Abstract
Background
Recent evidence suggests that parathyroid hormone (PTH) has effects on vascular smooth muscle cells, the renin-angiotensin system and kidney function, but less is known about its role in the development of hypertension. The distribution of serum PTH also varies by race.
Methods and results
Therefore, we examined the relation between PTH and incident hypertension and tested for interaction by race among 7,504 ARIC participants (1,264 black, 6,240 white, median age 56 years) without initial hypertension in 1990–1992. During a median follow-up of 6 years, 1,487 white and 509 black participants developed hypertension. In the overall study population, PTH was not associated with incident hypertension after adjustment for demographics and behavioral risk factors [HR highest vs. lowest quintiles, 95%CI: 1.11 (0.96–1.28); P for linear trend 0.02]. Although the interaction was not statistically significant (P= 0.60), there was some evidence that the PTH-hypertension association differed by race. Among blacks, PTH was positively associated with incident hypertension, independent of demographics and behavioral risk factors (P for linear trend 0.003). Among whites, PTH was not associated with hypertension risk. Results were similar when comparing participants with elevated versus non-elevated PTH (≥65 vs. <65 pg/mL): HR in blacks: 1.24 (1.02–1.54); HR in whites: 0.95 (0.78–1.16).
Conclusions
In this large community-based cohort, PTH levels, overall, were not independently associated with the risk of hypertension. However, we found some evidence that PTH may be associated with hypertension in blacks. Future research should continue to explore potential race differences in the PTH-hypertension association.
Keywords: hypertension, parathyroid hormone, cohort study, race disparity
INTRODUCTION
Parathyroid hormone (PTH) is a regulatory factor in bone health and mineral homeostasis. Elevated concentrations of PTH are observed in individuals with vitamin D deficiency, low calcium intake, and kidney disease[1]. Converging evidence suggests that PTH has vascular effects and may therefore alter blood pressure. Endothelial dysfunction is one mechanism thought to link PTH to vascular changes. Specifically, PTH may increase serum levels of endothelin-1 and interleukin-6[2, 3], and may stimulate the vascular smooth muscle cells to produce factors including collagen and beta-1 integrin which could, in turn, remodel the peripheral vasculature[4]. Also, PTH may increase renin release and activate the renin-angiotensin system[5, 6]; a complex process mediated by serum calcium, renal 1-alpha-hydroxylase, and resultant changes in 1,25(OH)2D[7, 8].
Epidemiologic evidence of the association between PTH and hypertension is inconclusive. Elevated PTH levels have been associated with hypertension in cross-sectional studies[9–11], and in a few prospective studies[12, 13]. These existing findings primarily come from white populations, though one study did included a multi-racial/ethnic sample[13]. Blacks are known to have a higher prevalence and incidence of hypertension, as compared to whites[14, 15]. Blacks also have higher levels of PTH, relative to whites[16], and PTH-related mineral metabolism may differ between blacks and whites [17–20]. Evidence from these studies raises interest in whether there are racial differences in the association between PTH and hypertension.
To enhance understanding of the association between PTH and incident hypertension we analyzed data from the Atherosclerosis Risk in Communities (ARIC) Study, a large, community-based cohort of mostly blacks and whites adults. We hypothesized that PTH would be positively associated with risk of incident hypertension and that the association may vary by race.
METHODS
Study population
The ARIC study is a prospective cohort originally designed to investigate risk factors for atherosclerosis and clinical cardiovascular disease[21]. The cohort included 15,792 men and women aged 45–64 years at baseline (1987–1989) who were recruited from four United States communities (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and suburban Minneapolis, Minnesota). Only blacks were recruited from Jackson, Mississippi, while the enrollment from the other communities was representative of the racial distribution in those communities (15% Black in Forsyth County, North Carolina, and almost exclusively white in Washington Co, Maryland and Minneapolis, Minnesota). Since baseline, four additional clinic visits have taken place: 1990–92 (visit 2), 1993–95 (visit 3), 1996–98 (visit 4), and 2011–13 (visit 5). Study protocols were approved by local Institutional Review Boards, and all participants gave written informed consent.
Visit 2, which was attended by 14,348 ARIC participants, serves as baseline for the present analysis. We successively excluded from our analysis 416 participants who at visit 2 had prevalent cardiovascular disease, 4,798 with prevalent hypertension (anti-hypertensive medication use, or systolic blood pressure (SBP) ≥140 mmHg, or diastolic blood pressure (DBP) ≥90 mmHg) or no information on hypertension, 3 who had PTH>200 pg/mL, 573 who did not attend Visits 3 or 4 or had no data on hypertension at the visits, 1,000 who were missing information on serum PTH, 30 who were neither black nor white, and 24 blacks from the Minnesota and Maryland centers. The final analytic sample included a total of 7,504 participants, 6,240 whites and 1,264 blacks.
Measurement of PTH and Covariates
Participants were asked to fast for 12 hours before their Visit 2 exam, and serum and plasma samples were obtained and stored at −80°C. Intact PTH was measured in previously unthawed serum on the Roche Elecsys 2010 analyzer using a sandwich immunoassay method (Roche Diagnostics, Indianapolis, Indiana, USA) in 2012–2013 at the Advanced Research and Diagnostic Laboratory, University of Minnesota, Minneapolis, Minnesota. Serum PTH by the Elecsys method has excellent stability long-term at −80°C[22]. Using duplicate samples collected at Visit 2 and stored, we estimated the coefficient of variation to be 9.7% for PTH.
Serum 25(OH)D, calcium and phosphorus levels were also measured in 2012–2013 using the same stored samples from ARIC Visit 2. Serum 25(OH)D2 and 25(OH)D3 were measured using a high sensitivity mass spectrometer (AB Sciex 5500). Total 25(OH)D was calculated as the sum of 25(OH)D2 and 25(OH)D3. Calcium and phosphate levels were measured (Modular P Chemistry Analyzer; Roche Diagnostics) using a colorimetric method.
Other co-variables were obtained using standardized questionnaires or exams. All covariate information came from Visit 2, unless otherwise noted. Body mass index was calculated as weight in kg divided by height in meters squared. Physical activity at Visit 1 (not measured at Visit 2) was assessed using the Baecke sports index[23, 24]. It was a semi-continuous index that ranged from 1 (low) to 5 (high), and was a function of the frequency, the duration, and an assigned intensity of the queried sports. Smoking status and alcohol intake status were both categorized into current, former, and never. Educational levels were categorized into 3 categories: < high school degree, high school degree, or > high school degree. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation which incorporates both creatinine and cystatin-C[25]. Diabetes was defined as fasting glucose ≥ 126 mg/dL, blood glucose ≥ 200 mg/dL at any time, the use of diabetic mediations or self-report of diabetes. Mother's and father's history of hypertension was reported at Visit 1, three years before the baseline.
Incident Hypertension Ascertainment
During the clinic visits, blood pressure was measured by trained technicians using protocols which were similar across Visits 2–4. At each visit, blood pressure was measured after 5 min rest, while participants were sitting, using a Hawksley random-zero sphygmomanometer[26]. The first and fifth phase Korotkoff sounds were the criteria for SBP and DBP, respectively. Technicians conducted these measurements after completing training with Korotkoff sound tapes and double stethoscope comparisons with the trainer. Training was targeted to reduce digit preference and systematic differences between technicians. At visits 2 and 3, three measurements were conducted; for the present analysis we used the mean of the second and third measurements. At visit 4 only two measurements were conducted; for analysis we use the mean of those two measurements. This approach is standard within ARIC.
The primary outcome was incident hypertension at either ARIC visit 3 or 4, defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or self-reported antihypertensive medication use within the previous 2 weeks[26]. Incident prehypertension was defined as systolic blood pressure 130–139 mmHg, or diastolic blood pressure 85–90 mmHg [26].
Data analysis
Participant characteristics at baseline (1990–1992) are presented stratified by PTH quintiles based on the overall population. Since the primary outcome, incident hypertension, was only ascertained at Visits 3 and 4, the precise date of hypertension diagnosis was unknown. Thus, we performed complementary log-log analysis to estimate hazard ratios for the association between PTH and incident hypertension[27, 28]. This interval-censored analysis approach assumes that the hazard is constant within each time interval (in this instance, at visit 3 and visit 4, respectively) but allows for variation of the hazard between intervals. The main analysis used quintiles of PTH as the exposure. Linear trend of baseline variables by quintiles of PTH were tested by modeling the median values of the quintiles as a continuous variable.
Our first model adjusted for age, sex, race, center and season (4 categories). Model 2, the main model, was additionally adjusted for educational level, physical activity, smoking status, alcohol intake, and body mass index. In an additional models we further adjusted, simultaneously, for 25(OH)D, eGFR, serum calcium, serum phosphorus, diabetes status and family history of hypertension. We explored the association between PTH (modeled as quintiles) and incident hypertension by race, age, sex, obesity status (body mass index [BMI] categories), eGFR, and low vitamin D status (<20 ng/ml). Given inherent interest, in addition to presenting results for the entire study population, race-stratified results are also presented, throughout. In separate analyses, we categorized PTH dichotomously, with participants as having clinically high level (≥ 65 pg/mL) or low levels (<65 pg/mL). Sensitivity analyses were conducted restricting our analysis to participants with normal kidney function (eGFR ≥90 ml/min/1.73 m2), excluding people with primary hyperparathyroidism defined by high levels of both serum PTH and calcium (PTH >65 pg/mL and calcium >10.2 mg/dL)[29], adjusting for baseline systolic blood pressure levels. Furthermore, the robustness of our analytic approach was checked by repeating the analyses of the relation between PTH and incident hypertension using Cox proportional hazard regression analysis. For these analyses person-time accrued until the development of incident hypertension, the date of the last visit in which the participant took part (visit 3 or 4) or death, whichever came first. Finally, we performed an additional analysis to explore the association between PTH and incident prehypertension.
RESULTS
At baseline, the 7,504 ARIC participants included in our analytic sample were on average 56.1 (SD 5.6) years old, 3,140 (42%) were male, and 1,264 (17%) were black. Baseline characteristics were presented by PTH quintiles in Table 1. The median (25th –75th percentiles) of PTH was 38.2 (30.5–47.6) pg/mL overall, 37.4 (30.1 –46.1) in whites, and 42.7 (33.8–54.2) in blacks. Participants in a higher PTH quintile group tended to be older, less often male, more often black, better educated, less often current drinkers or smokers, have lower mean levels of physical activity, serum calcium, 25(OH)D (adjusted for seasonality), and phosphorus, and have higher mean levels of blood pressure, body mass index, C-reactive protein, and eGFR. Race-stratified baseline characteristics are presented in Supplemental Table 1.
Table 1.
Baseline characteristics by quintiles of parathyroid hormone: The ARIC Study (1990–1992)
| Characteristics a | Quintile 1 (N=1,501) |
Quintile 2 (N=1,498) |
Quintile 3 (N=1,503) |
Quintile 4 (N=1,501) |
Quintile 5 (N=1,501) |
Linear trend Pc |
|---|---|---|---|---|---|---|
| Parathyroid hormones, pg/mL | ||||||
| Median | 24.9 | 32.0 | 38.2 | 45.2 | 58.1 | |
| Range | 3.2 – 28.8 | 28.9 – 34.9 | 35.0 – 41.5 | 41.6 – 50.1 | 50.2 – 162.6 | |
| Demographics | ||||||
| Age, years | 55.6 (5.5) | 56.0 (5.6) | 56.3 (5.6) | 56.1 (5.7) | 56.4 (5.6) | <0.001 |
| Male, % | 45.5 | 43.3 | 45.6 | 39.8 | 34.9 | <0.001 |
| Race, % | <0.001 | |||||
| White | 88.7 | 87.7 | 84.2 | 82.9 | 72.2 | |
| Black | 11.3 | 12.3 | 15.8 | 17.1 | 27.8 | |
| Education, % | 0.019 | |||||
| < High school | 16.2 | 15.0 | 17.3 | 14.6 | 16.4 | |
| high school | 46.3 | 44.4 | 41.4 | 41.4 | 40.9 | |
| > high school | 37.5 | 40.6 | 41.3 | 44.0 | 42.7 | |
| Behavioral Factors | ||||||
| Smoking status, % | <0.001 | |||||
| Current | 36.7 | 24.5 | 20.6 | 15.2 | 12.5 | |
| Past | 29.3 | 35.5 | 40.1 | 41.2 | 38.6 | |
| Never | 34.0 | 40.1 | 39.3 | 43.6 | 48.9 | |
| Drinking status, % | <0.001 | |||||
| Current | 63.8 | 64.2 | 62.1 | 60.5 | 56.3 | |
| Past | 19.3 | 16.9 | 18.6 | 16.5 | 17.6 | |
| Never | 16.8 | 18.9 | 19.3 | 23.0 | 26.1 | |
| Physical activity score | 2.5 (0.8) | 2.6 (0.8) | 2.6 (0.8) | 2.5 (0.8) | 2.4 (0.8) | <0.001 |
| Physiologic Characteristics | ||||||
| Systolic blood pressure, mmHg | 112 (13) | 113 (12) | 114 (12) | 115 (12) | 115 (12) | <0.001 |
| Diastolic blood pressure, mmHg | 68.0 (8.2) | 68.4 (8.3) | 69.4 (8.5) | 69.9 (8.1) | 70.5 (8.2) | <0.001 |
| Body mass index, kg/m2 | 25.9 (4.2) | 26.4 (4.3) | 26.8 (4.5) | 27.3 (4.7) | 28.5 (5.7) | <0.001 |
| Serum calcium, mg/dL | 9.38 (0.40) | 9.32 (0.38) | 9.29 (0.42) | 9.27 (0.39) | 9.26 (0.45) | <0.001 |
| Serum 25(OH)D, ng/dLb | 27.6 (8.5) | 26.6 (8.6) | 25.6 (8.0) | 24.1 (7.7) | 21.9 (7.9) | <0.001 |
| Serum phosphorus, mg/dL | 3.64 (0.49) | 3.59 (0.46) | 3.55 (0.47) | 3.51 (0.46) | 3.43 (0.47) | <0.001 |
| C-reactive protein, mg/L | 3.55 (6.14) | 3.43 (5.93) | 3.01 (4.85) | 3.71 (7.01) | 4.10 (8.01) | <0.001 |
| eGFR (SD), ml/min/1.73 m2 | 97.1 (14.4) | 97.1 (14.3) | 97.6 (14.1) | 98.5 (15.0) | 98.2 (15.7) | 0.038 |
GFR: glomerular filtration rate
For a continuous variable, mean (SD) was expressed unless specified
Adjusted for season by adding residuals, obtained from regression with 25(OH)D on season of visit, to overall mean value
Tested by modeling median values of the quintiles as a continuous variable
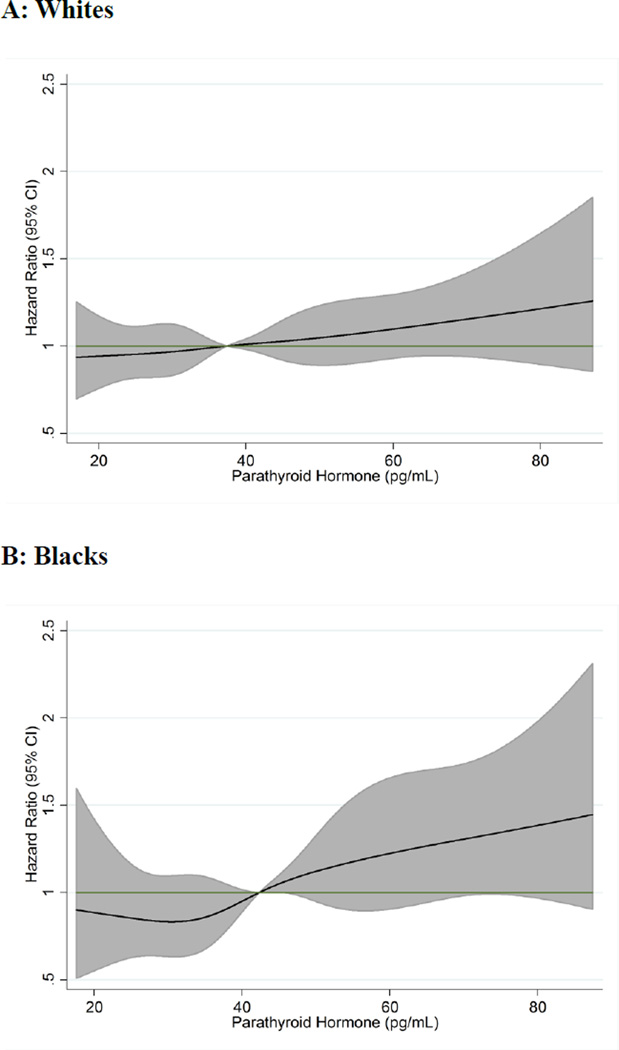
A total of 1,996 incident hypertension events accrued over a maximum follow-up of 8.8 years (median 5.9 years). Among whites 1,487 events occurred, and the crude incidence rate was 43.7 per 1,000 person-years. In blacks there were 509 events and the crude incidence rate was 81.1 per 1,000 person-years. A linear relationship between PTH and risk of incident hypertension was indicated by cubic spline analyses, in both race subgroups, after adjustment for age, sex, race and season (Figure 1). Adjusted for age, sex, race, center, and season (Model 1), high PTH was associated with greater risk of hypertension [HR (95%CI) for Quintile 5 vs. Quintile 1: 1.25 (1.08–1.44); P for linear trend: <0.001] (Table 2). With additional adjustment for educational level, physical activity, smoking status, alcohol intake, and body mass index (Model 2), the association of PTH with hypertension was attenuated [1.11 (0.96–1.28); P for linear trend: 0.02]. Results were similar when Model 2 was further adjusted for eGFR, serum calcium, phosphorus, 25(OH)D [1.09 (0.91–1.27); P for linear trend: 0.044]. In this model, where PTH and other related biomarkers were adjusted for simultaneously, 25(OH)D was not significantly associated with incident hypertension (P=0.19). Results were also similar when Model 2 was additionally adjusted for diabetes status, and family history of hypertension [1.11 (0.95–1.29); P for linear trend: 0.07]. No significant interaction between PTH and age, sex, obesity status or eGFR was detected. Participants with clinically elevated PTH (≥65 pg/mL) did not have significantly higher risk of incident HTN in the overall sample after accounting for behaviors and BMI (Table 3).
Figure 1. Association of Parathyroid Hormone with Risk of Incident Hypertension: The ARIC Study 1990–1998.
Table 2.
Adjusted hazard ratio (95% confidence interval) by complementary log-log analysis for the association between parathyroid hormone level and incident hypertension over a median of 6 years of follow-up: The ARIC Study (1990–1999)
| Quintile 1(ref) |
Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Linear trend P |
|
|---|---|---|---|---|---|---|
| Overall (N=7,504) | ||||||
| PTH Median, pg/mL | 24.9 | 32.0 | 38.2 | 45.2 | 58.1 | |
| PTH Range, pg/mL | 3.2 – 28.8 | 28.9 – 34.9 | 35.0 – 41.5 | 41.6 – 50.1 | 50.2 – 162.6 | |
| No. with incident hypertension | 350 | 344 | 400 | 426 | 476 | |
| Crude IR (95% CI), 1000 person-yr | 42.8 (38.6, 47.6) |
42.3 (38.0, 47.0) |
49.5 (44.9, 54.6) |
52.7 (47.9, 57.9) |
60.9 (55.5, 66.6) |
|
| Model 1 | 1 | 0.95 (0.82, 1.11) |
1.10 (0.95, 1.27) |
1.17 (1.01, 1.35) |
1.25 (1.08, 1.44) |
<0.001 |
| Model 2 | 1 | 0.92 (0.80, 1.07) |
1.05 (0.91, 1.22) |
1.11 (0.96, 1.28) |
1.11 (0.96, 1.28) |
0.020 |
| Whites (N=6,240) | ||||||
| No. with incident hypertension | 296 | 285 | 304 | 317 | 285 | |
| Crude IR (95% CI), 1000 person-yr | 40.5 (36.1, 45.4) |
39.6 (35.3, 44.5) |
44.1 (39.4, 49.4) |
46.6 (41.7, 52.0) |
48.9 (43.6, 54.9) |
|
| Model 1 | 1 | 0.95 (0.81, 1.12) |
1.06 (0.90, 1.25) |
1.12 (0.95, 1.31) |
1.15 (0.98, 1.36) |
0.017 |
| Model 2 | 1 | 0.93 (0.79, 1.09) |
1.03 (0.87, 1.21) |
1.06 (0.90, 1.24) |
1.02 (0.86, 1.21) |
0.36 |
| Blacks (N=1,264) | ||||||
| No. with incident hypertension | 54 | 59 | 96 | 109 | 191 | |
| Crude IR (95% CI), 1000 person-yr | 63.0 (48.3, 82.3) |
62.2 (48.2, 80.3) |
80.7 (66.1, 98.6) |
84.7 (70.2, 102.2) |
95.8 (83.1, 110.4) |
|
| Model 1 | 1 | 0.98 (0.67, 1.41) |
1.29 (0.92, 1.80) |
1.38 (1.00, 1.92) |
1.53 (1.12, 2.08) |
<0.001 |
| Model 2 | 1 | 0.91 (0.62, 1.32) |
1.19 (0.85, 1.67) |
1.33 (0.95, 1.86) |
1.38 (1.01, 1.89) |
0.003 |
CI: confidence interval; IR: incidence rate; PTH: parathyroid hormone
Model 1: Adjusted for age, sex, race-center and season; not adjusted for race-center in analyses stratified by race
Model 2: Adjusted for Model 1 plus educational attainment, physical activity, smoking status, alcohol intake, and body mass index
Table 3.
Adjusted hazard ratios and 95% confidence intervals by complementary log-log regression for incident hypertension according to elevated vs non-elevated parathyroid hormone (>=65 vs <65 pg/m), the ARIC Study (1990 to 1999)
| Parathyroid hormone | ||
|---|---|---|
| Normal (< 65.0 pg/mL) | Elevated (≥ 65.0 pg/mL) | |
| Overall (N=7,504) | ||
| N (%) | 7060 (94.1%) | 444 (5.9%) |
| Model 1 | 1 | 1.19 (1.04, 1.37) |
| Model 2 | 1 | 1.06 (0.92, 1.22) |
| Whites (N=6,240) | ||
| N (%) | 5956 (95.5%) | 284 (4.6%) |
| Model 1 | 1 | 1.08 (0.89, 1.31) |
| Model 2 | 1 | 0.95 (0.78, 1.16) |
| Blacks (N=1,264) | ||
| N (%) | 1104 (87.3%) | 160 (12.7%) |
| Model 1 | 1 | 1.34 (1.09, 1.65) |
| Model 2 | 1 | 1.24 (1.02, 1.54) |
Model 1: Adjusted for age, sex, race-center and season; not adjusted for race-center in analyses stratified by race
Model 2: Adjusted for Model 1 plus educational attainment, physical activity, smoking status, alcohol intake, and body mass index
Although there was no statistically significant interaction between race and PTH for hypertension risk (P for interaction: 0.60), as shown in Table 2, results of analyses varied qualitatively by race. In blacks higher PTH levels were associated with greater risk of incident hypertension [HR (95%CI) for Quintile 5 vs. Quintile 1: 1.53 (1.12–2.08); P for linear trend: <0.001] in Model 1, and after further adjustment for behavioral characteristics and BMI in Model 2 [1.38 (1.01–1.89); P for linear trend: 0.003]. Additional adjustment for eGFR, serum calcium, phosphorus, and 25(OH)D did not meaningfully alter the association [1.37 (0.99–1.90); P for linear trend: 0.004], and in this multivariate adjusted model 25(OH)D was not independently associated with hypertension risk. Further adjustment of Model 2 for diabetes status and family history of hypertension also yielded similar results [1.44 (1.03–2.01); P for linear trend: 0.002]. Likewise, when PTH was defined according to the clinical cut-point (≥65 vs. <65 pg/mL), blacks in the clinically high PTH group were at significantly greater risk of incident hypertension: HR (95%CI) Model 1: 1.34 (1.09–1.65), Model 2: 1.24 (1.02–1.54). Results were similar with further adjustment for eGFR, serum calcium, phosphorus and 25(OH)D:1.18 (0.94–1.47), and also with adjustment for diabetes status, and family history of hypertension: 1.22 (0.94–1.59).
In contrast, in whites, a higher PTH level was associated with greater risk of incident hypertension in Model 1, but the linear association became non-significant in Model 2 after adjustment for additional covariates. Results were also null with additional adjustment for related biomarkers, and (separately) for diabetes status and family history of hypertension (data not shown). Similarly, whites who had clinically high levels of PTH did not have excessive risk of hypertension compared to those with clinically low PTH levels.
In sensitivity analyses the results were similar when we excluded people with eGFR < 90 ml/min/1.73 m2 (N=2,143) and, separately, when we excluded those (N=69) with potential primary hyperparathyroidism (data not shown). Results were also similar when we adjusted for baseline levels of systolic blood pressure (data not shown). Cox proportional hazard models yielded results that were consistent with those obtained from the complemented log-log models (Supplemental Tables 2 and 3). In analyses of incident self-reported hypertension over longer follow-up (a median of 12 years; maximum 22 years), there was little evidence that PTH quintiles were associated with self-reported hypertension. Black participants who had clinically high levels of PTH had marginally significantly higher risk of self-reported hypertension than those with clinically low PTH levels (Supplemental Tables 4 and 5). We also conducted analyses of incident prehypertension. For these analyses, we further excluded 1,996 participants who developed hypertension during the follow-up and 299 participants with prehypertension at baseline, for a sample of 5,209. PTH was not significantly associated with incident prehypertension (data not shown).
DISCUSSION
In this large prospective cohort study, PTH was associated with risk of incident hypertension in blacks but not whites. Among middle-aged black adults in this community-based population, a positive linear association was observed between PTH levels and risk of incident hypertension. Furthermore, black participants with clinically elevated PTH levels (≥65 pg/mL) were at greater risk of incident hypertension, relative to blacks with normal PTH levels. In contrast, we found no evidence for an association of PTH with incident hypertension among white adults.
Few studies have prospectively evaluated the association between PTH and hypertension risk. A case-control study nested within the Health Professionals Follow-up Study[12] and a study of administrative data from the Intermountain Healthcare system both found that PTH was positively associated with hypertension[30]. Both of these study populations were predominately white, and the Health Professionals Study participants had a very high prevalence of kidney stones (>50%)[12], while the Intermountain Healthcare system sample included only patients for whom 25(OH)D levels were drawn for clinical indications (e.g. osteoporosis risk)[30]. In the population-based Multi-Ethnic Study of Atherosclerosis, which included 1,206 whites, 588 blacks, 418 Chinese and 692 Hispanics participants, elevated PTH (≥65 pg/ml) was associated with approximately 30% greater risk of incident hypertension[13]. There was no evidence in this study that the association varied by race/ethnicity, but they did observe an interaction whereby the association between high PTH and incident hypertension was stronger among obese individuals. The obesity interaction was not replicated in ARIC. PTH was also associated with measured blood pressure in cross-sectional studies of elderly individuals in France and China[31, 32]. However, in a population-based cross-sectional study of Chinese individuals aged 20–83 years, PTH was not independently associated with blood pressure or risk of hypertension[33].
In the present analysis of 6,240 whites and 1,264 blacks, as expected, PTH levels were higher in blacks than in whites[16]. Furthermore, the association between PTH and incident HTN was seemingly stronger among blacks than whites. The physiology of markers of mineral metabolism, such as PTH, calcium and 25(OH)D, is intricately intertwined, and may vary by race[16, 34, 35]. As such, it is possible that racial differences in these interrelations may underlie our observed interaction. Suppression of PTH has been shown to induce increases in ionized calcium and decrease vascular tone[36, 37]. Blacks are believed to be more efficient than whites in absorbing dietary calcium, preserving calcium in the bones, and retaining calcium in the kidney[17–20]. However, blacks are also known to consume lower levels of dietary calcium than other racial/ethnic groups[38]. As has been reviewed elsewhere, population studies indicate that high dietary calcium intake is associated with lower risk of developing hypertension, whereas meta-analyses of clinical trials have shown a modest reduction in BP with calcium supplementation[39–43]. Potential benefits of calcium supplementation may vary by individual characteristics; the effects of supplementation on blood pressure appear to be more pronounced in blacks than whites, as well as individuals who are salt-sensitive, and those who have low (≤800 mg/day) basal calcium intakes [36, 43].
There are also racial differences in the interplay between PTH and 25(OH)D. Among whites, an inverse association between PTH and 25(OH)D has been observed across the range of 25(OH)D, whereas in blacks there is no association between 25(OH)D and PTH above the threshold commonly used to define vitamin D deficiency (20 ng/mL) whereas below that threshold an inverse association is present15. However, the PTH-hypertension association was not modified by vitamin D deficiency status in our data. Low 25(OH)D has been associated with greater risk of incident hypertension in some studies[16, 44–46] but no such association was seen in others [31–33]. In our data, after accounting for PTH, 25(OH)D was not associated with risk of incident hypertension. The inconsistency between studies may be due to differences in methodology, the racial/ethnic distributions of the study populations, or possibly differences in levels of PTH and 25(OH)D in the study populations. Though our finding of a qualitatively stronger association between PTH and hypertension among blacks than whites was unexpected, the interrelation of markers of mineral metabolism clearly varies by race, and how these racial differences influence health outcomes needs further elucidation.
This study has several limitations. PTH was measured once and any changes over time or within person variability likely would have attenuated the hazard ratios observed[47]. Additionally, as with all observational studies, residual confounding may have occurred, despite adjustment for known risk factors for hypertension. For example, information on 24-h sodium or potassium urinary excretion was not available. Related, since this is observational research, it is unclear whether the observed associations are causal. Furthermore, meta-analyses of clinical trials supplementing related nutrients – namely calcium and vitamin D - have shown only modest reductions in BP[42, 43, 48]. Thus, conclusions from this study should be interpreted with caution. Since the study included black and white participants aged 45–65 years at baseline, generalizability of our findings to other racial/ethnic groups and age-ranges is uncertain. Nevertheless, the study has several strengths, including the prospective design, population-based sample, objective ascertainment of hypertension, high-quality of data collection, large number of events, and corresponding power for subgroup analyses.
In conclusion, in this large, population-based cohort, elevated PTH was associated with hypertension in black but not white adults. Future studies of PTH should consider potential interactions by race and be focused on race differences in etiology.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank the staff and participants of the ARIC study for their important contributions.
Source of funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Measurement of PTH and some of the related biomarkers was supported through National Institutes of Health grants R01 HL103706, R01 NS072243, and R01 DK089174.
Footnotes
Previous presentation: Poster presentation at the American Heart Association Epidemiology and Prevention/Nutrition, Physical Activity and Metabolism Scientific Sessions, Baltimore, MD, March 3–6, 2015.
Conflicts of interest: None.
REFERENCES
- 1.Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 2.Rashid G, Bernheim J, Green J, Benchetrit S. Parathyroid hormone stimulates endothelial expression of atherosclerotic parameters through protein kinase pathways. Am J Physiol Renal Physiol. 2007;292:F1215–F1218. doi: 10.1152/ajprenal.00406.2006. [DOI] [PubMed] [Google Scholar]
- 3.Isales CM, Sumpio B, Bollag RJ, Zhong Q, Ding KH, Du W, et al. Functional parathyroid hormone receptors are present in an umbilical vein endothelial cell line. Am J Physiol Endocrinol Metab. 2000;279:E654–E662. doi: 10.1152/ajpendo.2000.279.3.E654. [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V, Hewitson TD, Kelynack KJ, Martic M, Tait MG, Becker GJ. Parathyroid hormone has a prosclerotic effect on vascular smooth muscle cells. Kidney Blood Press Res. 2003;26:27–33. doi: 10.1159/000069761. [DOI] [PubMed] [Google Scholar]
- 5.Pilz S, Tomaschitz A. Role of vitamin D in arterial hypertension. Expert Rev Cardiovasc Ther. 2010;8:1599–1608. doi: 10.1586/erc.10.142. [DOI] [PubMed] [Google Scholar]
- 6.Grant FD, Mandel SJ, Brown EM, Williams GH, Seely EW. Interrelationships between the renin-angiotensin-aldosterone and calcium homeostatic systems. J Clin Endocrinol Metab. 1992;75:988–992. doi: 10.1210/jcem.75.4.1400892. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick LA, Bilezikian JP, Silverberg SJ. Parathyroid hormone and the cardiovascular system. Curr Osteoporos Rep. 2008;6:77–83. doi: 10.1007/s11914-008-0014-8. [DOI] [PubMed] [Google Scholar]
- 8.Beierwaltes WH. The role of calcium in the regulation of renin secretion. Am J Physiol Renal Physiol. 2010;298:F1–F11. doi: 10.1152/ajprenal.00143.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snijder MB, Lips P, Seidell JC, Visser M, Deeg DJ, Dekker JM, van Dam RM. Vitamin D status and parathyroid hormone levels in relation to blood pressure: a population-based study in older men and women. J Intern Med. 2007;261:558–565. doi: 10.1111/j.1365-2796.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhao G, Ford ES, Li C, Kris-Etherton PM, Etherton TD, Balluz LS. Independent associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with blood pressure among US adults. J Hypertens. 2010;28:1821–1828. doi: 10.1097/HJH.0b013e32833bc5b4. [DOI] [PubMed] [Google Scholar]
- 11.Jorde R, Sundsfjord J, Haug E, Bonaa KH. Relation between low calcium intake, parathyroid hormone, and blood pressure. Hypertension. 2000;35:1154–1159. doi: 10.1161/01.hyp.35.5.1154. [DOI] [PubMed] [Google Scholar]
- 12.Taylor EN, Curhan GC, Forman JP. Parathyroid hormone and the risk of incident hypertension. J Hypertens. 2008;26:1390–1394. doi: 10.1097/HJH.0b013e3282ffb43b. [DOI] [PubMed] [Google Scholar]
- 13.van Ballegooijen AJ, Kestenbaum B, Sachs MC, de Boer IH, Siscovick DS, Hoofnagle AN, et al. Association of 25-Hydroxyvitamin D and Parathyroid Hormone With Incident Hypertension: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63:1214–1222. doi: 10.1016/j.jacc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon SS, Burt V, Louis T, Carroll MD. Hypertension among adults in the United States, 2009–2010. NCHS Data Brief. 2012;(107):1–8. [PubMed] [Google Scholar]
- 15.Quinones AR, Liang J, Ye W. Racial and ethnic differences in hypertension risk: new diagnoses after age 50. Ethn Dis. 2012;22:175–180. [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22:1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell NH, Yergey AL, Vieira NE, Oexmann MJ, Shary JR. Demonstration of a difference in urinary calcium, not calcium absorption, in black and white adolescents. J Bone Miner Res. 1993;8:1111–1115. doi: 10.1002/jbmr.5650080912. [DOI] [PubMed] [Google Scholar]
- 18.Bryant RJ, Wastney ME, Martin BR, Wood O, McCabe GP, Morshidi M, et al. Racial differences in bone turnover and calcium metabolism in adolescent females. J Clin Endocrinol Metab. 2003;88:1043–1047. doi: 10.1210/jc.2002-021367. [DOI] [PubMed] [Google Scholar]
- 19.Cosman F, Morgan DC, Nieves JW, Shen V, Luckey MM, Dempster DW, et al. Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res. 1997;12:958–966. doi: 10.1359/jbmr.1997.12.6.958. [DOI] [PubMed] [Google Scholar]
- 20.Heaney RP. The importance of calcium intake for lifelong skeletal health. Calcif Tissue Int. 2002;70:70–73. doi: 10.1007/s00223-001-0032-3. [DOI] [PubMed] [Google Scholar]
- 21.Anonymous The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 22.Cavalier E, Delanaye P, Hubert P, Krzesinski JM, Chapelle JP, Rozet E. Estimation of the stability of parathyroid hormone when stored at-80 degrees C for a long period. Clin J Am Soc Nephrol. 2009;4:1988–1992. doi: 10.2215/CJN.03970609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 24.Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc. 1997;29:901–909. doi: 10.1097/00005768-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 27.Allison P. Discrete-Time Methods for the Analysis of Event Histories. Sociol Methods Res. 1982;15:61–98. [Google Scholar]
- 28.Prentice RL, Gloeckler LA. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- 29.Eastell R, Arnold A, Brandi ML, Brown EM, D'Amour P, Hanley DA, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:340–350. doi: 10.1210/jc.2008-1758. [DOI] [PubMed] [Google Scholar]
- 30.Anderson JL, Vanwoerkom RC, Horne BD, Bair TL, May HT, Lappe DL, Muhlestein JB. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: dependent or independent risk factors? Am Heart J. 2011;162:331–339.e2. doi: 10.1016/j.ahj.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Chan R, Chan D, Woo J, Ohlsson C, Mellstrom D, Kwok T, Leung P. Serum 25-hydroxyvitamin D and parathyroid hormone levels in relation to blood pressure in a cross-sectional study in older Chinese men. J Hum Hypertens. 2012;26:20–27. doi: 10.1038/jhh.2010.126. [DOI] [PubMed] [Google Scholar]
- 32.Mateus-Hamdan L, Beauchet O, Bouvard B, Legrand E, Fantino B, Annweiler C. High parathyroid hormone, but not low vitamin D concentrations, expose elderly inpatients to hypertension. Geriatr Gerontol Int. 2013;13:783–791. doi: 10.1111/j.1447-0594.2012.00945.x. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Yin X, Yao C, Zhu X, Wu X. Vitamin D, parathyroid hormone and their associations with hypertension in a Chinese population. PLoS One. 2012;7:e43344. doi: 10.1371/journal.pone.0043344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuleihan GE, Gundberg CM, Gleason R, Brown EM, Stromski ME, Grant FD, Conlin PR. Racial differences in parathyroid hormone dynamics. J Clin Endocrinol Metab. 1994;79:1642–1647. doi: 10.1210/jcem.79.6.7989469. [DOI] [PubMed] [Google Scholar]
- 35.Isakova T. Racial differences in parathyroid hormone levels in CKD. Nephrol Dial Transplant. 2012;27:2616–2617. doi: 10.1093/ndt/gfs173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowers JR, Zemel MB, Zemel PC, Standley PR. Calcium metabolism and dietary calcium in salt sensitive hypertension. Am J Hypertens. 1991;4:557–563. doi: 10.1093/ajh/4.6.557. [DOI] [PubMed] [Google Scholar]
- 37.Resnick LM, Oparil S, Chait A, Haynes RB, Kris-Etherton P, Stern JS, et al. Factors affecting blood pressure responses to diet: the Vanguard study. Am J Hypertens. 2000;13:956–965. doi: 10.1016/s0895-7061(00)01221-8. [DOI] [PubMed] [Google Scholar]
- 38.O'Neil CE, Nicklas TA, Keast DR, Fulgoni VL. Ethnic disparities among food sources of energy and nutrients of public health concern and nutrients to limit in adults in the United States: NHANES 2003–2006. Food Nutr Res. 2014;58:15784. doi: 10.3402/fnr.v58.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houston MC, Harper KJ. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. J Clin Hypertens (Greenwich) 2008;10:3–11. doi: 10.1111/j.1751-7176.2008.08575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ascherio A, Rimm EB, Giovannucci EL, Colditz GA, Rosner B, Willett WC, et al. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992;86:1475–1484. doi: 10.1161/01.cir.86.5.1475. [DOI] [PubMed] [Google Scholar]
- 41.Ascherio A, Hennekens C, Willett WC, Sacks F, Rosner B, Manson J, et al. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension. 1996;27:1065–1072. doi: 10.1161/01.hyp.27.5.1065. [DOI] [PubMed] [Google Scholar]
- 42.Dickinson HO, Nicolson DJ, Cook JV, Campbell F, Beyer FR, Ford GA, Mason J. Calcium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006;(2):CD004639. doi: 10.1002/14651858.CD004639.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Mierlo LA, Arends LR, Streppel MT, Zeegers MP, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2006;20:571–580. doi: 10.1038/sj.jhh.1002038. [DOI] [PubMed] [Google Scholar]
- 44.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 46.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28:205–221. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 47.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 48.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27:1948–1954. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.