Abstract
The microaerophilic protist parasite Trichomonas vaginalis is occurring globally and causes infections in the urogenital tract in humans, a condition termed trichomoniasis. In fact, trichomoniasis is the most prevalent non-viral sexually transmitted disease with more than 250 million people infected every year. Although trichomoniasis is not life threatening in itself, it can be debilitating and increases the risk of adverse pregnancy outcomes, HIV infection, and, possibly, neoplasias in the prostate and the cervix. Apart from its role as a pathogen, T. vaginalis is also a fascinating organism with a surprisingly large genome for a parasite, i. e. larger than 160 Mb, and a physiology adapted to its microaerophilic lifestyle. In particular, the hydrogenosome, a mitochondria-derived organelle that produces hydrogen, has attracted much interest in the last few decades and rendered T. vaginalis a model organism for eukaryotic evolution.
This review will give a succinct overview of the major advances in the T. vaginalis field in the last few years.
Keywords: Trichomonas vaginalis, trichomoniasis, non-viral sexually transmitted disease, hydrogenosome, metronidazole
Introduction
Trichomonas vaginalis (Tv) is a globally occurring anaerobic/microaerophilic protist parasite which colonizes the epithelium of the human urogenital tract. Although often asymptomatic, Tv infections can cause inflammation in the cervix, the vagina, and the urethra. Based on estimates of the World Health Organization (WHO) from 2008 1, trichomoniasis constitutes the most prevalent non-viral sexually transmitted disease (STD) worldwide, affecting more than 276 million people every year. Women and men are infected with comparable frequency, but in men symptoms are normally mild and infections are cleared by the host’s immune system within weeks. In women, however, Tv infections can persist for many years, and symptoms, mainly pruritus caused by inflammation and odorous vaginal discharge, can attain a severity which is debilitating. As trichomoniasis is not a life-threatening disease, it was often belittled as a “nuisance infection” in the past. A large number of studies from the last 20 years or so, however, have shown that underlying Tv infections increase the risk of adverse pregnancy outcomes and contagion with HIV virus 2. Given the fact that HIV and Tv are often jointly epidemic in many parts of the world, this is an alarming finding. Moreover, a higher risk of developing prostate cancer due to Tv infection has been suggested 2.
Before the development of the 5-nitroimidazole drug metronidazole in 1960, trichomoniasis was notoriously difficult to treat. Nowadays, most patients can be successfully treated with metronidazole or another more effective 5-nitroimidazole derivative, tinidazole. However, resistance to 5-nitroimidazoles does occur and seems to be on the rise 3. In addition, allergic reactions to nitroimidazoles have been reported and side effects of nitroimidazole treatment can be disturbing.
Apart from its role as a pathogen, Tv has attracted great interest from geneticists, biochemists, and evolutionary biologists after the discovery of the hydrogenosome 4, a mitochondrion-like organelle which generates hydrogen. Due to its microaerophilic lifestyle, Tv does not have the ability to generate ATP by oxidative phosphorylation but depends on substrate-level phosphorylation. Originally, it was assumed that the hydrogenosome is an ancestral form of the mitochondrion 5, which kindled interest in Tv as an assumed archaic eukaryote. This notion, however, has been thoroughly revised after publication of the Tv genome in 2007 6. It is now apparent, based on phylogenetic studies, that the hydrogenosome constitutes a reduced form of fully developed mitochondria. Nevertheless, from the evolutionary and cell biologist’s point of view, the hydrogenosome remains an intriguing organelle, and the extraordinary size of the Tv genome, exceeding 160 Mb, will certainly provoke further research in the years to come.
In this review, I will give a brief but comprehensive overview of the advances in the research on Tv from the last 5 years or so, spanning from the epidemiology to the infection biology, treatment, and cell biology of this fascinating parasite.
Epidemiology
Although Tv is a worldwide occurring parasite, prevalence rates differ very strongly in different parts of the world. In the Americas, for example, its incidence is calculated to be as high as 180 per 1000 men and women, whereas in South-East Asia estimates are much lower, with 40 to 50 per 1000 men and women 7. In total, 276 million infections with Tv are believed to occur worldwide and per annum 1. These numbers are very high indeed, but it is estimated that most Tv infections (up to 80%) are asymptomatic 8. Importantly, men are infected equally frequently, but 89% of trichomoniasis cases are actually diagnosed in women because of their higher incidence of symptoms, which are sometimes severe and debilitating. The main concern about Tv infections, however, is their predisposing nature for other diseases or sequelae 2, and a number of new studies give justification to this concern. For example, Tv was found to be associated with human papilloma virus infections and cervical cytological abnormalities 9. Moreover, in a meta-analytical study, strong statistical evidence was presented for an association of an underlying Tv infection and preterm birth 10. Most importantly, however, evidence for a predisposition for infection with HIV in Tv-infected individuals is mounting. In a meta-study on 31 studies, it was concluded that the risk of HIV acquisition is increased 2- to 3-fold in Tv carriers 11, and it was found that Tv infection increased the risk of HIV infection 2.5-fold in macaques, which serve as a non-human primate model. Accordingly, it was calculated that annual screening for Tv would save US$553 per woman and lifetime in the prevention of new HIV infections to susceptible male partners in the United States alone 12.
In order to get a picture of the genetic diversity of Tv, a large-scale study 13 was conducted, subjecting 235 Tv isolates, collected from all around the globe, to microsatellite genotyping 14. Intriguingly, Tv was found to group into two distinct clusters or “types”. Both types occur worldwide with comparable frequency, although type 1 is presumably the older clade 13. Interestingly, the presence of Tv virus (TVV) is unequally distributed within the two types, with about 70% of all type 1 isolates, but only 30% of type 2 isolates, carrying the virus. Conversely, metronidazole resistance is far more prevalent in type 2 isolates.
Treatment
Since 1960, metronidazole and other 5-nitroimidazoles, such as tinidazole, have been the mainstay of Tv treatment 3. 5-nitroimidazoles have been reported to damage DNA, form adducts with proteins (partly with inhibiting consequences 15), and cause oxidative damage in the cell by depleting thiol pools 15. 5-nitroimidazoles are in fact prodrugs, which have to be reduced at their nitro groups in order to become toxic. This reaction, however, takes place quantitatively only in microaerophilic/anaerobic organisms and has been suggested to be catalyzed by several enzymes and factors such as ferredoxin 16, nitroreductase 17, and thioredoxin reductase 15. Resistance to 5-nitroimidazoles in clinical Tv isolates does occur, sometimes leading to extended and complex treatment regimens 18. Clinical metronidazole resistance is based on decreased oxygen scavenging in the cell, leading to higher intracellular oxygen concentrations 19. Accordingly, expression of flavin reductase 1, an enzyme that uses flavin mononucleotide (FMN) to reduce molecular oxygen to H 2O 2, has been described to be downregulated or even shut-off in metronidazole-resistant Tv 20, 21. In addition, a correlation between metronidazole resistance and mutations in the genes for nitroreductase 4 and 6 was found 22.
Due to the occurrence of Tv strains refractory to 5-nitroimidazole treatment, the search for alternative treatments has never stopped. In recent years, a number of promising alternatives were presented, including pentamycin 23, boric acid 24, N-chlorotaurine 25, and drug-free chitosan 26, all of which would have be to administered intravaginally and not systemically, as is the case with 5-nitroimidazoles. Further, a combination of metronidazole and miconazole, administered intravaginally, was shown to greatly reduce adverse side effects often reported for systemic metronidazole treatment 27.
Pathogenicity
The last few years have seen a number of major advances in our understanding of Tv pathogenicity. In a number of studies, including proteomic and glycobiological approaches, several key components of the Tv cell surface were described. First, a detailed chemical structure of Tv lipoglycan, a surface molecule strongly binding to human galectin-1 and -3 28, was published 29. Further, a large surface proteome study was performed 30, identifying 261 putative membrane proteins, including ABC transporters and 11 BspA proteins. BspA proteins constitute a huge surface protein family in the Tv genome comprising 911 members 31. They could bind to proteins of the extracellular matrix of the host epithelium, e. g. fibronectin, and elicit strong immune responses. In addition, this proteomic study revealed the existence of two hypothetical proteins which seem to enhance adhesion of Tv to the host epithelium 30. Another proteomic study was performed using exosome-enriched cellular fractions of Tv, leading to the identification of 215 proteins, putatively localizing to exosome vesicles 32. Among these proteins were one BspA-like protein and one tetraspanin. Tetraspanins are a protein family known to be involved in cell adhesion, and proteins that had before been suggested to be involved in adhesion of Tv to the epithelium, such as glyceraldehyde 3-phosphate dehydrogenase 33, enolase 34, succinyl-CoA synthetase 35, and GP63 protease 36. Importantly, a large-scale transcriptomic deep sequencing study (RNAseq) with Tv during early infection performed by another work group corroborated many of these observations 37. Exosomes also contain large amounts of short RNA molecules (25–200 nucleotides) and enhance adhesion to vaginal ectocervical cells (VECs) when added extraneously to Tv strains with poor adhesion capacity 32. It is important to note that cell adhesion is an absolutely necessary prerequisite for the lysis of host cells by Tv 38. After cell adhesion has taken place, several factors are assumed to be involved in host cell lysis, including metalloproteases 39, 40, cysteine proteases 41– 43, a rhomboid protease (TvROM1) 44, and phospholipase A2 45. Tv also secretes a migration inhibition factor (TvMIF) 46 which can replace human migration factor (HuMIF) to trigger proinflammatory cytokine release. Possibly, this contributes to the increased risk of developing prostate cancer in Tv-infected men 3.
The detection of tetraspanins in Tv exosomes prompted further research on this protein family 47, 48. Of the tetraspanins studied, all but one (TvTsp2) were strongly upregulated upon contact with VECs 48. TvTsp6 changes its localization in the cell upon VEC contact and migrates from the flagellum to the plasma membrane. The C-terminal tail was found to be necessary for correct localization. Intriguingly, one tetraspanin, TvTSP8, seems to mediate Tv aggregation rather than VEC adhesion 48. Contact with VECs also triggers a reorganization of the actin cytoskeleton and enables the rapid transition of flagellate to amoeboid morphology 49. This process is mediated by TvFIM1, the only fimbrin found to be expressed in Tv.
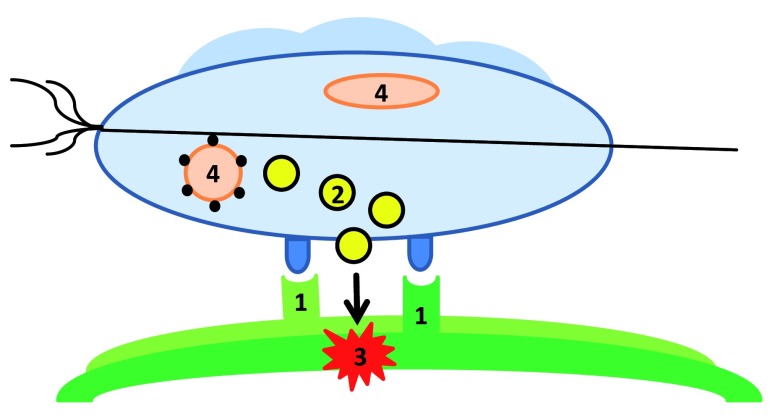
When discussing the pathogenicity of Tv, it is also important to take into account other microorganisms that coincide with the parasite, especially Mycoplasma hominis and TVV. In the presence of M. hominis, Tv infection triggers a far more pronounced proinflammatory reaction than in its absence 50. The enhancing effect of TVV (which resides in about half of all Tv isolates) on the proinflammatory response seems to be even stronger 51, as TVV is sensed by Toll-like receptor 3 on the surface of VECs. Especially worrying is the observation that metronidazole treatment, accompanied by the release of large amounts of virus particles from necrotic Tv, further amplifies this adverse response. The contents of this section are visualized in Figure 1.
Figure 1. Model of Trichomonas vaginalis (Tv) pathogenicity.
In order to exert a cytopathic effect, it is necessary 37 that Tv (light blue) binds ( 1) to the extracellular matrix (light green) or the host epithelium (dark green). Binding is accomplished by several surface proteins and other surface molecules that bind to a structure on the host’s cell surface. Among these are lipoglycan 27, BspA 28, 30, tetraspanins 28, 45, 46 and several others, such as glyceraldehyde 3-phosphate dehydrogenase 32, enolase 33, and succinyl-CoA synthetase 34 on the Tv surface, galectins-1 and -3 28 on the host cell surface, and fibronectin 32 in the extracellular matrix. Several Tv factors necessary for adhesion to the host epithelium reach the Tv surface or the epithelium surface via exosomes 31 ( 2). Damage to the host cell is caused by several effectors ( 3), including cysteine proteases, metalloproteases, rhomboid proteases, and phospholipase A2. Tv migration inhibition factor might favor the development of neoplasia in the prostate 44. In the presence of Mycoplasma hominis 48 and Tv virus 49 ( 4), symptoms might be exacerbated.
Biochemistry and cell biology
The last few years have seen several transcriptomic and proteomic studies addressing the impact of growth and culture conditions on gene expression in Tv. Deep sequencing of RNA libraries was applied to identify genes that are differentially expressed under oxidative stress 37 and glucose restriction 52. Oxidative stress led to an upregulation of expression of 218 genes after 2 hours, including peroxiredoxins (Prx), thioredoxin reductase, thioredoxins, superoxide dismutases (SODs), rubrerythrin, and ferredoxins 37. Upregulation of SOD and Prx upon oxidative stress at the protein level had already been reported before 53, underpinning the validity of the transcriptomic approach. Interestingly, glucose starvation also led to upregulation of SOD, Prx, and rubrerythrin, resulting in a more H 2O 2-resistant phenotype 52. Most glycolytic enzymes, however, were downregulated in glucose-starved cells, accompanied by a strong upregulation of glutamate dehydrogenase, which produces α-ketoglutarate by oxidative deamination of glutamate. Also, autophagy was observed in glucose-starved cells, and autophagy markers, i.e. autophagy-related genes (atg), were upregulated in expression 52. In a phosphoproteomic study, 82 phosphoproteins were discovered in Tv 54, a number conspicuously low given that more than 1000 genes for kinases exist in the Tv genome 54, 55.
The glycobiology of Tv has received considerable attention recently, and a comprehensive study on N-glycan composition in four Tv strains was published 56. In all strains, a major core structure, a truncated oligomannose form (Man5GlcNAc2) with α1,2-mannose residues, could be identified. In contrast, modifications with phosphoethanolamine and terminal N-acetyllactosamine varied depending on the strain studied. Moreover, the core structure is often decorated with xylose 29, 56, which has been described as typical for trematodes and plants. Indeed, Tv encodes a functional UDP-xylose synthase 57, the first to be described in a unicellular parasite. Further, asparagine-linked N-glycans of Tv were found to bind human mannose-binding lectin and retroviral lectins 58.
Naturally, the hydrogenosome, as a model for organelle evolution, has remained one of the major focuses in the Tv field. Again, proteomic studies provided a deeper insight into hydrogenosome biology. A study on hydrogenosomal membrane proteins, for example, demonstrated that hydrogenosomes and mitochondria have important core membrane components in common which are responsible for protein import and metabolite transport 59. Hydrogenosomes also contain a dynamin-like protein which is likely to be involved in hydrogenosomal fission 60. Nevertheless, essential differences with mitochondria also exist which can be attributed to the microaerophilic lifestyle and evolutionary adaptations of Tv and other related parasites. This is also reflected in the much lower number of proteins in the hydrogenosome 61 as compared to mitochondria, i. e. about 500 vs. 1000–1500. The proteome’s composition is also rather variable, as the expression levels of many hydrogenosomal proteins were found to depend on available iron concentrations 62, 63. This is in line with the high abundance of iron-sulfur cluster proteins such as pyruvate:ferredoxin oxidoreductase, hydrogenase, and ferredoxin in this organelle. Unfortunately, it is hard to predict the localization of proteins to the hydrogenosome based on sequence information alone because protein import seems to depend on as yet poorly defined internal sequences, rather than on N-terminal targeting sequences. The latter seem, if at all existent, to be dispensable in most cases, likely due to the loss of the electrochemical gradient 61, 64, 65. This difficulty can, however, be partly overcome by applying sophisticated machine learning approaches 66. Also, other recent findings are difficult to put into perspective, e. g. the obvious functional redundancy of one of the most abundant proteins in the hydrogenosomal membrane, Tvhmp23 67, or the localization of arginine deiminase to the hydrogenosome while other key enzymes of the arginine dihydrolase pathway reside in the cytosol 68.
Genomics and gene expression
The Tv genome is extremely large for a protist and might be even larger than originally anticipated, i. e. 175 Mb in size 69 rather than 160 Mb 6. Intriguingly, as much as 65% of its content consists of repetitive sequences, including transposable elements such as representatives of the types Maverick and Tc1/mariner 70, and microRNA 71. The expansion of gene families is a common phenomenon in Tv, so that the vast number of 60,000 genes has accumulated in the genome 6. On the other hand, the proportion of pseudogenes seems to be extraordinarily large as well, with, for example, as much as 46% of the 123 transmembrane adenylyl cyclases being truncated or having nonsense mutations 72. However, many pseudogenes are being transcribed, leading to a high representation of pseudogene mRNA in the long non-coding RNA pool 73. In total, only about half of the annotated genes are being expressed but almost all gene families are represented 73. It is likely that Tv harnesses this fluctuant nature of its genome to adaptive innovation, i. e. evolution. This flexibility might apply to annotated, functional genes as well. For example, seven full-length isoforms of the enzyme flavin reductase (FR1-7) with varying relatedness to each other are present in the genome 21, but only FR1 has a Km for FMN which is low enough to be of plausible physiological importance. Three other FRs have high Vmax but also high Km, and the remaining three have low Vmax and high Km. Nevertheless, all of the less specific isoforms are expressed, if not in all strains, and can, at very high expression levels, partly substitute for FR1.
The last few years have also seen several advances in our understanding of gene expression in Tv. Especially well studied are the Myb-like transcription factors tvMyb1-3, which are known to bind to the promoter sites MRE-1/MRE-2r and MRE-2f of the hydrogenosomal malate dehydrogenase gene, also known as ap65-1 74. In the case of tvMyb3, the DNA-binding site was crystallized and its structure determined 75. In a suite of excellent studies, the same group also revealed the mechanism of nuclear import of all three tvMybs 76– 78. Further, core promoter elements in Tv 79 and polyadenylation signals 80 were described. Finally, Tv mRNA was found to possess a metazoan/plant-like cap structure and a metazoan/plant-like capping enzyme 81.
Conclusion
In recent years, considerable progress was achieved in the Tv field. Although there are still many open questions regarding Tv’s epidemiology, particularly in the context of facilitated HIV contagion and cancer, our understanding of Tv’s pathogenesis made a large leap forward and the picture is becoming ever more complete. In the treatment of Tv, several interesting alternatives, especially topical treatments, might eventually replace metronidazole, which potentially has worrying side effects. Finally, the genome of Tv has remained a fascinating colossus, whose complexity will trigger plenty of further research in the years to come.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Sven Gould, Institute for Molecular Evolution, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany
Robert Hirt, Institute for Cell and Molecular Biosciences, Newcastle University, Newcastle upon Tyne, NE2 4HH, UK
Funding Statement
This work was supported by project J3492 of the Austrian science fund (FWF).
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. WHO: Global incidence and prevalence of selected curable sexually transmitted infections – 2008.2016. Reference Source [PubMed] [Google Scholar]
- 2. Kissinger P: Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infect Dis. 2015;15:307. 10.1186/s12879-015-1055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Sobel R, Sobel JD: Metronidazole for the treatment of vaginal infections. Expert Opin Pharmacother. 2015;16(7):1109–1115. 10.1517/14656566.2015.1035255 [DOI] [PubMed] [Google Scholar]
- 4. Lindmark DG, Müller M: Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J Biol Chem. 1973;248(22):7724–7728. [PubMed] [Google Scholar]
- 5. Martin W, Müller M: The hydrogen hypothesis for the first eukaryote. Nature. 1998;392(6671):37–41. 10.1038/32096 [DOI] [PubMed] [Google Scholar]
- 6. Carlton JM, Hirt RP, Silva JC, et al. : Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science. 2007;315(5809):207–212. 10.1126/science.1132894 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Hirt RP, Sherrard J: Trichomonas vaginalis origins, molecular pathobiology and clinical considerations. Curr Opin Infect Dis. 2015;28(1):72–79. 10.1097/QCO.0000000000000128 [DOI] [PubMed] [Google Scholar]
- 8. Poole DN, McClelland RS: Global epidemiology of Trichomonas vaginalis. Sex Transm Infect. 2013;89(6):418–422. 10.1136/sextrans-2013-051075 [DOI] [PubMed] [Google Scholar]
- 9. Donders GGG, Depuydt CE, Bogers J, et al. : Association of Trichomonas vaginalis and cytological abnormalities of the cervix in low risk women. PLoS One. 2013;8(12):e86266. 10.1371/journal.pone.0086266 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Silver BJ, Guy RJ, Kaldor JM, et al. : Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis. 2014;41(6):369–76. 10.1097/OLQ.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 11. Sexton J, Garnett G, Røttingen J: Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sex Transm Dis. 2005;32(6):351–357. 10.1097/01.olq.0000154504.54686.d1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Lazenby GB, Unal ER, Andrews AL, et al. : Cost-effectiveness analysis of annual Trichomonas vaginalis screening and treatment in HIV-positive women to prevent HIV transmission. Sex Transm Dis. 2014;41(6):353–358. 10.1097/OLQ.0000000000000008 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Conrad MD, Gorman AW, Schillinger JA, et al. : Extensive genetic diversity, unique population structure and evidence of genetic exchange in the sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis. 2012;6(3):e1573. 10.1371/journal.pntd.0001573 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Conrad M, Zubacova Z, Dunn LA, et al. : Microsatellite polymorphism in the sexually transmitted human pathogen Trichomonas vaginalis indicates a genetically diverse parasite. Mol Biochem Parasitol. 2011;175(1):30–38. 10.1016/j.molbiopara.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leitsch D, Kolarich D, Binder M, et al. : Trichomonas vaginalis: metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system: Implications for nitroimidazole toxicity and resistance. Mol Microbiol . 2009;72(2):518–536. 10.1111/j.1365-2958.2009.06675.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Kulda J: Trichomonads, hydrogenosomes and drug resistance. Int J Parasitol. 1999;29(2):199–212. 10.1016/S0020-7519(98)00155-6 [DOI] [PubMed] [Google Scholar]
- 17. Pal D, Banerjee S, Cui J, et al. : Giardia, Entamoeba, and Trichomonas enzymes activate metronidazole (nitroreductases) and inactivate metronidazole (nitroimidazole reductases). Antimicrob Agents Chemother. 2009;53(2):458–464. 10.1128/AAC.00909-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldman LM, Upcroft JA, Workowski K, et al. : Treatment of metronidazole-resistant Trichomonas vaginalis. Sex Health. 2009;6(4):345–347. 10.1071/SH09064 [DOI] [PubMed] [Google Scholar]
- 19. Yarlett N, Yarlett NC, Lloyd D: Metronidazole-resistant clinical isolates of Trichomonas vaginalis have lowered oxygen affinities. Mol Biochem Parasitol. 1986;19(2):111–116. 10.1016/0166-6851(86)90115-5 [DOI] [PubMed] [Google Scholar]
- 20. Leitsch D, Drinić M, Kolarich D, et al. : Down-regulation of flavin reductase and alcohol dehydrogenase-1 (ADH1) in metronidazole-resistant isolates of Trichomonas vaginalis. Mol Biochem Parasitol. 2012;183(2):177–183. 10.1016/j.molbiopara.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leitsch D, Janssen BD, Kolarich D, et al. : Trichomonas vaginalis flavin reductase 1 and its role in metronidazole resistance. Mol Microbiol. 2014;91(1):198–208. 10.1111/mmi.12455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paulish-Miller TE, Augostini P, Schuyler JA, et al. : Trichomonas vaginalis metronidazole resistance is associated with single nucleotide polymorphisms in the nitroreductase genes ntr4 Tv and ntr6 Tv. Antimicrob Agents Chemother. 2014;58(5):2938–2943. 10.1128/AAC.02370-13 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Kranzler M, Syrowatka M, Leitsch D, et al. : Pentamycin shows high efficacy against Trichomonas vaginalis. Int J Antimicrob Agents. 2015;45(4):434–437. 10.1016/j.ijantimicag.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 24. Brittingham A, Wilson WA: The antimicrobial effect of boric acid on Trichomonas vaginalis. Sex Transm Dis. 2014;41(12):718–722. 10.1097/OLQ.0000000000000203 [DOI] [PubMed] [Google Scholar]
- 25. Fürnkranz U, Nagl M, Gottardi W, et al. : In vitro activity of N-chlorotaurine (NCT) in combination with NH 4Cl against Trichomonas vaginalis. Int J Antimicrob Agents. 2011;37(2):171–173. 10.1016/j.ijantimicag.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pradines B, Bories C, Vauthier C, et al. : Drug-free chitosan coated poly(isobutylcyanoacrylate) nanoparticles are active against Trichomonas vaginalis and non-toxic towards pig vaginal mucosa. Pharm Res. 2015;32(4):1229–1236. 10.1007/s11095-014-1528-7 [DOI] [PubMed] [Google Scholar]
- 27. Schwebke JR, Lensing SY, Sobel J: Intravaginal metronidazole/miconazole for the treatment of vaginal trichomoniasis. Sex Transm Dis. 2013;40(9):710–714. 10.1097/01.olq.0000431069.38601.d5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Fichorova RN, Yamamoto HS, Fashemi T, et al. : Trichomonas vaginalis Lipophosphoglycan Exploits Binding to Galectin-1 and -3 to Modulate Epithelial Immunity. J Biol Chem. 2016;291(2):998–1013. 10.1074/jbc.M115.651497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ryan CM, Mehlert A, Richardson JM, et al. : Chemical structure of Trichomonas vaginalis surface lipoglycan: a role for short galactose (β1-4/3) N-acetylglucosamine repeats in host cell interaction. J Biol Chem. 2011;286(47):40494–40508. 10.1074/jbc.M111.280578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Miguel N, Lustig G, Twu O, et al. : Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol Cell Proteomics. 2010;9(7):1554–1566. 10.1074/mcp.M000022-MCP201 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Noël CJ, Diaz N, Sicheritz-Ponten T, et al. : Trichomonas vaginalis vast BspA-like gene family: evidence for functional diversity from structural organisation and transcriptomics. BMC Genomics. 2010;11:99. 10.1186/1471-2164-11-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Twu O, de Miguel N, Lustig G, et al. : Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host:parasite interactions. PLoS Pathog. 2013;9(7):e1003482. 10.1371/journal.ppat.1003482 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Lama A, Kucknoor A, Mundodi V, et al. : Glyceraldehyde-3-phosphate dehydrogenase is a surface-associated, fibronectin-binding protein of Trichomonas vaginalis. Infect Immun. 2009;77(7):2703–2711. 10.1128/IAI.00157-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mundodi V, Kucknoor AS, Alderete JF: Immunogenic and plasminogen-binding surface-associated alpha-enolase of Trichomonas vaginalis. Infect Immun. 2008;76(2):523–531. 10.1128/IAI.01352-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mundodi V, Kucknoor AS, Alderete JF: Antisense RNA decreases AP33 gene expression and cytoadherence by T. vaginalis. BMC Microbiol. 2007;7:64. 10.1186/1471-2180-7-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma L, Meng Q, Cheng W, et al. : Involvement of the GP63 protease in infection of Trichomonas vaginalis. Parasitol Res. 2011;109(1):71–79. 10.1007/s00436-010-2222-2 [DOI] [PubMed] [Google Scholar]
- 37. Gould SB, Woehle C, Kusdian G, et al. : Deep sequencing of Trichomonas vaginalis during the early infection of vaginal epithelial cells and amoeboid transition. Int J Parasitol. 2013;43(9):707–719. 10.1016/j.ijpara.2013.04.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Lustig G, Ryan CM, Secor WE, et al. : Trichomonas vaginalis contact-dependent cytolysis of epithelial cells. Infect Immun. 2013;81(5):1411–1419. 10.1128/IAI.01244-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quan JH, Kang BH, Cha GH, et al. : Trichonomas vaginalis metalloproteinase induces apoptosis of SiHa cells through disrupting the Mcl-1/Bim and Bcl-xL/Bim complexes. PLoS One. 2014;9(10):e110659. 10.1371/journal.pone.0110659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quintas-Granados LI, Villalpando JL, Vázquez-Carrillo LI, et al. : TvMP50 is an immunogenic metalloproteinase during male trichomoniasis. Mol Cell Proteomics. 2013;12(7):1953–1964. 10.1074/mcp.M112.022012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cárdenas-Guerra RE, Arroyo R, Rosa de Andrade I, et al. : The iron-induced cysteine proteinase TvCP4 plays a key role in Trichomonas vaginalis haemolysis. Microbes Infect. 2013;15(13):958–968. 10.1016/j.micinf.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 42. Cárdenas-Guerra RE, Ortega-López J, Flores-Pucheta CI, et al. : The recombinant prepro region of TvCP4 is an inhibitor of cathepsin L-like cysteine proteinases of Trichomonas vaginalis that inhibits trichomonal haemolysis. Int J Biochem Cell Biol. 2015;59:73–83. 10.1016/j.biocel.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 43. Puente-Rivera J, Ramón-Luing Lde L, Figueroa-Angulo EE, et al. : Trichocystatin-2 (TC-2): an endogenous inhibitor of cysteine proteinases in Trichomonas vaginalis is associated with TvCP39. Int J Biochem Cell Biol. 2014;54:255–265. 10.1016/j.biocel.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 44. Riestra AM, Gandhi S, Sweredoski MJ, et al. : A Trichomonas vaginalis Rhomboid Protease and Its Substrate Modulate Parasite Attachment and Cytolysis of Host Cells. PLoS Pathog. 2015;11(12):e1005294. 10.1371/journal.ppat.1005294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Escobedo-Guajardo BL, González-Salazar F, Palacios-Corona R, et al. : Trichomonas vaginalis acidic phospholipase A2: isolation and partial amino acid sequence. Acta Parasitol. 2013;58(4):519–526. 10.2478/s11686-013-0166-2 [DOI] [PubMed] [Google Scholar]
- 46. Twu O, Dessí D, Vu A, et al. : Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc Natl Acad Sci U S A. 2014;111(22):8179–8184. 10.1073/pnas.1321884111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Miguel N, Riestra A, Johnson PJ: Reversible association of tetraspanin with Trichomonas vaginalis flagella upon adherence to host cells. Cell Microbiol. 2012;14(12):1797–1807. 10.1111/cmi.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Coceres VM, Alonso AM, Nievas YR, et al. : The C-terminal tail of tetraspanin proteins regulates their intracellular distribution in the parasite Trichomonas vaginalis. Cell Microbiol. 2015;17(8):1217–1229. 10.1111/cmi.12431 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Kusdian G, Woehle C, Martin WF, et al. : The actin-based machinery of Trichomonas vaginalis mediates flagellate-amoeboid transition and migration across host tissue. Cell Microbiol. 2013;15(10):1707–1721. 10.1111/cmi.12144 [DOI] [PubMed] [Google Scholar]
- 50. Fiori PL, Diaz N, Cocco AR, et al. : Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes. Sex Transm Infect. 2013;89(6):449–454. 10.1136/sextrans-2012-051006 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Fichorova RN, Lee Y, Yamamoto HS, et al. : Endobiont viruses sensed by the human host - beyond conventional antiparasitic therapy. PLoS One. 2012;7(11):e48418. 10.1371/journal.pone.0048418 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Huang KY, Chen YY, Fang YK, et al. : Adaptive responses to glucose restriction enhance cell survival, antioxidant capability, and autophagy of the protozoan parasite Trichomonas vaginalis. Biochim Biophys Acta. 2014;1840(1):53–64. 10.1016/j.bbagen.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 53. Leitsch D, Kolarich D, Duchêne M: The flavin inhibitor diphenyleneiodonium renders Trichomonas vaginalis resistant to metronidazole, inhibits thioredoxin reductase and flavin reductase, and shuts off hydrogenosomal enzymatic pathways. Mol Biochem Parasitol. 2010;171(1):17–24. 10.1016/j.molbiopara.2010.01.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Yeh YM, Huang KY, Richie Gan RC, et al. : Phosphoproteome profiling of the sexually transmitted pathogen Trichomonas vaginalis. J Microbiol Immunol Infect. 2013;46(5):366–373. 10.1016/j.jmii.2012.07.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Hirt RP, de Miguel N, Nakjang S, et al. : Trichomonas vaginalis pathobiology new insights from the genome sequence. Adv Parasitol. 2011;77:87–140. 10.1016/B978-0-12-391429-3.00006-X [DOI] [PubMed] [Google Scholar]
- 56. Paschinger K, Hykollari A, Razzazi-Fazeli E, et al. : The N-glycans of Trichomonas vaginalis contain variable core and antennal modifications. Glycobiology. 2012;22(2):300–313. 10.1093/glycob/cwr149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rosenberger AF, Hangelmann L, Hofinger A, et al. : UDP-xylose and UDP-galactose synthesis in Trichomonas vaginalis. Mol Biochem Parasitol. 2012;181(1):53–56. 10.1016/j.molbiopara.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chatterjee A, Ratner DM, Ryan CM, et al. : Anti-Retroviral Lectins Have Modest Effects on Adherence of Trichomonas vaginalis to Epithelial Cells In Vitro and on Recovery of Tritrichomonas foetus in a Mouse Vaginal Model. PLoS One. 2015;10(8):e0135340. 10.1371/journal.pone.0135340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rada P, Doležal P, Jedelský PL, et al. : The core components of organelle biogenesis and membrane transport in the hydrogenosomes of Trichomonas vaginalis. PLoS One. 2011;6(9):e24428. 10.1371/journal.pone.0024428 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Wexler-Cohen Y, Stevens GC, Barnoy E, et al. : A dynamin-related protein contributes to Trichomonas vaginalis hydrogenosomal fission. FASEB J. 2014;28(3):1113–1121. 10.1096/fj.13-235473 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Schneider RE, Brown MT, Shiflett AM, et al. : The Trichomonas vaginalis hydrogenosome proteome is highly reduced relative to mitochondria, yet complex compared with mitosomes. Int J Parasitol. 2011;41(13–14):1421–1434. 10.1016/j.ijpara.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Beltrán NC, Horváthová L, Jedelský PL, et al. : Iron-induced changes in the proteome of Trichomonas vaginalis hydrogenosomes. PLoS One. 2013;8(5):e65148. 10.1371/journal.pone.0065148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Horváthová L, Šafaríková L, Basler M, et al. : Transcriptomic identification of iron-regulated and iron-independent gene copies within the heavily duplicated Trichomonas vaginalis genome. Genome Biol Evol. 2012;4(10):1017–1029. 10.1093/gbe/evs078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Garg S, Stölting J, Zimorski V, et al. : Conservation of Transit Peptide-Independent Protein Import into the Mitochondrial and Hydrogenosomal Matrix. Genome Biol Evol. 2015;7(9):2716–2726. 10.1093/gbe/evv175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rada P, Makki AR, Zimorski V, et al. : N-Terminal Presequence-Independent Import of Phosphofructokinase into Hydrogenosomes of Trichomonas vaginalis. Eukaryot Cell. 2015;14(12):1264–1275. 10.1128/EC.00104-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burstein D, Gould SB, Zimorski V, et al. : A machine learning approach to identify hydrogenosomal proteins in Trichomonas vaginalis. Eukaryot Cell. 2012;11(2):217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brás XP, Zimorski V, Bolte K, et al. : Knockout of the abundant Trichomonas vaginalis hydrogenosomal membrane protein TvHMP23 increases hydrogenosome size but induces no compensatory up-regulation of paralogous copies. FEBS Lett. 2013;587(9):1333–1339. 10.1016/j.febslet.2013.03.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Morada M, Smid O, Hampl V, et al. : Hydrogenosome-localization of arginine deiminase in Trichomonas vaginalis. Mol Biochem Parasitol. 2011;176(1):51–54. 10.1016/j.molbiopara.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith A, Johnson P: Gene expression in the unicellular eukaryote Trichomonas vaginalis. Res Microbiol. 2011;162(6):646–654. 10.1016/j.resmic.2011.04.007 [DOI] [PubMed] [Google Scholar]
- 70. Bradic M, Warring SD, Low V, et al. : The Tc1/mariner transposable element family shapes genetic variation and gene expression in the protist Trichomonas vaginalis. Mob DNA. 2014;5:12. 10.1186/1759-8753-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Huang PJ, Lin WC, Chen SC, et al. : Identification of putative miRNAs from the deep-branching unicellular flagellates. Genomics. 2012;99(2):101–107. 10.1016/j.ygeno.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 72. Cui J, Das S, Smith TF, et al. : Trichomonas transmembrane cyclases result from massive gene duplication and concomitant development of pseudogenes. PLoS Negl Trop Dis. 2010;4(8):e782. 10.1371/journal.pntd.0000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Woehle C, Kusdian G, Radine C, et al. : The parasite Trichomonas vaginalis expresses thousands of pseudogenes and long non-coding RNAs independently from functional neighbouring genes. BMC Genomics. 2014;15:906. 10.1186/1471-2164-15-906 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Jiang I, Tsai CK, Chen SC, et al. : Molecular basis of the recognition of the ap65-1 gene transcription promoter elements by a Myb protein from the protozoan parasite Trichomonas vaginalis. Nucleic Acids Res. 2011;39(20):8992–9008. 10.1093/nar/gkr558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wei SY, Lou YC, Tsai JY, et al. : Structure of the Trichomonas vaginalis Myb3 DNA-binding domain bound to a promoter sequence reveals a unique C-terminal β-hairpin conformation. Nucleic Acids Res. 2012;40(1):449–460. 10.1093/nar/gkr707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chu CH, Chang LC, Hsu HM, et al. : A highly organized structure mediating nuclear localization of a Myb2 transcription factor in the protozoan parasite Trichomonas vaginalis. Eukaryot Cell. 2011;10(12):1607–1617. 10.1128/EC.05177-11 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Hsu HM, Chu CH, Wang YT, et al. : Regulation of nuclear translocation of the Myb1 transcription factor by TvCyclophilin 1 in the protozoan parasite Trichomonas vaginalis. J Biol Chem. 2014;289(27):19120–19136. 10.1074/jbc.M114.549410 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Hsu HM, Lee Y, Hsu PH, et al. : Signal transduction triggered by iron to induce the nuclear importation of a Myb3 transcription factor in the parasitic protozoan Trichomonas vaginalis. J Biol Chem. 2014;289(42):29334–29349. 10.1074/jbc.M114.599498 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Smith AJ, Chudnovsky L, Simoes-Barbosa A, et al. : Novel core promoter elements and a cognate transcription factor in the divergent unicellular eukaryote Trichomonas vaginalis. Mol Cell Biol. 2011;31(7):1444–1458. 10.1128/MCB.00745-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fuentes V, Barrera G, Sánchez J, et al. : Functional analysis of sequence motifs involved in the polyadenylation of Trichomonas vaginalis mRNAs. Eukaryot Cell. 2012;11(6):725–734. 10.1128/EC.05322-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Simoes-Barbosa A, Hirt RP, Johnson PJ: A metazoan/plant-like capping enzyme and cap modified nucleotides in the unicellular eukaryote Trichomonas vaginalis. PLoS Pathog. 2010;6(7):e1000999. 10.1371/journal.ppat.1000999 [DOI] [PMC free article] [PubMed] [Google Scholar]