Abstract
STUDY QUESTION
Does semen quality improve during early adulthood?
SUMMARY ANSWER
Semen variables change little during the third decade of life, however some improvement in sperm morphology and motility may occur.
WHAT IS KNOWN ALREADY
A suspicion of deteriorating semen quality has been raised in several studies. The longitudinal development of semen quality in early adulthood is insufficiently understood.
STUDY DESIGN, SIZE, DURATION
A longitudinal follow-up of two cohorts of volunteer young adult Finnish men representing the general population was carried out. Cohorts A (discovery cohort, born 1979–1981, n = 336) and B (validation cohort, born 1983, n = 197) were followed up from the age of 19 years onward for 10 years.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Inclusion criteria included that both the men and their mothers were born in Finland. Semen analysis was performed in cohorts A and B at 2–4 year intervals over a period of 10 years. Semen volume, sperm concentration, total sperm count, motility, total motile count and morphology were the variables assessed in the analysis. A physical examination was carried out at each visit to detect any significant andrological abnormalities. The overall participation rate was 13.4%.
MAIN RESULTS AND THE ROLE OF CHANCE
During the follow-up, the percentage of sperm with normal morphology and the percentage of motile sperm increased significantly both in the discovery (A) (P < 0.001 at 19 versus 29 years for both) and validation (B) (P < 0.001 and P = 0.03 at 19 versus 29 years, respectively) cohort. Sperm concentration and total sperm count showed a significant increase with age only in cohort B (P = 0.03 at 21 versus 29 years, P = 0.009 at 19 versus 29 years, respectively).
LIMITATIONS, REASONS FOR CAUTION
A limited number of men participated both in the first round and in the final fourth round (cohort A, n = 111 and cohort B, n = 90 men) and in all four rounds (cohort A, n = 61 and cohort B, n = 52).
WIDER IMPLICATIONS OF THE FINDINGS
Almost full spermatogenic capacity is reached by the age of 19 years. However, the improvement in sperm motility and morphology during early adulthood may slightly improve male fecundity.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the European Commission (QLK4-CT-1999-01422, QLK4-CT-2001-00269, QLK4-2002-0063, FP7/2008-2012: DEER 212844), The Danish Medical Research Council (9700833, 9700909), Danish Agency for Science (Technology and Innovation 09-067180), the Svend Andersen's Foundation, Velux Foundation, and Novo Nordisk Foundation, the Turku University Hospital, Sigrid Jusélius Foundation and the Academy of Finland. There are no conflicts of interest.
Keywords: spermatogenesis, sperm, cohort, reproductive health, maturation, fertility, testis, germ cell, morphology, motility
Introduction
Finnish men used to have better semen quality than men in many other European countries (Jørgensen et al., 2001, 2002). However, the recent studies of Finnish men have shown a trend of deteriorating semen quality (Jørgensen et al., 2011). Such a decrease in semen quality has not been observed among recent cohorts of young Danish men, whereas it seems that in the Danish population semen quality has reached already a plateau at a low level (Jørgensen et al., 2012). The deterioration of male reproductive health overall is worrying. Parallel to declining semen quality, the incidence of testicular cancer has increased over the last few decades (Carlsen et al., 1992; Chia et al., 2010; Jørgensen et al., 2011; Rolland et al., 2013). During the past 10 years the incidence of testicular cancer in Finland has increased by 3.0% every year (NORDCAN, Association of the Nordic Cancer Registries). We have been assessing the semen quality of young Finnish men in cross-sectional studies since the 1990s (Jørgensen et al., 2002, 2011). The surprisingly poor semen quality in different cohorts of young Finnish men led us to question whether semen quality at the early adult age of 19 years represents that of fully mature men. To examine whether semen quality improves beyond this age, we have followed up longitudinally two cohorts of men for semen quality at 2–4 year intervals for up to 10 years.
Materials and Methods
Study population
All Finnish men are required to attend a medical examination at the age of 18 years prior to their recruitment to military service. The military authorities provided us with the list of conscripts living in the city of Turku and the surrounding cities of Raisio and Kaarina (in South West Finland). Inclusion criteria included that both the men and their mothers were born in Finland. These men represent the general population of Finnish men.
All the men were invited to take part in the study irrespective of their medical fitness for military service. For those willing to participate, an appointment for the examination at the Turku University Andrology Unit was made. The participation rate was 13.4% (Jørgensen et al., 2011). Participants were instructed to abstain from ejaculation for at least 48 h before providing the semen sample. Participants received financial reimbursement for their participation (40–50€).
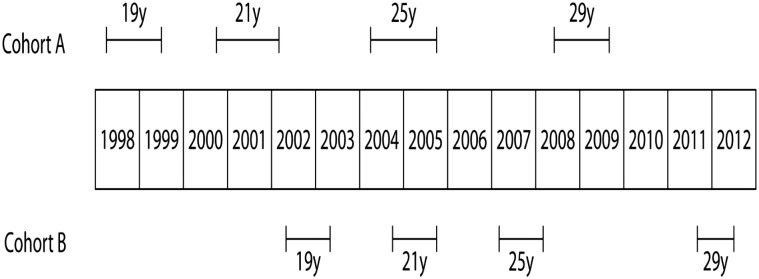
Volunteers were enrolled to the study between 1998 and 2003. The study population consisted of two cohorts. Cohort A included men born in 1979–1981 and cohort B men born in 1983. Men in both cohorts were invited for the physical examination and semen study four times in total, at the age of 19, 21, 25 and 29 years (Fig. 1). Cohort A was used as a discovery cohort and cohort B was used for validation of the findings in cohort A.
Figure 1.
The schedule of study visits for the two cohorts of men during the follow-up period through young adulthood.
In cohort A (born 1979–1981) 336 men took part in the first visit of the study at the age of 19 years. Ten years later 111 men participated in the final fourth visit. Sixty-one of these men were evaluated on all four rounds of this study. In cohort B (born 1983) 197 men were evaluated in the first round and 10 years later 90 men were examined. Fifty-two men in cohort B were evaluated on all four rounds (Fig. 1).
The baseline semen results of cohorts A and B have been previously described in a study reporting a secular cohort trend in semen quality (Jørgensen et al., 2011).
Semen samples
Semen samples were produced by masturbation in the privacy of a designated room next to the laboratory or at home, kept at 37°C and delivered within 1 h to the laboratory. The abstinence period was assessed as the time between current and previous ejaculation as reported by the individual participant.
Semen volume was estimated by weighing the collection tube with semen sample and subtracting the predetermined weight of the empty tube, assuming that 1 ml = 1 g. For sperm motility assessment, 10 μl of well-mixed semen was placed on a clean glass slide that had been kept at 37°C and covered with 22 × 22 mm cover slip. The preparation was observed on the heated stage of a microscope at 37°C and immediately examined at × 400 magnification. The sperm were classified as either motile (classes A+B+C, World Health Organization (WHO) 1992, 1999) or immotile (class D, WHO 1992 and 1999) and the percentage of motile sperm was reported. The motility counting was repeated on a second aliquot of semen, and the average of the two evaluations was used.
For the assessment of the sperm concentration the samples were diluted in a solution of 0.6 mol/l NaHCO3 and 0.4% (v/v) formaldehyde in distilled water. The sperm concentration was subsequently assessed using an improved Neubauer haemocytometer. Only sperm with tails were counted. The same technician assessed semen volume, sperm motility and concentration during all the study years.
For the morphological evaluation of the semen, samples smears were prepared, Papanicolaou stained and assessed according to strict criteria (Menkveld et al., 1990). The samples were originally assessed for morphology at intervals over a long period of several years. To reduce the intra-observer variation over time, all samples of 61 men in cohort A and 52 men in cohort B who participated in all four examinations were pooled (samples from cohort A and B were pooled separately). Thereafter, the samples of each pool were assessed for morphology in a random order during a period of a few weeks. Only the morphology data of these samples are reported. Assessment of the sperm morphology was performed entirely by a single investigator in each cohort (M.V. in cohort A and W.R. in cohort B).
A continuous external quality control programme for assessment of sperm concentration was performed during the entire study period (Jørgensen et al., 2002). Five semen samples were annually sent to the participating centres, including the Turku laboratory, 8–10 times and assessed as described above. The quality control programme showed that the results of the technician of the Turku laboratory stayed at a constant level over the years when compared with other participants in the programme.
Physical examination
Physical examination included evaluation of Tanner stage of pubic hair, testicular size by Prader orchidometer and ultrasound measurement. The presence of vas deference was confirmed by palpation. Also the existence of varicocele and its grade was estimated by Valsalva testing.
Questionnaires
A previously standardized questionnaire was sent to the participants before their attendance at the Andrology Unit. The questionnaire included information on age, previous and current diseases, including any known history of pregnancy/fertility. The participants were asked to fill in the questionnaire in collaboration with their parents.
Statistical analysis
Semen volume, sperm concentration, total sperm counts, total motile count and percentage of normal sperm were normalized by square root transformation due to skewed distribution of residuals. Mixed model repeated measures analyses were used to test for mean differences in the outcome variables between visits in both cohort groups. P-values for pairwise comparisons were adjusted using the Tukey–Kramer method. Abstinence time and quadratic term for abstinence time were added as covariates into the models. Motility was also adjusted for the time between ejaculation and start of semen analysis. A value of P < 0.05 was considered statistically significant. Statistical analyses were performed using SAS for Windows version 9.2 (Cary, NC, USA).
Ethics
The study was approved by the Joint Ethics Committee of the University of Turku and Turku University Hospital, Finland. All participants gave their written informed consent for the study. The study was performed according to the Helsinki II declaration.
Results
The clinical findings and the self-reported information of the cohorts A and B at the age of 19 years are reported in Table I. Semen variables of the cohorts A and B during the follow-up of 10 years are shown in Tables II–V. A multivariate analysis was performed to adjust P-values for the abstinence. Cigarette smoking did not influence semen quality in our analysis (data not shown).
Table I.
Clinical findings (varicocele and hydrocele) and self-reported conditions in young men from the Turku area in Finland (all men participated in the first round)1.
| Cohort A, n= 336 (%) | Cohort B, n= 197 (%) | |
|---|---|---|
| Clinical findings: | ||
| Varicocele | 22.3 | 20 |
| Hydrocele | 0 | 1.5 |
| Been diagnosed as having: | ||
| Epididymitis | 0.6 | 0 |
| Chlamydia | 1.2 | 1.5 |
| Prostatitis | 0.9 | 0.5 |
| Cystitis or pyelonephritis | 2.1 | 2 |
| Diabetes | 0 | 0 |
| Thyroid disease | 0.3 | 1 |
| Been treated for: | ||
| Varicocele | 1.5 | 0.5 |
| Cryptorchidism | 0.9 | 1.5 |
| Testicular torsion | 0.9 | 1 |
| Testicular cancer | 0 | 0 |
| Other diseases of penis, urethra or scrotum | 1.2 | 1 |
| Inguinal hernia | 6.8 | 3 |
| Has: | ||
| Experienced fertility problems | 0.9 | 0 |
| Caused a pregnancy | 2.1 | 6.1 |
| Taken any medication during past 3 months | 14.6 | 20 |
| Subgroup of men not affected by any of the above mention conditions | 53.9 | 55.3 |
1We performed additional analyses and excluded from the analysis participants with andrological conditions mentioned in Table I (varicocele, hydrocele, patients treated for cryptorchidism and patients who experienced fertility problems). This additional analysis did not change the results.
Table II.
Semen variables for men in cohort A at different ages.
| Age (years) | 19 | 21 | 25 | 29 | P-value |
|---|---|---|---|---|---|
| n | 336 | 179 | 181 | 111 | |
| Semen volume (ml) | 3.0 (3.3) 0.5–8.5 |
3.4 (3.5) 0.6–9.9 |
3.5 (3.8) 0.7–9.9 |
3.5 (3.8) 0.2–8.7 |
0.06 19y versus 21y <0.001 19y versus 25y <0.001 19y versus 29y 0.09 21y versus 25y 0.21 21y versus 29y 1.00 25y versus 29y |
| Sperm concentration (million/ml) | 60 (72) 0–515 |
56 (65) 0–377 |
51 (64) 0–225 |
70 (71) 0–195 |
0.37 19y versus 21y 0.61 19y versus 25y 0.81 19y versus 29y 0.98 21y versus 25y 0.20 21y versus 29y 0.27 25y versus 29y |
| Total sperm count (million) | 193 (219) 0–1380 |
184 (217) 0–753 |
187 (239) 0–1345 |
219 (251) 0–966 |
1.00 19y versus 21y 0.46 19y versus 25y 0.06 19y versus 29y 0.52 21y versus 25y 0.08 21y versus 29y 0.63 25y versus 29y |
| Motility (abc, %) | 66 (64) 1–87 |
72 (70) 15–92 |
78 (76) 19–95 |
82 (79) 36–94 |
<0.001 19y versus 21y <0.001 19y versus 25y <0.001 19y versus 29y <0.001 21y versus 25y <0.001 21y versus 29y 0.21 25y versus 29y |
| Total motile count (million) | 124 (144) 0–800 |
128 (154) 0–538 |
138 (182) 0–942 |
184 (199) 0–763 |
0.47 19y versus 21y 0.002 19y versus 25y <0.001 19y versus 29y 0.13 21y versus 25y <0.001 21y versus 29y 0.23 25y versus 29y |
P-value <0.05 is considered significant, shown in bold. Results are shown as median (mean) and range. Mixed model of repeated measures analyses following a square root transformation was used. P-values for pairwise comparisons were adjusted using the Tukey–Kramer methods. For other adjustments see statistical methods.
Table III.
Semen variables for the men in cohort A, who participated at all ages.
| Age (years) | 19 | 21 | 25 | 29 | P-value |
|---|---|---|---|---|---|
| n | 61 | 61 | 61 | 61 | |
| Semen volume (ml) | 3.2 (3.5) 0.9–8.0 |
3.7 (3.7) 0.8–8.7 |
3.6 (4.1) 1.0–8.2 |
3.9 (4.0) 0.2–7.9 |
0.69 19y versus 21y 0.01 19y versus 25y 0.02 19y versus 29y 0.19 21y versus 25y 0.26 21y versus 29y 1.00 25y versus 29y |
| Sperm concentration (million/ml) | 54 (68) 0–309 |
47 (64) 0–377 |
56 (67) 0–225 |
66 (70) 0–195 |
0.79 19y versus 21y 0.99 19y versus 25y 0.99 19y versus 29y 0.70 21y versus 25y 0.77 21y versus 29y 1.00 25y versus 29y |
| Total sperm count (million) | 177 (222) 0–651 |
182 (224) 0–753 |
219 (254) 0- 880 |
220 (264) 0–966 |
1.00 19y versus 21y 0.41 19y versus 25y 0.43 19y versus 29y 0.3 21y versus 25y 0.31 21y versus 29y 1.00 25y versus 29y |
| Motility (abc, %) | 67 (65) 30–81 |
72 (71) 31–87 |
80 (78) 37–95 |
82 (79) 41–92 |
<0.001 19y versus 21y <0.001 19y versus 25y <0.001 19y versus 29y <0.001 21y versus 25y <0.001 21y versus 29y 0.94 25y versus 29y |
| Normal morphology (%) | 7.5 (8.2) 4–11 |
7.0 (7.9) 4–11 |
8.0 (8.7) 4–12 |
10 (10.6) 6–14 |
0.57 19y versus 21y 0.99 19y versus 25y <0.001 19y versus 29y 0.44 21y versus 25y <0.001 21y versus 29y <0.001 25y versus 29y |
| Total motile count (million) | 126 (145) 0–417 |
140 (162) 0–538 |
163 (198) 0–689 |
184 (212) 0–763 |
0.93 19y versus 21y 0.01 19y versus 25y 0.008 19y versus 29y 0.03 21y versus 25y 0.02 21y versus 29y 0.99 25y versus 29y |
P-value <0.05 is considered significant, shown in bold. Results are shown as median (mean) and range. Mixed model of repeated measures analyses following a square root transformation was used. P-values for pairwise comparisons were adjusted using the Tukey–Kramer methods. For other adjustments see statistical methods.
Table IV.
Semen variables for the men in cohort B at different ages.
| Age (years) | 19 | 21 | 25 | 29 | P-value |
|---|---|---|---|---|---|
| n | 197 | 110 | 96 | 90 | |
| Semen volume (ml) | 3.4 (3.5) 0.6–9.0 |
3.5 (3.6) 0.4–10.9 |
3.7 (3.9) 1.0–8.9 |
3.5(3.8) 0.8–8.9 |
0.51 19y versus 21y 0.09 19y versus 25y 0.59 19y versus 29y 0.79 21y versus 25y 1.00 21y versus 29y 0.96 25y versus 29y |
| Sperm concentration (million/ml) | 50 (63) 0–274 |
57 (63) 0–263 |
46 (57) 0–195 |
62 (71) 0–171 |
0.96 19y versus 21y 0.43 19y versus 25y 0.09 19y versus 29y 0.76 21y versus 25y 0.03 21y versus 29y 0.007 25y versus 29y |
| Total sperm count (million) | 172 (199) 0–785 |
171 (209) 0–975 |
168 (217) 0–1361.7 |
225 (264) 0–1077 |
1.00 19y versus 21y 1.00 19y versus 25y 0.009 19y versus 29y 0.02 21y versus 29y 1.00 21y versus 25y 0.02 25y versus 29y |
| Motility (abc, %) | 76 (73) 18–91 |
79 (76) 21–93 |
82 (77) 21–94 |
81 (76) 18–96 |
0.02 19y versus 21y 0.002 19y versus 25y 0.03 19y versus 29y 0.85 21y versus 25y 0.90 21y versus 29y 1.00 25y versus 29y |
| Total motile count (million) | 130 (148) 0–573 |
132 (162) 0–711 |
138 (174) 0–1034 |
183 (210) 0–717 |
0.88 19y versus 21y 0.73 19y versus 25y <0.001 19y versus 29y 0.99 21y versus 25y 0.01 21y versus 29y 0.02 25y versus 29y |
P-value <0.05 is considered significant, shown in bold. Results are shown as median (mean) and range. Mixed model of repeated measures analyses following a square root transformation was used. P-values for pairwise comparisons were adjusted using the Tukey–Kramer methods. For other adjustments see statistical methods.
Table V.
Semen variables of the men in the cohort B, who participated at all ages.
| Age (years) | 19 | 21 | 25 | 29 | P-value* |
|---|---|---|---|---|---|
| n | 52 | 52 | 52 | 52 | |
| Volume (ml) | 3.8 (3.8) 1.1–8.7 |
3.8 (4.0) 0.4–10.9 |
4.0 (4.1) 1.6–8.9 |
3.4 (3.9) 0.8–7.9 |
0.94 19y versus 21y 0.86 19y versus 25y 1.00 19y versus 29y 1.00 21y versus 25y 0.93 21y versus 29y 0.86 25y versus 29y |
| Concentration (million/ml) | 54 (65) 0–266 |
59 (61) 0–160 |
57 (56) 0–153 |
69 (74) 0–160 |
0.99 19y versus 21y 0.56 19y versus 25y 0.12 19y versus 29y 0.53 21y versus 25y 0.03 21y versus 29y 0.005 25y versus 29y |
| Total count (million) | 202 (233) 0–701 |
192 (229) 0–928 |
193 (222) 0–1362 |
233 (280) 0–1077 |
1.00 19y versus 21y 0.72 19y versus 25y 0.16 19y versus 29y 0.79 21y versus 25y 0.13 21y versus 29y 0.02 25y versus 29y |
| Motility (abc, %) | 76 (74) 47–88 |
82 (78) 21–93 |
83 (79) 44–92 |
82 (78) 18–94 |
0.04 19y versus 21y 0.05 19y versus 25y 0.20 19y versus 29y 1.00 21y versus 25y 0.99 21y versus 29y 1.00 25y versus 29y |
| Normal morphology (%) | 7.3 (7.6) 0–19 n= 51 |
8.5 (8.4) 0–19 n= 49 |
8.0 (8.5) 1–21 n= 49 |
9.0 (9.7) 0–20.5 n= 51 |
0.22 19y versus 21y 0.59 19y versus 25y 0.001 19y versus 29y 0.91 21y versus 25y 0.04 21y versus 29y 0.02 25y versus 29y |
| Total motile count (million) | 142 (173) 0–558 |
161 (183) 0–711 |
153 (180) 0–1034 |
193 (192) 0–979 |
1.00 19y versus 21y 0.92 19y versus 25y 0.005 19y versus 29y 0.81 21y versus 25y 0.04 21y versus 29y 0.007 25y versus 29y |
P-value <0.05 is considered significant, shown in bold. Results are shown as median (mean) and range. Mixed model of repeated measures analyses following a square root transformation was used. P-values for pairwise comparisons were adjusted using the Tukey–Kramer methods. For other adjustments see statistical methods.
The (median) percentage of motile spermatozoa increased from 66 to 82% (P < 0.001) and from 76 to 81% (P = 0.03) during the 10-year follow-up in the cohorts A and B, respectively (Tables II and IV). A significant improvement in number of motile sperm was also observed in the subgroups of men in cohort A attending all follow-up visits (Table III). In the cohort B, in the subgroup of men attending all study visits, the increase in motility was significant only between ages 19 and 21 years (P = 0.04) (Table V). Number of motile sperm increased also in the subgroup of cohort B.
Semen volume increased significantly from the age of 19 to 29 years only in the cohort A (P < 0.001). This increase was also statistically significant within the subgroup of those 61 men in the cohort A (19 years versus 29 years, P = 0.02), who participated in all four rounds of the study (Table III).
In both cohorts, sperm morphology was analysed only in subgroups of men who participated at all ages. The proportion of sperm with normal morphology increased significantly during the study period (Tables III and V).
Sperm concentration showed some variation between the age groups. Neither the sperm concentration nor total sperm counts changed significantly over the study period in the cohort A (Tables II and III), although there was a slight tendency towards higher total sperm counts on the later study rounds. Azoospermia was established in nine samples from six men (all in the cohort A).
In the cohort B we observed a more pronounced improvement in sperm counts. The median total sperm count increased from 172 million at 19 years to 225 million at 29 years (P = 0.009) (Table IV). Sperm concentration also increased from 50 × 106/ml to 62 × 106/ml (medians) between age 19 and 29 years, however this change was not statistically significant (P = 0.09). Statistically significant differences in sperm concentrations were observed in pair-wise comparisons between ages 21 and 29 years (P = 0.03) as well as between ages 25 and 29 (P = 0.007) (Table IV). Within the subgroup of men participating in all follow-up visits (52 men), we observed a similar tendency for improvement in sperm concentrations (Table V), i.e. significant changes between ages 21 and 29 (P = 0.03) as well as between ages 25 and 29 (P = 0.05). In the same subgroup, the change in total sperm count between ages 19 and 29 was not significant (P = 0.16).
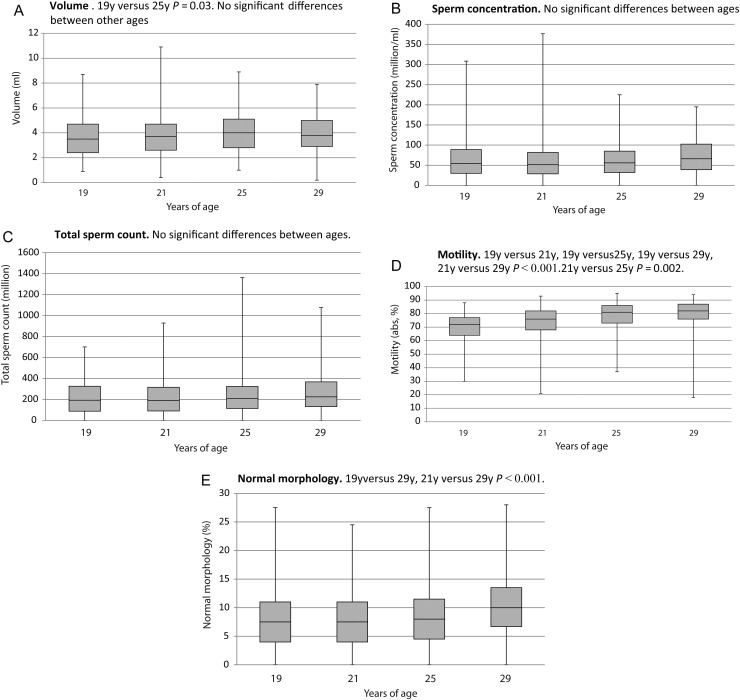
The semen parameters of those 113 men who participated at all ages of follow-up (both cohorts A and B) are presented in Fig. 2.
Figure 2.
The semen parameters for all Finnish men from cohorts A and B who participated in all visits during the 10-year follow-up (n = 113). Mixed model repeated measures analyses were used to test for mean differences in the outcome variables between visits in both cohort groups. P-values for pairwise comparisons were adjusted using the Tukey–Kramer method. (A) Semen volume, (B) sperm concentration, (C) total sperm count, (D) motility (WHO motility classification a, b and c, %) and (E) morphology (normal %).
Discussion
This study showed that sperm production increases only marginally after the age of 19 years whilst sperm motility and morphology improve slightly. The findings in cohort B validated the findings in cohort A. Thus our results suggest that full spermatogenic capacity has almost been reached by the age of 19 years.
There is one previous longitudinal study of semen quality. Carlsen et al. (2005) followed up semen quality of 158 19-year-old Danish men over 4 years with similar methodology as here. No changes in sperm concentration, total sperm count or percentage of morphologically normal sperm was observed. However, the increase in semen volume and in the percentage of rapid progressive motile sperm, as well as a decrease in the percentage of non-progressive and immotile sperm, was significant (only for men <23 years of age). The shorter follow-up time of the Danish men may explain why the sperm morphology did not change as it did over 10 years in the Finnish men.
Studies on the effect of age on semen quality typically cover a relatively long age range from 20 to 60 years with the emphasis mostly on fertility of aging men. There are little data on semen quality development in young adult men between the ages of 20 and 30 years. Levitas et al. (2007) compared sperm variables in men of different ages and observed the highest semen volumes in men at 30–35 years of age, and thereafter the volumes decreased. The best motility was found in men under 35 years of age, while the lowest sperm concentration was seen in the youngest group (under 25 years of age), and the older groups had slightly but significantly higher concentrations. The percentage of normal sperm morphology tended to be highest in the group of 25- to 29-year-old men. The Levitas et al. (2007) study was cross-sectional, and therefore it did not give information on longitudinal changes in semen quality. In another cross-sectional study on semen quality of Chinese men aged 20–60 years, Zhu et al. observed that rapid progressive motility and percentage of normal sperm started to decrease slowly after the age of 30 years (Zhu et al., 2011). In a third cross-sectional study, 21- to 25-year-old fertile men were observed to have a lower percentage of motile (post-thaw) spermatozoa and a lower percentage of normal spermatozoa as compared with fertile men aged 26–35 years (Schwartz et al., 1983). A similar trend was also observed for sperm concentration, semen volume and total sperm count (Schwartz et al., 1983). The results by Schwartz et al. (1983) are similar to our longitudinal follow-up results. Eskenazi et al. (2003) analysed semen quality in 97 men aged 22–80 years, and showed that semen volume and sperm motility decreased over this age span in this cross-sectional study. Recently, a meta-analysis on the effect of age on semen quality was performed (Johnson et al., 2015). Significant negative associations between age and semen quality variables (except for sperm concentration) were reported, but age thresholds for such declines were not estimated (Johnson et al., 2015). Stone et al. have performed such a threshold analysis using material from an infertility clinic and they suggested that semen quality starts to decline only after the mid-30s (Stone et al., 2013).
Accessory sex glands (seminal vesicle, prostate, bulbourethral and urethral glands) decisively contribute to semen volume. Thus, although the physical examination of the men born in 1979–1981 showed that most of these men had the mature stage of pubic hair, adult-size testis and adult testosterone levels by the age of 19 years (Jørgensen et al., 2002), late final maturation of the accessory organs could contribute to the increase in semen volume observed in one of the cohorts as well as to the improved motility.
As far as we are aware, this follow-up study is the one of the most extensive covering the maturation of early adulthood and semen quality. It is of special interest that although sperm production at 19 years of age appears to have reached almost mature adult capacity (concentration and total count), sperm morphology is still improving during this period of maturation. In addition to the changes in function of accessory sex glands and the consequent increase in seminal plasma, the ‘check-up’ mechanisms that ensure normal structure of the sperm may improve with age. Sertoli cells in the seminiferous epithelium serve as the structural, metabolic, and endocrine support for spermatogenic cells. Each Sertoli cell can support only a finite number of germ cells, and therefore the spermatogenic capacity depends on the number of Sertoli cells. Once spermatogenesis starts at puberty, the Sertoli cells stop dividing and therefore the maximum spermatogenic capacity is set by this time (Sharpe, 2012). Our findings are well in line with this physiological principle. Spermatogenic capacity of the 19-year-old man reflects well his sperm production for the rest of his life. However, other features of semen quality, such as sperm motility and morphology, do improve during young adulthood, giving the man possibly improved fecundity.
We observed recently a secular decline in the sperm count (concentration and total count) of 19-year-old men in Finland, i.e. the men born later had lower sperm counts than the older birth cohorts (Jørgensen et al., 2011). Furthermore, the sperm concentration of the young men was only 2/3 of that in the fertile men (mean age 30 years) (Jørgensen et al., 2001, 2002; Virtanen et al., 2013). It is clear from our present results that this previous finding (Jørgensen et al., 2011) is not explained by the young age of study subjects (19 years). A common limitation of semen studies is a low participation rate. In the study of Andersen et al. (2000), men who delivered a semen sample were compared with those who did not, and their reproductive hormone levels (including inhibin B) were similar suggesting that they are unlikely to be different in their reproductive capacity. Our study started as parallel to that of Andersen et al. (2000) and was therefore very similar. The young men in our study had no previous information of their fertility, which also makes it unlikely that the study group would be biased towards fertility problems. Despite the rather low participation rate, a significant selection bias is therefore considered unlikely.
The numeral findings of a sperm analysis are difficult to translate into ‘clinical fertility’. Certainly sperm counts do not directly translate into fertility. However, sperm concentration does influence fertility according to time to pregnancy (TTP) studies. At sperm concentrations below 40 million/ml the chance of a pregnancy is clearly decreased and the TTP prolonged (Bonde et al., 1998). The threshold in a more recent study was even higher, at 55 million/ml (Slama et al., 2002). As the sperm concentrations of the large proportion of young Finnish men are under these threshold levels there is good reason for concern regarding their future fertility. On the other hand, the finding that both the motility and morphology appear to improve with age suggests that the best fecundity may not be reached until well after puberty has been completed.
In conclusion, semen improves qualitatively during young adulthood, but nearly full spermatogenic capacity is reached before the age of 19 years, and the sperm counts at that age already reflect the sperm production capacity of the man for his lifetime.
Authors' roles
A.P., S.S., H.E.V., M.V., N.J., N.E.S. and J.T. designed the study. A.P., S.S., R.R. and H.E.V examined the men. A.P., S.S., R.R., H.E.V., W.R., N.J., N.E.S. and J.T. took part in the evaluation of the data. W.R. and M.V. were responsible for the sperm morphology and semen analysis. A.P., S.S., R.R., H.E.V., W.R. and J.T. drafted and revised the manuscript. All authors approved the final manuscript for submission.
Funding
This study was supported by the European Commission (QLK4-CT-1999-01422, QLK4-CT-2001-00269, QLK4-2002-0063, FP7/2008-2012: DEER 212844), The Danish Medical Research Council (9700833, 9700909), Danish Agency for Science (Technology and Innovation 09-067180), the Svend Andersen's Foundation, Velux Foundation, and Novo Nordisk Foundation, the Turku University Hospital, Sigrid Jusélius Foundation and the Academy of Finland. Funding to pay the Open Access publication charges for this article was provided by Turku University Hospital.
Conflict of interest
None declared.
Acknowledgements
We wish to thank Jaakko Matomäki for performing the statistical analysis of the study. We also thank lab technician Mrs Johanna Järvi for recruiting the study subjects and performing the semen analyses.
References
- Andersen AG, Jensen TK, Carlsen E, Jørgensen N, Andersson AM, Krarup T, Keiding N, Skakkebaek NE. High frequency of sub-optimal semen quality in an unselected population of young men. Hum Reprod 2000;15:366–372. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, Henriksen TB, Scheike T, Giwercman A, Olsen J, Skakkebaek NE. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 1998;352:1172–1177. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ 1992;305:609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E, Swan SH, Petersen JH, Skakkebaek NE. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod 2005;20:942–949. [DOI] [PubMed] [Google Scholar]
- Chia VM, Quraishi SM, Devesa SS, Purdue MP, Cook MB, McGlynn KA. International trends in the incidence of testicular cancer, 1973–2002. Cancer Epidemiol Biomarkers Prev 2010;19:1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S. The association of age and semen quality in healthy men. Hum Reprod 2003;18:447–457. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev 2015;19:22–33. [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Andersen AG, Eustache F, Irvine DS, Suominen J, Petersen JH, Andersen AN, Auger J, Cawood EH, Horte A et al. . Regional differences in semen quality in Europe. Hum Reprod 2001;16:1012–1019. [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Carlsen E, Nermoen I, Punab M, Suominen J, Andersen AG, Andersson AM, Haugen TB, Horte A, Jensen TK et al. . East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod 2002;17:2199–2208. [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Vierula M, Jacobsen R, Pukkala E, Perheentupa A, Virtanen HE, Skakkebaek NE, Toppari J. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. Int J Androl 2011;34:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, Juul A, Andersson AM, Carlsen E, Petersen JH et al. . Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2012;2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitas E, Lunenfeld E, Weisz N, Friger M, Potashnik G. Relationship between age and semen parameters in men with normal sperm concentration: analysis of 6022 semen samples. Andrologia 2007;39:45–50. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod 1990;5:586–592. [DOI] [PubMed] [Google Scholar]
- Rolland M, Le Moal J, Wagner V, Royère D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod 2013;28:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D, Mayaux MJ, Spira A, Moscato ML, Jouannet P, Czyglik F, David G. Semen characteristics as a function of age in 833 fertile men. Fertil Steril 1983;39:530–535. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Sperm counts and fertility in men: a rocky road ahead. Science & Society Series on Sex and Science . EMBO Rep 2012;13:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, Eustache F, Ducot B, Jensen TK, Jørgensen N, Horte A, Irvine S, Suominen J, Andersen AG, Auger J et al. . Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum Reprod 2002;17:503–515. [DOI] [PubMed] [Google Scholar]
- Stone BA, Alex A, Werlin LB, Marrs RP. Age thresholds for changes in semen parameters in men. Fertil Steril 2013;100:952–958. [DOI] [PubMed] [Google Scholar]
- Virtanen HE, Sadov S, Vierula M, Toppari J. Finland is following the trend-sperm quality in Finnish men. Asian J Androl 2013;15:162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QX, Meads C, Lu ML, Wu JQ, Zhou WJ, Gao ES. Turning point of age for semen quality: a population-based study in Chinese men. Fertil Steril 2011;96:572–576. [DOI] [PubMed] [Google Scholar]