Abstract
BACKGROUND
Extracellular vesicles (EVs) are membrane-bound vesicles, found in biofluids, that carry and transfer regulatory molecules, such as microRNAs (miRNAs) and proteins, and may mediate intercellular communication between cells and tissues. EVs have been isolated from a wide variety of biofluids, including plasma, urine, and, relevant to this review, seminal, follicular and uterine luminal fluid. We conducted a systematic search of the literature to review and present the currently available evidence on the possible roles of EVs in follicular growth, resumption of oocyte development and maturation (meiosis), sperm maturation, fertilization and embryo implantation.
METHODS
MEDLINE, Embase and Web of Science databases were searched using keywords pertaining to EVs, including ‘extracellular vesicles’, ‘microvesicles’, ‘microparticles’ and ‘exosomes’, combined with a range of terms associated with the period of development between fertilization and implantation, including ‘oocyte’, 'sperm’, 'semen’, 'fertilization’, ‘implantation’, ‘embryo’, ‘follicular fluid’, ‘epididymal fluid’ and ‘seminal fluid’. Relevant research articles published in English (both animal and human studies) were reviewed with no restrictions on publication date (i.e. from earliest database dates to July 2015). References from these articles were used to obtain additional articles.
RESULTS
A total of 1556 records were retrieved from the three databases. After removing duplicates and irrelevant titles, we reviewed the abstracts of 201 articles, which included 92 relevant articles. Both animal and human studies unequivocally identified various types of EVs in seminal, follicular and ULFs. Several studies provided evidence for the roles of EVs in these biofluids. In men, EVs in seminal fluid were linked with post-testicular sperm maturation, including sperm motility acquisition and reduction of oxidative stress. In women, EVs in follicular fluid were shown to contain miRNAs with potential roles in follicular growth, resumption of oocyte meiosis, steroidogenesis and prevention of polyspermy after fertilization. EVs were also detected in the media of cultured embryos, suggesting that EVs released from embryos and the uterus may mediate embryo-endometrium cross-talk during implantation. It is important to note that many of the biologically plausible functions of EVs in reproduction discussed in the current literature have not yet been substantiated by conclusive experimental evidence.
CONCLUSIONS
A detailed understanding of the contributions of EVs in the series of events from gametogenesis to fertilization and then on to implantation, in both normal and pathological cases, may enable the development of valuable tools to advance reproductive health. Because of the early stage of the field, it is unsurprising that the current literature includes not only growing experimental evidence, but also as-yet unproven hypotheses pertaining to the roles of EVs in key reproductive processes. In this review, we present a comprehensive survey of the rapidly expanding literature on this subject, highlighting both relevant findings and gaps in knowledge.
Keywords: extracellular vesicles, oocyte, sperm, fertilization, exosomes, ovarian follicle, embryo, implantation
Introduction
Interest in intercellular communication has risen in recent years, with an increasing awareness of the complexity of its contributions to diverse physiological processes, including regulation of cell proliferation, differentiation, gametogenesis, embryogenesis and development. In particular, the identification of extracellular vesicles (EVs) as novel mediators of intercellular communication has re-focused research efforts in the field. Traditionally, intercellular communication has included three mechanisms, i.e. contact-dependent signaling via membrane-bound signaling molecules (receptors) or gap junctions, short-range paracrine signaling via secreted soluble molecules, such as cytokines and chemokines, and long-range endocrine signaling via secreted hormones. Recent studies have uncovered the existence of EVs, which are released by cells into the extracellular environment and can serve as vehicles for the transfer of proteins, lipids and RNAs between cells both locally (autocrine and paracrine) and remotely (Zhang et al., 2009; Raposo and Stoorvogel, 2013). EVs are released by a wide range of cell types under both normal and pathological conditions. EVs and their cargoes may play key roles in numerous aspects of biology, including reproduction, as candidate biomarkers of health and disease, and as potential targets for therapeutic interventions (Gould and Raposo, 2013).
Gametogenesis, fertilization, implantation and early embryo development are complex processes that are highly dependent on communication between cells and organs. Oogenesis is a multistep process that occurs over an extended timeframe (decades in the human), and involves interactions between the developing oocyte and the cumulus and granulosa cells that surround it in the follicle. Sperm undergo maturation, capacitation and the acrosome reaction, which facilitate their binding and fusion with the released oocyte, thus enabling fertilization to occur. After the developing embryo migrates into the uterus, apposition and subsequent adherence of the blastocyst to the endometrial luminal epithelium, followed by endometrial invasion, must occur to result in successful implantation (Cuman et al., 2014). Detection of EVs in the reproductive biofluids points to possible roles for them in the intercellular communication necessary for and after conception (Table I). The aim of this review is to present the currently available evidence on the roles of EVs in conception and implantation and demonstrate key gaps in our present knowledge.
Table I.
Key studies implying a role for extracellular vesicles in reproduction.
| Process | Main findings | Species | Reference |
|---|---|---|---|
| Sperm maturation | EVs from epididymal fluid (epididymosomes) contain proteins associated with sperm maturation, such as macrophage migration inhibitory factor (MIF) and aldose reductase (AKRB1) | Bovine | Frenete et al. (2003) |
| Prevention of premature acrosome reaction and premature capacitation | The sperm membrane becomes enriched with cholesterol, sphingomyelin, and saturated glycophospholipids after fusion with EVs (prostasomes) in the seminal fluid. The fluidity of the sperm membrane decreases, preventing premature acrosomal reaction | Human |
Arienti et al. (1998a, b) Carlini et al. (1997) |
| EVs (prostasomes) in seminal fluid inhibit early capacitation and spontaneous acrosome reaction | Human | Pons-Rejraji et al. (2011) | |
| EVs (epididymosomes) contain GPX5, which protects the sperm against premature acrosome reaction | Bovine | Rejraji et al. (2002) | |
| Capacitation, acrosome reaction, and fertilization | CD9-labeled EVs from the plasma membranes of oocytes are able to transfer proteins to the fertilizing sperm in the perivitelline space (PVS) before fertilization. Transfer of these EVs is crucial for the reorganization of sperm membrane and fusion with the oocyte | Mice | Barraud-Lange et al. (2007) |
| A significant increase in the acrosome reaction occurs in sperm incubated with EVs isolated from seminal plasma compared with control sperm | Porcine | Siciliano et al. (2008) | |
| Prevention of polyspermy | After fertilization, Juno is shed from the oolemma and is redistributed in EVs. These EVs can bind and neutralize acrosome-reacted sperm and prevent polyspermy | Mice | Bianchi and Wright, (2014) |
| Communication between embryos | Co-culture of porcine embryos significantly improves the in vitro development of cloned embryos. Labeled EVs from porcine embryos are internalized by the NT embryos | Porcine | Saadeldin et al. (2014) |
| Endometrial embryo cross-talking | EVs are present in the uterine fluid. EVs isolated from the uterine fluid of pregnant sheep can transfer RNAs including endogenous beta-retroviruses RNAs, which play a role in the regulation of conceptus trophectoderm development, to other cells | Sheep | Burns et al. (2014) |
EVs: extracellular vesicles; NT: nuclear transfer.
Methods
For this review, we performed a systematic online literature search of MEDLINE, Embase and Web of Science databases. We searched all articles published since database inception through July 2015. We used the following query: (‘extracellular vesicles’ OR ‘microvesicles’ OR ‘microparticles’ OR ‘exosomes’ OR ‘epididymosomes’ OR ‘prostatosomes’ OR ‘oviductosomes’ OR ‘uterosomes’) AND (‘oocyte’ OR ‘sperm’ OR ‘semen’ OR ‘capacitation’ OR ‘nidation’ OR ‘fertilization’ OR ‘fertilisation’ OR ‘implantation’ OR ‘embryo’ OR ‘follicular fluid’ OR ‘epididymal fluid’ OR ‘seminal fluid’). MeSH and EMTREE terms were also used where applicable. The terms ‘apoptotic bodies’ and ‘apoptotic blebs’ were excluded as these types of EVs might have functions independent of reproductive processes (Caselles et al., 2014). Both animal and human studies were considered eligible for this review. We applied database filters for ‘English language’ and ‘full length articles’ and eliminated any duplicate articles. After application of the search limits defined above, we reviewed the titles and abstracts of each publication. Studies found to be irrelevant after screening their titles and/or abstracts were excluded. The remaining full-length articles were retrieved. Only studies whose scope included EVs of all types plus conception/reproduction were included. References from these articles were also used to obtain additional articles. A total of 1556 records were retrieved from the three databases. After removing duplicates and irrelevant titles, the abstracts of 201 articles were reviewed and 92 were considered relevant and included in this review.
Extracellular vesicles
EVs are membrane-bound vesicles released by every prokaryotic (Kim et al., 2015) and eukaryotic (Regente et al., 2009; Oliveira et al., 2010; Mantel and Marti, 2014; Cocucci and Meldolesi, 2015) cell type that has been studied to date. Different terms have been used to classify subtypes of EVs, which have been often intentionally or unintentionally employed to designate overlapping categories of EVs (Gould and Raposo, 2013). Here we report a commonly used nomenclature, recognizing that it is not universally adopted or enforced (Gould and Raposo, 2013), largely because it is usually not possible to determine the specific biogenesis mechanism of any given population of EVs. Exosomes, which are typically 40–100 nm in diameter (Crescitelli et al., 2013), are formed within cells by inward budding of late endosomes, called multi-vesicular bodies, and are then released into the extracellular environment by fusion of the multi-vesicular body with the plasma membrane (Denzer et al., 2000; Laulagnier et al., 2004). Microvesicles bud directly from the plasma membrane, and are usually 50–1000 nm in diameter. Apoptotic bodies have a particularly wide range of size, having been described to range from 800 to 5000 nm (Thery et al., 2009; Crescitelli et al, 2013; EL Andaloussi et al., 2013; Traver et al., 2014). Given the overlapping size ranges, it is well appreciated that EV preparations are generally quite heterogeneous and it is difficult to distinguish between the different subtypes of EVs. Historically, the term ‘exosomes’ has been commonly used to describe any type of vesicle found in an extracellular biofluid, but we prefer the term EV (Lötvall et al., 2014). EVs have also been labeled according to the tissue/biofluid in which they are detected. Based on this nomenclature, prostatosomes or prostasomes, epididymosomes, oviductosomes and uterosomes have been used to indicate vesicles isolated from seminal fluid, epidydimal, oviduct and uterine fluids, respectively (Ronquist and Brody, 1985; Saez et al., 2003; Griffiths et al., 2008; Al-Dossary et al., 2013). EVs have been shown to contain proteins, lipids (specifically high levels of sphingomyelins), DNA, and a variety of RNA species, including microRNAs (miRNAs) and mRNA fragments (Thery et al., 2009). Proteins commonly found on EV membranes include tetraspanins, specifically CD63, CD9, CD81, heat-shock proteins (HSP70) and glycophosphatidylinositol-anchored proteins. Tissue-specific molecular mediators characteristic of the parent cell can be found both on EV membranes and within EVs (Wubbolts et al., 2003; Laulagnier et al., 2004; Subra et al., 2007; Valadi et al., 2007; Luo et al., 2009; Simpson et al., 2009; Record et al., 2011; Sullivan and Saez, 2013; van der Grein and Nolte-'t Hoen, 2014). Molecules on the surface of EVs promote interaction with other cells through adhesion of the vesicles to lipids and ligands on the surface of the recipient cell, internalization of the whole vesicle into recipient cells, or fusion of the EV membrane with the plasma membrane of the recipient cell (Thery et al., 2009; Record et al., 2011).
Extracellular vesicles and sperm maturation
The ability of sperm to fertilize an oocyte is gradually acquired during transit through the epididymis, interaction with the seminal fluid during ejaculation, passage in the vagina, contact with the epithelium of the oviduct and fusion with the oocyte (Caballero et al., 2010). The epididymis has several functions, including sperm transport, sperm maturation and storage of gametes (Cooper, 1996; Jones, 2004). The epididymis is divided into three main segments: the caput, the corpus and the cauda. Each segment forms its own microenvironment with different protein secretions and gene expression, optimized for each stage of sperm maturation (Dacheux et al., 2012; Belleannee et al., 2013). The caput and the corpus are responsible for sperm maturation, while the cauda acts as a sperm reservoir (Sullivan et al., 2005; Sullivan and Saez, 2013). During their transit through the epididymis, sperm lose their cytoplasmic droplets and their plasma membrane surface proteins undergo remodeling (i.e. changes in phospholipid composition and in the cholesterol/phospholipid ratio, an increase in total negative charges, and modification of surface proteins). Upon ejaculation, sperm is mixed with seminal fluid, which consists of secretions from the prostate, seminal vesicles and bulbo-urethral glands (Aalberts et al., 2013). From these secretions, the sperm acquire the ability to survive and move in the hostile acidic vagina, bind the zona pellucida and fuse with the oocyte plasma membrane. The changes in sperm morphology and function all result from interactions between the sperm and the intraluminal fluid along the epididymis, and depend on the secretion and uptake of proteins and lipids from the surrounding microenvironment (Caballero et al., 2010).
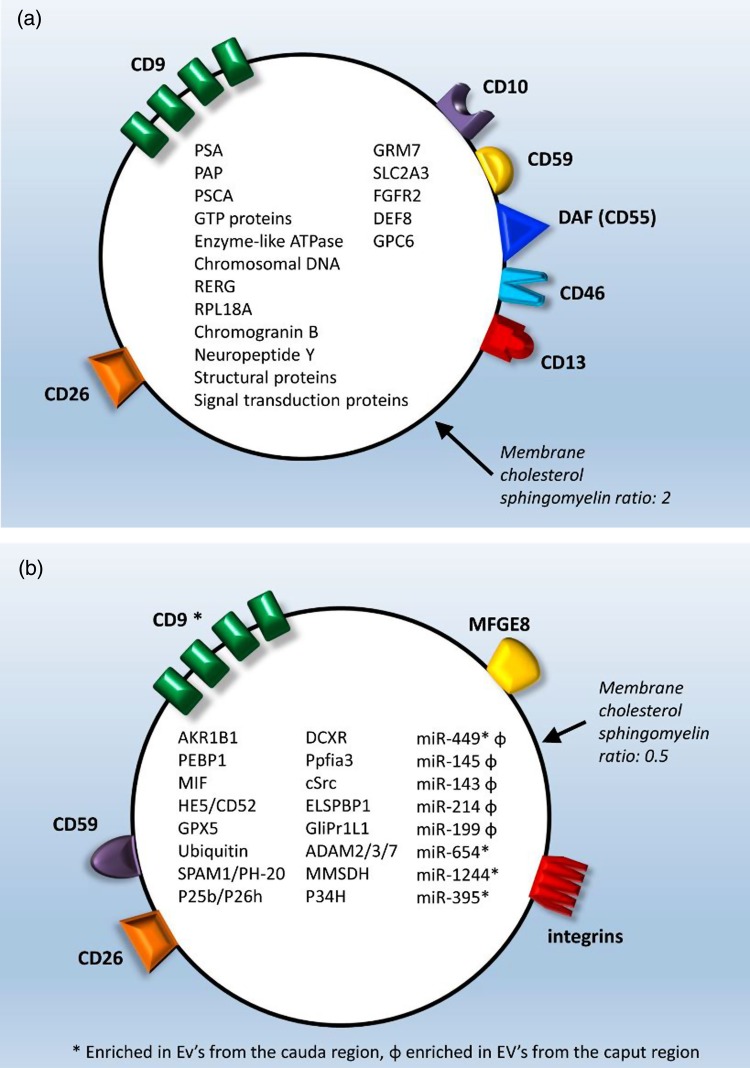
Prevailing data demonstrate that proteins and miRNAs in the epididymal fluid associated with post-testicular sperm maturation are transferred to the sperm by EVs. EVs were first identified in the human seminal fluid as organelles expelled with prostatic secretions, and named ‘prostasomes’ (Ronquist and Brody, 1985; Ronquist et al., 1978a, b; Ronquist et al., 2009), which measure 50–500 nm and contain proteins, lipids and nucleic acids. Human prostasomes transport at least 140 proteins, including prostate-specific proteins (e.g. PSA and PAP), prostate stem cell antigen, structural proteins, signal transduction proteins, Guanosine triphosphates proteins and Adenosine triphosphates (Utleg et al., 2003; Ronquist et al., 2012) (Fig. 1a). These proteins have antimicrobial, antioxidant and immune-regulatory effects (Rooney et al., 1993; Saez et al., 1998, 2000; Carlsson et al., 2000; Pons-Rejraji et al., 2011; Li et al., 2013; Madison et al., 2014). Prostasomes have an active role in controlling the timing of capacitation and acrosome reaction. In particular, prostasomes participate in avoiding premature sperm capacitation and premature acrosome reaction. In fact, the prostasome membrane is enriched in cholesterol and sphingomyelin, with a high cholesterol/phospholipid ratio, which contributes to its stability in the acidic vaginal environment (Arvidson et al., 1989; Fabiani and Ronquist, 1995; Arienti et al., 1998a, b, 1999; Kravets et al., 2000; Carlsson et al., 2003; Pons-Rejraji et al., 2011). Furthermore, in vitro experiments have shown that prostasomes also contribute to capacitation and acrosome reaction. In vitro incubation at a slightly acidic pH results in fusion of human sperm with prostasomes (Carlini et al., 1997), which results in decreased sperm membrane fluidity, making the sperm receptive to subsequent fertilization signals (Carlini et al., 1997; Ikawa et al., 2010).
Figure 1.
(a) Prostasome structure and content. (b) Epididymosome structure and content.
Epididymosomes are EVs that are released from epididymal epithelial cells via apocrine secretion (Yanagimachi et al., 1985; Sullivan et al., 2005; Belleannee et al., 2013; Sullivan and Saez, 2013). Epididymosomes have been isolated from hamster, rat, ram, mouse and bovine species, as well as from humans (Yanagimachi et al., 1985; Fornes et al., 1995; Frenette and Sullivan, 2001; Rejraji et al., 2002; Gatti et al., 2005; Sullivan and Saez, 2013). Epididymosomes contain adhesion molecules, such as tetraspanins, integrins and milk fat globule-epidermal growth factor 8 protein (MFGE8) (Thimon et al., 2008; Thery et al., 2009; Girouard et al., 2011) (Fig. 1b). Bovine epididymosomes contain several proteins that participate in the acquisition of sperm motility, fertilization ability and protection against reactive oxygen species (Frenette et al., 2006a, b; Frenette et al., 2004; Vernet et al., 2004). These include aldo-keto reductase family 1, member B1 (aldose reductase) (AKR1B1), phosphatidylethanolamine binding protein 1 (PEBP1), macrophage migration inhibitory factor (MIF), enzymes of the polyol pathway, HE5/CD52, type 5 glutathione peroxidase (GPX5), ubiquitin, sperm adhesion molecule 1(SPAM1)/PH-20 and P25b/P26h (known in humans as dicarbonyl/L-xylulose reductase (DCXR) or P34H) (Fig. 1b). These proteins are involved in sperm maturation and fertilization: MIF and enzymes from the polyol pathway promote sperm motility (Frenette et al., 2003, 2004, 2006a, b); GPX5 prevents premature acrosome reaction (Rejraji et al., 2002) and, together with ubiquitin, protects sperm against oxidative stress. AKR1B1 and PEBP1, found in caudal epididymosomes, help to maintain epididymal sperm in a quiescent state during transit (Frenette et al., 2010). SPAM1/PH20 is a hyaluronidase that increases sperm penetration through the cumulus cell layer around the oocyte and is involved in sperm-zona pellucida adhesion (Zhang and Martin-DeLeon, 2003; Chen et al., 2006; Kimura et al., 2009). P25b/P26 h is important for the binding of sperm to the zona pellucida (Berube and Sullivan, 1994; Frenette and Sullivan, 2001; Sullivan et al., 2007).
In the bovine system, epididymosomes from different epididymis regions carry different cargoes (Frenette et al., 2010; Belleannee et al., 2013). Some miRNAs, for instance, such as miR-449, are enriched in epididymosomes from both the caput and cauda regions, while others are differentially expressed between these regions, with miR-145, miR-143, miR-214 and miR-199 being more abundant in epididymosomes from the caput, while miR-654, miR-1224 and miR-395 are expressed mostly in epididymosomes from the cauda region (Belleannee et al., 2013).
EVs in the seminal fluid are less well studied, although they originate from several cell types in the male genital tract (Renneberg et al., 1997; Vojtech et al., 2014) and fuse with the sperm membrane to transfer molecules that assist with sperm survival in the acidic environment of the vagina (Arienti et al., 1997, 1999).
Interactions between sperm and EVs are pH-dependent (Arienti et al., 1997; Carlini et al., 1997; Frenette et al., 2002). This fits well with the known alkaline pH of prostatic secretions, which prevents early EV/sperm fusion, and the acidic pH of the vagina, which acts as a trigger for fusion.
These animal and human studies provide solid evidence for the contribution of EVs to sperm maturation. However, there is as-yet limited evidence for the roles of specific types of EVs.
Extracellular vesicles, communication in the ovarian follicle and oocyte maturation
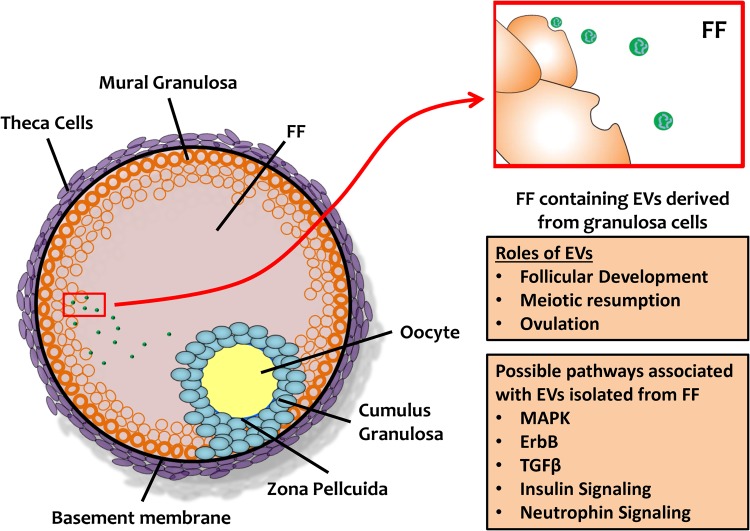
The mature ovarian follicle is composed of the oocyte, the somatic cells (cumulus granulosa, mural granulosa and theca cells) and follicular fluid (FF) (Fig. 2). FF derives from constituents of circulating plasma that cross the blood–follicular barrier via theca capillaries, as well as from granulosa and theca cell products, including hormones, proteins, amino acids and anti-apoptotic factors (Fortune, 1994; Revelli et al., 2009). Bidirectional communication between gametes and granulosa cells in the follicle occurs either directly by a network of gap junctions or through paracrine, autocrine and endocrine signaling factors in the FF (Eppig et al., 1997; Matzuk et al., 2002). These interactions are critical for normal follicular growth, proliferation and differentiation of granulosa cells, oocyte maturation, modulation of transcriptional activity, fertilization and preimplantation embryonic development (Brower and Schultz, 1982; Buccione et al., 1990; Adashi, 1994; Chesnel et al., 1994; Eppig, 2001; Eppig et al., 2002; Matzuk et al., 2002; Senbon et al., 2003; Hamel et al., 2008). EVs add an additional layer of transmission and control, as they carry miRNAs that are predicted to target key elements in pathways related to follicular growth and oocyte maturation in mammals, such as wingless signaling pathway (WNT), transforming growth factor beta (TGFβ), mitogen-activated protein kinase (MAPK), neurotrophin, epidermal growth factor receptor (ErbB) pathways and ubiquitin-mediated pathways (da Silveira et al., 2012; Sohel et al., 2013; Santonocito et al., 2014). WNT molecules are glycoproteins involved in follicular growth, luteogenesis and steroidogenesis. Members of the TGFβ superfamily, including inhibin, activin, bone morphogenetic protein 15 (BMP15) and growth differentiation factor 9 (GDF9), are expressed in the oocyte from very early stages and are responsible for follicular growth and oocyte maturation (Knight and Glister, 2003, 2006; Boyer et al., 2010; da Silveira et al., 2012). The MAPK pathway stimulates granulosa cell proliferation and cumulus expansion. Furthermore, together with the ERbB pathway, the MAPK pathway promotes the resumption of meiosis in the oocyte (Zhang et al., 2009; Conti et al., 2012). The ubiquitin-mediated pathway modulates oocyte meiotic maturation (Huo et al., 2004) and early mitotic division in embryos (Suzumori et al., 2003), while the neurotrophin-signaling pathway regulates oogenesis and follicle formation (Dissen et al., 2009).
Figure 2.
Extracellular vesicles and the ovarian follicle. EV: extracellular vesicles; FF: follicular fluid.
Equine, bovine and human studies have all demonstrated the presence of EVs in FF (da Silveira et al., 2012; Sohel et al., 2013; Santonocito et al., 2014). Within the ovarian follicle, EVs transport RNAs, miRNAs and proteins to recipient cells (da Silveira et al., 2012; Sohel et al., 2013; Santonocito et al., 2014). Some miRNAs are found only in FF EVs, while others can be detected also in the non-vesicular fraction of FF (da Silveira et al., 2012; Sohel et al., 2013). Interestingly, both equine and bovine EVs found in the FF contain miRNAs predicted to target transcripts of proteins associated with focal adhesion and regulation of the actin skeleton (da Silveira et al., 2012; Sohel et al., 2013). In the bovine system, relevant pathways associated with exosomal miRNAs include the ubiquitin-mediated pathway, MAPK signaling, insulin signaling and neurotrophin signaling (Sohel et al., 2013). In the equine, miRNAs related to the MAPK pathway are found in larger EVs, i.e. microvesicles rather than exosomes (da Silveira et al., 2012). In humans, most of the EV miRNAs are predicted to act on targets that regulate the WNT, ErbB, MAPK and TGFβ signaling pathways, all of which operate across the different compartments in the ovarian follicle and can contribute to follicular development, meiotic resumption and ovulation. In general, granulosa and/or cumulus cells can take up miRNAs from the FF EVs both in vivo and in vitro (Sohel et al., 2013). Table II lists the miRNAs detected in FF EVs to date.
Table II.
Expression of miRNAs in exosomes and microvesicles in the follicular fluid and their target pathways.
| Species | miRNAs Enriched in Exosomes | Pathways | Reference |
|---|---|---|---|
| Human | miR-10b, miR-21-5p, miR-31, miR-95, miR-99b-3p, miR-134, miR-135b, miR-140-3p, miR-190b, miR-203, miR-218, miR-337-5p, miR-339-3p, miR-370, miR-449a, miR-455-5p, miR-483-5p, miR-489, miR-493, miR-503, miR-542-5p, miR-654-3p, miR-874, miR-886-5p, miR-887 |
WNT MAPK ErbB TGFβ |
Santonocito et al. (2014) Diez-Fraile et al. (2014) |
| Bovine | miR-21, miR-26b, miR-30b, miR-33a, miR-132, miR-155, miR-191, miR-199a-5p, miR-221, miR-324-3p, miR-363, miR-373, miR-449b, miR-450b-3p, miR-451, miR-491-5p, miR-526b, miR-573, miR-582-5p, miR-640, miR-654-5p, miR-873, miR-1272, has-let-7c |
Ubiquitin-mediated Neutrophin MAPK Insulin signaling |
Sohel et al. (2013) |
| Equine | miR-20a, miR-20b, miR-22, miR-23a, miR-23b, miR-30b, miR-93, miR-101, miR-152, miR-215, miR-223, miR-378, miR-433, miR-483-3p,miR-499-3p, miR-513a-3p miRNAs enriched both in exosomes and microvesicles miR-17, miR-19b, miR-21, miR-24, miR-25, miR-30a, miR-92a, miR-92b, miR-132, miR-135a, miR-135b, miR-181a, miR-181c, miR-191, miR-192, miR-195, miR-197, miR-320a, miR-323-5p, miR-363-5p, miR-375, miR-421, miR-485-3p, miR-486-5p, miR-523 |
WNT MAPK Focal adhesion TGFβ |
da Silveira et al. (2012) |
TGFβ: transforming growth factor beta; MAPK: mitogen-activated protein kinase; ErbB: epidermal growth factor receptor.
Data linking EVs with follicular maturation and oocyte competence are currently associative and descriptive. A recent bovine study contrasted the profiles of EV-encapsulated miRNAs from follicles containing mature oocytes against those of follicles with immature oocytes. EVs from FF surrounding immature oocytes contained higher numbers of up-regulated miRNAs, suggesting the presence of a higher transcriptional activity during oocyte growth (Sohel et al., 2013). It is unclear whether the higher numbers of miRNA detected results from either an increase in the release of exosomes-contained RNA during oocyte growth or from an increase in miRNA concentrations. The predicted targets for these up-regulated miRNAs include the ubiquitin, neurotrophin, MAPK and insulin signaling pathways, which are linked with ovarian follicular growth, oocyte meiotic maturation and mitotic division of early embryos (Sohel et al., 2013). In equine FF, miRNAs from exosomes can regulate gene expression of the TGFβ superfamily in granulosa cells during oocyte maturation (da Silveira et al., 2014). In several species, FF exosomes contain miRNAs, such as miR-30b, let-7, miR-181A, miR-375, miR-503 and miR-513a-3p, that may contribute to follicular growth and oocyte maturation (Murchison et al., 2007; Nagaraja et al., 2008; Lei et al., 2010; Xu et al., 2011; da Silveira et al., 2012). A recent human study compared the exosomal profile of plasma and pooled FF from 15 women younger than 35 years who underwent in vitro fertilization (IVF) due to male factor infertility (Santonocito et al., 2014). The authors identified 37 miRNAs upregulated in FF compared with plasma, 32 of them carried by exosomes. Some of the miRNA isolated from FF exosomes are also found in cumulus and granulosa cells. Pathway analysis revealed that these miRNAs targeted factors, such as WNT, MAPK, ERbB and TGFβ, that contribute to follicular development and meiotic resumption. This study, however, analyzed pooled FF, which is expected to contain follicles from different maturation stages, and therefore could not provide information on the role of FF EVs across the different steps of oocyte maturation. Describing the dynamic transitions of FF EVs miRNAs throughout follicle maturation requires further studies investigating EVs from follicles at each stage of oocyte maturation. These studies require invasive sampling procedures and cannot be easily performed in the general population. Valuable information could be obtained from animal experiments or, in humans, by recruiting patients undergoing IVF and obtaining FF and paired data on oocyte maturation from oocyte retrievals.
The catalog of the macromolecules contained in FF and FF EVs is continuously expanding, yet direct evidence for EV communication within the ovarian follicle is still lacking. Current studies have several limitations. Because of the relatively high costs of FF harvesting and analysis, to contain laboratory costs, in vivo studies of FF EVs typically have very small sample sizes and/or have pooled together FF samples from multiple follicles. These studies therefore often lack statistical power to draw conclusive results. Further, the in vivo studies conducted to date are mostly observational in nature, have generated descriptive information, and do not provide experimental evidence on the functions of EVs within the FF. In vitro functional studies will be fundamental to elucidate FF EV functions. The EV community has used in vitro studies to investigate the transfer of EVs from source into recipient cells. Similar studies are urgently needed for FF EVs. For instance, future investigations could harvest EVs from granulosa cells cultured in vitro and transfer them to an immature oocyte to determine whether EVs can induce maturation and fertilization. Findings from in vivo descriptive studies can directly inform experimental investigations. Several groups have reported or are actively identifying molecular mediators encapsulated in FF EVs in vivo. The growing capabilities for engineering EVs with predetermined contents could be leveraged to increase fertilization rates and improve embryo quality with the ultimate goal of increasing IVF success rates.
Other studies have examined the miRNA content of FF EVs in relation to female age. In equines, exosomal miRNA expression profiles vary with animal age and are associated with differences in fertility. Two studies reported high levels of miR-181A, miR-375 and miR-513a-3p in exosomes from older versus younger mares. These three miRNAs all target TGFβ, can suppress the TGFβ pathway, and lead to impaired oocyte maturation in older females (Knight and Glister, 2006; da Silveira et al., 2012). A human study also observed different miRNA profiles of EVs isolated from FF of three younger (<31 years old) compared with three older women (>38 years old) (Diez-Fraile et al., 2014). Specifically, four miRNAs were differentially expressed in FF of young versus older women: miR-21-5p was expressed only in the FF from young women, miR-190b and miR-99b-3p were exclusively in EVs from older patients, and miR-134 was significantly enriched in EVs from older patients. The observed correlations between miRNAs in FF EVs and female age open new fundamental questions: do miRNAs transported across the FF contribute, or even just track along, oocyte aging? If FF EVs were found to influence oocyte viability, could we use FF EVs miRNAs that are detected in younger women to develop new therapeutic interventions and attenuate the effects of age on fertility? These are forward-looking questions, yet easily testable in vitro. If the miRNAs that differ between younger and older patients also had a role in fertilization and embryo quality, the addition of EVs from younger women to the FF of older women, or preferably the addition of the relevant miRNAs or other biomolecules that may be involved in maintaining oocyte viability in younger women, could potentially improve fertilization as well as the rates of top quality embryos in older patients.
Extracellular vesicles in fertilization
Fertilization consists of sequential events, including cumulus cells expansion facilitating sperm passage through them, followed by sperm binding to the zona pellucida (Jin et al., 2011). The interaction of capacitated sperm with the zona pellucida induces the acrosome reaction, resulting in biochemical changes to the sperm head membrane and the release of proteases and hyaluronidase from the acrosome, enabling the sperm to recognize the zona pellucida and to enter the perivitelline space (PVS) (Chang, 1951; Austin, 1952; Kirchhoff et al., 1997; Aitken and Nixon, 2013). Recently, PMCA4a, a protein that helps maintain sperm Ca2+ homeostasis, was isolated from CD9-positive EVs in the oviduct (oviductosomes) and uterus (uterosomes). In vitro, sperm acquired PMCA4a after incubation with isolated exosomes from the luminal fluid. These findings suggest that EVs may prevent premature sperm capacitation (Al-Dossary et al., 2013).
After the acrosome reaction, the inner acrosomal membrane adheres to the areas of the oocyte that are covered with microvilli, and the sperm fuses with the oocyte membrane (Kaji et al., 2000). These microvilli display CD9 (Runge et al., 2007). Fusion of the sperm and oocyte leads to depolarization of the oolemma and triggers the release of cortical granules that are found under the oocyte surface, blocking the penetration of other sperm. The molecular basis underlying fusion remains obscure. In an in vitro porcine model, EVs are capable of fusing with sperm and inducing an acrosome reaction (Siciliano et al., 2008). Barraud-Lange et al. (2007) have shown in a mouse model that EVs are transferred from the oocyte to the sperm in the PVS before direct interaction between the oocyte and the sperm. The presence of EVs in the PVS was confirmed also by using electron microscopy (Barraud-Lange et al., 2012).
CD9-positive EVs are found on the plasma membrane of the oocyte, especially on the oocyte microvilli at the sperm attachment site. CD9 also localizes on the surface of the fertilizing sperm at the time of membrane binding and fusion and is required for sperm-oocyte fusion. CD9 null oocytes have altered microvilli and are unable to fuse with sperm (Runge et al., 2007). In a mouse study, fusion between the sperm and CD9 null oocytes was rescued when they were co-incubated wild-type oocytes. The authors concluded that fusion between the sperm and the oocyte is mediated by exosome-like vesicles containing CD9, which are released from the oocyte into the PVS and then transferred to the sperm (Miyado et al., 2008). However, two other studies in hamster and mice did not find any increase in the ability of CD9 null oocytes to fuse with sperm when they were inseminated in the presence of wild-type oocytes or medium that contained CD9-associated EVs (Gupta et al., 2009; Barraud-Lange et al., 2012).
Another tetraspanin, CD81, primarily produced by cumulus cells and localized mainly in the inner region of the zona pellucida, may also participate in fertilization, mainly in fusion-related events prior to membrane fusion and specifically in acrosome reaction (Tanigawa et al., 2008). Both CD81 and CD9 appear to be transferred to the sperm via EVs when the sperm penetrates the PVS (Miyado et al., 2008; Ohnami et al., 2012). CD81 may help transfer CD9 from the oocyte into the sperm membrane before sperm-oocyte fusion (Ohnami et al., 2012). In 2005, a new protein called Izumo was identified on the surface of sperm that had undergone an acrosome reaction (Inoue et al., 2005). Sperm lacking this protein are unable to fuse with oocytes. During oocyte and sperm fusion, Izumo1 binds to a folate receptor on the oocyte called Juno (Bianchi and Wright, 2014). Surprisingly, after fertilization, Juno, which is highly expressed on oocytes before fertilization, is shed from the oocyte membrane and redistributed in the PVS as EVs that are likely derived from the microvillus-rich oolemma. EVs may bind and neutralize subsequent acrosome-reacted sperm, providing a possible mechanism to prevent polyspermy (Bianchi and Wright, 2014; Bianchi et al., 2014b). In vitro studies have shown that Juno-deficient oocytes are unable to be fertilized (Bianchi and Wright, 2014). These findings provide evidence that the Izumo1 and Juno interaction facilitates the adhesion between the sperm and oocyte required for fertilization (Bianchi and Wright, 2014).
Extracellular vesicles and paracrine communication among embryos
Although controversial, it has been reported that in vitro culture of embryos is more successful when the embryos are kept in large groups during the whole culture period (Ferry et al., 1994; Hoelker et al., 2009). Embryos may generate their own microenvironment by secreting growth factors, which constitute a ‘secretome’ with both autocrine and paracrine effects (Katz-Jaffe et al., 2006; Ratajczak et al., 2006; Saadeldin et al., 2014). Cloned embryos cultured with porcine parthenogenetic embryos show a significant increase in their developmental competency (i.e. increased number of blastomeres and better blastocyst formation) compared with cloned embryos cultured alone. Analysis of culture media from porcine embryos cultured individually detected 30–120 mm vesicles differing in size according to the embryo's age (<40 mm in cultures from two-cell embryos and <120 mm in cultures from blastocysts). These vesicles express the exosomal marker CD9 and contain mRNAs, including OCT4, SOX2 and KLF4, which vary according to the stages of embryo development. Further experiments have shown that these exosomes/microvesicles can pass through the zona pellucida and are internalized by blastomeres (Saadeldin et al., 2014). The potential functional roles of EVs in embryo development have yet to be demonstrated. In humans, replicating the results from porcine studies have been challenging since the culture media used for in vitro fertilization contains significant quantities of EVs derived from the synthetic serum substitutes that are used to supplement the developing embryo, making it difficult to isolate EVs that are secreted by the embryo (Tannetta et al., 2014).
Extracellular vesicles and endometrial-embryo cross-talk during implantation
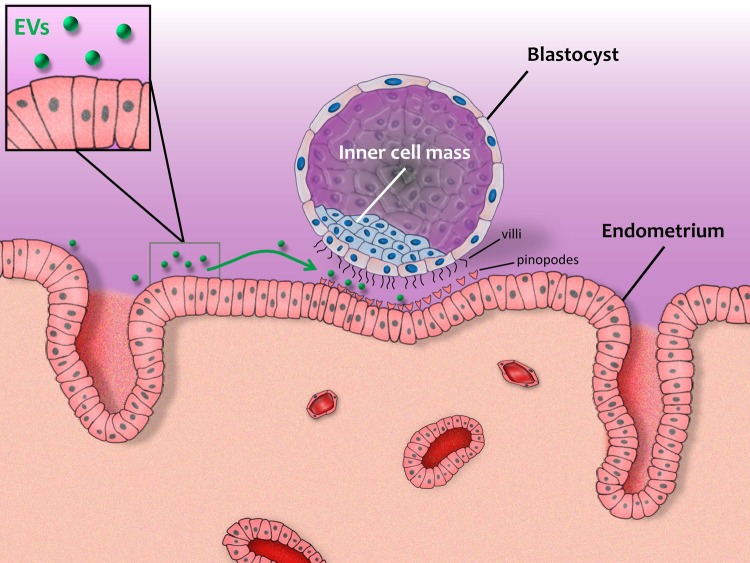
Successful implantation is dependent on coordination between the embryo and the endometrium, and EVs may participate in this required cross-talk (Fig. 3). It has been suggested that the endometrial epithelium release EVs that are involved in the transfer of signaling miRNAs and adhesion molecules either to the blastocyst or to the adjacent endometrium into the uterine cavity, which in turn can affect endometrial receptivity and implantation. EVs positive for the exosomal markers CD63 and HSP70 have been isolated from the uterine luminal fluid (ULF) of cyclic and pregnant sheep (Burns et al., 2014). Analysis of these vesicles revealed miRNAs and proteins expressed both by the conceptus trophectoderm and by the endometrial epithelium, such as cathepsin L1 and prostaglandin synthase 2. In the sheep, endogenous beta-retroviruses (enJSRVs) play a critical role in regulating conceptus trophectoderm development and placental growth (Varela et al., 2009; Burns et al., 2014). In vitro studies have shown that EVs isolated from the ULF of pregnant sheep can transfer RNAs (including enJSRV RNA) to other cells. These findings indicate that EVs present in the ULF are likely to have a biological role in the interaction between the embryo and the endometium. In human studies, EVs have been isolated from uterine fluid from women in different phases of the menstrual cycle. Both the luminal and glandular apical surfaces of endometrial epithelial cells express CD9 and CD63 exosomal markers, thereby showing that the endometrial epithelium are possible source of the exosomes found in the uterine cavity (Ng et al., 2013). The identification of EVs in the uterine fluid suggests a possible role of EVs in transferring information from the endometrium during implantation, although this hypothesis still needs to be proven.
Figure 3.
Extracellular vesicles and the cross-talk between the blastocyst and endometrium during implantation.
Possible future clinical implications
The study of EVs in reproduction has the potential for expanding our current understanding of the normal physiology of reproduction, i.e. identifying high-quality sperm and oocytes or pathological conditions such as implantation failure. Specifically, EVs have potential for identifying non-invasive biomarkers and for developing novel therapies to increase reproductive success.
In the last decade, multiple efforts have been performed to develop non-invasive methods to assess oocyte quality, top quality embryos and blastocyst formation and to identify the right embryo(s) to transfer in order result in a successful pregnancy. New techniques such as morpho-kinetic parameters, and attempts to establish proteins or secreted metabolomes in the culture medium have been added either clinically or experimentally to the classic morphological parameters for embryo selection, however, birth rates after assisted reproduction technologies have remained almost unchanged (Barkalina et al., 2014; Rodgaard et al., 2015). Recently several studies have focused on miRNAs in FF or culture media as possible biomarkers for increasing IVF success rates (Rosenbluth et al., 2014). EV-encapsulated miRNAs, in particular, are shielded from degradation and are remarkably stable in biological fluids. This property may greatly facilitate the translation of the growing understanding of miRNA biology into clinical applications. Investigating EV-encapsulated miRNAs also has key biological advantages over analysis of total miRNAs. While total miRNAs in human biofluids or supernatants from cell cultures may be released from apoptotic cells or cell debris, EV miRNAs are actively released by viable cells and are expected to represent an active means of communication between cells and tissues locally or systemically. Specifically miRNAs that are encapsulated by EVs might have a different role compared with miRNAs in biofluids as they transfer biological information to recipient cells.
A question of particular interest is about whether EVs carry and transport DNA from the developing embryo and the fetus to the maternal circulation. In the past few years, non-invasive prenatal testing using cell-free DNA (cfDNA) in maternal plasma has modified the clinical paradigm of prenatal screening for the common fetal aneuploidies and Y-chromosome (Bianchi et al., 2015). If cfDNA were to be carried by EVs, and embryonic and/or fetal DNA can be identified either in the culture media or maternal circulation, EVs could be used as biomarkers for Y-chromosome specific DNA. Further, EVs could be used for non-invasive prenatal genetic diagnostic techniques to discover aneuploidy before embryo transfer or in the very early stages of pregnancy, instead of embryo biopsy as in today's practice (Wright and Burton, 2009; Kahlert et al., 2014; Saadeldin et al., 2015).
EVs are being actively investigated for their potential clinical applications, particularly for targeted drug delivery as an alternative to nanoparticle-mediated delivery. In reproduction, nanoparticles have been used experimentally to load sperm with exogenous genetic material that is transferred to the oocyte during fertilization (Kim et al., 2010; Campos et al., 2011). However, the long-term consequences of using exogenous nanoparticles during conception are unknown. In this respect, EVs, as endogenous carriers of biomolecules, may be particularly advantageous. EVs are naturally present in human biofluids, but may also be engineered for tissue-specific transfers, such as the transfer of selected compounds into gametes and embryos to increase reproductive success (Barkalina et al., 2015).
Conclusions
Research in the field of intercellular communication in the reproductive tract has increased exponentially in the last decade. EVs and their contents, including miRNAs and proteins, have been characterized in several relevant body fluids. While in many cases, their functional roles are not entirely understood, there is growing evidence in the literature that intercellular communication mediated by EVs may contribute to oocyte and sperm maturation, fertilization, prevention of polyspermy and embryo implantation. Most studies of EVs in reproduction have used animal models, although relevant human research is emerging. As discussed throughout this review, much of the current published work is correlative. There is a critical need for further functional and mechanistic studies to provide conclusive experimental evidence for EVs and their cargoes as mediators of intercellular communication. In human reproduction, a major challenge is presented by limitations in relevant biological materials, which limits our ability to perform studies that utilize perturbations of pathways to confirm cause and affect relationships.
Authors’ roles
R.M. designed the review, performed the literature research and wrote the manuscript. L.L. designed the review, performed revisions and critically discussed the complete manuscript. A.A.B. designed the manuscript, supervised, and critically reviewed the complete manuscript.
Funding
R.M. and A.A.B. research on extracellular vesicles has been funded by P30ES00002 and R21ES024236 from the National Institute of Environmental Health Research and by grant award no. RPGA1301 from the Environment and Health Fund Israel. L.C.L. and A.A.B. receive support from the Common Fund Extracellular RNA Communication Consortium (ERCC) project U01HL126494 from the National Institutes of Health. L.C.L. also receives support from the ERCC project NIH UH2 TR000906.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aalberts M, Sostaric E, Wubbolts R, Wauben MWM, Nolte THENM, Gadella BM, Stout TAE, Stoorvogel W. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim Biophys Acta 2013;1834:2326–2335. [DOI] [PubMed] [Google Scholar]
- Adashi EY. Endocrinology of the ovary. Hum Reprod 1994;9:815–827. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Nixon B. Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod 2013;19:785–793. [DOI] [PubMed] [Google Scholar]
- Al-Dossary AA, Strehler EE, Martin-DeLeon PA. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS One 2013;8:e80181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arienti G, Carlini E, Palmerini CA. Fusion of human sperm to prostasomes at acidic pH. J Membr Biol 1997;155:89–94. [DOI] [PubMed] [Google Scholar]
- Arienti G, Carlini E, De Cosmo AM, Di Profio P, Palmerini CA. Prostasome-like particles in stallion semen. Biol Reprod 1998a;59:309–313. [DOI] [PubMed] [Google Scholar]
- Arienti G, Carlini E, Polci A, Cosmi EV, Palmerini CA. Fatty acid pattern of human prostasome lipid. Arch Biochem Biophys 1998b;358:391–395. [DOI] [PubMed] [Google Scholar]
- Arienti G, Carlini E, Nicolucci A, Cosmi EV, Santi F, Palmerini CA. The motility of human spermatozoa as influenced by prostasomes at various pH levels. Biol Cell 1999;91:51–54. [PubMed] [Google Scholar]
- Arvidson G, Ronquist G, Wikander G, Ojteg AC. Human prostasome membranes exhibit very high cholesterol/phospholipid ratios yielding high molecular ordering. Biochim Biophys Acta 1989;984:167–173. [DOI] [PubMed] [Google Scholar]
- Austin CR. The capacitation of the mammalian sperm. Nature 1952;170:326. [DOI] [PubMed] [Google Scholar]
- Barkalina N, Charalambous C, Jones C, Coward K. Nanotechnology in reproductive medicine: emerging applications of nanomaterials. Nanomedicine 2014;10:921–938. [DOI] [PubMed] [Google Scholar]
- Barkalina N, Jones C, Wood MJ, Coward K. Extracellular vesicle-mediated delivery of molecular compounds into gametes and embryos: learning from nature. Hum Reprod Update. 2015;21:627–639. [DOI] [PubMed] [Google Scholar]
- Barraud-Lange V, Naud-Barriant N, Bomsel M, Wolf JP, Ziyyat A. Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J 2007;21:3446–3449. [DOI] [PubMed] [Google Scholar]
- Barraud-Lange V, Boissonnas CC, Serres C, Auer J, Schmitt A, Lefevre B, Wolf JP, Ziyyat A. Membrane transfer from oocyte to sperm occurs in two CD9-independent ways that do not supply the fertilising ability of Cd9-deleted oocytes. Reproduction 2012;144:53–66. [DOI] [PubMed] [Google Scholar]
- Belleannee C, Calvo E, Caballero J, Sullivan R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol Reprod 2013;89:30. [DOI] [PubMed] [Google Scholar]
- Berube B, Sullivan R. Inhibition of in vivo fertilization by active immunization of male hamsters against a 26-kDa sperm glycoprotein. Biol Reprod 1994;51:1255–1263. [DOI] [PubMed] [Google Scholar]
- Bianchi E, Wright GJ. Izumo meets Juno: preventing polyspermy in fertilization. Cell Cycle 2014;13:2019–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014b;508:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer A, Goff AK, Boerboom D. WNT signaling in ovarian follicle biology and tumorigenesis. Trends Endocrinol Metab: TEM 2010;21:25–32. [DOI] [PubMed] [Google Scholar]
- Brower PT, Schultz RM. Intercellular communication between granulosa cells and mouse oocytes: existence and possible nutritional role during oocyte growth. Dev Biol 1982;90:144–153. [DOI] [PubMed] [Google Scholar]
- Buccione R, Schroeder AC, Eppig JJ. Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol Reprod 1990;43:543–547. [DOI] [PubMed] [Google Scholar]
- Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS One 2014;9:e90913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero J, Frenette G, Sullivan R. Post testicular sperm maturational changes in the bull: important role of the epididymosomes and prostasomes. Vet Med Int 2011;757194:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos VF, de Leon PM, Komninou ER, Dellagostin OA, Deschamps JC, Seixas FK, Collares T. NanoSMGT: transgene transmission into bovine embryos using halloysite clay nanotubes or nanopolymer to improve transfection efficiency. Theriogenology 2011;76:1552–1560. [DOI] [PubMed] [Google Scholar]
- Carlini E, Palmerini CA, Cosmi EV, Arienti G. Fusion of sperm with prostasomes: effects on membrane fluidity. Arch Biochem Biophys 1997;343:6–12. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Pahlson C, Bergquist M, Ronquist G, Stridsberg M. Antibacterial activity of human prostasomes. Prostate 2000;44:279–286. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Nilsson O, Larsson A, Stridsberg M, Sahlen G, Ronquist G. Characteristics of human prostasomes isolated from three different sources. Prostate 2003;54:322–330. [DOI] [PubMed] [Google Scholar]
- Caselles AB, Miro-Moran A, Morullo Rodriguez A, Gallardo Bolanos JM, Ortega-Ferrusola C, Salido GM, Pena FJ, Tapia JA, Aparicio IM. Identification of apoptotic bodies in equine semen. Reprod Domest Anim 2014;49:254–262. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951;168:697–698. [DOI] [PubMed] [Google Scholar]
- Chen H, Griffiths G, Galileo DS, Martin-DeLeon PA. Epididymal SPAM1 is a marker for sperm maturation in the mouse. Biol Reprod 2006;74:923–930. [DOI] [PubMed] [Google Scholar]
- Chesnel F, Wigglesworth K, Eppig JJ. Acquisition of meiotic competence by denuded mouse oocytes: participation of somatic-cell product(s) and cAMP. Dev Biol 1994;161:285–295. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends in Cell Biology 2015;25:364–372. [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol 2012;356:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG. Epididymis and sperm function. Andrologia 1996;28:57–59. [PubMed] [Google Scholar]
- Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, Buzas EI, Lotvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles 2013;2:20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuman C, Menkhorst E, Winship A, Van Sinderen M, Osianlis T, Rombauts LJ, Dimitriadis E. Fetal-maternal communication: the role of Notch signalling in embryo implantation. Reproduction 2014;147:R75–R86. [DOI] [PubMed] [Google Scholar]
- Dacheux JL, Belleannee C, Guyonnet B, Labas V, Teixeira-Gomes AP, Ecroyd H, Druart X, Gatti JL, Dacheux F. The contribution of proteomics to understanding epididymal maturation of mammalian spermatozoa. Systems Biol Reprod Medicine 2012;58:197–210. [DOI] [PubMed] [Google Scholar]
- da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod 2012;86:71. [DOI] [PubMed] [Google Scholar]
- da Silveira JC, Carnevale EM, Winger QA, Bouma GJ. Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod Biol Endocrinol 2014;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000;113:3365–3374. [DOI] [PubMed] [Google Scholar]
- Diez-Fraile A, Lammens T, Tilleman K, Witkowski W, Verhasselt B, De Sutter P, Benoit Y, Espeel M, D'Herde K. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum Fertil 2014;17:90–98. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Garcia-Rudaz C, Ojeda SR. Role of neurotrophic factors in early ovarian development. Semin Reprod Med 2009;27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013;12:347–357. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001;122:829–838. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Chesnel F, Hirao Y, O'Brien MJ, Pendola FL, Watanabe S, Wigglesworth K. Oocyte control of granulosa cell development: how and why. Hum Reprod 1997;12:127–132. [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci USA 2002;99:2890–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani R, Ronquist G. Association of some hydrolytic enzymes with the prostasome membrane and their differential responses to detergent and PIPLC treatment. Prostate 1995;27:95–101. [DOI] [PubMed] [Google Scholar]
- Ferry L, Mermillod P, Massip A, Dessy F. Bovine embryos cultured in serum-poor oviduct-conditioned medium need cooperation to reach the blastocyst stage. Theriogenology 1994;42:445–453. [DOI] [PubMed] [Google Scholar]
- Fornes MW, Barbieri A, Cavicchia JC. Morphological and enzymatic study of membrane-bound vesicles from the lumen of the rat epididymis. Andrologia 1995;27:1–5. [DOI] [PubMed] [Google Scholar]
- Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod 1994;50:225–232. [DOI] [PubMed] [Google Scholar]
- Frenette G, Sullivan R. Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol Reprod Dev 2001;59:115–121. [DOI] [PubMed] [Google Scholar]
- Frenette G, Lessard C, Sullivan R. Selected proteins of “prostasome-like particles” from epididymal cauda fluid are transferred to epididymal caput spermatozoa in bull. Biol Reprod 2002;67:308–313. [DOI] [PubMed] [Google Scholar]
- Frenette G, Lessard C, Madore E, Fortier MA, Sullivan R. Aldose reductase and macrophage migration inhibitory factor are associated with epididymosomes and spermatozoa in the bovine epididymis. Biol Reprod 2003;69:1586–1592. [DOI] [PubMed] [Google Scholar]
- Frenette G, Lessard C, Sullivan R. Polyol pathway along the bovine epididymis. Mol Reprod Dev 2004;69:448–456. [DOI] [PubMed] [Google Scholar]
- Frenette G, Thabet M, Sullivan R. Polyol pathway in human epididymis and semen. J Androl 2006a;27:233–239. [DOI] [PubMed] [Google Scholar]
- Frenette G, Girouard J, Sullivan R. Comparison between epididymosomes collected in the intraluminal compartment of the bovine caput and cauda epididymidis. Biol Reprod 2006b;75:885–890. [DOI] [PubMed] [Google Scholar]
- Frenette G, Girouard J, D'Amours O, Allard N, Tessier L, Sullivan R. Characterization of two distinct populations of epididymosomes collected in the intraluminal compartment of the bovine cauda epididymis. Biol Reprod 2010;83:473–480. [DOI] [PubMed] [Google Scholar]
- Gatti JL, Metayer S, Belghazi M, Dacheux F, Dacheux JL. Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol Reprod 2005;72:1452–1465. [DOI] [PubMed] [Google Scholar]
- Girouard J, Frenette G, Sullivan R. Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int J Androl 2011;34:e475–e486. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2013;2 doi:10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GS, Galileo DS, Reese K, Martin-Deleon PA. Investigating the role of murine epididymosomes and uterosomes in GPI-linked protein transfer to sperm using SPAM1 as a model. Mol Reprod Development 2008;75:1627–1636. [DOI] [PubMed] [Google Scholar]
- Gupta S, Primakoff P, Myles DG. Can the presence of wild-type oocytes during insemination rescue the fusion defect of CD9 null oocytes? Mol Reprod Dev 2009;76:602. [DOI] [PubMed] [Google Scholar]
- Hamel M, Dufort I, Robert C, Gravel C, Leveille MC, Leader A, Sirard MA. Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum Reprod 2008;23:1118–1127. [DOI] [PubMed] [Google Scholar]
- Hoelker M, Rings F, Lund Q, Ghanem N, Phatsara C, Griese J, Schellander K, Tesfaye D. Effect of the microenvironment and embryo density on developmental characteristics and gene expression profile of bovine preimplantative embryos cultured in vitro. Reproduction 2009;137:415–425. [DOI] [PubMed] [Google Scholar]
- Huo LJ, Fan HY, Zhong ZS, Chen DY, Schatten H, Sun QY. Ubiquitin-proteasome pathway modulates mouse oocyte meiotic maturation and fertilization via regulation of MAPK cascade and cyclin B1 degradation. Mech Dev 2004;121:1275–1287. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm's journey to and interaction with the oocyte. J Clin Invest 2010;120:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005;434:234–238. [DOI] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA 2011;108:4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. Sperm survival versus degradation in the Mammalian epididymis: a hypothesis. Biol Reprod 2004;71:1405–1411. [DOI] [PubMed] [Google Scholar]
- Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 2014;289:3869–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet 2000;24:279–282. [DOI] [PubMed] [Google Scholar]
- Katz-Jaffe MG, Schoolcraft WB, Gardner DK. Analysis of protein expression (secretome) by human and mouse preimplantation embryos. Fertil Steril 2006;86:678–685. [DOI] [PubMed] [Google Scholar]
- Kim TS, Lee SH, Gang GT, Lee YS, Kim SU, Koo DB, Shin MY, Park CK, Lee DS. Exogenous DNA uptake of boar spermatozoa by a magnetic nanoparticle vector system. Reprod Domest Anim 2010;45:e201–e206. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Seminars in Cell & Developmental Biology 2015;40:97–104. [DOI] [PubMed] [Google Scholar]
- Kimura M, Kim E, Kang W, Yamashita M, Saigo M, Yamazaki T, Nakanishi T, Kashiwabara S, Baba T. Functional roles of mouse sperm hyaluronidases, HYAL5 and SPAM1, in fertilization. Biol Reprod 2009;81:939–947. [DOI] [PubMed] [Google Scholar]
- Kirchhoff C, Pera I, Derr P, Yeung CH, Cooper T. The molecular biology of the sperm surface. Post-testicular membrane remodelling. Adv Exp Med Biol 1997;424:221–232. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. Local roles of TGF-beta superfamily members in the control of ovarian follicle development. Anim Reprod Sci 2003;78:165–183. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006;132:191–206. [DOI] [PubMed] [Google Scholar]
- Kravets FG, Lee J, Singh B, Trocchia A, Pentyala SN, Khan SA. Prostasomes: Current concepts. Prostate 2000;43:169–174. [DOI] [PubMed] [Google Scholar]
- Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, Kobayashi T, Salles JP, Perret B, Bonnerot C et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J 2004;380:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Jin S, Gonzalez G, Behringer RR, Woodruff TK. The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol 2010;315:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, Liu J, Pan T, Chen J, Wu M et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol 2013;14:793–803. [DOI] [PubMed] [Google Scholar]
- Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;22:26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod 2009;81:717–729. [DOI] [PubMed] [Google Scholar]
- Madison MN, Roller RJ, Okeoma CM. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 2014;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel PY, Marti M. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell Microbiology 2014;16:344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 2002;296:2178–2180. [DOI] [PubMed] [Google Scholar]
- Miyado K, Yoshida K, Yamagata K, Sakakibara K, Okabe M, Wang X, Miyamoto K, Akutsu H, Kondo T, Takahashi Y et al. The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc Natl Acad Sci USA 2008;105:12921–12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ. Critical roles for Dicer in the female germline. Genes Dev 2007;21:682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen R, Han DY, Zhu H, Agno JE, Gunaratne PH, DeMayo FJ et al. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol 2008;22:2336–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YH, Rome S, Jalabert A, Forterre A, Singh H, Hincks CL, Salamonsen LA. Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One 2013;8:e58502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnami N, Nakamura A, Miyado M, Sato M, Kawano N, Yoshida K, Harada Y, Takezawa Y, Kanai S, Ono C et al. CD81 and CD9 work independently as extracellular components upon fusion of sperm and oocyte. Biol Open 2012;1:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, Nakayasu ES, Joffe LS, Guimaraes AJ, Sobreira TJ, Nosanchuk JD, Cordero RJ, Frases S, Casadevall A, Almeida IC et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One 2010;5:e11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons-Rejraji H, Artonne C, Sion B, Brugnon F, Canis M, Janny L, Grizard G. Prostasomes: inhibitors of capacitation and modulators of cellular signalling in human sperm. Int J Androl 2011;34:568–580. [DOI] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006;20:847–856. [DOI] [PubMed] [Google Scholar]
- Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 2011;81:1171–1182. [DOI] [PubMed] [Google Scholar]
- Regente M, Corti-Monzon G, Maldonado AM, Pinedo M, Jorrin J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Letters 2009;583:3363–3366. [DOI] [PubMed] [Google Scholar]
- Rejraji H, Vernet P, Drevet JR. GPX5 is present in the mouse caput and cauda epididymidis lumen at three different locations. MolReprod Dev 2002;63:96–103. [DOI] [PubMed] [Google Scholar]
- Renneberg H, Konrad L, Dammshauser I, Seitz J, Aumuller G. Immunohistochemistry of prostasomes from human semen. Prostate 1997;30:98–106. [DOI] [PubMed] [Google Scholar]
- Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol 2009;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgaard T, Heegaard PM, Callesen H. Non-invasive assessment of in-vitro embryo quality to improve transfer success. Reprod Biomed Online 2015;31:585–592. [DOI] [PubMed] [Google Scholar]
- Ronquist G, Brody I. The prostasome: its secretion and function in man. Biochim Biophys Acta 1985;822:203–218. [DOI] [PubMed] [Google Scholar]
- Ronquist G, Brody I, Gottfries A, Stegmayr B. An Mg2+ and Ca2+-stimulated adenosine triphosphatase in human prostatic fluid: part I. Andrologia 1978a;10:261–272. [DOI] [PubMed] [Google Scholar]
- Ronquist G, Brody I, Gottfries A, Stegmayr B. An Mg2+ and Ca2+-stimulated adenosine triphosphatase in human prostatic fluid: part II. Andrologia 1978b;10:427–433. [DOI] [PubMed] [Google Scholar]
- Ronquist G, Carlsson L, Larsson A. Human prostasomes contain chromosomal DNA. Prostate 2009;69:737–743. [DOI] [PubMed] [Google Scholar]
- Ronquist GK, Larsson A, Stavreus-Evers A, Ronquist G. Prostasomes are heterogeneous regarding size and appearance but affiliated to one DNA-containing exosome family. Prostate 2012;72:1736–1745. [DOI] [PubMed] [Google Scholar]
- Rooney IA, Atkinson JP, Krul ES, Schonfeld G, Polakoski K, Saffitz JE, Morgan BP. Physiologic relevance of the membrane attack complex inhibitory protein CD59 in human seminal plasma: CD59 is present on extracellular organelles (prostasomes), binds cell membranes, and inhibits complement-mediated lysis. J Exp Med 1993;177:1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth EM, Shelton DN, Wells LM, Sparks AET, Van Voorhis BJ. Human embryos secrete microRNAs into culture media—a potential biomarker for implantation. Fertil Steril 2014;101:1493–1500. [DOI] [PubMed] [Google Scholar]
- Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, Primakoff P, Myles DG. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol 2007;304:317–325. [DOI] [PubMed] [Google Scholar]
- Saadeldin IM, Kim SJ, Choi YB, Lee BC. Improvement of cloned embryos development by co-culturing with parthenotes: a possible role of exosomes/microvesicles for embryos paracrine communication. Cell Reprogram 2014;16:223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadeldin IM, Oh HJ, Lee BC. Embryonic-maternal cross-talk via exosomes: potential implications. Stem Cells Cloning Adv Appl 2015;8:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez F, Motta C, Boucher D, Grizard G. Antioxidant capacity of prostasomes in human semen. Mol Hum Rep 1998;4:667–672. [DOI] [PubMed] [Google Scholar]
- Saez F, Motta C, Boucher D, Grizard G. Prostasomes inhibit the NADPH oxidase activity of human neutrophils. Mol Hum Reprod 2000;6:883–891. [DOI] [PubMed] [Google Scholar]
- Saez F, Frenette G, Sullivan R. Epididymosomes and prostasomes: their roles in posttesticular maturation of the sperm cells. J Androl 2003;24:149–154. [DOI] [PubMed] [Google Scholar]
- Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa M, Barbagallo D, Borzi P, Rizzari S, Maugeri M et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril 2014;102:1751–1761: e1751. [DOI] [PubMed] [Google Scholar]
- Senbon S, Hirao Y, Miyano T. Interactions between the oocyte and surrounding somatic cells in follicular development: lessons from in vitro culture. J Reprod Dev 2003;49:259–269. [DOI] [PubMed] [Google Scholar]
- Siciliano L, Marciano V, Carpino A. Prostasome-like vesicles stimulate acrosome reaction of pig spermatozoa. Reprod Biol Endocrinol 2008;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics 2009;6:267–283. [DOI] [PubMed] [Google Scholar]
- Sohel MM, Hoelker M, Noferesti SS, Salilew-Wondim D, Tholen E, Looft C, Rings F, Uddin MJ, Spencer TE, Schellander K et al. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLoS One 2013;8:e78505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 2007;89:205–212. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction 2013;146:R21-R35. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Saez F, Girouard J, Frenette G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol Dis 2005;35:1–10. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl 2007;9:483–491. [DOI] [PubMed] [Google Scholar]
- Suzumori N, Burns KH, Yan W, Matzuk MM. RFPL4 interacts with oocyte proteins of the ubiquitin-proteasome degradation pathway. Proc Natl Acad Sci USA 2003;100:550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa M, Miyamoto K, Kobayashi S, Sato M, Akutsu H, Okabe M, Mekada E, Sakakibara K, Miyado M, Umezawa A et al. Possible involvement of CD81 in acrosome reaction of sperm in mice. Mol Reprod Dev 2008;75:150–155. [DOI] [PubMed] [Google Scholar]
- Tannetta D, Dragovic R, Alyahyaei Z, Southcombe J. Extracellular vesicles and reproduction-promotion of successful pregnancy. Cell Mol Immunol 2014;11:548–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009;9:581–593. [DOI] [PubMed] [Google Scholar]
- Thimon V, Frenette G, Saez F, Thabet M, Sullivan R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum Reprod 2008;23:1698–1707. [DOI] [PubMed] [Google Scholar]
- Traver S, Assou S, Scalici E, Haouzi D, Al-Edani T, Belloc S, Hamamah S. Cell-free nucleic acids as non-invasive biomarkers of gynecological cancers, ovarian, endometrial and obstetric disorders and fetal aneuploidy. Hum Reprod Update 2014;20:905–923. [DOI] [PubMed] [Google Scholar]
- Utleg AG, Yi EC, Xie T, Shannon P, White JT, Goodlett DR, Hood L, Lin B. Proteomic analysis of human prostasomes. Prostate 2003;56:150–161. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654–659. [DOI] [PubMed] [Google Scholar]
- van der Grein SG, Nolte-'t Hoen EN. “Small Talk” in the innate immune system via RNA-containing extracellular vesicles. Front Immunol 2014;5:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela M, Spencer TE, Palmarini M, Arnaud F. Friendly viruses: the special relationship between endogenous retroviruses and their host. Ann N Y Acad Sci 2009;1178:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet P, Aitken RJ, Drevet JR. Antioxidant strategies in the epididymis. Mol Cell Endocrinol 2004;216:31–39. [DOI] [PubMed] [Google Scholar]
- Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R, Tewari M et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucl Acids Res 2014;42:7290–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CF, Burton H. The use of cell-free fetal nucleic acids in maternal blood for non-invasive prenatal diagnosis. Hum Reprod Update 2009;15:139–151. [DOI] [PubMed] [Google Scholar]
- Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 2003;278:10963–10972. [DOI] [PubMed] [Google Scholar]
- Xu YW, Wang B, Ding CH, Li T, Gu F, Zhou C. Differentially expressed micoRNAs in human oocytes. J Assist Reprod Genet 2011;28:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R, Kamiguchi Y, Mikamo K, Suzuki F, Yanagimachi H. Maturation of spermatozoa in the epididymis of the Chinese hamster. Am J Anat 1985;172:317–330. [DOI] [PubMed] [Google Scholar]
- Zhang H, Martin-DeLeon PA. Mouse Spam1 (PH-20) is a multifunctional protein: evidence for its expression in the female reproductive tract. Biol Reprod 2003;69:446–454. [DOI] [PubMed] [Google Scholar]
- Zhang M, Ouyang H, Xia G. The signal pathway of gonadotrophins-induced mammalian oocyte meiotic resumption. Mol Hum Reprod 2009;15:399–409. [DOI] [PubMed] [Google Scholar]