Abstract
STUDY QUESTION
What is the association between daily preconception-initiated low-dose aspirin (LDA) treatment and very early pregnancy losses or euploid (chromosomally normal) losses among women with one to two prior losses?
SUMMARY ANSWER
Daily LDA initiated preconception was not associated with the rate or type of pregnancy loss among women with a history of one to two prior pregnancy losses.
WHAT IS KNOWN ALREADY
LDA is often used to treat recurrent pregnancy loss with reductions in pregnancy loss generally only observed among women with antiphospholipid antibodies, and null associations observed among women without antiphospholipid antibodies. We previously evaluated the association between LDA and pregnancy loss overall among women with one to two prior losses in the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial and found no association, though did not distinguish between potential effects at different stages of pregnancy loss, including implantation failure, or between euploid and aneuploid losses.
STUDY DESIGN, SIZE, DURATION
The EAGeR trial was a multi-site prospective block-randomized double-blind placebo-controlled trial. In total, 1228 women were randomized to daily LDA (81 mg/day) plus folic acid (400 mcg/day), or placebo plus folic acid. Participants were assigned study drug for less than or equal to six menstrual cycles or if they conceived, throughout pregnancy with study drug discontinued at 36 weeks gestation. This analysis includes additional outcome information obtained from chart abstractions after the completion of the trial, as well as testing of stored urine for measurement of hCG and detection of very early pregnancy losses, and karyotyping of the products of conception for assessment of aneuploidy of the losses.
PARTICIPANTS, SETTING, METHODS
Women aged 18–40 with a history of one to two prior losses and actively trying to conceive were randomized (n = 615 LDA and n = 613 placebo) at four clinical centers in the USA (2007–2011). Log-binomial regression was used to estimate risk ratios under the intent-to-treat approach.
MAIN RESULTS AND THE ROLE OF CHANCE
Daily LDA initiated preconception was not associated with clinically recognized pregnancy losses or implantation failures among women with proved fecundity and a history of one to two prior losses. Specifically, 1088 (88.6%) women completed the trial with 797 having an hCG detected pregnancy (64.9%). Overall there were 133 clinical losses (12.7% LDA versus 11.8% placebo, P = 0.71) and 55 implantation failures (5.2% LDA versus 4.9% placebo, P = 0.89). No differences were found in rate of euploid losses (RR 1.11, 95% confidence interval: 0.99, 1.26).
LIMITATIONS, REASONS FOR CAUTION
Generalizability of these findings is limited to women with a history of one to two prior losses, and may further be limited to women of white race with higher socioeconomic status as given the rigors of the study protocol participants tended to be white and have higher incomes and more education. We were also missing karyotype information on approximately one-third of the clinically recognized pregnancy losses, which may limit our power to detect effects on euploid losses, though detailed sensitivity analysis showed similar results.
WIDER IMPLICATIONS OF THE FINDINGS
Our data do not support the general use of LDA to decrease pregnancy loss and further demonstrate no increased risk of loss for women on LDA treatment.
STUDY FUNDING/COMPETING INTERESTS
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (Contract Nos. HHSN267200603423, HHSN267200603424, HHSN267200603426). The authors have no conflicts of interest.
TRIAL REGISTRATION NUMBER
The trial was registered at ClinicalTrials.gov #NCT00467363.
TRIAL REGISTRATION DATE
27 April 2007.
DATE OF FIRST PATIENT'S ENROLLMENT
15 June 2007.
Keywords: low-dose aspirin, conception, pregnancy loss, fertility, live birth
Introduction
Pregnancy loss is estimated to occur in up to 30% of conceptions (Wilcox et al., 1999). For women with recurrent pregnancy loss, therapies often include aspirin and heparin despite several meta-analyses indicating no effects on preventing pregnancy loss in women without antiphospholipid syndrome (Tulppala et al., 1997; Rai et al., 2000; Empson et al., 2002, 2005; Farquharson et al., 2002; Li et al., 2003; Gris et al., 2004; Di Nisio et al., 2005; Kaandorp et al., 2009, 2010). We previously reported that low-dose aspirin (LDA) treatment initiated preconception was not associated with live birth or clinically recognized pregnancy loss in the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial (Schisterman et al., 2014). However, we were unable to distinguish between potential therapeutic effects during different stages of early pregnancy loss, including implantation failure, which cannot be detected using conventional clinical tools. In addition, whether the lack of effect of LDA treatment that we observed was affected by losses due to aneuploidy has not been examined.
Preconception-initiated LDA has the potential to influence very early pregnancy losses not previously distinguished in prior studies, given its effects on endometrial vascularization and placentation, and thus its effects may differ by stage and karyotype of the loss. Specifically, while treatment with LDA is less likely to influence aneuploid losses, it may reduce euploid losses at very early gestational ages through its anti-inflammatory and antithrombotic properties (Silver and Branch, 1999; Vane and Botting, 2003).
The purpose of this analysis was to evaluate the effects of preconception-initiated LDA on specific subtypes of pregnancy loss (i.e. stages of pregnancy loss), including clinically undetected implantation failures, clinically recognized pregnancy losses and euploid versus aneuploid losses.
Materials and Methods
Design and target population
EAGeR was a multi-center, block-randomized, double-blind, placebo-controlled clinical trial at four clinical centers in the USA (2007–2011). Each participating center's Institutional Review Board approved the study, and participants provided written informed consent. The trial was registered on ClinicalTrials.gov (#NCT00467363), and a Data Safety and Monitoring Board (DSMB) provided oversight.
The study design, methods, inclusion and exclusion criteria have been described in detail elsewhere (Schisterman et al., 2013). In brief, women aged 18–40 with a history of one or two prior pregnancy losses, no history of infertility, actively trying to conceive and with regular menstrual cycles of 21–42 days during the past year were eligible. Participants were recruited using both community and health care center-based strategies. Participants were questioned about the details of prior losses, and prior losses were verified using reports of serum hCG results, sonogram findings, physician reports, medical records and histology reports. Women were excluded if they had a major medical problem or known contraindication to aspirin, indication for anticoagulant therapy, or had sought treatment for infertility.
Study protocol
Eligible women were block-randomized by study center and eligibility stratum to daily LDA (81 mg; standard low dose in the USA) plus folic acid (400 mcg) or placebo plus folic acid. Study pills were taken daily until completion of six menstrual cycles while attempting pregnancy or 36 weeks' gestation for those who became pregnant. Adherence was self-reported daily and additionally assessed by weighing medication bottles at each study visit. Participants provided daily first-morning urine collection for the first two cycles of follow-up. Fertility monitors were used to assist with timing of intercourse and study visits (Clearblue Easy Fertility Monitor; Inverness Medical).
Pregnancy was initially verified by a clinic and/or home urine pregnancy test [with the majority (89%) of women having both] and then confirmed by ultrasound at 6–7 weeks' gestation. If evidence of pregnancy was not apparent on the sonogram after a positive pregnancy test, the case was considered a peri-conception loss and the participant continued on the non-pregnancy follow-up schedule. Participation in the study ended when a woman either completed six menstrual cycles in the trial without becoming pregnant, had two peri-conception losses or other pregnancy outcome. Participants who became pregnant provided daily first-morning urine collected at home for the first month of pregnancy and spot urine samples at monthly study visits until the pregnancy ended.
Randomization and masking
Participants were randomized at each clinic according to a computerized randomization algorithm developed by the Data Coordinating Center (DCC), and based on a permuted block design with blocks of length six or eight, within each stratum (original/expanded) and by center. Study medications were manufactured to be identical by appearance and weight [over-coated tablets (UPM pharmaceutical, Baltimore, MD for the first batch only) or over-encapsulated tablets (ThermoFisher, Rockville, MD for subsequent batches)], with folic acid given separately as a supplement (generic) to all participants. Participants, trial staff and investigators remained blinded to the treatment assignment throughout the trial.
Outcome measures
The primary outcome for this analysis was early pregnancy loss, including both implantation failures and clinically recognized pregnancy losses. Gestational age was based on the early study sonogram performed at 6−7 weeks' of gestation. For participants with a pregnancy loss before the early sonogram, gestational age was determined using menstrual cycle dating from home-based fertility monitors provided by the study and the measured hCG levels from first-morning urine samples.
Implantation failures were identified in two ways. First, a positive urine pregnancy test at the clinical site (Quidel Quickvue, Quidel Corporation, San Diego, CA, sensitive to 25 mIU/ml hCG) followed by the absence of signs of clinical pregnancy (e.g. the initial sonogram showed a gestation sac, clinical documentation of fetal heart tones or a later stage confirmation of pregnancy) with or without missed menses indicated an implantation failure. Secondly, we also identified additional early implantation failures from batched augmented urine hCG testing that was performed later in the laboratory on the last 10 days of each woman's first and second cycle of study participation (using daily first-morning urine collected at home) and on spot urine samples collected at all post-cycle visits. Free beta hCG was measured in these urine samples to enable more sensitive detection of very early pregnancy than possible with conventional urine pregnancy testing. Two laboratory assays for free beta hCG (catalogue no. 4221-16, Diagnostic Automation Inc., Calabasas, CA, USA; catalogue no. RIS0011R, BioVendor, Asheville, NC, USA) were sequentially employed to determine, first, ‘potentially positive’ values (n = 164), out of which 21 were verified as positive tests for early hCG detected pregnancy.
Clinically recognized pregnancy losses were defined as a pregnancy loss detected by the woman or her primary care provider after clinical recognition of pregnancy by early ultrasound at ∼6.5 weeks of gestation. Clinically recognized pregnancy losses were then further classified by type as: pre-embryonic (loss at <10 weeks' gestation in which embryonic development does not occur—mean gestational sac diameter of ≥16 mm without an embryo, mean gestational sac diameter of ≥8 mm without a yolk sac, no visible embryo 2 weeks after detection of gestational sac of any size, or positive serum hCG with missed menses and no sac visible on sonogram); embryonic (loss occurring before 10 weeks gestation with an embryo with crown rump length ≤30 mm with no cardiac activity, or if an embryo was documented with a crown rump length ≤30 mm and cardiac activity present, and a subsequent loss occurred prior to 10 weeks' gestation and no further ultrasound was done, then the loss was presumed to have occurred at the embryonic stage); fetal pregnancy loss (loss at 10–20 weeks' gestation or death of a fetus with crown rump length of >30 mm and composite mean gestational age based on biparietal diameter, abdominal circumference and femur length of ≤20 weeks' gestation); stillbirth (loss occurring at or after 20 weeks' gestation, with the complete expulsion or extraction from its mother of a product of conception in which there is no breathing, beating of the heart, pulsation of the umbilical cord or unmistakable movement of voluntary muscle); ectopic (a pregnancy that has implanted somewhere other than the uterus as confirmed by sonography, laparoscopy or laparotomy); or pregnancy of unknown stage (Silver et al., 2011).
When possible, products of conception were collected and karyotyping or chromosomal microarray of the tissue was performed at two of the clinical sites. Participants were given labeled sterile specimen containers and clean gloves when they had a positive pregnancy test or at their 6.5 week ultrasound, and instructed in the event of a pregnancy loss to take some of the tissue, place it in a specimen container, keep it chilled and contact the research nurse as soon as possible. Some samples were also obtained after a dilation and curettage. Samples were then sent to a local cytogenetics laboratory for attempted karyotype. Chromosomal microarray was performed if karyotype failed (Romero et al., 2014). Results were classified as normal, abnormal, or unable to determine. Of note, only aneuploidy by microarray was considered abnormal; copy number variants were considered non-informative.
Statistical analysis
Descriptive statistics were used to compare characteristics between women in the LDA and placebo groups. Outcomes in the LDA and placebo groups were compared using Fisher's exact test and t-tests and were based on the intent-to-treat principle, including all women who completed the original trial (n = 1078) as well as women with outcome information obtained after the trial via chart abstraction and augmented urine hCG testing (n = 10; total n = 1088; Figs 1 and 2). Risk ratios (RRs) and 95% confidence intervals (CIs) for pregnancy losses, overall and by type, were estimated using log-binomial regression. Results were also stratified by number of prior losses, gestational age of the prior loss and time from last loss to randomization.
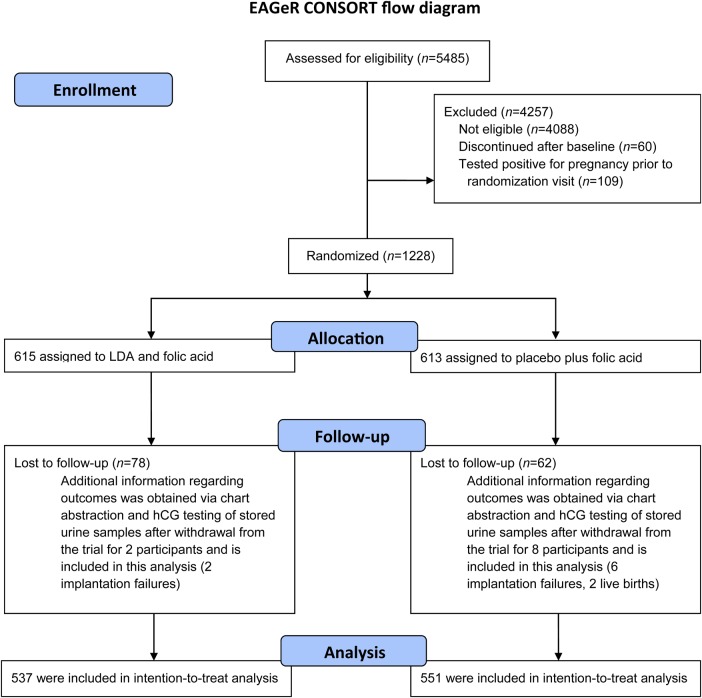
Figure 1.
Participant flow for the EAGeR trial.
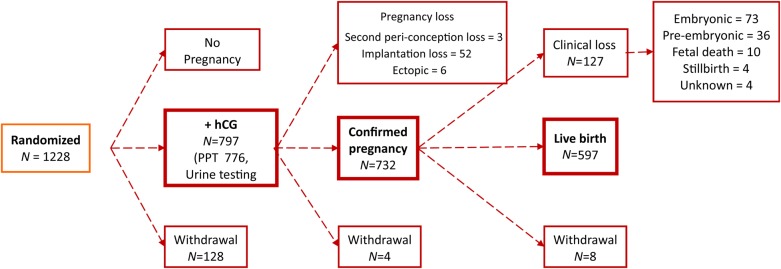
Figure 2.
Participant outcomes for the EAGeR trial. Notes: (1) Information regarding outcomes for 10 women who originally withdrew from the study was obtained via chart abstraction and hCG testing of stored urine samples (eight implantation failures and two live births). (2) There are a total of 55 women with implantation failures: 34 from the original trial (including 3 second peri-conception losses and 31 women with a positive pregnancy test without ultrasound confirmation of which 5 withdrew following the loss) and 21 newly detected implantation failures based on hCG testing of stored urine samples. (3) There are a total of 133 women with a clinical losses (127 clinical losses, 6 ectopic pregnancies). Upon further chart review, four losses initially classified as ‘probable ectopic’ were reclassified as one ectopic, one embryonic, one pre-embryonic and one unknown; three previously unknown losses were classified as one ectopic, one embryonic, one fetal.
As pregnancy loss is conditional upon becoming pregnant, we next restricted our analysis to women with any hCG detected pregnancy. In order to correctly estimate the effect of LDA versus placebo on pregnancy loss, inverse probability weights were used to control for potential selection bias introduced by restricting the analytical cohort post-randomization to only women with an hCG detected pregnancy (n = 785; LDA n = 405, placebo n = 380; Robins et al., 2000; Hernan et al., 2004). Weights were based on factors associated with becoming pregnant, including maternal age, parity, marital status, number of prior losses and treatment assignment. Weighted log-binomial regression was used to estimate RRs and 95% CIs. Results are presented overall and for each loss type.
We further evaluated the effect of aspirin on euploid losses and present the karyotype results by pregnancy loss type and treatment assignment. As having a euploid loss is also conditional upon having a pregnancy loss, we further restricted our analytical cohort to women with a pregnancy loss (n = 188; LDA n = 96, placebo n = 92). Weighted log-binomial regression was used to estimate RRs and 95% CIs using inverse probability weights that took this additional restriction into account, and multiple imputation was used to account for missing data on karyotype.
Sensitivity analyses were performed to evaluate the potential impact of early participant withdrawal. In addition, we also evaluated the impact of missing or indeterminate genetic testing results for 133 of the 188 losses in the trial. We compared results from scenarios where we assumed the same proportion of aneuploidy losses among those that were missing as were observed in the trial, as well as scenarios where we assumed all possible combinations of proportions of aneuploidy losses for the missing karyotype analysis. Results of these sensitivity analyses were drawn based on a priori assumptions for plausible values of the percent of withdrawals that resulted in a loss and the percent of losses with an abnormal karyotype: the percent of losses or abnormal karyotypes of missing data (i) could vary from the observed value in the placebo to the observed value in the treatment group; and (ii) in the treatment group was greater than or equal to that of the placebo group. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Trial recruitment took place between 15 June 2007 and 15 July 2011, at four medical centers in the USA and follow-up continued through 2012. Participant flow is shown in Figs 1 and 2. A total of 1228 women were block-randomized (615 LDA, 613 placebo) by center and stratum, and 1088 participants were included in the analysis (537 LDA, 551 placebo), including all women who completed the original trial (n = 1078), as well as women with outcome information obtained after the trial via chart abstraction and augmented urine hCG testing (n = 10; total n = 1088). Of the hCG detected pregnancies overall in the study (n = 797), 97% (n = 776) were detected by positive clinic pregnancy test and 3% (n = 21) were detected by hCG testing of stored spot/daily urine samples. A total of 785 pregnancies were included in this analysis, as 12 women withdrew prior to an observed pregnancy outcome. Thus, this analysis includes an additional 21 implantation failures identified using the hCG testing of stored urine samples, as well as two live births identified via chart abstraction for women who originally withdrew from the study.
Demographic and reproductive characteristics of participants, by treatment arm and number of prior losses, are shown in Table I. Women were on average 28.7 years of age with a mean body mass index of 26.4 kg/m2, were predominantly white (95%; 1162/1228), and most had above a high school education (86%; 1057/1228). Most women had only one prior pregnancy loss (67%; 825/1228), with the loss occurring at or before 9 weeks gestation (56%; 684/1228) and for 53% (651/1228) of women the interval between the most recent pregnancy loss and randomization was <4 months. Treatment arms were similar with regard to all characteristics assessed. Women with a single prior loss were more likely to be employed, have more than a high school education and be nulliparous.
Table I.
Demographics and baseline characteristics by treatment arm: the EAGeR trial.
| Characteristics, N (%) | Total | LDA | Placebo |
|---|---|---|---|
| N = 1228 | N = 615 | N = 613 | |
| Age, years: Mean ± SD | 28.7 ± 4.8 | 28.8 ± 4.9 | 28.7 ± 4.7 |
| BMI, kg/m2: Mean ± SD | 26.4 ± 6.6 | 26.3 ± 6.8 | 26.5 ± 6.4 |
| White race | 1162 (95) | 576 (94) | 586 (96) |
| >High School Education | 1057 (86) | 526 (86) | 531 (87) |
| Household income (annual) | |||
| ≥$100 000 | 491 (40) | 241 (39) | 250 (41) |
| $75 000–$99 999 | 149 (12) | 84 (14) | 65 (11) |
| $40 000–$74 999 | 181 (15) | 91 (15) | 90 (15) |
| $20 000–$39 999 | 312 (25) | 147 (24) | 165 (27) |
| ≤$19 999 | 94 (8) | 51 (8) | 43 (7) |
| Employed | 895 (73) | 451 (73) | 444 (72) |
| Time from last loss to randomization (months) | |||
| ≤4 months | 651 (53) | 331 (54) | 320 (52) |
| 5–8 months | 222 (18) | 103 (17) | 119 (20) |
| 9–12 months | 99 (8) | 50 (8) | 49 (8) |
| >12 months | 237 (19) | 119 (19) | 118 (19) |
| Number of previous pregnancy losses | |||
| 1 | 825 (67) | 422 (69) | 403 (66) |
| 2 | 403 (33) | 193 (31) | 210 (34) |
| Number of previous pregnancies, not including losses | |||
| 0 | 526 (43) | 263 (43) | 263 (43) |
| 1 | 433 (35) | 212 (34) | 221 (36) |
| 2 | 247 (20) | 127 (21) | 120 (20) |
| 3 | 22 (2) | 13 (2) | 9 (1) |
| Number of previous live births | |||
| 0 | 571 (47) | 283 (46) | 288 (47) |
| 1 | 443 (36) | 221 (36) | 222 (36) |
| 2 | 214 (17) | 111 (18) | 103 (17) |
| Gestational age of prior loss | |||
| ≤9 weeks gestation | 684 (56) | 341 (55) | 343 (56) |
| 10–19 weeks gestation | 347 (28) | 168 (27) | 179 (29) |
| ≥20 weeks gestation | 32 (3) | 21 (3) | 11 (2) |
| Smoking in past year | |||
| Never | 1067 (87) | 529 (86) | 538 (88) |
| <6 times/week | 87 (7) | 41 (7) | 46 (7) |
| Daily | 63 (5) | 38 (6) | 25 (4) |
| Alcohol consumption in past year | |||
| Often | 26 (2) | 18 (3) | 8 (1) |
| Sometimes | 380 (31) | 187 (30) | 193 (31) |
| Never | 806 (66) | 398 (65) | 408 (67) |
Data on covariates were missing for education (n = 1), income (n = 1), employment (n = 44), time from last loss to randomization (n = 19), gestational age of prior loss (n = 165), smoking (n = 11) and alcohol (n = 16).
Overall, 1088 women completed the trial and were included in this analysis. There were 45 women who had multiple pregnancies during the trial: four had two implantation failures, two had an implantation failure followed by a clinical loss, one had two implantation failures followed by a clinical loss and 38 women had an implantation failure and then went on to have a live birth during follow-up. Analyses are limited to the final outcome observed for these women. Thus, pregnancy loss occurred in 17.3% of women (188/1088), and 23.9% of pregnancies (188/785) during the trial (when outcomes from multiple pregnancies were included, the loss rate was 28.2%, 234/831). These included 55 implantation failures (5.1% of women) and 133 clinically recognized pregnancy losses (12.2% of women). The mean gestational age of the clinically recognized pregnancy losses was 9.7 weeks (median 8.7 weeks), with a range from 3.3 to 35.4 weeks.
In the intent-to-treat analysis including all women who completed the trial, the proportion of pregnancy losses in the LDA group was 17.9% (96/537) compared with a similar proportion of 16.7% (92/551) in the placebo group (RR 1.07, 95% CI: 0.83, 1.39, Table II). Similar null findings were observed when evaluating the effect of LDA on implantation failures and clinically recognized pregnancy losses (Table II). Effects were further evaluated by type of clinically recognized pregnancy loss, and no associations were observed between LDA and any type/stage of pregnancy loss (e.g. pre-embryonic, embryonic, etc.). Results also did not differ when stratified by number of prior losses and the gestational age of the prior loss (data not shown).
Table II.
Pregnancy loss by treatment arm overall and among women who became pregnant: the EAGeR trial.
| Outcomes: N (%)a | Total | LDA | Placebo | Overall RR (95% CI) | Among pregnanciesb RR (95% CI) |
|---|---|---|---|---|---|
| Women who completed the trial | N = 1088 | N = 537 | N = 551 | — | — |
| hCG detected pregnancy | N = 785 | N = 405 | N = 380 | — | — |
| Any pregnancy loss | 188 (17.3) | 96 (17.9) | 92 (16.7) | 1.07 (0.83, 1.39) | 0.99 (0.78, 1.27) |
| Implantation failure | 55 (5.1) | 28 (5.2) | 27 (4.9) | 1.06 (0.64, 1.78) | 0.97 (0.59, 1.60) |
| PPT, no confirmation | 34 (3.1) | 18 (3.4) | 16 (2.9) | 1.15 (0.59, 2.24) | 1.10 (0.57, 2.12) |
| hCG, no PPT | 21 (1.9) | 10 (1.9) | 11 (2.0) | 0.93 (0.40, 2.18) | 0.80 (0.35, 1.82) |
| Clinical loss | 133 (12.2) | 68 (12.7) | 65 (11.8) | 1.07 (0.78, 1.48) | 1.00 (0.73, 1.36) |
| Pre-embryonic | 36 (3.3) | 17 (3.2) | 19 (3.5) | 0.92 (0.48, 1.75) | 0.91 (0.48, 1.71) |
| Embryonic | 73 (6.7) | 39 (7.3) | 34 (6.2) | 1.18 (0.75, 1.84) | 1.07 (0.69, 1.65) |
| Fetal pregnancy loss (<20 weeks) | 10 (0.9) | 4 (0.7) | 6 (1.1) | 0.68 (0.19, 2.41) | 0.61 (0.17, 2.23) |
| Stillbirth | 4 (0.4) | 2 (0.4) | 2 (0.4) | 1.03 (0.15, 7.26) | 0.97 (0.14, 6.74) |
| Ectopic | 6 (0.6) | 3 (0.6) | 3 (0.5) | 1.03 (0.21, 5.06) | 1.00 (0.19, 5.23) |
| Unknown | 4 (0.4) | 3 (0.6) | 1 (0.2) | 3.08 (0.32, 29.50) | 2.54 (0.28, 23.41) |
aPercentages calculated out of women who completed the trial.
bInverse probability weights were used to account for different rates of confirmed pregnancies by intervention arm and were based on factors associated with becoming pregnant, including maternal age, parity, marital status, number of prior losses and treatment assignment. Weighted log-binomial regression was used to estimate RRs and 95% CIs.
Among women with an hCG detected pregnancy, we also did not observe any effects of LDA on pregnancy loss, or any type of pregnancy loss. These results take into account the post-randomization stratification through the use of inverse probability weights.
Karyotype or chromosomal microarray was performed on a total of 82 of the clinically recognized pregnancy losses (61.7%). Of those tested, 29 (35.4%) were abnormal (19 LDA, 10 placebo), 26 (31.7%) normal (13 LDA, 13 placebo) and 27 (32.9%) were non-informative (14 LDA, 13 placebo) (usually due to culture failure; Table III). LDA was not associated with an abnormal karyotype (RR 1.11, 95% CI: 0.99, 1.26). Sensitivity analysis showed that under reasonable assumptions for genetic assessment of the losses where genetic testing was not performed or unable to be determined, LDA was not significantly associated with aneuploidy (Table IV).
Table III.
Karyotyping results and associations with LDA among losses in the EAGeR trial.
| Karyotype results | Total | Abnormal karyotype | Normal | Unable to determine | Not tested |
|---|---|---|---|---|---|
| N (N LDA/N Placebo) | 1228 (615/613) | 31 (20/11) | 30 (15/15) | 28 (14/14) | 1139 (566/573) |
| Live birth | 597 (309/288) | 2 (1/1) | 4 (2/2) | 1 (0/1) | 590 (306/284) |
| Any pregnancy loss | 188 (96/92) | 29 (19/10) | 26 (13/13) | 27 (14/13) | 106 (50/56) |
| Implantation failure | 55 (28/27) | 0 | 0 | 0 | 55 |
| Clinical loss type | 133 (68/65) | 29 (19/10) | 26 (13/13) | 27 (14/13) | 51 (22/29) |
| Pre-embryonic | 36 (17/19) | 7 (4/3) | 4 (1/3) | 8 (4/4) | 17 (8/9) |
| Embryonic | 73 (39/34) | 18 (14/4) | 15 (8/7) | 18 (10/8) | 22 (7/15) |
| Fetal death | 10 (4/6) | 2 (1/1) | 5 (2/3) | 1 (0.1) | 2 (1/1) |
| Stillbirth | 4 (2/2) | 2 (0/2) | 2 (2/0) | 0 | 0 |
| Ectopic | 6 (3/3) | 0 | 0 | 0 | 6 (3/3) |
| Unknown | 4 (3/1) | 0 | 0 | 0 | 4 (3/1) |
Table IV.
Sensitivity analysis for missing karyotype information displaying significance of comparison between LDA and placebo groups under possible allocation of losses with missing karyotype (missing karyotype for 133 of 188 losses; 64 LDA, 69 placebo).
| Allocation of losses with missing karyotype | LDA abnormal (n) | LDA normal (n) | Placebo abnormal (n) | Placebo normal (n) | RR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Complete case | 19 | 13 | 10 | 13 | 1.37 (0.79, 2.36) | 0.2832 |
| Allocation to poor outcome | 83 | 13 | 79 | 13 | 1.01 (0.90, 1.96) | 1.000 |
| Allocation to good outcome | 19 | 77 | 10 | 82 | 1.82 (0.89, 3.70) | 0.1078 |
| Extreme case favoring LDA | 19 | 77 | 79 | 13 | 0.23 (0.15, 0.35) | <0.0001 |
| Extreme case favoring Placebo (worst case) | 83 | 13 | 10 | 82 | 7.95 (4.41, 14.36) | <0.0001 |
We further observed similar effects of LDA on pregnancy loss in sensitivity analyses under reasonable assumptions for the risk of pregnancy loss for the women who withdrew early from the study (data not shown).
Discussion
Overall, pregnancy loss occurred in 23.9% of pregnancies among women with a history of proved fecundity and one or two prior pregnancy losses, and preconception-initiated treatment with LDA was not significantly associated with risk of pregnancy loss, regardless of stage of the loss. Specifically, LDA treatment did not reduce the risk of pregnancy loss overall as previously reported (Schisterman et al., 2014), or by type of loss, including implantation failure, clinically recognized pregnancy loss, pre-embryonic, embryonic, fetal pregnancy loss <20 weeks gestation or stillbirth as presented here. Moreover, LDA was not associated with a reduction in euploid losses. Thus, our data do not support the general use of LDA to decrease the risk of pregnancy loss, and further show no increased risk of loss for women on LDA treatment.
These results among a population of women with one or two prior pregnancy losses are consistent with multiple trials completed among women with a history of recurrent pregnancy loss (two or more losses). Typically, previous studies initiated aspirin therapy post-conception and reported no reduction in pregnancy loss rates for women treated with LDA (Kaandorp et al., 2009, 2010). As the prior null findings could potentially be due to the late initiation of LDA, a call for studies utilizing preconception-initiated treatment was made, motivating the current trial (Kaandorp et al., 2009). However, even among studies of couples undergoing IVF, preconception-initiated LDA was not associated with improved pregnancy rates (Groeneveld et al., 2011; Siristatidis et al., 2011, 2012; Dentali et al., 2012). Moreover, we previously reported that LDA treatment initiated preconception was not associated with live birth or clinically recognized pregnancy loss (Schisterman et al., 2014). Here, we extend those findings to evaluate subtypes of pregnancy loss, including very early implantation failures and stages of clinically recognized pregnancy losses, while additionally accounting for loss karyotype, and observed no effect of LDA on any type of pregnancy loss or on rates of euploid losses. Our study is the first to show that LDA does not decrease any type of pregnancy loss, either implantation failures or clinically recognized losses, when initiated preconception among women with a history of only one to two prior pregnancy losses. Taken together with previous findings, LDA alone has not been shown to reduce rates of pregnancy loss.
The EAGeR trial is unique in that LDA treatment was initiated preconception. Moreover, trial outcomes were well-defined and well-documented, and we were able to follow a large number of women and obtain detailed information during frequent scheduled study visits, and early ultrasounds to precisely document pregnancy and pregnancy loss. An added benefit of the current trial is the measurement of hCG from daily first-morning urines from the first cycle attempting pregnancy. Thus, we were able to detect very early pregnancy losses that would have otherwise gone unobserved. It is important to note that our power may have been limited in the smallest subtypes of losses, though many of the point estimates showed very little differences between groups and suggest that beta errors were unlikely. This paper also includes additional chart abstraction to further refine outcome assessment. These additional efforts improved the methodological rigor of this work, although the additional data did not materially change any of the results or conclusions. The design of this study further lends strength to the findings, as this was a large, block-randomized, placebo-controlled, double-blind trial. However, the generalizability of our study is limited to women with only one to two prior losses. In addition, given the rigors of the study protocol, participants tended to be white and have higher incomes and more education, and these results may thus be further limited to white women with higher socioeconomic status. It should also be noted that the dose of aspirin may have been too low to exert an effect on pregnancy loss outcomes. Further, we were able to analyze products of conception for genetic abnormalities in early pregnancy loss to evaluate effects of LDA on euploid pregnancy loss in a subgroup of the participants. This analysis not only included karyotype, but also chromosomal microarray that allows for additional differentiation of genetic abnormalities in early losses (Romero et al., 2014). Rates of maternal cell contamination in this study were similar to that observed in other studies (Lathi et al., 2014). Lastly, we were missing karyotype information on approximately one-third of the clinically recognized pregnancy losses, which may limit our power to detect effects, though multiple imputation techniques were used along with additional detailed sensitivity analysis showing similar results.
In summary, preconception-initiated LDA was not associated with pregnancy loss among women with proved fecundity and a history of one to two prior losses (Schisterman et al., 2014). There were also no differences based on type of loss, including both implantation failures and clinically recognized pregnancy losses, or on abnormal karyotypes. Our data do not support the general use of LDA to decrease pregnancy loss and further demonstrate no increased risk of loss for women on LDA treatment.
Authors' roles
E.F.S. conceived the idea and designed the study. R.M.S., L.A.S., J.W.-W., J.M.T., A.M.L., N.G., L.L.L., D.F., N.J.P., K.C.S., S.M.Z. contributed to the study design, enrollment of patients and interpretation of the data. N.G., D.F., E.F.S., N.J.P., S.L.M. analyzed the study and contributed to the interpretation of the data. S.L.M., R.M.S., N.G., N.J.P., E.F.S. drafted the report, but all listed contributors edited and revised the report. The DCC, led by Drs Galai and Faraggi, had full access to the data throughout the trial, and performed analysis as requested by the DSMB. Upon trial completion, NICHD investigators (S.L.M., N.J.P. and E.F.S.) and DCC investigators (N.G. and D.F.) had full access to complete study data. All authors take responsibility for the integrity of the data and accuracy of data analysis, and approved the final manuscript. E.F.S. is the guarantor.
Funding
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (Contract Nos. HHSN267200603423, HHSN267200603424, HHSN267200603426).
Conflict of interest
The authors have no conflicts of interest.
Acknowledgements
We would like to especially thank the EAGeR participants for their extraordinary commitment to the study, all of the EAGeR investigators and staff who devoted their time and energy to the success of this trial, and the Data Safety and Monitoring Board members for ongoing oversight, constant support and advice throughout the trial. The authors have no conflicts of interest to disclose.
References
- Dentali F, Ageno W, Rezoagli E, Rancan E, Squizzato A, Middeldorp S, Margaglione M, Grandone E. Low-dose aspirin for in vitro fertilization or intracytoplasmic sperm injection: a systematic review and a meta-analysis of the literature. J Thromb Haemost 2012;10:2075–2085. [DOI] [PubMed] [Google Scholar]
- Di Nisio M, Peters L, Middeldorp S. Anticoagulants for the treatment of recurrent pregnancy loss in women without antiphospholipid syndrome. Cochrane Database Syst Rev 2005;2:CD004734. [DOI] [PubMed] [Google Scholar]
- Empson M, Lassere M, Craig JC, Scott JR. Recurrent pregnancy loss with antiphospholipid antibody: a systematic review of therapeutic trials. Obstet Gynecol 2002;99:135–144. [DOI] [PubMed] [Google Scholar]
- Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev 2005;1:CD002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol 2002;100:408–413. [DOI] [PubMed] [Google Scholar]
- Gris JC, Mercier E, Quere I, Lavigne-Lissalde G, Cochery-Nouvellon E, Hoffet M, Ripart-Neveu S, Tailland ML, Dauzat M, Mares P. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 2004;103:3695–3699. [DOI] [PubMed] [Google Scholar]
- Groeneveld E, Broeze KA, Lambers MJ, Haapsamo M, Dirckx K, Schoot BC, Salle B, Duvan CI, Schats R, Mol BW et al. . Is aspirin effective in women undergoing in vitro fertilization (IVF)? Results from an individual patient data meta-analysis (IPD MA). Hum Reprod Update 2011;17:501–509. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004;15:615–625. [DOI] [PubMed] [Google Scholar]
- Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin or anticoagulants for treating recurrent miscarriage in women without antiphospholipid syndrome. Cochrane Database Syst Rev 2009;1:CD004734. [DOI] [PubMed] [Google Scholar]
- Kaandorp SP, Goddijn M, van der Post JA, Hutten BA, Verhoeve HR, Hamulyak K, Mol BW, Folkeringa N, Nahuis M, Papatsonis DN et al. . Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med 2010;362:1586–1596. [DOI] [PubMed] [Google Scholar]
- Lathi RB, Gustin SL, Keller J, Maisenbacher MK, Sigurjonsson S, Tao R, Demko Z. Reliability of 46,XX results on miscarriage specimens: a review of 1222 first-trimester miscarriage specimens. Fertil Steril 2014;101:178–182. [DOI] [PubMed] [Google Scholar]
- Li DK, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. Br Med J 2003;327:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Backos M, Baxter N, Chilcott I, Regan L. Recurrent miscarriage-an aspirin a day? Hum Reprod 2000;15:2220–2223. [DOI] [PubMed] [Google Scholar]
- Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- Romero ST, Geiersbach KB, Paxton CN, Rose NC, Schisterman EF, Branch DW, Silver RM. Differentiation of genetic abnormalities in early pregnancy loss. Ultrasound Obstet Gynecol 2014;45:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, Lesher LL, Faraggi D, Wactawski-Wende J, Browne RW et al. . A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatr Perinat Epidemiol 2013;27:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski-Wende J, Townsend JM, Lynch AM, Perkins NJ, Mumford SL, Galai N. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014;384:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RM, Branch DW. Sporadic and recurrent pregnancy loss. In: Reece EA, Hobbins JC (eds). Medicine of the Fetus and Mother, 2nd edn Philadelphia, PA: J.B. Lippincott Company, 1999, 195–216. [Google Scholar]
- Silver RM, Branch DW, Goldenberg R, Iams JD, Klebanoff MA. Nomenclature for pregnancy outcomes: time for a change. Obstet Gynecol 2011;118:1402–1408. [DOI] [PubMed] [Google Scholar]
- Siristatidis CS, Dodd SR, Drakeley AJ. Aspirin for in vitro fertilisation. Cochrane Database Syst Rev 2011;8:CD004832. [DOI] [PubMed] [Google Scholar]
- Siristatidis CS, Dodd SR, Drakeley AJ. Aspirin is not recommended for women undergoing IVF. Hum Reprod Update 2012;18:233. [DOI] [PubMed] [Google Scholar]
- Tulppala M, Marttunen M, Soderstrom-Anttila V, Foudila T, Ailus K, Palosuo T, Ylikorkala O. Low-dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Hum Reprod 1997;12:1567–1572. [DOI] [PubMed] [Google Scholar]
- Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res 2003;110:255–258. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999;340:1796–1799. [DOI] [PubMed] [Google Scholar]