Abstract
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal disorders worldwide. The economic impact of IBS on the health care system is substantial, as is the personal impact on patients. Patients with diarrhea-predominant IBS (IBS-D) comprise a substantial proportion of the overall IBS population. Primary care providers are often the first point of contact for patients with IBS-D and can accurately diagnose IBS after a careful history and examination without extensive diagnostic tests. Several pharmacologic treatments (eg, loperamide, alosetron, and antidepressants) and non-pharmacologic treatments (eg, dietary modification and probiotics) are available for IBS-D, but restrictions on use (eg, alosetron) or the lack of controlled trial data showing reductions in both global and individual IBS-D symptoms (eg, bloating, pain and stool frequency) emphasize the need for alternative treatment options. Two newer medications (eluxadoline and rifaximin) were approved in May 2015 for the treatment of IBS-D, and represent new treatment options for this common gastrointestinal condition.
Keywords: abdominal pain, antibiotic, bloating, diarrhea, irritable bowel syndrome
Introduction
Irritable bowel syndrome (IBS) is a prevalent functional gastrointestinal (GI) disorder that affects up to 15% of the general global population.1–5 IBS is characterized by symptoms of abdominal pain or discomfort that are relieved by defecation, and is associated with disordered bowel function (ie, constipation, diarrhea, or alternating constipation and diarrhea).6 IBS can be diagnosed using Rome III criteria and subclassified according to patients’ predominant stool pattern using the Bristol Stool Form Scale as either constipation- or diarrhea-predominant, mixed type, or unsubtyped/unclassified (ie, insufficient abnormality of stool consistency to meet criteria for the other IBS subtypes; Table 1).6 Up to 40% of patients with IBS have diarrhea as the predominant bowel symptom (IBS-D subtype).1
Table 1.
Diagnostic criteria for IBS*
| IBS (any subtype) | IBS-D | IBS-C | IBS-M | IBS-U |
|---|---|---|---|---|
| Recurrent abdominal pain or discomfort** ≥3 days/month in the preceding 3 months | Loose or watery stool*** ≥25% of bowel movements | Hard or lumpy stool§ ≥25% of bowel movements | Hard or lumpy stool§ ≥25% of bowel movements | Insufficient alteration of stool consistency to be classified as one of the other subtypes |
| Must be associated with ≥2 of the following: • Symptom improvement with defecation • Symptom onset with altered frequency of stool • Onset of symptoms associated with altered stool form |
Hard or lumpy stool§ <25% of bowel movements | Loose or watery stool*** <25% of bowel movements | Loose or watery stool*** ≥25% of bowel movements |
Notes:
Rome III criteria. Criteria should be met for the previous 3 months, with onset of symptoms ≥6 months before diagnosis;
uncomfortable sensation that is not described as pain;
Bristol Stool Form Score 6–7;
Bristol Stool Form Score 1–2. Data from Longstreth et al.6
Abbreviations: IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; IBS-M, mixed-symptom IBS; IBS-U, unclassified IBS.
IBS significantly reduces the quality of life of patients, their partners, and caregivers and impairs daily functioning.3,7 It places a substantial financial burden on society, owing to impaired work productivity and increased use of health care resources.3 At least some of the increased costs are related to additional medical tests and medical and non-medical therapies, which are of uncertain value.4 In a database search of patients with and without IBS, patients with IBS were more likely to undergo standard laboratory blood and stool testing and endoscopic testing than other patients.8
Because the majority of IBS diagnoses originate from primary care physicians (PCPs),8 PCPs are in a unique position to facilitate proper diagnosis and management techniques that may reduce the overall personal and societal burden of IBS. Diagnosis and management of IBS by PCPs varies substantially throughout the United States and is inconsistently applied across all patient age groups.9 Patient misinformation concerning the proper diagnosis of IBS may result in PCPs performing extensive diagnostic testing to provide reassurance;10 however, there is little evidence that such additional testing alters the final diagnosis.11 Facilitating understanding and effective communication of diagnosing IBS among PCPs and patients may solidify standard of care practices, thereby eliminating extraneous tests and reducing health care costs.
This article provides an overview of the pathophysiologic mechanisms underlying IBS-D, discusses IBS-D diagnosis, and highlights newer treatment advances for patients with IBS-D.
Pathophysiology of IBS-D
Dietary factors
Ingestion of a meal stimulates a complex set of reactions that allow processing, absorption, and movement of the ingested food through the GI tract.12 These processes are coordinated via bidirectional neural, hormonal, and immunologic communication between the GI tract and the brain.13 The possible relationship between diet and IBS is ill defined, but diet-induced alterations in the gut microbiota, permeability, and proinflammatory responses (either directly via food sensitivity/intolerance or indirectly via changes in microbiota composition) have been proposed and may contribute to the increased visceral hypersensitivity and disruption in brain-GI tract crosstalk associated with IBS.14
Accelerated transit
The GI tract is controlled, in part, by the enteric nervous system (ENS), which uses neurotransmitters (eg, serotonin; also known as 5-hydroxytryptamine) to modulate motility, mucosal secretion and absorption activities, mucosal homeostasis, blood flow, and immune function.15 Serotonin receptors are located throughout the ENS and are involved in the regulation of reflexes that control GI motility and secretion, as well as visceral sensation.16 Disruptions in serotonin signaling, including a greater number of serotonin-positive enterochromaffin cells in the crypt epithelium, increased spontaneous release of serotonin from descending colon mucosal cells, decreased concentration of colonic serotonin, and diminished expression of serotonin reuptake transporters, have been described in patients with IBS-D.17,18 An increased number of brief (<15 seconds) colonic contractions, accelerated colonic transit, and alterations in small bowel motor function (eg, increased frequency of migrating motor complexes, duodenal and jejunal contractions, and ileal distension) have been observed in patients with IBS-D;19–21 however, whether these disruptions are a direct result of alterations in serotonin signaling or a consequence of other pathophysiologic processes is unclear.
Visceral hypersensitivity
Some patients with IBS have reduced thresholds for the sensation of bloating throughout the GI system compared with unaffected individuals; however, visceral perception is not altered in all patients and does not necessarily correlate with a particular symptom profile.22 Sensitization of neurons involved in the perception or transmission of sensory information from the ENS to the central nervous system (CNS) is likely involved in IBS-related visceral hypersensitivity.15 Alterations in sensory neuromediators (eg, inflammatory mediators) that occur as a result of changes in gut microbiota, which might also be associated with a previous episode of gastroenteritis,19 may contribute to the visceral hypersensitivity and abdominal pain observed in some patients with IBS-D.23 Studies have also implicated alterations in CNS innervation and processing of GI tract stimulation in patients with IBS compared with healthy controls.15,24,25 In patients with IBS-D, increased visceral activity has been associated with an increased number of colonic mast cells, closer proximity of mast cells to nerves, and augmented activation of pain and motor areas in the brain.24,26 Taken together, these data suggest that a variety of factors contribute to enhanced GI tract reactivity in patients with IBS-D.
Gut microbiota
The gut microbiota is responsible for a variety of structural, metabolic, and immune functions27 and is relatively stable in healthy individuals.28–30 Because of the plethora of functions of the gut microbiota in human health, its disruption (ie, dysbiosis) causes aberrations in multiple interconnected pathways, including brain-gut crosstalk, regulation of the neuroendocrine system, and immune system homeostasis, and may contribute to the symptoms of IBS-D. Several lines of evidence support a role of the gut microbiota in the pathophysiology of IBS-D. For example, patients who experience gastroenteritis are more likely to develop IBS (ie, postinfectious IBS), including the IBS-D subtype, than patients without a history of gastroenteritis.31 In addition, the composition and diversity of the gut microbiota in patients with IBS-D is altered compared with that in healthy individuals and patients with other IBS subtypes.32–35 Additional clinical studies are needed to fully understand the consequences of the gut microbiota dysbiosis associated with IBS-D, but the efficacy of agents that may impact the gut microbiota (eg, probiotics and non-systemic antibiotics) suggest at least a symptomatic role.
Diagnosis of IBS-D
Patients with IBS-D typically present with abdominal pain associated with frequent loose stools and cramping, urgency that is not relieved by defecation, and mucus in the stool.36 The occurrence of additional comorbid functional GI and extraintestinal symptoms or disorders (eg, gastroesophageal reflux disease,37 dyspepsia,38 interstitial cystitis,39 migraine headaches,38 and fibromyalgia39) may increase the probability of a positive IBS diagnosis. Symptoms may be mild and infrequent, moderate and somewhat bothersome, or severe enough to interfere with daily functioning, but all typically require an ongoing management strategy to control symptoms.40,41
The diagnosis of IBS is often first made by PCPs, particularly by internists and family practitioners, and usually without the need for specialized tests (eg, endoscopic or radiologic tests).8 The diagnosis may be established via a symptom-based strategy using a combination of patient history, exclusion of alarm symptoms/warning signs, and use of the Rome III criteria (Table 2)36 or the American College of Gastroenterology (ACG) definition of IBS (ie, abdominal pain or discomfort that occurs in association with altered bowel habits for ≥3 months).42–44 Diagnostic testing for celiac disease may be warranted in patients presenting with diarrhea as their predominant symptom, but extensive diagnostic testing is unnecessary for patients without alarm symptoms. PCPs and other non-IBS specialists tend to perform a greater number of diagnostic tests before informing the patient of an IBS diagnosis;45 however, at present, there appears to be little diagnostic value in some of these tests (eg, complete blood count, C-reactive protein, serum chemistries, stool testing for intestinal parasites, and sigmoidoscopy with biopsies).36,46 Addressing disease-related concerns, discussing reasonable treatment goals and expectations, educating and empowering patients, and addressing somatization issues with patients may provide greater benefit than extensive testing.45
Table 2.
Differential diagnosis in patients presenting with symptoms of IBS and alarm symptoms/warning signs
| Alarm symptoms/warning signs | Potential differential diagnosis |
|---|---|
| Anemia | Celiac disease, colon cancer |
| Family history of colorectal cancer, inflammatory bowel disease, or celiac disease | Celiac disease, IBD, colon cancer |
| Nocturnal diarrhea that awakens the patient | Colorectal cancer, IBD, microscopic colitis |
| Onset >50 years of age | Microscopic colitis, colon cancer |
| Recent antibiotic use | Clostridium difficile colitis |
| Rectal bleeding | IBD, colon cancer, hemorrhoids, ischemic colitis |
| Unintentional weight loss (>10% of body weight) | Ischemic colitis, IBD, celiac disease, colon cancer |
| Persistent frequent diarrhea without hematochezia | Bile acid malabsorption |
| Persistent bloating and diarrhea unresponsive to dietary interventions | Small intestinal bacterial overgrowth |
Note: Data from Wilkins et al.36
Abbreviations: IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
Treatment options for IBS-D
Antimotility agents
Loperamide
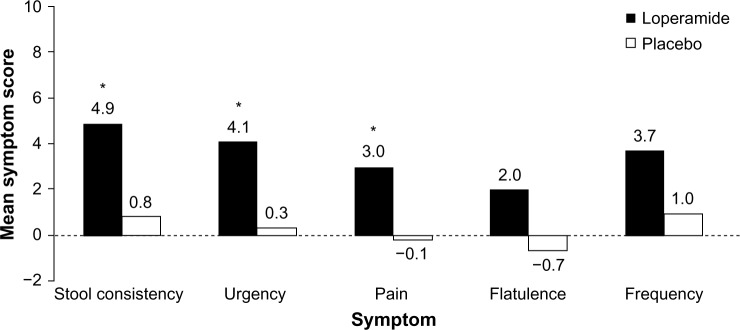
Loperamide is an over-the-counter opioid receptor agonist that reduces peristalsis, increases intestinal transit time, and diminishes loss of fluid and electrolytes. In four randomized, placebo-controlled, double-blind studies, patients with IBS-D who received loperamide experienced a significant improvement in individual symptoms of stool frequency,47,48 stool consistency/number of unformed stools,47–50 and urgency47 but no improvement in bloating, a frequently bothersome IBS symptom (Figure 1).48,49 Abdominal pain, a cardinal symptom of IBS, was not relieved with the use of loperamide compared with placebo in most studies.47,48,50 Loperamide was well tolerated by patients with IBS-D;47–49 the only adverse effects reported were constipation, swollen fingers, trouble sleeping, and oral blisters (n=1 each).47,49 Loperamide 2 mg/day to 8 mg/day may be useful in some patients with IBS-D, but it is not currently recommended by the ACG because of a lack of high-quality evidence.4
Figure 1.
Symptom improvement with loperamide.
Notes: Patients with IBS-D (N=25) received loperamide 2 mg/day for 1 week, followed by a 4-week dose titration period in which patients could increase the dose by 2 mg/day each week until the response was satisfactory (maximum dose, 8 mg/day). The treatment period was 13 weeks. Data are accumulated mean scores ascertained at 5, 9, and 13 weeks of treatment. *P<0.05 vs placebo. Data from Lavö et al.49
Abbreviation: IBS-D, diarrhea-predominant irritable bowel syndrome.
Eluxadoline
Eluxadoline, a mixed μ-opioid receptor agonist and δ-opioid receptor antagonist that reduces contractility and secretion in the GI tract, was approved in 2015 by the US Food and Drug Administration (FDA) for daily administration in patients with IBS-D.51 However, because the FDA has recommended to the US Drug Enforcement Administration that eluxadoline be classified as a controlled substance and the designation is pending, eluxadoline is not anticipated to be available until the first quarter of 2016. In two separate Phase 3 trials in patients with IBS-D (pooled population, >2,400 patients), currently only published in abstract form, eluxadoline 75 mg or 100 mg twice daily significantly improved abdominal pain and stool consistency (P<0.005 vs placebo), number of daily bowel movements (P<0.05), and urgency (P<0.05).52 These benefits were observed in the overall population and in patients for whom loperamide had provided insufficient relief.9 The most commonly reported adverse events (AEs) were constipation (7.4% and 8.3% for 75 mg and 100 mg eluxadoline, respectively, compared with 2.4% for placebo) and nausea (7.8% and 7.3% for 75 mg and 100 mg eluxadoline, respectively, compared with 4.8% for placebo).52
Probiotics
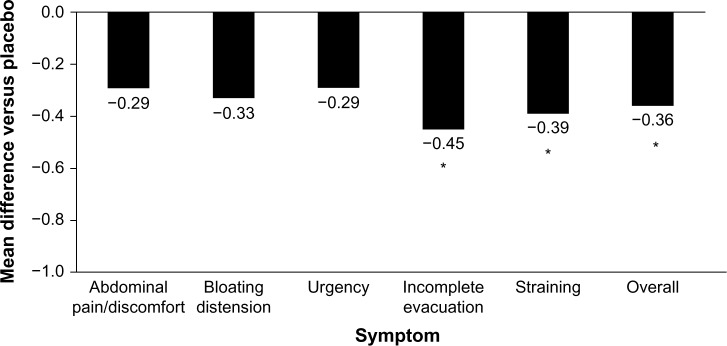
Although probiotics may be an intuitive treatment option for the treatment of IBS-D, few have been studied clinically. In addition, the quality and consistency of probiotic preparations on store shelves is questionable because of shelf-life limitations and a lack of government-sanctioned quality-control standards. The limited clinical trial data published for probiotics in IBS-D are contradictory and strain-dependent. In a double-blind, placebo controlled study, VSL#3® (Sigma-Tau Pharmaceuticals, Inc., Gaithersburg, MD, USA) for 8 weeks did not improve small bowel or colonic transit and had no effect on bowel dysfunction, abdominal pain, flatulence, or urgency compared with placebo in patients with IBS-D (n=25).53 In contrast, in a subset of patients with IBS-D, treatment with Bifidobacterium infantis 1×108 cfu/mL capsule once daily for 4 weeks (n=49) significantly improved incomplete evacuation (P=0.008), straining (P=0.007), bowel habit satisfaction (P=0.014), and overall IBS symptoms (P=0.028) vs placebo (n=56; Figure 2).54 Based on these limited data and the favorable benefit/risk ratio, B. infantis 1×108 cfu/mL may alleviate symptoms in some patients; however, health care providers should be aware of the small sample size of the trials and the limited number of results available compared with those of other currently available agents. In addition, health care providers should weigh the potential benefits against the cost to the patients.4
Figure 2.
Reduction of IBS symptoms with Bifidobacterium infantis capsule administered once daily vs placebo.
Notes: Females with IBS-D recruited from primary care centers were randomized to receive B. infantis 35624 1×108 (n=49) or placebo (n=56) once daily for 4 weeks. Data are least-squares mean changes from baseline at week 4. *P<0.03 vs placebo. Data from Whorwell et al.54
Abbreviations: IBS, irritable bowel syndrome; IBS-D, diarrhea-predominant IBS.
Dietary modification
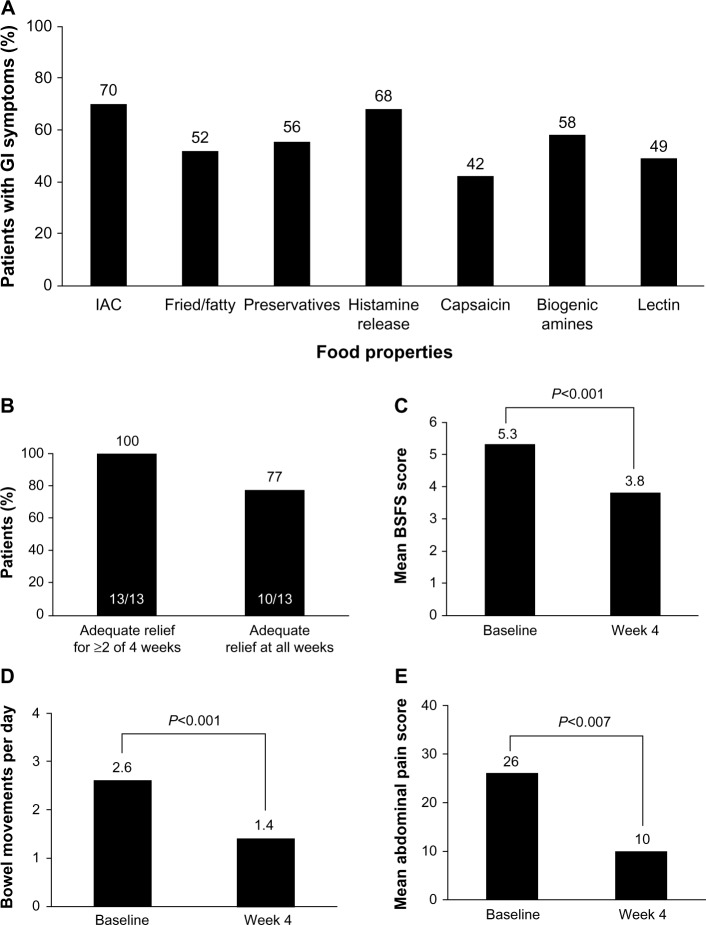
Many patients with IBS report the onset of GI symptoms after ingestion of specific food types (eg, fried or fatty foods; Figure 3A).55 Rigorous trials of dietary manipulations in patients with IBS are lacking; however, potential benefits have been observed with some dietary modifications.4,56 A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) may decrease symptoms in patients with IBS,57–59 particularly those with IBS-D.57 In addition, two small trials of a gluten-free or very low carbohydrate diet showed improved stool frequency,60,61 stool consistency,60 and abdominal pain60 in patients with IBS-D (Figure 3B–E).60 Given the lack of strong evidence for dietary adjustment and the high patient adherence requirements, dietary modification plans are not strongly recommended by the ACG.4
Figure 3.
Percentage of patients reporting GI symptoms.
Notes: (A) Specific foods or food-related mediators often associated with GI symptoms. (B–E) Efficacy of a 4-week very low-carbohydrate (maximum, 20 g/day) diet in patients with IBS-D (n=17). Data from Böhn et al and Austin et al.55,60
Abbreviations: BSFS, Bristol Stool Form Scale; GI, gastrointestinal; IAC, incompletely absorbed carbohydrates; IBS-D, diarrhea-predominant irritable bowel syndrome.
Serotonergic agents
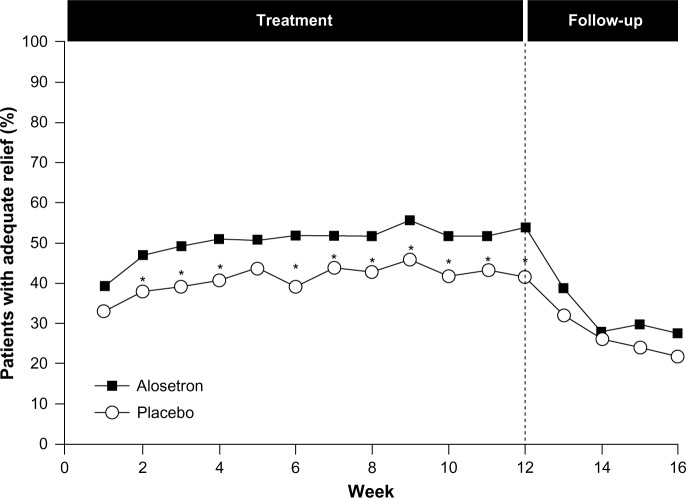
Given the complex role of serotonin in the GI tract, serotonin antagonists have been evaluated for the treatment of IBS-D; however, only alosetron, a serotonin receptor antagonist, is available, under a risk management program, in the United States.4 A number of randomized, placebo-controlled trials have been conducted with alosetron in patients with IBS, including those with the IBS-D subtype. Most of these trials showed improvement in stool consistency, stool frequency, and overall IBS symptoms in females during treatment; however, daily therapy is required, and effects diminish rapidly with treatment cessation (Figure 4).62–67 Evidence on the efficacy of alosetron in males has been conflicting; early dose-ranging studies showed a lack of consistent efficacy in males despite beneficial effects in females.65,67 However, in a 2005 trial, alosetron treatment resulted in an increased percentage of males with adequate relief of pain and discomfort and improved stool consistency, despite a lack of effect on stool frequency, urgency, incomplete evacuation, or bloating.68
Figure 4.
Efficacy of alosetron in females with severe IBS-D.
Notes: Females with predominantly IBS-D vs other subtypes were randomized to receive either placebo (n=323) or alosetron 1 mg twice daily (n=324) for 12 weeks and were followed for an additional 4 weeks. Adequate relief of pain was ascertained weekly by patient response (yes/no) to the following question: “In the past seven days, have you had adequate relief of your irritable bowel syndrome pain and discomfort?” *P<0.05 vs placebo. Reprinted from The Lancet, 355(9209), Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW, Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial, 1035–1040., Copyright (2000), with permission from Elsevier.62
Abbreviations: IBS, irritable bowel syndrome; IBS-D, diarrhea-predominant IBS.
Alosetron is associated with several AEs, predominately constipation.69 In 2001, alosetron was voluntarily withdrawn from the US market because of concerns of reports of ischemic colitis,70 which occurs in approximately 0.1% of patients.71 It was reintroduced into the US market in 2002 but only for females with severe IBS-D and with required participation in a risk-management program.4
Antidepressants
Tricyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs) modulate the activity of serotonin in the GI tract and reduce global IBS symptoms and abdominal pain in patients with IBS (number need to treat =4 for both agents).4 Because tricyclic antidepressants increase intestinal transit time, they may be preferable to SSRIs for the treatment of patients with IBS-D.72 However, data on the efficacy of these agents specifically for IBS-D are limited. A small trial of amitriptyline in patients with IBS-D (n=50) reported improvement in the feeling of incomplete defecation and loose stools compared with placebo.73 AEs (eg, drowsiness and dry mouth) are more common with anti-depressants than placebo (number needed to harm =9) and may limit patient tolerability. Currently, the ACG does not strongly recommend antidepressants for IBS-D and notes the potential for AEs, the greater costs of some medications (especially SSRIs), and potential patient reluctance to take antidepressants.4
Fecal microbiota transplant
The recognition of the importance of gut microflora modulation in IBS has prompted the investigation of a non-traditional means of restoring the gut microbiota (ie, fecal microbiota transplant [FMT]). FMT involves the administration of healthy donor stool into the colon of the symptomatic patients.74 Benefits of this therapy have been described for other GI diseases associated with alterations in the gut microbiota (eg, Clostridium difficile infection and inflammatory bowel disease);74 however, there are few published data on the efficacy of this therapy in patients with IBS-D (n=9).75 Therefore, FMT should be considered as an investigational therapy because its usefulness as a therapeutic option for IBS-D is unknown. In addition, there are notable safety concerns with FMT, including transient GI complaints (eg, abdominal cramping) and the potential for development of autoimmune disorders.
Non-systemic antibiotics (rifaximin)
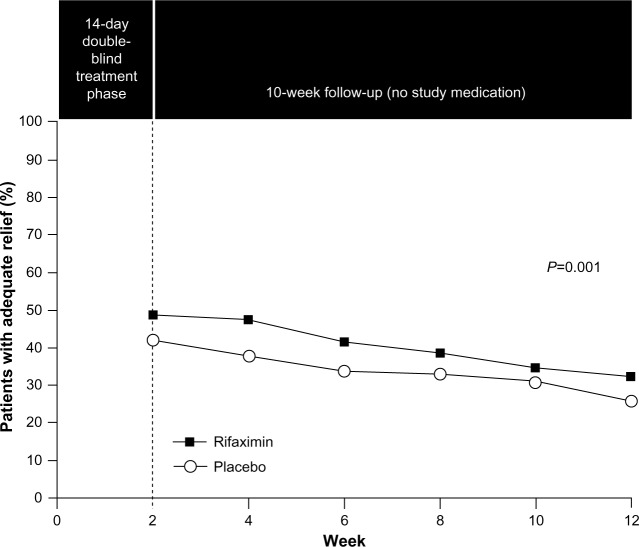
The role of the gut microbiota in IBS, including interactions with CNS processes (eg, pain sensitivity), suggests that modulation of the gut microbiota might improve IBS symptoms. Rifaximin, a non-systemic antibiotic, was approved in May 2015 for the treatment of IBS-D in adults. In a combined analysis of two separate Phase 3 trials, a 14-day course of rifaximin 550 mg three times daily, in patients with IBS-D, significantly increased the percentage of patients who had adequate relief of global IBS symptoms (P<0.001; pooled; Figure 5),76 and improved IBS-related bloating (P<0.001; pooled) and abdominal pain/discomfort and loose or watery stools (P=0.008; pooled) compared with placebo for up to 10 weeks post-treatment.76 In a Phase 3 retreatment study of patients initially responding to open-label rifaximin who experienced symptom recurrence, the percentage of responders (pain and stool consistency improvements) to randomized, repeat treatment was significantly greater with rifaximin vs placebo (33% vs 25%, P=0.02).77 The AE profile of rifaximin in patients with IBS-D was generally similar to that observed with placebo. AEs (≥2% of patients) reported more often with rifaximin vs placebo were nausea (4.3% vs 3.8%), diarrhea (4.3% vs 3.5%), sinusitis (2.7% vs 2.5%), vomiting (2.4% vs 1.4%), and cough (2.1% vs 1.4%).76 Data suggest that 1 patient would experience an AE with rifaximin for every 846 patients who would benefit (number needed to harm =8,971 and number needed to treat =10.6).76,78,79 As noted in the Warnings and Precautions section of the rifaximin prescribing information, there is a risk of C. difficile infection with use of nearly all antibacterial agents including rifaximin, and severity may vary from symptoms of mild diarrhea to fatal colitis. Based on its favorable efficacy and safety profiles, rifaximin is a viable treatment option for patients with IBS-D; however, health care providers should be aware that response may not be ubiquitous.4
Figure 5.
Efficacy of rifaximin for patients with IBS-D.
Notes: Patients were randomized to receive placebo (n=635) or rifaximin 550 mg three times daily (n=625) for 2 weeks followed by a 10-week follow-up period. Adequate relief of symptoms was assessed weekly by patient response (yes/no) to the following question: “In regard to all of your symptoms of IBS, as compared with the way you felt before you started the study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms?” From The New England Journal of Medicine, Pimentel M, Lembo A, Chey WD, et al, Rifaximin therapy for patients with irritable bowel syndrome without constipation, 364(1), 22–32. Copyright © (2011) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.76
Abbreviation: IBS, irritable bowel syndrome.
Conclusion
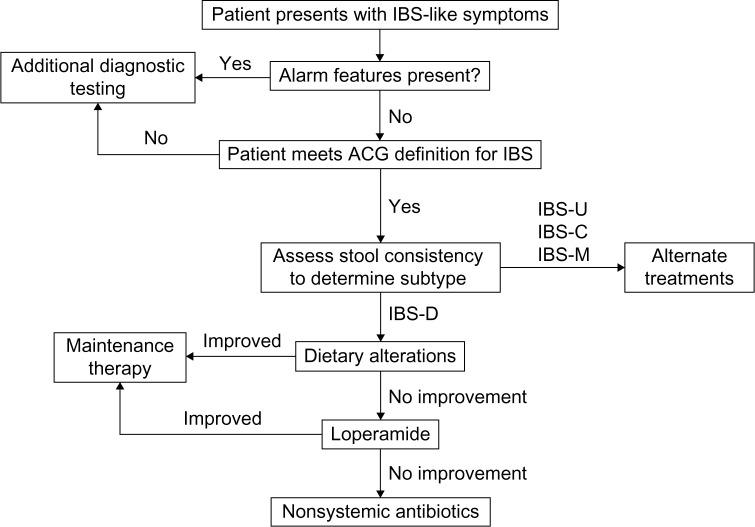
IBS-D is a common GI condition that imposes substantial personal and societal burdens. Whereas the etiology and pathophysiology of IBS-D continue to be investigated, interconnected alterations in visceral sensitivity, GI motility, and gut microbiota likely play a role in the disorder. PCPs are often the first point of contact for patients with IBS-D and are capable of identifying and treating IBS-D. The diagnosis and treatment of IBS-D should involve a symptom-based strategy consisting of exclusion of alarm signs, evaluation of IBS subtype, and selection of treatment based on risk/benefit profile (Figure 6). Extensive diagnostic testing in the absence of alarm symptoms is unnecessary and should be avoided to minimize costs as appropriate. Several treatment options are available for IBS-D; however, some therapies (eg, diet modulation and probiotics) require additional clinical safety and efficacy data and therefore have not been strongly recommended. Two drugs approved in the United States in May 2015 for IBS-D (eluxadoline and rifaximin) appear to be efficacious and well-tolerated treatment alternatives.
Figure 6.
Diagnostic and treatment algorithm for IBS-D.
Abbreviations: ACG, American College of Gastroenterology; IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; IBS-M, mixed-symptom IBS; IBS-U, unclassified IBS.
Acknowledgments
Technical editorial and medical writing support was provided, under the direction of the author, by Mary Beth Moncrief, PhD, and Jillian Gee, PhD, Synchrony Medical Communications, LLC, West Chester, PA. Funding for this support was provided by Salix, a Division of Valeant Pharmaceuticals North America LLC, Bridgewater, NJ, USA. The author was not provided funding for development of this review.
Footnotes
Author contribution
The author contributed toward data analysis, drafting and revising the paper and agrees to be accountable for all aspects of the work.
Disclosure
Dr Lacy is a co-investigator for the NIH Functional Dyspepsia Treatment Trial and has served on the scientific advisory boards of Ironwood, Takeda, and Prometheus.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Wilson S, Roberts L, Roalfe A, Bridge P, Singh S. Prevalence of irritable bowel syndrome: a community survey. Br J Gen Pract. 2004;54(504):495–502. [PMC free article] [PubMed] [Google Scholar]
- 3.Hungin APS, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21(11):1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2–S26. doi: 10.1038/ajg.2014.187. [DOI] [PubMed] [Google Scholar]
- 5.Ng KS, Nassar N, Hamd K, Nagarajah A, Gladman MA. Prevalence of functional bowel disorders and faecal incontinence: an Australian primary care survey. Colorectal Dis. 2015;17(2):150–159. doi: 10.1111/codi.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 7.Wong RK, Drossman DA, Weinland SR, et al. Partner burden in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(2):151–155. doi: 10.1016/j.cgh.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Ladabaum U, Boyd E, Zhao WK, et al. Diagnosis, comorbidities, and management of irritable bowel syndrome in patients in a large health maintenance organization. Clin Gastroenterol Hepatol. 2012;10(1):37–45. doi: 10.1016/j.cgh.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacy B, Patel H, Guérin A, et al. Regional variation of care for irritable bowel syndrome in the United States. Am J Gastroenterol. 2014;109(Suppl 2):S529. [Google Scholar]
- 10.Lacy BE, Weiser K, Noddin L, et al. Irritable bowel syndrome: patients’ attitudes, concerns and level of knowledge. Aliment Pharmacol Ther. 2007;25(11):1329–1341. doi: 10.1111/j.1365-2036.2007.03328.x. [DOI] [PubMed] [Google Scholar]
- 11.Cash BD, Schoenfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol. 2002;97(11):2812–2819. doi: 10.1111/j.1572-0241.2002.07027.x. [DOI] [PubMed] [Google Scholar]
- 12.Cuomo R, Andreozzi P, Zito FP, Passananti V, De Carlo G, Sarnelli G. Irritable bowel syndrome and food interaction. World J Gastroenterol. 2014;20(27):8837–8845. doi: 10.3748/wjg.v20.i27.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes PA, Fraher MH, Quigley EM. Irritable bowel syndrome: the role of food in pathogenesis and management. Gastroenterol Hepatol (NY) 2014;10(3):164–174. [PMC free article] [PubMed] [Google Scholar]
- 14.Ringel Y, Maharshak N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2013;305(8):G529–G541. doi: 10.1152/ajpgi.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermeulen W, De Man JG, Pelckmans PA, De Winter BY. Neuroanatomy of lower gastrointestinal pain disorders. World J Gastroenterol. 2014;20(4):1005–1020. doi: 10.3748/wjg.v20.i4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141(8):1285–1293. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126(7):1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Cremon C, Carini G, Wang B, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106(7):1290–1298. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- 19.Spiller R, Aziz Q, Creed F, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56(12):1770–1798. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitehead WE, Engel BT, Schuster MM. Irritable bowel syndrome: physiological and psychological differences between diarrhea-predominant and constipation-predominant patients. Dig Dis Sci. 1980;25(6):404–413. doi: 10.1007/BF01395503. [DOI] [PubMed] [Google Scholar]
- 21.Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22(3):293–e282. doi: 10.1111/j.1365-2982.2009.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Posserud I, Ersryd A, Simrén M. Functional findings in irritable bowel syndrome. World J Gastroenterol. 2006;12(18):2830–2838. doi: 10.3748/wjg.v12.i18.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thabane M, Simunovic M, Akhtar-Danesh N, Garg AX, Clark WF, Marshall JK. Clustering and stability of functional lower gastrointestinal symptom after enteric infection. Neurogastroenterol Motil. 2012;24(6):546–552. e252. doi: 10.1111/j.1365-2982.2012.01898.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53(11):1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson MB, Tillisch K, Craig AD, et al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142(3):463–472. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 27.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 28.Martínez I, Muller CE, Walter J. Long-term temporal analysis of the human fecal microbiota revealed a stable core of dominant bacterial species. PLoS One. 2013;8(7):e69621. doi: 10.1371/journal.pone.0069621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kajander K, Myllyluoma E, Rajilic-Stojanovic M, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27(1):48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 30.Vanhoutte T, Huys G, De Brandt E, Swings J. Temporal stability analysis of the microbiota in human feces by denaturing gradient gel electrophoresis using universal and group-specific 16S rRNA gene primers. FEMS Microbiol Ecol. 2004;48(3):437–446. doi: 10.1016/j.femsec.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Cremon C, Stanghellini V, Pallotti F, et al. Salmonella gastroenteritis during childhood is a risk factor for irritable bowel syndrome in adulthood. Gastroenterology. 2014;147(1):69–77. doi: 10.1053/j.gastro.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Jalanka-Tuovinen J, Salojärvi J, Salonen A, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63(11):1737–1745. doi: 10.1136/gutjnl-2013-305994. [DOI] [PubMed] [Google Scholar]
- 33.Lyra A, Rinttilä T, Nikkilä J, et al. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J Gastroenterol. 2009;15(47):5936–5945. doi: 10.3748/wjg.15.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malinen E, Rinttilä T, Kajander K, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100(2):373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 35.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24(6):521–530. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkins T, Pepitone C, Alex B, Schade RR. Diagnosis and management of IBS in adults. Am Fam Physician. 2012;86(5):419–426. [PubMed] [Google Scholar]
- 37.Yarandi SS, Nasseri-Moghaddam S, Mostajabi P, Malekzadeh R. Overlapping gastroesophageal reflux disease and irritable bowel syndrome: increased dysfunctional symptoms. World J Gastroenterol. 2010;16(10):1232–1238. doi: 10.3748/wjg.v16.i9.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faresjo A, Grodzinsky E, Hallert C, Timpka T. Patients with irritable bowel syndrome are more burdened by comorbidity and worry about serious diseases than healthy controls – eight years follow-up of IBS patients in primary care. BMC Public Health. 2013;13:832. doi: 10.1186/1471-2458-13-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 40.Saito YA, Schoenfeld P, Locke GR., III The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97(8):1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 41.Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35(3):350–359. doi: 10.1111/j.1365-2036.2011.04948.x. [DOI] [PubMed] [Google Scholar]
- 42.Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology. 2002;122(6):1701–1714. doi: 10.1053/gast.2002.33741. [DOI] [PubMed] [Google Scholar]
- 43.Engsbro AL, Begtrup LM, Kjeldsen J, et al. Patients suspected of irritable bowel syndrome-cross-sectional study exploring the sensitivity of Rome III criteria in primary care. Am J Gastroenterol. 2013;108(6):972–980. doi: 10.1038/ajg.2013.15. [DOI] [PubMed] [Google Scholar]
- 44.Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Suppl 1):S8–S28. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 45.Spiegel BM, Farid M, Esrailian E, Talley J, Chang L. Is irritable bowel syndrome a diagnosis of exclusion?: a survey of primary care providers, gastroenterologists, and IBS experts. Am J Gastroenterol. 2010;105(4):848–858. doi: 10.1038/ajg.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Begtrup LM, Engsbro AL, Kjeldsen J, et al. A positive diagnostic strategy is noninferior to a strategy of exclusion for patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(8):956–962. doi: 10.1016/j.cgh.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 47.Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS) Dig Dis Sci. 1984;29(3):239–247. doi: 10.1007/BF01296258. [DOI] [PubMed] [Google Scholar]
- 48.Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl. 1987;130:81–84. doi: 10.3109/00365528709091004. [DOI] [PubMed] [Google Scholar]
- 49.Lavö B, Stenstam M, Nielsen AL. Loperamide in treatment of irritable bowel syndrome – a double-blind placebo controlled study. Scand J Gastroenterol Suppl. 1987;130:77–80. doi: 10.3109/00365528709091003. [DOI] [PubMed] [Google Scholar]
- 50.Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol. 1996;31(5):463–468. doi: 10.3109/00365529609006766. [DOI] [PubMed] [Google Scholar]
- 51.Dove LS, Lembo A, Randall CW, et al. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology. 2013;145(2):329–338. doi: 10.1053/j.gastro.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Lembo A, Dove S, Andrae D, et al. Eluxadoline for the treatment of diarrhea-predominant irritable bowel syndrome: results of 2 randomized, double-blind, placebo-controlled phase 3 clinical trials of efficacy and safety. Gastroenterology. 2014;146(Suppl 1, issue 5):S159. [Google Scholar]
- 53.Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 54.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101(7):1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 55.Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–641. doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 56.Lacy BE. The science, evidence and practice of dietary interventions in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2015;13(11):1899–1906. doi: 10.1016/j.cgh.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 57.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146(1):67–75. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 58.de Roest RH, Dobbs BR, Chapman BA, et al. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67(9):895–903. doi: 10.1111/ijcp.12128. [DOI] [PubMed] [Google Scholar]
- 59.Staudacher HM, Lomer MC, Anderson JL, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142(8):1510–1518. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 60.Austin GL, Dalton CB, Hu Y, et al. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7(6):706–708. doi: 10.1016/j.cgh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144(5):903–911. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355(9209):1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 63.Krause R, Ameen V, Gordon SH, et al. A randomized, double-blind, placebo-controlled study to assess efficacy and safety of 0.5 mg and 1 mg alosetron in women with severe diarrhea-predominant IBS. Am J Gastroenterol. 2007;102(8):1709–1719. doi: 10.1111/j.1572-0241.2007.01282.x. [DOI] [PubMed] [Google Scholar]
- 64.Lembo T, Wright RA, Bagby B, et al. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96(9):2662–2670. doi: 10.1111/j.1572-0241.2001.04128.x. [DOI] [PubMed] [Google Scholar]
- 65.Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther. 1999;13(9):1149–1159. doi: 10.1046/j.1365-2036.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 66.Camilleri M, Chey WY, Mayer EA, et al. A randomized controlled clinical trial of the serotonin type 3 receptor antagonist alosetron in women with diarrhea-predominant irritable bowel syndrome. Arch Intern Med. 2001;161(14):1733–1740. doi: 10.1001/archinte.161.14.1733. [DOI] [PubMed] [Google Scholar]
- 67.Bardhan KD, Bodemar G, Geldof H, et al. A double-blind, randomized, placebo-controlled dose-ranging study to evaluate the efficacy of alosetron in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2000;14(1):23–34. doi: 10.1046/j.1365-2036.2000.00684.x. [DOI] [PubMed] [Google Scholar]
- 68.Chang L, Ameen VZ, Dukes GE, McSorley DJ, Carter EG, Mayer EA. A dose-ranging, phase II study of the efficacy and safety of alosetron in men with diarrhea-predominant IBS. Am J Gastroenterol. 2005;100(1):115–123. doi: 10.1111/j.1572-0241.2005.40365.x. [DOI] [PubMed] [Google Scholar]
- 69.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin Ther. 2008;30(5):884–901. doi: 10.1016/j.clinthera.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Tong K, Nicandro JP, Shringarpure R, Chuang E, Chang L. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol. 2013;6(5):344–357. doi: 10.1177/1756283X13491798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heading R, Bardhan K, Hollerbach S, Lanas A, Fisher G. Systematic review: the safety and tolerability of pharmacological agents for treatment of irritable bowel syndrome – a European perspective. Aliment Pharmacol Ther. 2006;24(2):207–236. doi: 10.1111/j.1365-2036.2006.02937.x. [DOI] [PubMed] [Google Scholar]
- 72.Olden KW. Targeted therapies for diarrhea-predominant irritable bowel syndrome. Clin Exp Gastroenterol. 2012;5:69–100. doi: 10.2147/CEG.S29023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vahedi H, Merat S, Momtahen S, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27(8):678–684. doi: 10.1111/j.1365-2036.2008.03633.x. [DOI] [PubMed] [Google Scholar]
- 74.Aroniadis OC, Brandt LJ. Intestinal microbiota and the efficacy of fecal microbiota transplantation in gastrointestinal disease. Gastroenterol Hepatol (NY) 2014;10(4):230–237. [PMC free article] [PubMed] [Google Scholar]
- 75.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single-center experience. Am J Gastroenterol. 2014;109(11):1831–1832. doi: 10.1038/ajg.2014.295. [DOI] [PubMed] [Google Scholar]
- 76.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 77.Lembo A, Pimentel M, Rao SS, et al. Efficacy and safety of repeat treatment with rifaximin for diarrhea-predominant irritable bowel syndrome (IBS-D): results of the TARGET 3 study; Presented at: American College of Gastroenterology (ACG) 2014 Annual Scientific Meeting; October 17–22 2014; Philadelphia, PA. [Google Scholar]
- 78.Shah E, Kim S, Chong K, Lembo A, Pimentel M. Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med. 2012;125(4):381–393. doi: 10.1016/j.amjmed.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 79.Schoenfeld P, Pimentel M, Chang L, et al. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment Pharmacol Ther. 2014;39(10):1161–1168. doi: 10.1111/apt.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]