Abstract
This study explored individuals' preferences for genetic testing for colorectal cancer (CRC) in a screening situation and their willingness to participate in genetic testing for Lynch syndrome, familial adenomatous polyposis (FAP), and familial colorectal cancer (FCC). For that purpose, 532 respondents aged 55–65 years completed a Discrete Choice Experiment. Using panel latent class models, the preferences for two screening situation characteristics (the probability of being genetically predisposed and the probability of developing CRC) and screening test characteristics (the frequency of preventive colonoscopies and CRC survival) were estimated. Based on these preferences, respondents' willingness to participate in the three screening initiatives was estimated. Lower-educated respondents and respondents who express serious anxiety and worries found colonoscopy frequency and the probability of developing CRC relatively more important and survival relatively less important compared with higher-educated respondents and respondents who express no anxiety and worries. These differences in preferences resulted in opposite preferences for participation in FCC and FAP screening. In conclusion, the general population is willing to participate in genetic screening for CRC. If individuals are suspected of genetic or familial CRC, they should at least be informed about their increased risk of being genetically predisposed and about the importance of participating in all preventive follow-up colonoscopies in order to maximize survival.
Introduction
Although genetic screening, in addition to population-based colorectal cancer (CRC) screening, may be beneficial for those who run a higher risk of developing CRC, there is a discussion about whether this additional form of screening is advisable and desirable.1, 2, 3, 4 CRC is one of the most commonly diagnosed cancers and the leading cause of death among all cancer types worldwide.5 Prognosis, treatment intensity and the 5-year survival rate significantly improve if CRC is diagnosed at an early stage.6, 7 Moreover, CRC can actually be prevented, because it is usually preceded by a slow progressive premalignant lesion (an adenomatous polyp), which may become cancer but can be detected and removed during colonoscopy.7 Therefore, population-based screening programs for CRC are recommended and widely implemented in Western countries. Within these programs, there is little attention for genetically predisposed individuals who run a higher risk of developing CRC. About 5% of all diagnosed CRCs is of genetic origin.8, 9, 10 This relatively small percentage actually reflects a substantial number of CRC patients given the high incidence of CRC in the general population. Offering genetic testing to participants in a population-based CRC screening program after a positive colonoscopy and/or with a familial cancer history (ie, screening situation) will identify genetically predisposed individuals and their families.11, 12 By including genetic screening in current population-based CRC screening programs, CRC-related morbidity and mortality may further decrease due to increased surveillance of cases and their relatives.11, 12, 13
However, genetic testing raises several ethical and counseling challenges.14, 15 For instance, knowing that one is at risk to develop cancer might induce fear of actually developing cancer, possibly with a negative impact on a person's quality of life.16, 17 Positive test results may also have a severe impact on the family of the tested individual,16, 17, 18 as they themselves might run a higher risk of developing cancer as well. Moreover, the general population often holds unrealistic expectations about the accuracy with which genetic screening tests can predict future disease status.17, 19
Despite these potential negative consequences, the general population shows great interest in genetic screening and has a positive attitude towards such screening initiatives.16, 20, 21, 22 Previous research shows that individuals are willing to take part in genetic screening when the test aims to identify an increased risk for a monogenic form of a common disease, when adequate treatment and/or prevention options are available and when clinicians recommend screening.21, 23, 24, 25
To date, no research has been conducted into studying the preferences of the general population for genetic testing for CRC specifically within a screening situation. Therefore, this study aims to explore individual preferences concerning genetic testing for CRC within a population-based CRC screening program. A further aim is to estimate whether individuals are willing to participate in genetic testing for (1) Lynch syndrome, (2) familial adenomatous polyposis (FAP) and (3) familial colorectal cancer (FCC) within a screening situation.
Materials and methods
Discrete choice experiment (DCE)
DCEs are increasingly being used to determine an individual's preferences regarding different characteristics of interventions or medical treatments.26 This method is based on the Random Utility Theory. This theory assumes that any intervention or treatment can be described by its characteristics or ‘attributes', such as the probability of a positive test outcome. The preferences of an individual for an intervention or treatment is determined on the basis of the ‘levels' of the attributes, such as 1, 3 or 15% probability that the test outcome is positive.26 Hypothetical situations are constructed by varying the levels of the attributes. Respondents are provided with a series of ‘choice tasks' that consist of at least two situations. They are asked to choose the situation they prefer most within every choice task.
DCE development
To construct the DCE used for this study, possible attributes were identified from previously published studies,21, 22, 23, 24, 27 six expert interviews (ie, a scientist with a specific interest in public health genomics, a scientist with a specific interest in ethics of genetics/genomics, a specialist in cancer genetics and three medical specialists in gastroenterology) and five group interviews (n=38) with the target population of men and women aged 55–65 years. These group interviews were conducted using the Nominal Group Technique.28 During these interviews, participants were asked to rank a number of potential attributes from most to least important, and the mean group ranking of the attributes was then discussed in the group, after which participants could change their original individual ranking.28 Finally, four attributes were selected for this DCE (Table 1). The levels that were used to describe the identified attributes were based on realistic numbers representing the three most common types of genetic and familial CRC: Lynch syndrome, FAP, and FCC. About 3% of all CRC patients are diagnosed with Lynch syndrome.4, 29, 30, 31 Without surveillance, these patients have a 70% probability of developing CRC during their lifetime.4, 29, 30, 31 Patients who are diagnosed with Lynch syndrome are offered a preventive colonoscopy every 2 years. On average, their 5-year survival rate is 92% if they are aware of their genetic predisposition and participate in biannual colonoscopies.4, 29, 30, 31 FAP is present in 1% of all CRC patients.29, 30, 31 The probability of developing CRC among these patients is 99% without surveillance and therefore they are advised to undergo an annual colonoscopy.29, 30, 31 This results in a 5-year survival rate of 80% if CRC is discovered.29, 30, 31 Finally, FCC is considered to be present in 15% of all CRC patients.29, 30, 31 These patients have an at least 15% probability of developing CRC based on the number and age of relatives with CRC. They are offered screening by means of a 5-yearly colonoscopy, which increases their 5-year survival rate to 98% if CRC is found.29, 30, 31
Table 1. Attributes and levels that were included in this DCE.
| Attributes | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| Probability of being genetically predisposed (genetic predisposition): the likelihood that you are genetically predisposed to develop colorectal cancer | |||
| 1%, 1 out of every 100 | 3%, 3 out of every 100 | 15%, 15 out of every 100 | |
| Probability of developing CRC (CRC risk): 5 out of every 100 (5%) Dutch individuals develop colorectal cancer. If you have a genetic predisposition to develop colorectal cancer and you do not participate in preventive colonoscopies, the likelihood that you will develop colorectal cancer is higher and varies between: | |||
| 15%, 15 out of every 100 | 70%, 70 out of every 100 | 99%, 99 out of every 100 | |
| Frequency of preventive colonoscopies (colonoscopy frequency): If the genetic test shows that you are genetically predisposed to develop colorectal cancer, you will be invited to participate in preventive colonoscopies. These colonoscopies are performed to prevent cancer from developing or to diagnose cancer in an early stage. These colonoscopies will be scheduled on a regular basis varying between: | |||
| Every year | Every 2 years | Every 5 years | |
| Probability of surviving CRC (survival): 60 out of every 100 (60%) Dutch individuals with colorectal cancer survive over the next 5 years. If you know you are genetically predisposed to develop colorectal cancer and if you participate in the preventive colonoscopies, the likelihood that you will survive colorectal cancer over the next 5 years will increase and varies between: | |||
| 80%, 80 out of every 100 | 92%, 92 out of every 100 | 98%, 98 out of every 100 | |
Abbreviation: CRC, colorectal cancer.
NGene 1.0 (ChoiceMetrics pty ltd, 2011, St. Leonards, NSW, Australia) software was used to develop a D-efficient design.26 The DCE consisted of nine unique choice tasks each containing two situations. Following each choice task, participants were asked whether they would actually participate in the chosen situation or not (ie, opt-out). Before participants were asked to complete the choice tasks, they received detailed information on the meaning of all attributes and levels as well as an explanation on how to complete a choice task, illustrated by an example (see Supplementary File). The draft questionnaire was pilot tested among a subgroup (n=90) of our target population. Four of these pilot tests were ‘think aloud' tests, during which a researcher was present when the participant completed the questionnaire, reading out loud. It was tested by means of this pilot whether correct wording was used and whether the target population understood the attributes, levels and choice tasks. Additionally, the attribute-level estimates that were retrieved from the pilot study served as input for the design of the final DCE questionnaire.
Questionnaire
The final questionnaire consisted of three parts. The first section of the questionnaire comprised 25 questions on demographics, such as gender, age, educational level, health literacy and ethnicity. Educational level was dichotomized into higher (ie, tertiary education) or lower education (ie, all other educational levels). Health literacy was measured by three validated Dutch questions of the Set of Brief Screening Questions.32 Participants scored these questions on a five-point Likert scale, from zero to four. An average score of ≤2 indicates inadequate health literacy, while an average score >2 indicates adequate health literacy.32 Furthermore, questions pertained to information on experience with other national cancer screening programs, experience with genetic screening and family cancer history. Respondents were asked to indicate to what extent they agreed or disagreed with several theorems about their attitude, social norm, self-efficacy and intention towards genetic screening for CRC. The second part of the questionnaire consisted of the actual DCE as explained above. The third part consisted of several theorems regarding the consequences of genetic testing, such as fear and worries, and on the possibility of incidental findings.
Study population
From 2014 onwards, all Dutch residents aged 55–75 years will receive a biannual invitation to participate in the national population-based screening program for CRC. Screening is carried out by means of the fecal immunochemical test. If the test result is positive, that is, blood is detected in the stool, a colonoscopy will be planned and participants are asked to complete a family cancer history questionnaire. At present, it is expected that genetic screening for CRC might only become part of the Dutch CRC screening program for individuals with a positive colonoscopy and/or a familial cancer history.
As it was expected that preferences for genetic screening for CRC are highly dependent on age and experience with CRC screening, individuals were eligible to participate in our study if they were aged 55–65 years and had not yet participated in the CRC screening program or one of the extensive pilot studies that preceded the decision to implement the Dutch population-based CRC screening. Respondents were recruited via an existing online panel of the general Dutch population. Respondents were selected to be representative for the entire target population with respect to age, gender and educational level. In total, 5500 individuals were invited to participate in this study and recruitment continued until at least 500 questionnaires were fully completed by a representative sample of the target population.
The Dutch Central Committee on Research involving Human Subjects concluded that formal testing by an Institutional Review Board was not necessary, as respondents were only required to complete an anonymous and non-invasive questionnaire once, which is in accordance with the Dutch legislation and guidelines laid down in the Declaration of Helsinki.
Statistical analysis
All results were considered statistically significant when P<0.05. All attributes were considered to be non-linear and were recoded using effect codes.26 This coding procedure codes the reference category as −1 and the sum of the effect-coded attribute levels is always 0.
Preferences for genetic screening for CRC
Nlogit 5.0 (Econometric Software Inc, 2012, Plainview, NY, USA) was used to conduct the panel latent class models for this study. Such models account for the multilevel structure of our data (ie, every respondent answered nine choice tasks). Moreover, by means of such models, it can be determined whether preferences differ across unobserved subgroups of the population. This modelling procedure identifies whether there are ‘classes' within the data based on respondents' answering patterns. Which respondents belong to what class is not assigned by researchers but is latent. Each respondent has a certain probability to belong to one of the identified classes. However, demographic characteristics can be incorporated into the modelling procedure, which provides insight into which respondents are more likely to belong to a certain class.
Based on model fit tests (AIC, Log likelihood), it was tested which model was most suitable for our data and how many classes could be identified within the data. This resulted in a two-class model based on the utility equation displayed below. The utility component (V) describes the utility that respondent ‘r' belonging to class ‘c' reported for alternative ‘a' in choice task ‘t'. β0 represents the constant of the model. The attribute-level estimates that indicate the relative importance of each attribute level are represented by β1−β8. A significant attribute estimate within a certain class indicates that this attribute contributes to the decision-making process of respondents who belong to that class.
Vrta|c=β0|c+β1|c genetic predisposition3% rta|c+β2|c genetic predisposition15% rta|c+β3|c CRC risk70% rta|c+β4|c CRC risk99% rta|c+β5|c colonoscopy frequency2years rta|c+β6|c colonoscopy frequency5years rta|c+β7|c survival92% rta|c+β8|c survival98% rta|c
After fitting the above-specified utility function, a class assignment model was fitted. All demographic variables and all theorems were tested for a significant contribution to the class assignment model, and the final class assignment utility function was:
Vrc=β0|c+β1|c high educational levelr+β2|c experience with genetic screeningr+β3|c being anxious and worried about CRC predispositionr
A significant estimate in this function indicates that this variable contributes to the class assignment (eg, if the higher education variable is positive and significant for class 1, this indicates that respondents with a higher educational level are more likely to belong to class 1).
Relative importance of the attributes
The relative importance of the attributes was estimated separately for both classes of the panel latent class models. The difference between the highest and lowest attribute-level estimate was calculated for each attribute. The largest difference value received an importance score of 1, representing the attribute that was deemed most important by respondents. The other difference values were divided by the largest difference value resulting in a relative distance between all other attributes and the most important attribute.
Utility scores for Lynch syndrome, FAP and FCC screening
For each of the three realistic screening scenarios, specific utility scores were calculated for both classes separately. The attribute levels that correspond with each of the three screening scenarios were entered into the utility function. The outcome (V) represents individuals' willingness to participate in one screening initiative compared with the other initiatives.
Results
Respondents' characteristics
Of the individuals initially invited (n=5500), 798 (14.5%) respondents started the questionnaire within the first 4 weeks of data collection. Complete data was gathered for 532 eligible respondents (66.7% of those who started the questionnaire) and data collection was closed.
Table 2 describes the demographic characteristics of the study population. The majority of the respondents reported that genetic screening for CRC is important for themselves as well as for their family (Table 3). Although about half of the respondents expect to become seriously anxious and worried about developing CRC due to a suspected genetic predisposition, 89.0% reported that they would participate in genetic screening for CRC if such a program would become available (Table 3).
Table 2. Demographic characteristics of the study population (n=532).
| Mean (SD) | Percentage | |
|---|---|---|
| Age, years | 59.5 (3.1) | |
| Gender | ||
| Female | 50.9 | |
| Educational level | ||
| Low | 26.3 | |
| Average | 37.1 | |
| High | 36.6 | |
| Health literacy | ||
| Inadequate | 3.4 | |
| Ethnicity | ||
| Dutch | 96.6 | |
| Previously participated in another cancer screening program | 48.9 | |
| Previously diagnosed with cancer | 14.0 | |
| Family member previously diagnosed with cancer | 24.6 | |
| Previously participated in genetic screening | 7.7 | |
Table 3. Proportion of respondents who agree with the provided theorems concerning genetic screening for CRC (n=532).
| Percentage | |
|---|---|
| I think genetic testing for CRC is useful | 89.1 |
| I think it is important to take part in genetic testing for CRC | 86.9 |
| I consider it self-evident to take part in CRC | 77.4 |
| It would not be difficult for me to take part in genetic testing for CRC | 76.7 |
| It is important that my family takes part in genetic screening for CRC | 70.3 |
| My family would take part in genetic screening for CRC | 69.1 |
| My family would consider it important that I take part in genetic screening for CRC | 71.2 |
| It is important to know whether I am genetically predisposed so my family can take precautions | 87.4 |
| I would inform my family if I was genetically predisposed to develop CRC | 89.1 |
| I would be seriously anxious if I was genetically predisposed to develop CRC | 43.7 |
| I would find it seriously worrying if I was genetically predisposed to develop CRC | 65.2 |
| I always want to know about incidental findings | 75.9 |
| I never want to know about incidental findings | 3.2 |
| I only want to know about incidental findings concerning diseases that can be prevented | 8.3 |
| I only want to know about incidental findings concerning diseases that can be treated | 12.6 |
| I would take part in genetic screening for CRC | 89.1 |
Abbreviation: CRC, colorectal cancer.
Preferences for genetic screening for CRC
The average probability of respondents belonging to either of the two latent classes was 65% and 35%, respectively, but this depended on educational level, experience with genetic screening tests and being worried and anxious about being predisposed to develop CRC (Table 4). Respondents with a higher educational level, respondents who had no experience with genetic screening tests and respondents who were less worried and anxious about their predisposition to develop CRC were more likely to belong to class 1. The probability of belonging to class 2 increased when respondents had a lower educational level, when they had experience with genetic screening tests and when they were worried and anxious about being predisposed to develop CRC.
Table 4. Preferences for genetic testing for colorectal cancer based on latent class analysisa.
| Class 1 | Class 2 | |||||
|---|---|---|---|---|---|---|
| Estimate | SE | RI | Estimate | SE | RI | |
| Constant | 0.21*** | 0.04 | 0.14 | 0.12 | ||
| Genetic predisposition | ||||||
| 1% (ref.) | −0.20*** | 0.04 | 4 | 0.18* | 0.11 | 4 |
| 3% | −0.07* | 0.04 | −0.31*** | 0.10 | ||
| 15% | 0.27*** | 0.04 | 0.13 | 0.10 | ||
| CRC risk | ||||||
| 15% (ref.) | −0.37*** | 0.04 | 3 | 0.63*** | 0.11 | 2 |
| 70% | 0.20*** | 0.04 | −0.22** | 0.10 | ||
| 99% | 0.17*** | 0.04 | −0.41*** | 0.11 | ||
| Colonoscopy frequency | ||||||
| Every year (ref.) | −0.29*** | 0.04 | 2 | 1.13*** | 0.12 | 1 |
| Every 2 years | 0.29*** | 0.04 | 0.61*** | 0.11 | ||
| Every 5 years | −0.00 | 0.05 | −1.74*** | 0.18 | ||
| Survival | ||||||
| 80% (ref.) | −0.57*** | 0.04 | 1 | −0.51*** | 0.16 | 3 |
| 92% | −0.01 | 0.04 | 0.10 | 0.08 | ||
| 98% | 0.58*** | 0.05 | 0.41** | 0.16 | ||
| Class probability model | ||||||
| Constant | 0.78*** | 0.18 | ||||
| Higher education | 1.02*** | 0.24 | ||||
| Experience with genetic screening | −0.87** | 0.39 | ||||
| Being worried and anxious | −0.88*** | 0.22 | ||||
| Average class probability | 0.65 | 0.35 | ||||
Abbreviations: CRC=colorectal cancer; RI=relative importance. *P<0.10; **P <0.05; ***P <0.01.
The attribute-level estimate of the reference categories can be calculated as: −1 × (sum of the other attribute-level estimates).
In both classes, respondents preferred a genetic screening test when their probability of being genetically predisposed to develop CRC was high and their survival rate due to screening would increase the most. Respondents in class 1 preferred a genetic screening when the probability that they would develop CRC due to their genetic predisposition was highest, while respondents in class 2 preferred a genetic screening test if the probability that they would develop CRC due to their genetic predisposition was lowest. Respondents in class 1 preferred to have a biannual colonoscopy, while respondents in class 2 preferred to have an annual preventive colonoscopy.
Relative importance of the attributes
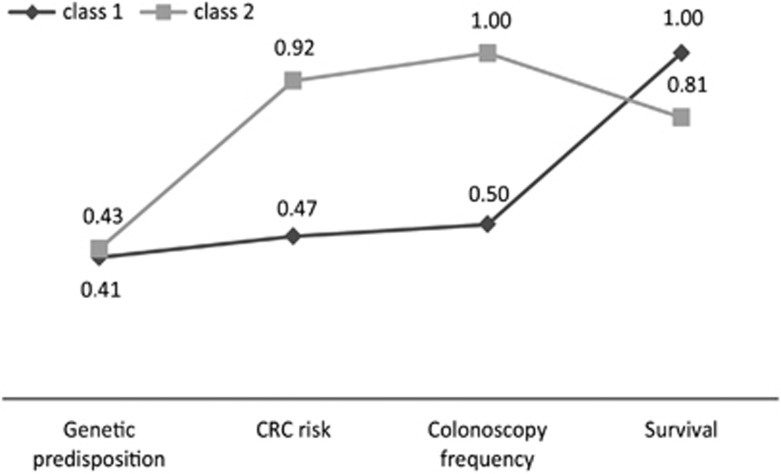
Respondents in both classes reported different preferences with respect to genetic screening for CRC, which indicates preference heterogeneity. Respondents in class 1 found survival to be most important, followed by colonoscopy frequency, CRC risk and genetic predisposition (Table 4). For respondents in class 2, colonoscopy frequency was most important (relative importance score of 1) followed by CRC risk, survival and genetic predisposition (Table 4). Figure 1 shows these results in more detail, here the values of the attributes display the relative distance of all attributes to the most important attribute on a scale of 0–1. The range in those distances is large in class 2, while for respondents in class 1 most attributes were approximately equally important.
Figure 1.
Relative importance of the attributes stratified by class. Values reflect the relative distance of all attributes to the most important attribute on a scale from 0 to 1 (1 indicating the most important attribute).
Utility scores for Lynch syndrome, FAP and FCC screening
Respondents in class 1 (higher education, no experience with genetic testing and who are less anxious and worried about CRC predisposition) preferred Lynch syndrome screening (V=0.62) over FAP screening (V=−0.68) and preferred FCC screening (V=0.69) over both the other screening initiatives. Respondents in class 2 (respondents with a lower education, experience with genetic screening tests and respondents who are anxious and worried about CRC predisposition) preferred Lynch syndrome screening (V=0.32) over FCC screening (V=−0.43) and preferred FAP screening (V=0.53) over both the other screening initiatives.
Discussion
This study shows that the probability of being genetically predisposed, the probability of developing CRC, the frequency of preventive follow-up colonoscopies and the probability of surviving CRC, all influence respondents' preferences for genetic testing for CRC. However, results also show heterogeneity in these preferences. Respondents with a lower education found colonoscopy frequency and the probability of developing CRC relatively more important and survival relatively less important than higher-educated respondents. These differences in preferences were also found among respondents who had some versus no experience with genetic screening tests and among respondents with serious or little anxiety and worries about being genetically predisposed to develop CRC. Because of the differences in preferences among subgroups in the population, their willingness to participate in specific genetic screening initiatives also differed. Respondents in class 1 preferred FCC screening most and FAP screening least, while the respondents in class 2 showed complete opposite preferences for the screening initiatives.
This is the first DCE that studied the preferences of the general population for genetic testing for CRC within a screening situation. However, previous studies did measure preferences for population-based CRC screening program characteristics (without genetic screening)33, 34, 35, 36 or preferences for genetic screening test characteristics in general (not specifically applied to CRC).24 Although these studies focused on different topics and different target populations, their results do provide face validity for the results of the current study.
Insights into the preferences of the target population for genetic screening for CRC provide clear recommendations for effective communication between counselors and counselees about genetic testing specifically within a screening situation.22, 37 Optimal communication may improve knowledge among the general population and may facilitate informed decision making among individuals who are offered genetic screening. First, respondents deemed survival probability as a highly important test characteristic of genetic screening. Although increased survival rates as a result of participation in genetic screening should be communicated to those eligible for screening, counselors should bear in mind to ensure that individuals understand that their survival rates will only increase if they participate consistently in preventive colonoscopies. Second, the current study shows that some of the respondents preferred annual preventive colonoscopies, in particular those who had a lower educational level and who expressed serious anxiety and worries about a genetic predisposition for CRC. These respondents might have reasoned that frequent screening will increase the likelihood of early cancer detection and therefore will increase their probability of surviving CRC. However, annual colonoscopies are, at present, only recommended for surveillance of individuals diagnosed with FAP;29, 30, 31 individuals with Lynch syndrome or FCC are usually screened less often (biannually and every 5 or 6 years, respectively) based on solid clinical evidence.4, 29, 30, 31 For respondents with a lower educational level or those who are anxious and worried, effective communication and counseling are necessary to reduce their anxiety and to explain that screening frequency depends on the specific type of genetic or familial CRC. Third, respondents preferred a genetic screening test when their probability of being genetically predisposed increased. This result supports the fact that participation in screening will increase if all individuals suspected of genetic or familial CRC are actively informed about their personal risk of being predisposed to develop CRC. As relative occurrence of FAP within all genetic or familial CRC is relatively small (about 1%), some participants appeared less interested in FAP screening. However, this is the most aggressive and severe genetic variant of CRC for which active screening is of utmost importance.38, 39 Fortunately, most FAP patients are aware of their predisposition from a young age due to a family history resulting in an acceptable surveillance compliance.40 However, when clinicians suspect individuals may have FAP without a clear family history, they are advised to (continue to) stress the importance of active screening once genetic predisposition is confirmed.
This study is subject to some limitations. First, generalizability of our results to non-Dutch individuals may be limited because the number of non-Dutch respondents in our study population is relatively low compared with Dutch national population figures. Second, some respondents probably perceived the choice tasks to be difficult. Respondents with a lower educational level preferred to participate in a genetic screening test for CRC if their risk of actually developing CRC as a result of their genetic predisposition would be lowest. They might have mistakenly interpreted this attribute as their probability of developing CRC in general. Third, in this study we used an unlabeled design and respondents were not informed about the different genetic or familial CRC diagnoses (FAP, Lynch syndrome, FCC). Therefore, we did not include any diagnosis-specific attributes or information. Future DCE studies should be conducted to determine whether preferences differ per diagnosis.
In conclusion, the current study suggests that the general population is willing to participate in genetic testing for CRC. Both screening situation characteristics and screening test characteristics influenced respondents' preferences for genetic screening for CRC. The increased survival rates as a result of genetic screening and preventive follow-up colonoscopies were the most important screening test characteristics for respondents with a higher educational level, respondents who have no experience with genetic testing and who are less anxious and worried about CRC predisposition. The frequency of colonoscopies was the most important screening test characteristic for respondents with a lower educational level, experience with genetic testing and who were anxious and worried about CRC predisposition. If individuals are suspected of genetic or familial CRC, counsellors should provide information about their increased risk of being genetically predisposed and about the importance of participating in all the preventive follow-up colonoscopies in order to maximize their survival. Specifically, individuals with a lower educational level and those who express worries or anxiety should be informed about the frequency of preventive colonoscopies that is appropriate for the genetic or familial CRC they are diagnosed with.
Acknowledgments
This study was funded by the Strategic Research RIVM (SOR) of the National Institute for Public Health and the Environment; sponsors were not part of the research group and therefore were not involved in conducting the research, writing the manuscript or submitting the final paper.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- 1Hickner J: Will screening open Pandora's box? J Fam Pract 2013; 62: 465. [PubMed] [Google Scholar]
- 2Yazdi SM, Robin NH: We need to know our limitations: genetic testing for complex traits. Curr Opin Pediatr 2013; 25: 643–644. [DOI] [PubMed] [Google Scholar]
- 3Di Lena M, Travaglio E, Altomare DF: New strategies for colorectal cancer screening. World J Gastroenterol 2013; 19: 1855–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Giardiello FM, Allen JI, Axilbund JE et al: Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol 2014; 109: 1159–1179. [DOI] [PubMed] [Google Scholar]
- 5Ferlay J, Soerjomataram II, Dikshit R et al: Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2014; 13: 29210. [DOI] [PubMed] [Google Scholar]
- 6Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN: Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 137: 132–141. [DOI] [PubMed] [Google Scholar]
- 7Walsh JM, Terdiman JP: Colorectal cancer screening: scientific review. JAMA 2003; 289: 1288–1296. [DOI] [PubMed] [Google Scholar]
- 8Bogaert J, Prenen H: Molecular genetics of colorectal cancer. Ann Gastroenterol 2014; 27: 9–14. [PMC free article] [PubMed] [Google Scholar]
- 9Haydon AM, Jass JR: Emerging pathways in colorectal-cancer development. Lancet Oncol 2002; 3: 83–88. [DOI] [PubMed] [Google Scholar]
- 10Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–767. [DOI] [PubMed] [Google Scholar]
- 11Jarvinen HJ, Renkonen-Sinisalo L, Aktan-Collan K, Peltomaki P, Aaltonen LA, Mecklin JP: Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol 2009; 27: 4793–4797. [DOI] [PubMed] [Google Scholar]
- 12Vasen HF, Abdirahman M, Brohet R et al: One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterol 2010; 138: 2300–2306. [DOI] [PubMed] [Google Scholar]
- 13Bellcross CA, Bedrosian SR, Daniels E et al: Implementing screening for Lynch syndrome among patients with newly diagnosed colorectal cancer: summary of a public health/clinical collaborative meeting. Genet Med 2012; 14: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Bredenoord AL, Onland-Moret NC, Van Delden JJ: Feedback of individual genetic results to research participants: in favor of a qualified disclosure policy. Hum Mutat 2011; 32: 861–867. [DOI] [PubMed] [Google Scholar]
- 15Lolkema MP, Gadellaa-van Hooijdonk CG, Bredenoord AL et al: Ethical, legal, and counseling challenges surrounding the return of genetic results in oncology. J Clin Oncol 2013; 31: 1842–1848. [DOI] [PubMed] [Google Scholar]
- 16Glanz K, Grove J, Lerman C, Gotay C, Le Marchand L: Correlates of intentions to obtain genetic counseling and colorectal cancer gene testing among at-risk relatives from three ethnic groups. Cancer Epidemiol Biomarkers Prev 1999; 8: 329–336. [PubMed] [Google Scholar]
- 17Finkler K, Skrzynia C, Evans JP: The new genetics and its consequences for family, kinship, medicine and medical genetics. Soc Sci Med 2003; 57: 403–412. [DOI] [PubMed] [Google Scholar]
- 18Burton AM, Hovick SR, Peterson SK: Health behaviors in patients and families with hereditary colorectal cancer. Clin Colon Rectal Surg 2012; 25: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Tambor ES, Bernhardt BA, Rodgers J, Holtzman NA, Geller G: Mapping the human genome: an assessment of media coverage and public reaction. Genet Med 2002; 4: 31–36. [DOI] [PubMed] [Google Scholar]
- 20Leventhal KG, Tuong W, Peshkin BN et al: "Is it really worth it to get tested?": primary care patients' impressions of predictive SNP testing for colon cancer. J Genet Couns 2013; 22: 138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Matro JM, Ruth KJ, Wong YN et al: Cost sharing and hereditary cancer risk: predictors of willingness-to-pay for genetic testing. J Genet Couns 2014; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Walsh J, Arora M, Hosenfeld C, Ladabaum U, Kuppermann M, Knight SJ: Preferences for genetic testing to identify hereditary colorectal cancer: perspectives of high-risk patients, community members, and clinicians. J Cancer Educ 2012; 27: 112–119. [DOI] [PubMed] [Google Scholar]
- 23Kuppermann M, Wang G, Wong S et al: Preferences for outcomes associated with decisions to undergo or forgo genetic testing for Lynch syndrome. Cancer 2013; 119: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Severin F, Schmidtke J, Muhlbacher A, Rogowski WH: Eliciting preferences for priority setting in genetic testing: a pilot study comparing best-worst scaling and discrete-choice experiments. Eur J Hum Genet 2013; 21: 1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Huang MY, Huston SA, Perri M: Consumer preferences for the predictive genetic test for Alzheimer disease. J Genet Couns 2014; 23: 172–178. [DOI] [PubMed] [Google Scholar]
- 26Hensher DA, Rose JM, Greene WH: Applied Choice Analysis: A Primer. Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- 27Hall J, Fiebig DG, King MT, Hossain I, Louviere JJ: What influences participation in genetic carrier testing? Results from a discrete choice experiment. J Health Econ 2006; 25: 520–537. [DOI] [PubMed] [Google Scholar]
- 28Hiligsmann M, van Durme C, Geusens P et al: Nominal group technique to select attributes for discrete choice experiments: an example for drug treatment choice in osteoporosis. Patient Prefer Adherence 2013; 7: 133–139.:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29American Gastroenterological Association: American Gastroenterological Association medical position statement: hereditary colorectal cancer and genetic testing. Gastroenterology 2001; 121: 195–197. [DOI] [PubMed] [Google Scholar]
- 30Dutch Society For Clinical Genetics: CBO Guideline Hereditary Colorectal Cancer 2008. Oisterwijk, The Netherlands: Van den Boogaard, 2008.
- 31Vasen HF, van der Meulen-de Jong AE, de Vos Tot Nederveen Cappel WH, Oliveira J, , Group EGW: Familial colorectal cancer risk: ESMO clinical recommendations. Ann Oncol 2009; 20: 51–53. [DOI] [PubMed] [Google Scholar]
- 32Fransen MP, Van Schaik TM, Twickler TB, Essink-Bot ML: Applicability of internationally available health literacy measures in the Netherlands. J Health Commun 2011; 16: 134–149. [DOI] [PubMed] [Google Scholar]
- 33Benning TM, Dellaert BG, Severens JL, Dirksen CD: The effect of presenting information about invasive follow-up testing on individuals' noninvasive colorectal cancer screening participation decision: results from a discrete choice experiment. Value Health 2014; 17: 578–587. [DOI] [PubMed] [Google Scholar]
- 34Groothuis-Oudshoorn CG, Fermont JM, van Til JA, Ijzerman MJ: Public stated preferences and predicted uptake for genome-based colorectal cancer screening. BMC Med Inf Decision Making 2014; 14: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Hol L, de Bekker-Grob EW, van Dam L et al: Preferences for colorectal cancer screening strategies: a discrete choice experiment. BJC 2010; 102: 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36van Dam L, Hol L, de Bekker-Grob EW et al: What determines individuals' preferences for colorectal cancer screening programmes? A discrete choice experiment. Eur J Cancer 2010; 46: 150–159. [DOI] [PubMed] [Google Scholar]
- 37Pieterse AH, Ausems MG, Van Dulmen AM, Beemer FA, Bensing JM: Initial cancer genetic counseling consultation: change in counselees' cognitions and anxiety, and association with addressing their needs and preferences. Am J Med Genet A 2005; 137: 27–35. [DOI] [PubMed] [Google Scholar]
- 38Nieuwenhuis MH, Vasen HF: Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Onco Hematol 2007; 61: 153–161. [DOI] [PubMed] [Google Scholar]
- 39Rozen P, Macrae F: Familial adenomatous polyposis: The practical applications of clinical and molecular screening. Fam Cancer 2006; 5: 227–235. [DOI] [PubMed] [Google Scholar]
- 40Douma KF, Bleiker EM, Aaronson NK et al: Long-term compliance with endoscopic surveillance for familial adenomatous polyposis. Colorectal Dis 12: 1198–1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.