Abstract
The non-fluent/agrammatic variant of primary progressive aphasia (nfvPPA) and the behavioral variant frontotemporal dementia (bvFTD) are focal neurodegenerative disorders belonging to the FTD-spectrum clinical syndromes. NfvPPA is characterized by effortful speech and/or agrammatism and left frontal atrophy, while bvFTD is characterized by social-emotional dysfunction often accompanied by right-lateralized frontal damage. Despite their contrasting clinical presentations, both disorders show prominent left anterior insula atrophy. We investigated differential patterns of insular subregion atrophy in nfvPPA and bvFTD. Based on knowledge of insular connectivity and physiology, we hypothesized that the left superior precentral region of the dorsal anterior insula (SPGI) would be more atrophic in nvfPPA due to its critical role in motor speech, whereas the ventral anterior region would be more atrophied in bvFTD reflecting its known role in social-emotional-autonomic functions. Early stage nfvPPA and bvFTD patients matched for disease severity, age, gender and education and healthy controls participated in the study. Detailed clinical history, neurological examination, neuropsychological screening evaluation, and high-resolution T1-weighted brain magnetic resonance images (MRI) were collected. Voxel-based morphometry (VBM) was applied to perform group comparisons across the whole brain and in bilateral insula region of interest (ROI). Correlation analyses between insular subregion atrophy and relevant clinical features were performed. Whole brain group comparisons between nfvPPA and bvFTD showed the expected predominantly left or right anterior insular atrophy pattern. ROI analysis of bilateral insula showed that the left SPGI was significantly more atrophied in nfvPPA compared to bvFTD, while the bilateral ventral anterior and right dorsal anterior insula subregions were more atrophied in bvFTD than nfvPPA.
Only left SPGI volume correlated with speech production abilities, while left and right ventral anterior insula volumes correlated with ratings of aberrant eating behavior. These two FTD clinical variants show different patterns of insular subregion atrophy in the left precentral dorsal anterior and bilateral ventral anterior regions, providing further evidence for the role of these subregions in speech production and social-emotional function.
Keywords: primary progressive aphasia, behavioral variant frontotemporal dementia, insula, speech production, voxel-based morphometry, apraxia of speech
1. Introduction
Frontotemporal dementia (FTD) is a heterogeneous group of neurodegenerative diseases characterized by atrophy of frontal, temporal, and insular brain regions. Clinically, FTD manifests either with prominent behavioral and personality changes (behavioral variant, bvFTD; Neary et al., 1998; Rascovsky et al., 2011) or with isolated language impairments (primary progressive aphasia, PPA; Mesulam, 1982, 2001).
The non-fluent/agrammatic variant PPA (nfvPPA) is characterized by agrammatism and/or effortful, halting speech consistent with apraxia of speech (AOS) (Gorno-Tempini et al., 2011; Grossman 1996; Ogar et al., 2007). Imaging studies in nfvPPA patients have shown left-lateralized atrophy mostly in the posterior frontal lobe, including Broca’s area and premotor cortex, as well as in the anterior insula (Nestor et al, 2003; Gorno-Tempini et al., 2004; Josephs et al., 2006; Rogalski et al., 2011; Rohrer et al., 2009).
BvFTD is characterized by prominent progressive changes in personality and social-emotional function. Typical symptoms include disinhibition, altered eating behavior, loss of empathy, apathy, compulsivity and emotional dysregulation. Aberrant eating behavior is one of the most common and distinctive symptoms of bvFTD (Bozeat et al., 2000). It occurs in over 80% of bvFTD patients over the course of the disease (Piquet et al., 2009) and is characterized by gluttonous and indiscriminant food consumption (Shinagawa et al. 2009; Snowdon et al., 2001). BvFTD neuroimaging studies have shown areas of atrophy in the ventromedial and posterior orbital frontal cortex, as well as the anterior insula and anterior cingulate cortex, striatum, and amygdala bilaterally but often more prominently on the right (Rosen et al., 2002; Boccardi et al., 2005; Ibach et al., 2004; Franceschi et al., 2005; Schroeter, Raczka, Neumann, & Yves von Cramon, 2007).
Despite the broad clinical and anatomical differences between bvFTD and nfvPPA, both disorders share regions of focal neurodegeneration in the insulae. Recent evidence highlights differential roles of anterior insular subregions in sensory-motor, cognitive, control and attentional, and behavioral functions (Nelson et al., 2010; Deen et al., 2011; Kurth et al., 2010; Mutschler et al., 2009; Touroutoglou et al., 2012). We predicted that the left superior precentral region of the dorsal anterior insula (SPGI), previously implicated in motor speech planning (Dronkers, 1996), would be most involved in nfvPPA, whereas the ventral anterior insula, previously linked to social-emotional and autonomic functions (Wooley, et al., 2007), would be more atrophied in bvFTD. To test these hypotheses, we compared the patterns of MRI-based regional grey matter (GM) atrophy between early-stage nfvPPA and bvFTD. In particular, we investigated distinct subregions of the insulae to isolate specific foci of focal GM atrophy associated with each clinical presentation. Correlation analyses between these specific sub-regional volumes and relevant clinical scores were performed.
2. Material and Methods
2.1 Subjects
We searched the University of California San Francisco (UCSF) Memory and Aging Center (MAC) database for patients who met the following inclusion criteria: a research diagnosis of bvFTD or nfvPPA, a high-resolution magnetic resonance imaging (MRI), and a Clinical Dementia Rating (CDR) score ≤ 1 (Morris, 1993) within 6 months of first diagnosis. Detailed clinical history, neurological examination, and neuropsychological screening were conducted as previously described (Rosen et al., 2002). NfvPPA patients also underwent a detailed speech and language evaluation (Gorno-Tempini et al., 2004). Recent research clinical criteria for bvFTD and nfvPPA were applied (Gorno-Tempini et al., 2011; Rascovsky et al., 2011). Eighty-eight patients met these initial inclusion criteria (40 nfvPPA and 48 bvFTD) but the groups significantly differed in age, gender and CDR. For this reason, we then matched the two groups of patients, seeking comparable distributions of age, gender, disease severity (CDR total and sum-of-boxes scores and Mini-Mental State Examination) and sample size. The resulting study population included 25 nfvPPA and 23 bvFTD patients (Table 1). Fifty healthy volunteers matched to the patient groups according to age, gender, education, and handedness were recruited as controls for the imaging analysis. An additional cohort of healthy subjects (n=34) who received the language battery were used as controls for the clinical comparison (Table 1). Procedures were approved by the UCSF Committee on Human Research, and informed consent was obtained from all participants according to the Declaration of Helsinki.
Table 1.
Demographic and clinical data comparison
| Controls (n=34) |
nfvPPA (n=25) |
bvFTD (n=23) |
|
|---|---|---|---|
| Number of males/females | 12/22 | 14/11 | 13/10 |
| Years of age | 62.3 ± 6.6 | 66.6 ± 7.7 | 62.9 ± 6.5 |
| Handedness (R/L) | 28/6 | 23/2 | 18/5 |
| Years of education | 16.4 ± 2.1 | 16.5 ± 3.0 | 16.1 ± 2.6 |
| Global cognition & functional level: | |||
| MMSE score (max score=30) | 28.4 ± 1.2 | 24.7 ± 4.8* | 26.6 ± 3.5 |
| CDR | NA | 0.6 ± 0.3 | 0.7 ± 0.3 |
| CDR box score sum | NA | 3.2 ± 1.7 | 4.1 ± 1.2 |
| Memory: | |||
| CVLT-SF, 4 trials, score (maximum score=36) | 29.6 ± 3.3 | 20.1 ± 7.2* | 23.1 ± 4.9* |
| CVLT-SF, 10-minute recall, score (maximum score=9) |
7.7 ± 1.5 | 4.6 ± 2.6* | 5.4 ± 2.4* |
| Modified Rey-Osterrieth delay score (maximum score=17) |
12.2 ± 2.2 | 9.2 ± 4.2 | 8.5 ± 3.9* |
| Visuospatial functioning: | |||
| Modified Rey-Osterrieth copy score (maximum score=17) |
15.4 ± 1.0 | 14.2 ± 1.2 | 14.5 ± 2.0 |
| VOSP Number Location (maximum score=10) | 9.1 ± 1.1 | 8.6 ± 1.9 | 8.2 ± 2.3 |
|
Attention, Speed, Working Memory &
Executive functions: |
|||
| Digit span forward score | 6.7 ± 1.2 | 5.0 ± 1.2* | 5.5 ± 1.3 |
| Digit span backward score | 5.3 ± 1.1 | 2.9 ± 1.4*,a | 4.0 ± 1.2* |
| Phonemic fluency score (D words generated in 1 min) |
15.6 ± 4.8 | 4.4 ± 2.9* | 8.7 ± 4.6* |
| Modified Trails B score (time in sec, max allowed=120) |
29.5 ± 14.6 | 89.5 ± 37.6*,a | 65.3 ± 38.9* |
| Modified Trails B score (correct lines in 120 secs) |
14.0 ± 0.0 | 9.6 ± 5.3 | 11.8 ± 4.2 |
| Design fluency score (correct in 1 min) | 10.1 ± 2.9 | 5.2 ± 2.8* | 6.2 ± 3.0* |
| Alternating M and N (best score=0; worst score=2) |
0.7 ± 0.9 | 0.9 ± 1.0 | 1.0 ± 0.9 |
| Stroop color naming task score (correct in 1 min) |
88.8 ± 16.9 | 36.6 ± 13.4*,a | 61.6 ± 25.6* |
| Stroop interference task score (correct in 1 min) |
50.7 ± 12.3 | 13.5 ± 14.2*,a | 33.2 ± 15.5* |
| Abstract reasoning (ma score=6) | 4.3 ± 1.1 | 2.5 ± 1.4* | 2.3 ± 1.6* |
| Calculations score (max score=5) | 4.5 ± 0.6 | 3.9 ± 1.2 | 4.0 ± 1.4 |
| Speech and Language: | |||
| Abbreviated BNT score (max score=15) | 14.3 ± 1.1 | 12.0 ± 2.9 | 12.8 ± 2.6 |
| Semantic fluency score (animals generated in 1 min) |
22.4 ± 5.9 | 9.0 ± 4.7* | 11.9 ± 4.5* |
| Repetition (max score=5) | 4.6 ± 0.6 | 2.7 ± 1.3*,a | 4.3 ± 0.9 |
| Verbal Agility (max score=6) | 5.8 ± 0.4 | 2.6 ± 1.3*,a | 4.8 ± 1.4 |
| Sentence comprehension - syntax (max score=5) |
4.8 ± 0.5 | 3.7 ± 0.9* | 4.1 ± 1.4* |
| PPVT-R Vocabulary comprehension (max score=16) |
15.5 ± 0.8 | 14.4 ± 2.1 | 14.2 ± 2.4 |
| Neuropsychiatric Inventory (NPI): | |||
| Delusions | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 1.7* |
| Hallucinations | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Agitation | 0.0 ± 0.0 | 1.6 ± 1.7* | 2.2 ± 3.7* |
| Anxiety | 0.0 ± 0.0 | 2.6 ± 2.9* | 1.3 ± 2.0* |
| Depression | 0.0 ± 0.0 | 0.1 ± 1.4 | 1.3 ± 2.5* |
| Euphoria | 0.0 ± 0.0 | 0.3 ± 0.9 | 2.5 ± 2.9*,a |
| Apathy | 0.0 ± 0.0 | 2.9 ± 3.4* | 6.3 ± 3.7*,a |
| Disinhibition | 0.0 ± 0.0 | 1.6 ± 2.5 | 5.0 ± 3.6*,a |
| Irritability | 0.0 ± 0.0 | 2.1 ± 2.5* | 2.4 ± 3.3* |
| Aberrant Motor | 0.0 ± 0.0 | 2.5 ± 3.7* | 5.7 ± 4.4*,a |
| Aberrant sleep | 0.0 ± 0.0 | 1.2 ± 2.5 | 2.6 ± 2.4* |
| Aberrant eating | 0.0 ± 0.0 | 0.8 ± 1.3 | 5.9 ± 3.5*,a |
| Total NPI | 0.0 ± 0.0 | 15.7 ± 13.6* | 35.7 ± 15.8*,a |
p<0.05 versus controls.
p<0.05 between patients.
Abbreviations: nfvPPA, non-fluent variant primary progressive aphasia; bvFTD, behavioral-variant fronto-temporal dementia; CDR, Clinical Dementia Rating; CVLT-SF, California Verbal Learning Test–Short Form; MMSE, Mini-Mental State Examination; BNT, Boston Naming Test; VOSP, Visual Object Space Perception; PPVT-R, Peabody Picture Vocabulary Test – Revised.
Data are presented as mean ±SD
2.4 Voxel-based morphometry
2.4.1 Data Acquisition
All participants (48 patients and 50 healthy controls) underwent structural brain MRI within 6 months of first diagnosis on either a 1.5 T or 3T scanner. For 13 bvFTD, 11 nfvPPA, and 25 control subjects T1 images were acquired on a 1.5T Siemens Magnetom VISION system (Siemens, Iselin, NJ) equipped with a standard quadrature head coil, using a magnetization prepared rapid gradient echo (MPRAGE) sequence (164 coronal slices; slice thickness = 1.5 mm; field of view = 256 mm2; matrix 256 × 256; voxel size 1.0 × 1.5 × 1.0 mm3; repetition time= 10 ms; echo time = 4 ms; inversion time = 300 ms; flip angle = 15°). For the remaining subjects, T1 images were acquired on a 3T Siemens TrioTim syngo equipped with an eight-channel transmit and receive head coil using a MPRAGE sequence (160 sagittal slices; slice thickness = 1 mm; field of view = 256 mm2; matrix = 256 × 240; voxel size 1.0 × 1.0 × 1.0 mm3; repetition time= 2300 ms; echo time = 2.98 ms; inversion time = 900 ms; flip angle = 9°).
2.4.2 Voxel-based morphometry (VBM) analysis
Image processing and statistical analysis were performed using Statistical Parametric Mapping (SPM8) software (Wellcome Trust Center for Neuroimaging, London, UK) running under Matlab R2013a (MathWorks). A custom template was created from a large dataset of 300 healthy controls available at UCSF MAC with both 1.5 T and 3 T scanners. All the structural images were first resampled at the same voxel size (1.5 × 1.5 × 1.5 mm). They were then segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) based on an adaptive maximum, which is a posterior technique to account for intensity inhomogeneity and for other local variations of intensity (Rajapakse, Giedd, & Rapoport, 1997), and a partial volume estimation (PVE) to account for partial volume effects (Tohka, Zijdenbos, & Evans, 2004). The images were spatially normalized to the custom template with a diffeomorphic exponentiated lie algebra (DARTEL) registration method (Ashburner, 2007). The images were then modulated by multiplying the voxel values by the Jacobian determinant derived from the spatial normalization to preserve the relative volumes of GM. Finally the images were normalized to the Montreal Neurological Institute (MNI) standard space and smoothed with a Gaussian kernel (8 mm FWHM).
2.4.3 Statistical analysis
Whole brain differences in GM were investigated using an ANOVA across groups, including age, gender, total intracranial volume, and scanner type as nuisance variables. We compared control subjects versus nfvPPA and bvFTD patients to confirm the expected pattern of atrophy in these two FTD variants. We then performed a direct comparison between nfvPPA and bvFTD. An ROI analysis within the insulae was performed to identify differential patterns of subregional insular atrophy between nfvPPA and bvFTD. The WFU PickAtlas toolbox (http://fmri.wfubmc.edu/software/PickAtlas) was used to create a mask of the insulae.
Statistical thresholds were set at p<0.05 corrected for family wise error (FWE) for the whole brain analysis and at p<0.001 uncorrected for the ROI analysis on the insulae.
3. Results
3.1 Group demographic and neuropsychological features
Consistent with our inclusion criteria, patient groups were similar in age, education, CDR score, box-score, and MMSE (Table 1). Neuropsychological assessment also revealed comparable general cognitive profiles between the two patient groups. Consistent with clinical diagnosis, bvFTD patients were significantly worse in their behavioral scores from the Neuropsychiatric Inventory (NPI) such as aberrant eating, disinhibition, apathy, euphoria, and aberrant motor scores. NfvPPA patients were significantly worse than bvFTD patients in tests involving language production, such as digits backward, oral repetition, verbal agility, phonemic and semantic fluency, and the Stroop test (both color naming and interference conditions) (Table 1). A speech and language evaluation, administered only to nfvPPA patients, showed AOS and dysarthria and impaired repetition, with preservation of semantic abilities and language comprehension (Table 2).
Table 2.
Speech and language evaluation results in nfvPPA patients
| Speech and language evaluation | Mean SD |
|---|---|
| WAB Spontaneous Speech Fluency (max score=10) | 6.24 ± 2.59 |
| WAB Spontaneous Speech Total (max score=20) | 13.96 ± 4.92 |
| WAB Repetition (max score=100) | 80.92 ± 20.61 |
| MSE Apraxia of Speech Rating (0-7, max score=7) | 2.96 ± 2.34 |
| MSE Dysarthria Rating (0-7, max score=7) | 2.04 ± 2.56 |
| WAB Auditory Word Recognition (max score=60) | 58.40 ± 4.49 |
| WAB Sequential Commands (max score=80) | 66.84 ± 15.19 |
| Pyramid and Palm Tree – pictures (max score=52) | 48.75 ± 4.57 |
Abbreviations: WAB, Western Aphasia Battery; MSE, Motor Speech Evaluation.
Data are presented as mean ±SD
3.2 Voxel-based morphometry
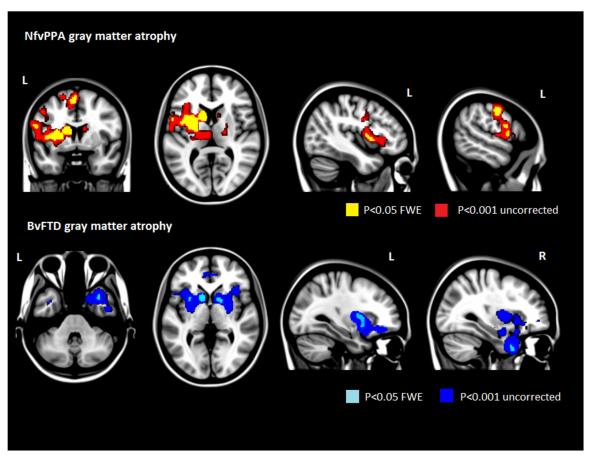
In the whole brain analysis, when compared with healthy controls, nfvPPA patients showed significantly more GM atrophy in the left cerebral cortex including the inferior frontal and precentral gyri, the supplementary motor area (SMA), and the anterior insula, as well as bilateral striatum (Fig.1 and Table 3). BvFTD patients showed significantly more bilateral GM atrophy involving orbitofrontal and ventromedial cortices, anterior cingulate cortex, mid- and anterior temporal cortices (right > left), anterior insula and striatum (Fig.1 and Table 4).
Figure 1.
Results of the whole brain with Voxel based morphometry (VBM). Statistical significance of loss of gray matter volume for nfvPPA compared to controls (upper row). In yellow the significance is corrected at p<0.05 Family wise corrected (FWE) and in red at p<0.001 without correction. Statistical significance of loss of gray matter volume for bvFTD compared to controls (lower row). In teal the significance is corrected at p<0.05 Family wise corrected (FWE) and in blue at p<0.001 without correction for multiple comparisons.
Table 3.
Regions of GM atrophy in nfvPPA versus control subjects
| Region | X, Y, Z | Z Score | P value |
|---|---|---|---|
| Left Precentral gyrus (BA6) | −26, −10, 55 | 5.67 | 0.001* |
| Left Precentral gyrus (BA6) | −48, 0, 42 | 5.03 | 0.02 * |
| Left Ventral Precentral gyrus | −62, −1, 24 | 5.49 | 0.003 * |
| Left Postcentral gyrus | −56, −9, 42 | 5.82 | 0.001 * |
| Left Supplementar Motor Area (SMA) | −3, 8, 55 | 6.41 | 0.000* |
| Left Inferior Frontal Operculum (BA44) | −56, 7, 9 | 5.11 | 0.02 * |
| Left Mid Cingulum | −9, 14, 43 | 5.14 | 0.02 * |
| Left Insula (Dorsal Anterior) | −38, 23, 1 | 5.09 | 0.02 * |
| Left Putamen | −29, 5, 12 | 6.73 | 0.000 * |
| Left Caudate | −9, 8, 13 | 6.52 | 0.000* |
| Left Thalamus | −12, −19, 15 | 5.46 | 0.003* |
P value data with the asterisk are corrected for family-wise error (FWE)
Table 4.
Regions of GM atrophy in bvFTD versus control subjects
| Region | X, Y, Z | Z Score | P value |
|---|---|---|---|
| Right Med Orbito-Frontal cortex | 2, 33, −12 | 4.84 | 0.000 |
| Left Med Orbito-Frontal cortex | −29, 35, −15 | 4.69 | 0.000 |
| Right Superior Frontal gyrus | 18, 23, 61 | 4.20 | 0.000 |
| Right Mid-Temporal Pole | 35, 11 ,−36 | 5.20 | 0.012 * |
| Right Inferior Temporal gyrus | 54, −12 ,−23 | 4.71 | 0.000 |
| Right Anterior Cingulum | 3, 45, 10 | 4.30 | 0.000 |
| Left Anterior Cingulum | −6, 45, 10 | 4.67 | 0.000 |
| Right Insula (Dorsal Anterior) | 39, 21, 0 | 4.08 | 0.012 * |
| Left Insula (Ventral Anterior) | −33, 14, −12 | 4.88 | 0.000 |
| Right Caudate | 11, 9, 9 | 5.40 | 0.005 * |
| Left Caudate | −9, 11, 9 | 6.19 | 0.000 * |
| Right Pallidum | 14, 6, −3 | 5.17 | 0.014 * |
| Left Pallidum | −21, −1, 7 | 5.31 | 0.007 * |
| Left Putamen | −26, 6, 9 | 5.30 | 0.008 * |
P value data with the asterisk are corrected for family-wise error (FWE)
The direct comparison between the two patient populations showed left-lateralized GM atrophy in Broca’s area (Brodmann area BA 44), the premotor regions, and the SMA in nfvPPA, and right-lateralized GM atrophy in the orbitofrontal, mid- and anterior temporal regions (right > left), and anterior insula in bvFTD at the p<0.001 uncorrected level.
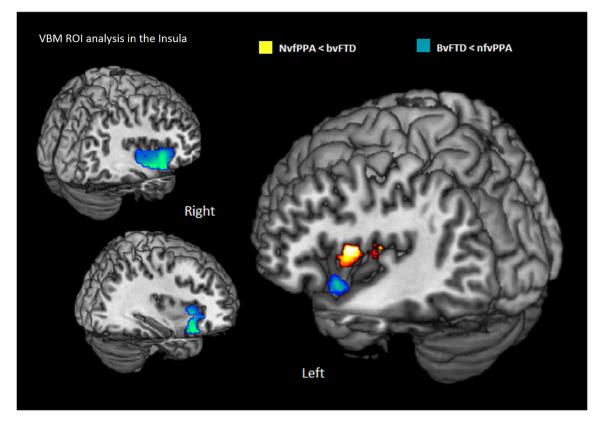
ROI analysis in the insulae showed greater atrophy in the left SPGI in nfvPPA compared to bvFTD (Fig. 2), and greater atrophy in the left ventral and right ventral and dorsal anterior insula in bvFTD compared to nfvPPA (Fig. 2) at the p<0.001 uncorrected level.
Figure 2.
Results of the region of interest analysis in the insulae with Voxel based morphometry (VBM). Statistical significance of loss of gray matter in the insula in nfvPPA compared to bvFTD are shown in red. Statistical significance of loss of gray matter in the insula in bvFTD compared to nfvPPA are shown in blue. Results are at p<0.001 without correction for multiple comparisons.
3.3 Post-hoc correlation between insular subregion volumes and verbal agility and aberrant eating
In this analysis, we investigated whether atrophy in the insular subregions differentially involved in nfvPPA and bvFTD correlated with relevant clinical symptoms, i.e. verbal agility for the left SPGI (Dronkers 1996), and aberrant eating behavior for the ventral anterior insula (Woolley et al., 2007). Relative volume for each subject was calculated by multiplying binarized significant clusters with normalized gray matter images. A clinical rating of general speech production abilities (0=no speech output to 6=normal speech) (Goodglass et al., 1983), and the aberrant eating score from the Neuropsychiatric Inventory (NPI; Cummings 1994) were considered. A subset of participants had both these scores: 9 nvfPPA, 11 bvFTD and 13 controls.
Results showed a significant positive correlation between left SPGI volume and verbal agility (R=0.46, P=0.007) but not with the aberrant eating score (R=−0.29, P=0.1). The opposite pattern was observed in the ventral anterior insula regions, which correlated with aberrant eating scores (right R=−0.58, P=0.000; left R=−0.45, P=0.008) but not with the verbal agility (right R=−0.01, P=0.96; left R=0.26, P=0.01). Within group correlations showed a trend of correlation in nfvPPA (R=0.66, P=0.05) for verbal agility only.
4. Discussion
This study evaluated patterns of brain atrophy in patients with early stage nfvPPA and bvFTD, with a particular focus on the insula. Consistent with their clinical profiles, nfvPPA and bvFTD showed distinctive atrophy patterns affecting the left and right anterior insula regions respectively. Within the insulae, nfvPPA patients showed greater atrophy in the left SPGI, while bvFTD patients showed greater atrophy in bilateral ventral anterior insular atrophy. Furthermore, volume changes in these regions suggested a trend of correlation with clinical relevant scores. We discuss these findings in relation to specific clinical symptoms and previous knowledge regarding insular structure and functions.
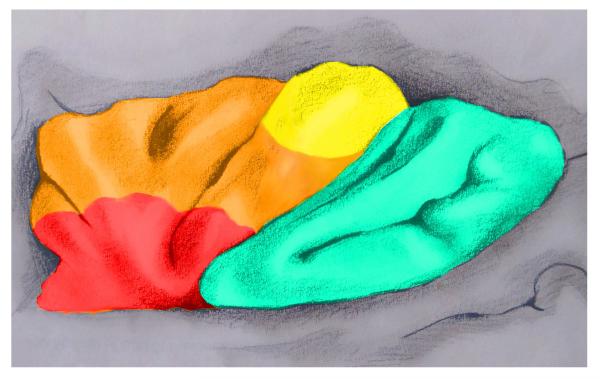
The insula is a portion of the cerebral cortex folded deep within the lateral sulcus (Fig. 3). Macroscopically, the central sulcus of the insula divides it into an anterior and a posterior part. Converging evidence from hodological studies on monkeys (Mesulam & Mufson, 1982), human cytoarchitectonics (von Economo & Kosinkas, 1925), intracerebral electrical stimulation in patients with insular epilepsy (Stephani, Fernandez-Baca Vaca, Maciunas, Koubeissi, & Luders, 2011; Penfield & Rasmussen, 1950), and neuroimaging studies (Nanetti et al., 2008, Mutschler et al 2009, Kurth, et al. 2010) point to anatomical and functional topographical organization of the insular lobe. While the most posterior part of the insula is associated with somatosensory representations, the anterior part can be divided into at least two distinct sub-regions: a ventral agranular region, at the basal transition from orbitofrontal cortex into insula, and a dorsal (dysgranular) region extending toward the frontal operculum.
Figure 3.
Anatomical picture of the insula and its sub-regions. The insula is divided by the central sulcus into an anterior and posterior part. The posterior part is represented in blue and the anterior is divided in a ventral (red) and dorsal (orange) region. The most caudal part of the dorsal insula comprises the superior precentral gyrus (yellow).
In humans, the ventral anterior insula is part of a large-scale network that includes the pregenual anterior cingulate cortex, amygdala, and striatum and is involved particularly in social-emotional functions (Craig, 2009; Critchley, 2005; Kurth et al., 2010; Mutschler et al., 2009; Seeley et al., 2007; Singer, Critchley, & Preuschoff, 2009). The dorsal anterior insula is a node in the cognitive control network along with dorsal anterior cingulate and lateral prefrontal cortex (Dosenbach et al. 2006; Dosenbach et al. 2007; Nelson et al., 2010; Deen et al., 2011). The most caudal part of the anterior insula comprising the superior precentral gyrus of the insula (SPGI), supports motor speech control such as the programming and/or the actual execution of vocal tract gestures (Ackermann & Riecker, 2004; Mutschler et al., 2009;). In particular, Riecker, Ackermann, Wildgruber, Dogil, and Grodd (2000) showed that activation of this region was specifically associated with overt as opposed to covert (silent) speech production, supporting its role in coordinating the movements of the many laryngeal and phonation muscles engaged in speech production, rather than in planning pre-articulatory processes of speech gestures. In a lesion-based study, Dronkers identified the invariant presence of SPGI lesions in patients AOS, suggesting that this area could be critical for coordination of the articulators during complex speech production (Dronkers, 1996).
In our study, greater atrophy in the left SPGI as part of the speech production network could thus be related to the early motor speech difficulties typical of nfvPPA but not seen in early bvFTD. This finding is also supported by the correlation analysis revealing that volume loss in the left SPGI is associated with lower verbal agility scores.
In addition to the left dorsal anterior insula and SPGI, we also showed focal areas of greater GM atrophy in the left posterior frontal regions in nfvPPA compared to bvFTD, namely the inferior frontal gyrus, the premotor cortex, the SMA, as well as in the striatum. These results are consistent with precedent neuroimaging studies in nfvPPA patients showing that the main target of neurodegeneration in this clinical population consists of a left-sided frontal and insular cortical and subcortical network relevant to speech production (Mandelli et al., 2014). The relative roles of specific regions and connections within this speech network in speech production remains to be established (Hillis et al., 2004).
The relative preservation of the SPGI and of the speech production network in bvFTD is consistent with the typical absence of speech production deficits in this population. Conversely, we found greater atrophy in the bilateral ventral anterior insula in bvFTD. Greater damage of this subregion in early bvFTD compared to nfvPPA is likely related to the social and emotional disorder typical of this syndrome which is absent in early nfvPPA. The ventral anterior insular region and the most rostral part of the dorsal anterior insula, collectively named Area Frontoinsulare or FI of von Economo (Seeley, 2010) are associated with social-emotional functioning (Critchley 2005; Kurth et al. 2010; Mutschler et al. 2009; Singer, Critchley, & Preuschoff, 2009), as well as gustatory processes such as taste perception (Krolak-Salmon et al., 2003; Prtichard et al., 1999; Rudenga et al., 2010) and food disgust (Calder et al., 2007, Deen et al., 2011). The FI is considered part of the “salience network” (Seeley et al., 2007) which is highly vulnerable in early bvFTD (Seeley et al., 2009). Consistent with preferential involvement of this salience network in bvFTD compared to nvfPPA, other salience network regions showed greater atrophy in bvFTD, including paralimbic (anterior cingulate, ventral anterior insula, temporal pole and orbitofrontal), and subcortical (ventral striatum) areas suggesting that sub-regional insular damage contributes to differential clinical symptoms in the FTD clinical variants.
The main limitation of this study is the small sample size of the participants due to the careful matching of the two study cohorts for possible confounding variables such as disease severity, age and gender.
5. Conclusion
In summary, this study showed that patients with early nfvPPA and bvFTD present differential patterns of atrophy in the speech production and salience networks in accordance to their typical language and behavioral presentations. We suggested, for the first time, differential involvement of dorsal precentral and ventral anterior insula subregions in nfvPPA and bvFTD respectively. The findings highlight these insular subregions as possible early imaging biomarkers of disease in these syndromes and provide neuropsychological evidence regarding their functional specificity.
Acknowledgements
The study was supported by grants from the National Institutes of Health (NINDS R01 NS050915, NIA P50 AG03006, NIA P50 AG023501, NIA P01 AG019724); State of California (DHS04-35516); Alzheimer’s Disease Research Centre of California (03-75271 DHS/ADP/ARCC); Larry L. Hillblom Foundation; John Douglas French Alzheimer’s Foundation; Koret Family Foundation; Consortium for Frontotemporal Dementia Research; and McBean Family Foundation and a Career Scientist Award (NFD) from the US Department of Veterans Affairs Clinical Sciences R&D Program. These supporting sources had no involvement in the study design, collection, analysis or interpretation of data, nor were they involved in writing the paper or the decision to submit this report for publication.
The authors thank the patients and their families for the time and effort they dedicated to the research.
The authors thank Mrs Nanza De Vito for her contribution for Figure 3 and Ms H Isabel Hubbard for collecting the cognitive scores in the control subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89(2):320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Wilkins DP, Ogar J, Willock S, Dronkers NF. Role of the precentral gyrus of the insula in complex articulation. Cortex. 2011;47(7):800–807. doi: 10.1016/j.cortex.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Sabattoli F, Laakso MP, Testa C, Rossi R, Beltramello A, Frisoni GB. Frontotemporal dementia as a neural system disease. Neurobiol Aging. 2005;26(1):37–44. doi: 10.1016/j.neurobiolaging.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Gregory CA, Ralph MAL, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? Journal of Neurology, Neurosurgery & Psychiatry. 2000;69(2):178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25(11):3422–8. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex. 2011;21(7):1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–8. doi: 10.1073/pnas.0704320104. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Franceschi M, Anchisi D, Pelati O, Zuffi M, Matarrese M, Moresco RM, Perani D. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol. 2005;57(2):216–225. doi: 10.1002/ana.20365. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination (BDAE) Lea and Febiger; Philadelphia: 1983. Distributed by Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D'Esposito M, Ding XS, Alavi A, Reivich M. Progressive Nonfluent Aphasia: Language, Cognitive, and PET Measures Contrasted with Probable Alzheimer's Disease. J Cogn Neurosci. 1996;8(2):135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(Pt 7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Ibach B, Poljansky S, Marienhagen J, Sommer M, Manner P, Hajak G. Contrasting metabolic impairment in frontotemporal degeneration and early onset Alzheimer's disease. Neuroimage. 2004;23(2):739–743. doi: 10.1016/j.neuroimage.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, Dickson DW, Jack CR, Jr, Petersen RC. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolak-Salmon P, Hénaff MA, Isnard J, Tallon-Baudry C, Guénot M, Vighetto A, Bertrand O, Mauguière F. An attention modulated response to disgust in human ventral anterior insula. Ann Neurol. 2003;53(4):446–53. doi: 10.1002/ana.10502. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5-6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli ML, Caverzasi E, Binney RJ, Henry ML, Lobach I, Block N, Amirbekian B, Dronkers N, Miller BL, Henry RG, Gorno-Tempini ML. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J Neurosci. 2014;34(29):9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11(6):592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49(4):425–432. [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212(1):1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009;457(2):66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Nanetti L, Cerliani L, Gazzola V, Renken R, Keysers C. Group analyses of connectivity-based cortical parcellation using repeated k-means clustering. Neuroimage. 2009;47(4):1666–77. doi: 10.1016/j.neuroimage.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126(Pt 11):2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord. 2007;21(4):S23–30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- Penfield, Rasmussen . The cerebral cortex of man. A clinical study of localization of function. MacMillan; New York: 1950. [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the processing of singe words. J Cogn Neurosci. 1989;1(2):153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Pritchard TC, Macaluso DA, Eslinger PJ. Taste perception in patients with insular cortex lesions. Behav Neurosci. 1999;113(4):663–71. [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16(2):176–186. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport. 2000;11(9):1997–2000. doi: 10.1097/00001756-200006260-00038. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, Mesulam MM. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31(9):3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, Ourselin S, Fox NC. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009;72(18):1562–1569. doi: 10.1212/WNL.0b013e3181a4124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rudenga K, Green B, Nachtigal D, Small DM. Evidence for an integrated oral sensory module in the human anterior ventral insula. Chemical senses. 2010 doi: 10.1093/chemse/bjq068. bjq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Raczka K, Neumann J, Yves von Cramon D. Towards a nosology for frontotemporal lobar degenerations-a meta-analysis involving 267 subjects. Neuroimage. 2007;36(3):497–510. doi: 10.1016/j.neuroimage.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW. Anterior insula degeneration in frontotemporal dementia. Brain Struct Funct. 2010;214(5-6):465–475. doi: 10.1007/s00429-010-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65(2):249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa S, Ikeda M, Nestor PJ, Shigenobu K, Fukuhara R, Nomura M, Hodges JR. Characteristics of abnormal eating behaviours in frontotemporal lobar degeneration: a cross-cultural survey. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(12):1413–1414. doi: 10.1136/jnnp.2008.165332. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Stephani C, Fernandez-Baca Vaca G, Maciunas R, Koubeissi M, Luders HO. Functional neuroanatomy of the insular lobe. Brain Struct Funct. 2011;216(2):137–149. doi: 10.1007/s00429-010-0296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23(1):84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- von Economo C, Kosinkas GN. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen. Springer; Berlin: 1925. [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60(4):1947–58. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, Miller B. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69(14):1424–33. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]