Abstract
TB is an underappreciated public health threat in developed nations. In 2014, an estimated 9.6 million TB cases and 1.5 million deaths occurred worldwide; 3.3% of these cases resulted from multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) strains. These figures underestimate the economic burden associated with MDR-TB and XDR-TB, as the cost of treating disease caused by these strains can be 9–25 times higher than treating drug-susceptible TB. Developing new drugs, improved diagnostics and new TB vaccines are critical components of a strategy to combat TB in general, and drug-resistant TB in particular. Because Mycobacterium tuberculosis (MTB) has demonstrated a capacity to develop resistance to drugs developed to combat it, it is unlikely that drug-resistant MTB would be ‘resistant’ to vaccines capable of preventing disease or established infection with drug-sensitive MTB strains. Accordingly, the development of TB vaccines represents an important long-term investment in preventing the spread of drug-resistant TB and achieving WHO's goal of ending the global TB epidemic by 2035. Our current understanding of the epidemiology of drug-resistant TB and the interventions needed to limit its spread, reviewed in this article, illustrates the need for increased financial support for developing new TB drugs, diagnostics and vaccines to meet the WHO goal of TB elimination by 2035.
Keywords: Extensively drug-resistant TB, Multidrug-resistant TB, Public health, Tuberculosis, Vaccines
Introduction
TB represents a vastly underappreciated public health threat in developed nations. WHO estimates that in 2014, 9.6 million people became ill with active TB and 1.5 million died from the disease, making it the world's leading cause of death by a single infectious agent.1 The majority of new TB cases occur in Asia (58% of new cases in 2014) and Africa (28% of new cases).1 In South Africa, TB represents the single largest cause of mortality, resulting in nearly 9% of all deaths in 2013.2 Additionally, TB is the number one cause of death of HIV-infected persons in Africa and a leading killer of HIV-infected persons worldwide, causing 400 000 of the 1.2 million HIV deaths reported in 2014.1 Women and children are not spared the deleterious effects of TB; in 2014, 140 000 children and 480 000 women died from the disease.1 Despite the enormous toll on global public health caused by TB, this ancient yet active disease received only 18% of total global neglected disease research and development (R&D) funding in 2013, compared to 34% for HIV/AIDS.3
While treatment options for TB are available at a cost of approximately US$2000 per patient for a standard 6-month course of therapy in most industrialized countries, the presence of drug-resistant TB complicates fully addressing the epidemic.4 Treatment for multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB strains can cost up to 25 times as much and take three times as long as for drug-susceptible TB.4,5 Moreover, only approximately 20% of MDR-TB cases are diagnosed as such, leading to increased morbidity and mortality among persons infected with these strains, and a greater chance that these drug-resistant strains will be spread to others.6 Given the extraordinary cost of treating MDR-TB and XDR-TB, effectively managing even a few of these cases is beyond the resource capabilities of many impoverished nations that are at the epicenter of the ongoing TB epidemic, and would strain the resources of the most developed countries. For example, in South Africa MDR-TB comprised approximately 2% of the total burden of TB cases, yet consumed more than 30% of the total national TB budget in 2011.7
In light of the serious individual and societal consequences of the spread of TB in general, and MDR-TB and XDR-TB in particular, a vaccine to prevent TB disease or infection with Mycobacterium tuberculosis (MTB), the bacterium that causes TB, is desperately needed. Currently, only one TB vaccine is licensed globally: Bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, the cause of TB-like disease in cattle. BCG, which was developed in France in the early part of the 20th century and first used in humans in 1921, has been shown to be efficacious in preventing severe, often deadly, forms of TB disease in infants.8 Although BCG vaccination provides a varying degree of protection against pulmonary TB disease in young children, it has minimal effectiveness in adolescents and adults in preventing TB disease or MTB infection.8 Additionally, the development of drug resistance would not be expected to reduce the effect of a vaccine otherwise effective at preventing TB disease or MTB infection, heightening the importance of the overall effort to develop new TB vaccines, as detailed below.9
This paper will review the scientific evidence available to support the developing of vaccines as a critical strategy to interrupt the spread of drug-resistant TB.
Drug-resistant TB
Two important types of drug-resistant TB have been identified; MDR-TB, which is resistant to both of the first line drugs of standard TB treatment, isoniazid and rifampicin, and XDR-TB, resistant to isoniazid, rifampin, at least one fluoroquinolone, and any of the second line injectable treatments (amikacin, capreomycin, kanamycin).10
Globally, 3.3% percent of new TB cases are MDR-TB.1 The proportion is much higher (20%) among patients who have previously received TB treatment.1 In 2014, there were 480 000 new cases of MDR-TB worldwide.1 In certain regions of the globe, such as Eastern Europe and Central Asia, the proportion of MDR-TB and XDR-TB cases is growing dramatically. One example is Belarus, where in 2013 nearly 40% of new TB cases were caused by MDR-TB strains.1 Moreover, approximately 9.7% of MDR-TB cases are caused by XDR-TB strains globally, with the highest proportions of XDR-TB among MDR-TB cases occurring in Belarus (29%), Lithuania (25%), Latvia (19%) and Georgia (15%) in 2014.1
In the US in 2014, 1.3% of reported TB cases were due to MDR-TB strains.11 In early 2015, a patient with XDR-TB was treated in a respiratory isolation unit at The National Institutes of Health (NIH) just outside the US Capital, an event that received heightened media attention due to the delayed diagnosis of her case, potentially putting many people at risk as she travelled by plane to a number of US cities (a person with active TB can spread the disease to 10–15 persons per year).12–15 This case illustrates the cost, disruption, and fear associated with these highly drug-resistant MTB strains, an impact that is further magnified by the increased spread of XDR-TB, which has been reported in 105 countries as of 2014.1
Drug resistance to MTB generally results from inadequate drug treatment of active TB.16 This may result from patient non-compliance, inappropriate drug levels, drug shortages or a number of other factors.16 Treating TB is challenging. Unlike most bacterial infections, where 1 or 2 weeks of orally ingested antibiotics are usually sufficient to result in a complete cure, treating even drug-sensitive TB takes at least 6 months.16 Due to the complex nature of TB pathogenesis, recurrence of TB disease following completion of a treatment regimen occurs in 20% of previously treated patients globally.1 Drug-resistant TB has been identified in more than 50% of previously treated patients in several Eastern European and Central Asian countries.1
Bedaquiline and delamanid are recently approved drugs targeted at drug-resistant MTB strains.17,18 While these drugs represent important new interventions against MTB, and while the development of additional drugs effective against drug-resistant TB strains are critically needed, the complex nature of MTB microbiology, metabolism and pathogenesis suggests that even combination drug therapy will not prevent drug resistance from occurring.10,17 The molecular changes to internal enzymes targeted by TB drugs that result in drug resistance, however, are unlikely to result in changes to antigenic proteins likely to be included in vaccine constructs, suggesting that vaccines capable of preventing TB disease or MTB infection with drug-sensitive MTB strains would be expected to have similar activity against drug-resistant strains as well.9 Additionally, a more effective vaccine could potentially shorten the current standard 6-month treatment therapy for drug-sensitive TB, in turn curtailing the development of drug-resistant strains.19
Cost of treating MDR-TB and XDR-TB
The spread of drug-resistant TB strains poses profound challenges to the health of individuals and populations, and to healthcare systems, based on the enormous costs associated with these infections. The treatment regimens for MDR-TBand XDR-TB are complex, often demanding 18 months or more of uninterrupted, multi-drug administration.10 Moreover, many of the second-line drugs are toxic, difficult and painful to administer, requiring frequent intramuscular or intravenous injections.20 Given the frequent and devastating adverse effects of drug therapy for MDR-TB and XDR-TB, it is easy to understand the difficulties inherent in ensuring the kind of prolonged, uninterrupted treatment necessary to avoid treatment failure and the further propagation of drug-resistant strains.
Due to intensive public health efforts to ensure compliance with drug treatment regimens, including directly observed therapy (DOT) programs, most patients with MDR-TB and XDR-TB in the US reported between 2005 and 2007 completed treatment with resultant elimination of culturable MTB from sputum cultures.5 Successful treatment of MDR-TB has also been demonstrated in a community-based, resource-poor setting in Peru, where early initiation of appropriate therapy was instrumental to preserving drug susceptibility.21 Globally, however, TB in general, and drug-resistant TB in particular, mainly occurs among the most impoverished citizens of otherwise impoverished nations, where the extraordinary cost of treating MDR-TB and XDR-TB often makes such interventions impossible.22
According to the most recent cost estimates for treating MDR-TB and XDR-TB in the US, direct treatment costs in 2015 averaged US$154 000 per MDR-TB patient and US$494 000 per XDR-TB patient compared to the estimated US$17 000 in direct costs (inpatient and outpatient) associated with treating drug-sensitive TB patients5 (study author S. Marks provided updated cost estimates; unpublished data). While the actual costs of treating TB in developing countries are lower than those reported for the US, the costs associated with treating MDR-TB and XDR-TB can be disproportionately higher. Accordingly, it is easy to see why providing drug treatment for a growing number of MDR-TB and XDR-TB cases is beyond the economic capacity of many developing countries, and why developing new vaccines to prevent TB disease and MTB infection, along with other infection control and prevention measures, for both drug sensitive and drug-resistant TB strains represents a critical public health imperative.
TB vaccine development: state of the field
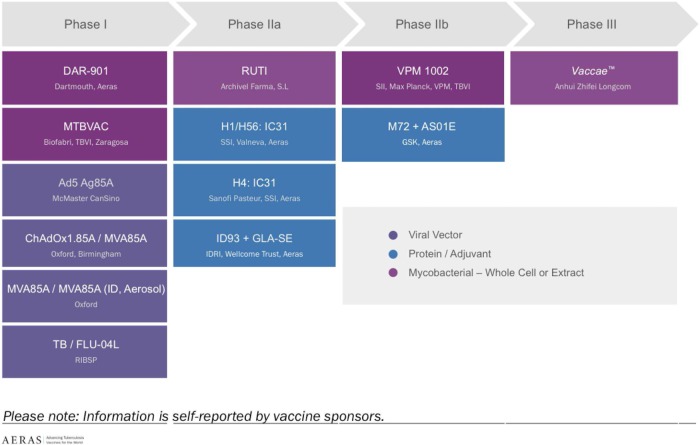
There has been a great deal of progress in TB vaccine development over the past decade. Currently, 13 candidates are undergoing clinical trials, with at least three candidates in advanced (Phase 2b and Phase 3) testing, and one (H4:IC31) undergoing a Phase 2 trial assessing its ability to protect high-risk South African adolescents from initial MTB infection (Figure 1). In contrast, only four TB vaccines were in clinical trials in 2005, all of them in early Phase 1 testing.
Figure 1.
Global TB vaccine pipeline, 2015 (Source: Aeras). This figure is available in black and white in print and in colour at Transactions online.
As noted, BCG represents the only TB vaccine that is licensed and in use in most countries worldwide. BCG has been successful in preventing life-threatening forms of MTB infection in infants, with efficacy ranging up to 80% in clinical studies.8 However, despite the extensive, global BCG vaccination of infants, approximately one out of three persons, or more than 2 billion people, are infected with MTB worldwide, of whom approximately 10%, or more than 200 million, will develop TB disease during their lifetimes.1 These figures demonstrate the lack of real world effectiveness of infant BCG vaccination to prevent MTB infection and TB disease in adolescents and adults.9,22 Accordingly, developing a new, effective TB vaccine represents a critical goal.
After disappointing results were obtained from a Phase 2b clinical trial of an MVA85A vaccine in BCG-vaccinated South African infants, increased attention is now being focused upon developing new vaccine candidates, primarily to prevent TB disease or established MTB infection in adolescents and adults.23 Unfortunately, funding for TB vaccine development significantly lacks the same support provided to other vaccine development efforts. For example, funding for the Ebola vaccine development effort ramped up quickly following the 2013–2014 epidemic in Sierra Leone, Guinea and Liberia, an outbreak that tragically killed approximately 11 400 persons.24 The global response to the outbreak has been enormous; the US Government alone has appropriated over US$5.4 billion for the Ebola emergency response for 2015, of which a large portion will be used for research, including developing Ebola vaccines.25 In comparison, the global TB vaccine development effort will receive only US$85–90 million in 2015 based on prior years' funding numbers, despite an estimated 29 000 people dying of TB per week (based on 2014 mortality estimates), with little sign of meaningful diminution in the global spread of MTB and the continuing spread of MDR-TB and XDR-TB.26,27
The need for a more effective vaccine
The WHO's End TB Strategy aims to end the global TB epidemic by 2035, reducing the global incidence of TB disease from the current annual rate of >100 cases/100 000 persons to <10 cases/100 000 persons.28 Mathematical modeling of the TB epidemic and the impact of drugs, diagnostics and vaccines suggests this goal cannot be reached without developing effective TB vaccines capable of greatly diminishing TB disease and new, established, MTB infection.29 Recent findings show a vaccine with an efficacy as low as 40% and a duration of effectiveness of only 5 years still could reduce approximately 24% of TB cases in low and middle-income countries if targeted at adolescents and adults. In contrast, a vaccine with 80% efficacy and lifelong duration could prevent approximately 70% of TB cases in these countries.29 Prevention of TB among these populations would dramatically reduce transmission. Modeling of the effects of vaccines targeted only at infants predicted that this approach would be far less effective at decreasing the TB disease burden (decrease of only 12%), and would not be cost-effective before 2050, whereas vaccines for adolescents/adults would be cost-effective and potentially cost-saving, assuming a duration of protection of 10 years or longer or an efficacy greater than or equal to 20%.29
These cost-saving estimates do not factor in the potential benefits of preventing the development of drug-resistant cases of TB. Vaccine prevention of drug-resistant TB would provide highly leveraged economic benefits given the extraordinary costs of caring for MDR-TB and XDR-TB cases cited above. These numbers likely represent an underestimate of the actual economic impact that an effective TB vaccine would provide given the weighty indirect costs of these devastating diseases, particularly considering that drug-resistant TB can take 2 years or more to treat, resulting in substantial loss of workplace and household productivity. An additional factor to consider is that, for diseases in which highly effective vaccines have been introduced, such as vaccines against Streptococcus pneumoniae, the overall rate of drug-resistant, invasive pneumococcal cases and pneumococcal pneumonia occurring in the population declined along with the general reduction in pneumococcal pneumonia incidence. The introduction of the S. pneumoniae conjugate vaccine appears to have created a virtuous cycle, whereby fewer cases of clinical disease resulted in diminished antibiotic pressure in the population, thereby reducing the selection of additional drug-resistant strains.30,31 In regions where the burden of MDR-TB continues to increase, such as Eastern Europe and Central Asia, the introduction of an effective TB vaccine could potentially precipitate a similar virtuous cycle.
Future directions and recommendations
When considering future strategies to address the spread of MDR-TB and XDR-TB, lessons can be learned from the 2013–2014 Ebola epidemic in West Africa, including the importance of having sufficient resources and pre-established plans available to strengthen health systems, engage the community, and implement emergency preparedness strategies.27
One of the most valuable lessons, however, is the importance of the commitment to support R&D of new health tools, including drugs, diagnostics and vaccines, in anticipation of future acceleration of disease spread, especially in the time of war, significant population migrations, or other social upheavals.
The worst Ebola outbreak in history might have been prevented had there been sufficient commitment to R&D efforts to counter the disease in the 40 years after its identification. When the outbreak began, there was no approved vaccine or treatment for the Ebola virus, both of which likely would have prevented thousands of deaths and curbed the outbreak before it turned into a larger epidemic.32 The spread of MDR-TB and XDR-TB already has resulted in a greater number of deaths than from Ebola, and will prove to be far more expensive as well. Even now, more people die of TB every 3 days than those who died during the entire 2013–2014 Ebola outbreak in Western Africa.1,24
Another lesson derived from the Ebola experience is that funding for developing disease interventions where there is high risk of a low return on investment will result in delayed development of drugs or vaccines unless sufficient funding and incentives are provided to minimize investment risk. Neglected diseases that affect mainly poor countries, including TB, are often underfunded due to this market failure, evidenced by the finding that only 37 of 850 new health products approved between 2000 and 2011 were focused on neglected diseases.33 Research programs to address predictable, looming health challenges involving neglected diseases, such as the spread of drug-resistant TB strains, require higher and continuous robust support from both private and public sector sources.
According to WHO, without developing new interventions against TB, and particularly without an effective TB vaccine, it will be impossible to meet the 2035 TB elimination target.34 Additionally, prevention of TB through vaccination is the most cost-effective tool for eradication, as is the case with every other major infectious disease.32 Greater political commitment, along with increased funding for TB R&D, including the development of new drugs, better diagnostics and new vaccines, is urgently needed to curtail this epidemic.
In 2014, US$1.4 billion globally went toward TB overall, indicating a drop of 9.2% since 2013.32 In comparison, total development assistance for HIV/AIDS was US$10.9 billion in 2014, and malaria received US$2.4 billion, despite TB being a leading cause of death from infectious disease in developing countries.32 The Global Fund is the main channel of TB funding, but its mandate currently does not include support of TB R&D. While the NIH is the largest funder for TB R&D efforts, especially basic TB research, the largest resource for translational TB vaccine and drug R&D is the Bill & Melinda Gates Foundation. Other funders include the Wellcome Trust, UK Department for International Development (DFID), the Dutch Ministry of Foreign Affairs (DGIS) and the Japanese Global Health Innovative Technology Fund (GHIT).l Private sector resources for TB R&D have also been decreasing, with several major pharmaceutical companies having disbanded their TB research programs in the last few years.26 Overall TB R&D spending decreased 11.8% from 2012 to 2013, and private sector contributions have dropped by one-third.26
Of the R&D resources spent on TB, 14% goes toward vaccines.26 The Stop TB Partnership's Global Plan 2011–2015 called for annual funding for TB vaccines of US$380 million. Donors contributed US$95.2 million in 2013, which left a funding gap of US$248.8 million.26 Current funding will not be sufficient to develop a vaccine capable of curbing an outbreak of MDR-TB and XDR-TB in a reasonable timeframe. The ultimate costs of vaccine development are uncertain. However, the costs from the MVA85A Phase 2b trial were estimated at US$30 million, plus an additional US$10 million for infrastructure. Using these estimates, it has been further supported that a new vaccine would be cost-effective.35
It is critical that public and private sector partners work together to accelerate TB R&D efforts, including funds for drugs and diagnostics, as well as for vaccine development efforts, before the spread of drug-resistant TB strains creates devastating health and economic consequences. Finally, the cost of a new vaccine would be significantly less than the financial burden of TB treatment and care globally.36
Conclusions
TB is a leading global infectious disease killer and demands more attention and resources to combat its spread. The proliferation of MDR-TB and XDR-TB strains greatly exacerbates the potentially devastating consequences to the health of individuals and populations, as well as to the health economies, of both developing and developed countries. New drugs and better diagnostics are needed to combat MDR-TB and XDR-TB in the short to medium term, while developing a vaccine capable of preventing TB disease and established MTB infection represents a critical long-term strategy to curb the spread of both drug-resistant and drug-sensitive MTB strains. Although important strides have been taken in the past decade to develop new TB vaccines, significant further investment is needed to increase the likelihood that a TB vaccine can be brought to market in time to stem the spread of this deadly infection and to avert the growing threat of the global spread of drug-resistant TB strains.
Acknowledgments
Authors' contributions: JM and LS conceived the study. JM, DE, SP and LS contributed to the development and critical review of the manuscript outline and validated the final version. JM and LS are guarantors of the paper.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.WHO. Global tuberculosis report 2015. Geneva: World Health Organization; 2015. http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1 [accessed 28 August 2015]. [Google Scholar]
- 2.Statistics South Africa. Causes of Death 2013. Pretoria: Statistics South Africa; 2015. http://www.statssa.gov.za/?page_id=737&id=3 [accessed 17 August 2015]. [Google Scholar]
- 3.Moran M, Guzman J, Chapman N et al. G-Finder 2014: Neglected disease research and development: Emerging trends. Sydney: Policy Cures; 2014. http://www.policycures.org/downloads/Y7%20GFINDER%20full%20report%20web%20.pdf [accessed 15 August 2015]. [Google Scholar]
- 4.WHO. Trade, foreign policy, diplomacy and health. Tuberculosis (TB). Geneva: World Health Organization; http://www.who.int/trade/glossary/story092/en/ [accessed 17 August 2015]. [Google Scholar]
- 5.Marks SM, Flood J, Seaworth B et al. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg Infect Dis 2014;20:812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marais BJ, Mlambo CK, Rastogi N et al. Epidemic spread of multidrug-resistant tuberculosis in Johannesburg, South Africa. J Clin Microbiol 2013;51:1818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pooran A, Pieterson E, Davids M et al. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLoS One 2013;8:e54587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkie MEM, McShane H. TB vaccine development: where are we and why is it so difficult? Thorax 2015;70:299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole ST, Eisenach KD, McMurray DN, Jacobs WR (editors). Tuberculosis and the tubercle bacillus. Washington, DC: ASM Press; 2005, p. 129–31. [Google Scholar]
- 10.Millard J, Ugarte-Gil C, Moore DA. Multidrug resistant tuberculosis. BMJ 2015;350:h882. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Tuberculosis (TB): Fact sheet. Trends in Tuberculosis, 2014. Atlanta: Centers for Disease Control and Prevention; http://www.cdc.gov/tb/publications/factsheets/statistics/tbtrends.htm [accessed 28 October 2015]. [Google Scholar]
- 12.WHO. What is TB? How is it treated? Geneva: World Health Organization; 2015. http://www.who.int/features/qa/08/en/ [accessed 28 October 2015]. [Google Scholar]
- 13.Hedgpeth D, Gebelhoff R. CDC, state officials track down people possibly exposed to rare form of TB. Washington Post. 9 June 2015. https://www.washingtonpost.com/national/health-science/woman-with-rare-form-of-tb-treated-at-nih-in-bethesda/2015/06/09/7f779e60-0e97-11e5-a0dc-2b6f404ff5cf_story.html [accessed 28 October 2015]. [Google Scholar]
- 14.Belt D. Woman with rare, drug-resistant form of TB treated at NIH. Bethesda-Chevy Chase Patch 10 June 2015. http://patch.com/maryland/bethesda-chevychase/woman-rare-drug-resistant-form-tb-treated-nih [accessed 28 October 2015]. [Google Scholar]
- 15.Grady D. Tuberculosis case prompts search for patient's fellow airline passengers. The New York Times 8 June 2015: A8. http://www.nytimes.com/2015/06/09/science/tuberculosis-case-prompts-search-for-patients-fellow-airline-passengers.html?_r%BC0&_r=0 [accessed 28 October 2015].
- 16.Cole ST, Eisenach KD, McMurray DN, Jacobs WR (editors). Tuberculosis and the tubercle bacillus. Washington, DC: ASM Press; 2005, p. 115. [Google Scholar]
- 17.Cox E, Laessig K. FDA approval of bedaquiline: the benefit-risk balance for drug-resistant tuberculosis. N Engl J Med 2014;371:689–91. [DOI] [PubMed] [Google Scholar]
- 18.Committee for Medicinal Products for Human Use (CHMP). Summary of opinion (initial authorisation): Deltyba (delamanid). London: European Medicines Agency; 2013. EMA/CHMP/713909/2013. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002552/WC500155458.pdf [accessed 28 October 2015]. [Google Scholar]
- 19.Prabowo SA, Groschel MI, Schmidt EDL et al. Targeting multidrug-resistant tuberculosis (MDR-TB) by therapeutic vaccines. Med Microbiol Immunol 2013;202:95–104. [DOI] [PubMed] [Google Scholar]
- 20.Sturdy A, Goodman A, Jose RJ et al. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother 2011;66:1815–20. [DOI] [PubMed] [Google Scholar]
- 21.Mitnick C, Bayona J, Palacios E et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. NEJM 2003;348:119–28. [DOI] [PubMed] [Google Scholar]
- 22.Barker LF, Leadman AE, Clagett B. The challenges of developing new tuberculosis vaccines. Health Affairs 2011;30:1073–9. [DOI] [PubMed] [Google Scholar]
- 23.Tameris M, McShane H, McClain B et al. Lessons learnt from the first efficacy trial of a new infant tuberculosis vaccine since BCG. Tuberculosis 2013;93:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC. Ebola (Ebola Virus Disease). 2014 Ebola Outbreak in West Africa - Case Counts. Atlanta: Centers for Disease Control and Prevention; http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html [accessed 17 August 2015]. [Google Scholar]
- 25.Campos LM, Rissetto CL, Helland R. Global Regulatory Enforcement Law Blog. Government Contracts & Grants. FY 2015 Ebola federal funding: Congressional increases and program support. Pittsburg: Reed Smith; http://www.globalregulatoryenforcementlawblog.com/2014/12/articles/government-contracts/fy-2015-ebola-federal-funding-congressional-increases-and-program-support/ [accessed 20 August 2015]. [Google Scholar]
- 26.Frick M. 2014 Report on TB Research Funding Trends, 2005–2013. 2nd ed. New York: Treatment Action Group (TAG); 2015. http://www.treatmentactiongroup.org/sites/g/files/g450272/f/201505/TAG_2014_TB_Funding_Report_2nd_Ed.pdf [accessed 20 August 2015]. [Google Scholar]
- 27.WHO. Emergencies preparedness, response. Ebola response: What needs to happen in 2015. Geneva: World Health Organization; 2015. http://www.who.int/csr/disease/ebola/one-year-report/response-in-2015/en/ [accessed 17 August 2015]. [Google Scholar]
- 28.WHO. Tuberculosis (TB): The End TB strategy. Geneva: World Health Organization; 2016. http://www.who.int/tb/strategy/end-tb/en/ [accessed 16 November 2015]. [Google Scholar]
- 29.Knight GM, Griffiths UK, Sumner T et al. Impact and cost-effectiveness of new tuberculosis vaccines in low- and middle-income countries. Proc Natl Acad Sci U S A 2014;111:15520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richter SS, Diekema DJ, Heilmann KP et al. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrob Agents Chemother 2014;58:6484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dagan R, Juergens C, Trammel J et al. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible streptococcus pneumonia. J Infect Dis 2015;211:1144–53. [DOI] [PubMed] [Google Scholar]
- 32.IHME. Financing Global Health 2014. Shifts in Funding as the MDG Era Closes. Seattle: Institute for Health Metrics and Evaluation; 2015. http://www.healthdata.org/sites/default/files/files/policy_report/2015/FGH2014/IHME_PolicyReport_FGH_2014_0.pdf [accessed 20 August 2015]. [Google Scholar]
- 33.GHTC. 2015 policy report: Meeting the challenge, seizing the opportunity. Washington, DC: Global Health Technologies Coalition; http://www.ghtcoalition.org/policy-report/2015/lessons-learned-from-ebola.php [accessed 20 August 2015]. [Google Scholar]
- 34.Moran M, Chapman N, Howard R et al. Tuberculosis: The Last Mile. Sydney: Policy Cures; 2014. http://policycures.org/downloads/GF_TBreport_final_web.pdf [accessed 20 August 2015]. [Google Scholar]
- 35.Ditkowsky JB, Schwartzmann K. Potential cost-effectiveness of a new infant tuberculosis vaccine in South Africa- implication for clinical trials: A decision analysis. PLoS ONE 2014;9:e83526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahsan MJ. Recent advances in the development of vaccines for tuberculosis. Ther Adv Vaccines 2015;3:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]