracellular delivery of carbon monoxide. Biomater Sci. 2015 doi: 10.1039/c5bm00210a. in press, doi: 10.1039/C5BM00210A. [DOI] [PubMed] [Google Scholar]
- 74.Yin H, Fang J, Liao L, Nakamura H, Maeda H. Styrene-maleic acid copolymer-encapsulated CORM2, a water-soluble carbon monoxide (CO) donor with a constant CO-releasing property, exhibits therapeutic potential for inflammatory bowel disease. J Control Release. 2014;187:14–21. doi: 10.1016/j.jconrel.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 75.Kunz PC, Meyer H, Barthel J, Sollazzo S, Schmidt AM, Janiak C. Metal carbonyls supported on iron oxide nanoparticles to trigger the CO-gasotransmitter release by magnetic heating. Chem Commun. 2013;49:4896–4898. doi: 10.1039/c3cc41411f. [DOI] [PubMed] [Google Scholar]
- 76.Govender P, Pai S, Schatzschneider U, Smith GS. Next generation photoCORMs: polynuclear tricarbonylmanganese(I)-functionalized polypyridyl metallodendrimers. Inorg Chem. 2013;52:5470–5478. doi: 10.1021/ic400377k. [DOI] [PubMed] [Google Scholar]
- 77.Brückmann NE, Wahl M, Reiß GJ, Kohns M, Wätjen W, Kunz PC. Polymer conjugates of photoinducible CO-releasing molecules. Eur J Inorg Chem. 2011;2011:4571–4577. [Google Scholar]
- 78.Atkin AJ, Fairlamb IJS, Ward JS, Lynam JM, Atkin AJ, Fairlamb IJS, Ward JS, Lynam JM. CO release from norbornadiene iron(0) tricarbonyl complexes. Importance of ligand dissociation. Organomet. 2012;31:5894–5902. [Google Scholar]
- 79.Romanski S, Kraus B, Guttentag M, Schlundt W, Rucker H, Adler A, Neudorfl JM, Alberto R, Amslinger S, Schmalz HG. Acyloxybutadiene tricarbonyl iron complexes as enzyme-triggered CO-releasing molecules (ET-CORMs): a structure-activity relationship study. Dalton Trans. 2012;41:13862–13875. doi: 10.1039/c2dt30662j. [DOI] [PubMed] [Google Scholar]
- 80.Botov S, Stamellou E, Romanski S, Guttentag M, Alberto R, Neudörfl J-M, Yard B, Schmalz H-G. Synthesis and performance of acyloxy-diene-Fe(CO)3 complexes with variable chain lengths as enzyme-triggered carbon monoxide-releasing molecules. Organomet. 2013;32:3587–3594. [Google Scholar]
- 81.Stamellou E, Storz D, Botov S, Ntasis E, Wedel J, Sollazzo S, Kramer BK, van Son W, Seelen M, Schmalz HG, Schmidt A, Hafner M, Yard BA. Different design of enzyme-triggered CO-releasing molecules (ET-CORMs) reveals quantitative differences in biological activities in terms of toxicity and inflammation. Redox Biol. 2014;2:739–748. doi: 10.1016/j.redox.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Romanski S, Rücker H, Stamellou E, Guttentag M, Neudörfl J-M, Alberto R, Amslinger S, Yard B, Schmalz H-G. Iron dienylphosphate tricarbonyl complexes as water-soluble enzyme-triggered CO-releasing molecules (ET-CORMs). Organomet. 2012;31:5800–5809. [Google Scholar]
- 83.Sitnikov NS, Li Y, Zhang D, Yard B, Schmalz HG. Design, synthesis, and functional evaluation of CO-releasing molecules triggered by penicillin G amidase as a model protease. Angew Chem Int Ed. 2015;54:12314–12318. doi: 10.1002/anie.201502445. [DOI] [PubMed] [Google Scholar]
- 84.Wang B, Siahaan T, Soltero R, editors. Drug delivery: Principles and applications. John Wiley and Sons; New York: 2005. [Google Scholar]
- 85.Borchardt RT, Kerns EH, Lipinski CA, Thakker DR, Wang B, editors. Pharmaceutical profiling in drug discovery for lead selection. AAPS Press; Arlington, VA: 2004. [Google Scholar]
- 86.Horspool WM, Khandelwal GD. Photo-decarbonylation of coumarandiones. J Chem Soc D. 1970;5:257–258. [Google Scholar]
- 87.Kuzmanich G, Garcia-Garibay MA. Ring strain release as a strategy to enable the singlet state photodecarbonylation of crystalline 1,4-cyclobutanediones. J Phys Org Chem. 2011;24:883–888. [Google Scholar]
- 88.Mikol GJ, Boyer JH. Photo-induced decarbonylation of β-styryl isocyanates. J Chem Soc Chem Commun. 1972;8:439–439. [Google Scholar]
- 89.Kuzmanich G, Gard MN, Garcia-Garibay MA. Photonic amplification by a singlet-state quantum chain reaction in the photodecarbonylation of crystalline diarylcyclopropenones. J Am Chem Soc. 2009;131:11606–11614. doi: 10.1021/ja9043449. [DOI] [PubMed] [Google Scholar]
- 90.L'Abbé G. Heterocyclic analogues of methylenecyclopropanes. Angew Chem Int Ed. 1980;19:276–289. [Google Scholar]
- 91.Mondal R, Shah BK, Neckers DC. Photogeneration of heptacene in a polymer matrix. J Am Chem Soc. 2006;128:9612–9613. doi: 10.1021/ja063823i. [DOI] [PubMed] [Google Scholar]
- 92.Uno H, Yamashita Y, Kikuchi M, Watanabe H, Yamada H, Okujima T, Ogawa T, Ono N. Photo precursor for pentacene. Tetrahedron Lett. 2005;46:1981–1983. [Google Scholar]
- 93.Yamada H, Kuzuhara D, Ohkubo K, Takahashi T, Okujima T, Uno H, Ono N, Fukuzumi S. Synthesis and photochemical properties of α-diketoporphyrins as precursors for π-expanded porphyrins. J Mater Chem. 2010;20:3011–3024. [Google Scholar]
- 94.Mondal R, Okhrimenko AN, Shah BK, Neckers DC. Photodecarbonylation of α-diketones: A mechanistic study of reactions leading to acenes. J Phys Chem B. 2008;112:11–15. doi: 10.1021/jp076738l. [DOI] [PubMed] [Google Scholar]
- 95.Carvalho JC, Ferreira LP, da Silva Santos L, Correa MJ, de Oliveira Campos LM, Bastos JK, Sarti SJ. Anti-inflammatory activity of flavone and some of its derivates from Virola michelli Heckel. J Ethnopharmacol. 1999;64:173–177. doi: 10.1016/s0378-8741(98)00109-3. [DOI] [PubMed] [Google Scholar]
- 96.Pillai SI, Subramanian SP, Kandaswamy M. Evaluation of antioxidant efficacy of vanadium-3-hydroxyflavone complex in streptozotocin-diabetic rats. Chem Biol Interact. 2013;204:67–74. doi: 10.1016/j.cbi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 97.Lang Q, Zhang H, Li J, Xie F, Zhang Y, Wan B, Yu L. 3-Hydroxyflavone inhibits endogenous Aurora B and induces growth inhibition of cancer cell line. Mol Biol Rep. 2010;37:1577–1583. doi: 10.1007/s11033-009-9562-y. [DOI] [PubMed] [Google Scholar]
- 98.Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F. Novel insights into the pharmacology of flavonoids. Phytother Res. 2013;27:1588–1596. doi: 10.1002/ptr.5023. [DOI] [PubMed] [Google Scholar]
- 99.Chen W, Wang D, Dai C, Hamelberg D, Wang B. Clicking 1,2,4,5-tetrazine and cyclooctynes with tunable reaction rates. Chem Commun. 2012;48:1736–1738. doi: 10.1039/c2cc16716f. [DOI] [PubMed] [Google Scholar]
- 100.Wang D, Chen W, Zheng Y, Dai C, Wang K, Ke B, Wang B. 3,6-Substituted-1,2,4,5-tetrazines: tuning reaction rates for staged labeling applications. Org Biomol Chem. 2014;12:3950–3955. doi: 10.1039/c4ob00280f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ji X, Ji K, Aghoghovbia RE, Chittavong V, Zhu M, Wang B. Carbon monoxide prodrugs with tunable release rates and a fluorescent “reporter” for real-time monitoring. 2015 Manuscript submmited. [Google Scholar]