Abstract
In women, clinical studies suggest that pain syndromes such as irritable bowel syndrome and interstitial cystitis, which are associated with visceral hyperalgesia, are often comorbid with endometriosis and chronic pelvic pain. One of the possible explanations for this phenomenon is viscerovisceral cross-sensitization, in which increased nociceptive input from an inflamed pelvic organ sensitizes neurons that receive convergent input to the same dorsal root ganglion (DRG) from an unaffected visceral organ. Nociception induces up-regulation of cellular mechanisms such as phosphorylated extracellular signal-regulated kinase (pERK) and substance P (SP), neurotransmitters associated with induced pain sensation. The purpose of this study was to determine, in a rodent model, whether uterine inflammation increased the number of pERK- and SP-positive neurons that received input from both the uterus and the colon. Cell bodies of colonic and uterine DRG were retrogradely labeled with fluorescent tracer dyes microinjected into the colon/rectum and into the uterus. Ganglia were harvested for fluorescent microscopy to identify positively stained neurons. Approximately 6% of neurons were colon specific and 10% uterus specific. Among these uterus- or colon-specific neurons, up to 3–5% of DRG neurons in the lumbosacral neurons (L1–S3 levels) received input from both visceral organs. Uterine inflammation increased the number of pERK- and SP-immunoreactive DRG neurons innervating specifically colon, or innervating specifically uterus, and those innervating both organs. These results suggest that a localized inflammation activates primary visceral afferents, regardless of whether they innervate the affected organ. This visceral sensory integration in the DRG may underlie the observed comorbidity of female pelvic pain syndromes.
Keywords: DRG, colon, uterus, pERK, substance P
The incidence of persistent, episodic, or chronic visceral pain disorders such as irritable bowel syndrome (IBS), interstitial cystitis (IC), and chronic pelvic pain (CPP), are more prevalent in females (Berkley, 1997; Lee et al., 2001). These syndromes are characterized by recurring symptoms of pain, abdominal discomfort, alterations in urinary and bowel habits, and menstrual cycle-associated pain either in the absence of detectable organic disease or out of proportion to the degree of anatomic pathology. Defining the organization of pain transmission at the level of sensory neurons may increase our understanding of female chronic pelvic pain syndromes that have defied explanation.
Nociception is a balance of pro- and antinociceptive inputs depending on the state of the organism and continues to be a subject of intensive research. Direct activation of chemosensitive receptors and ion channels on peripheral terminals of sensory neurons or modulation of neural excitability activates extrinsic primary afferent neurons, whose cell bodies are located in the dorsal root ganglia (DRG). Our previous studies suggest that one such mechanism may be the convergence of nociceptive stimuli in the primary afferent neurons that innervate uterus and colon in the rat (Chaban et al., 2007). Analogous results were recently reported for the bladder and colon (Christianson et al., 2007), suggesting that pathology in one organ can influence sensory output and physiology in other nearby organs. Thus, visceral hyperalgesia and pain in one organ could be referred to an unaffected organ and elicit symptoms in the second organ without de novo pathology. Alteration in signal transduction of primary afferent neurons that innervate different visceral organs can induce enhanced perception of visceral sensation, resulting in elevated pain perception common in patients with functional disorders (Berkley, 1997, 2005).
Neural integration among the pelvic viscera is important for regulation of both reproductive and digestive functions. It is possible that these different neuronal pathways are cross-sensitized, but these afferent mechanisms have not been extensively studied in terms of viscerovisceral sensitization between colonic and uterine DRG. Viscerovisceral convergence and cross-sensitization is a powerful concept that may explain referred pain, and the phenomenon of inappropriate sensation from unaffected viscera (Berkeley, 2005). Viscerovisceral convergence may be an emergent property of spinal organization. For example, sensory afferents from several viscera innervate the same second-order spinal neuron, thus integrating the information. There is another possible site of viscerovisceral convergence, the DRG. First, we and others have demonstrated that DRG neurons can dichotomize and innervate at least two different organs (Chaban et al., 2007; Christianson et al., 2007). Dichotomizing neurons that innervate different organs represent 5–15% of the DRG neurons and have been identified in a number of different species (McNeill and Burden, 1986; Malykhina et al., 2006). Second, intraganglionic release of nociceptive agents [e.g., substance P (SP) and ATP] in response to peripheral nociceptive stimuli may involve the whole ganglion through volume transmission (Tang et al., 2006; Sculptoreanu and de Groat, 2007). Either mechanism provides a pathway through which inflammation in one visceral organ could lead to neurogenic sensitization in another, unaffected organ.
Visceral pain is different from somatic pain based on clinical, neurophysiological, and pharmacological characteristics (Chang and Heitkemper, 2002) and often characterized by allodynia (pain sensations from stimuli that are not normally painful) and hyperalgesia (extreme sensitivity to pain). Visceral nociceptive C-fibers activated by SP have been implicated as mediators of noxious stimuli (Burnstock, 2000). SP and its neurokinin receptors are ubiquitously expressed in the gastrointestinal and reproductive tracts and represent an endogenous system regulating inflammatory, immune responses, and visceral hypersensitivity (Mowa et al., 2003; Saban et al., 2007). Moreover, SP afferents play an important role in the pathogenesis of IBS (Wang et al., 2006). A marker of nociceptive activation of DRG neurons is the phosphorylation of extracellular signal-regulated kinase (ERK), one of the mitogen-activated protein kinases (MAPKs) involved in the sensitization of neurons in the DRG (Dai et al., 2002; Doya et al., 2005). Phospho-ERK (pERK) increased in DRG neurons involved in transmission of various noxious signals (Dai et al., 2002) and contributed to the allodynia that is common in the patients with functional pain-associated diseases.
The aim of the present study was to test the hypothesis that uterine inflammation leads to an increased expression of SP and pERK in uterine afferents and affects dichotomizing afferents innervating both uterus and colon. Such an increase would be consistent with our model of viscerovisceral convergence at the level of the DRG and may provide basic information regarding the etiology of pelvic pain syndromes.
MATERIALS AND METHODS
Retrograde Labeling
Retrograde labeling was used to identify uterus-specific and colon-specific DRG and sensory neurons innervating both visceral organs, uterus and colon. For colonic afferents, the descending colon of adult female ovariectomized Long-Evans rats (200–250 g) was exposed under deep anesthesia with isoflurane. Fluorogold (FG; 12 µl 5% in 0.9% saline) was injected into the colonic wall at four to seven different sites using a Hamilton syringe (Hamilton Co., Reno, NV) with a 26-gauge needle. The syringe was retracted 0.1 mm and held in this position for 2 min and then fully retracted over 2 min. Injection sites were carefully swabbed, and the colon was extensively rinsed with 0.9% sodium chloride solution and sealed with New Skin to prevent dye leakage. Next, during laparotomy, tetramethylrhodamine (TMR; 6 µl, 1.5% in 0.9% saline) was injected into the uterine wall. In control experiments, rather than injecting into organs, TMR was sprayed on the uterus, FG on colon, and FG and TMR on both organs in the same animal. Immediately after surgery, all animals were injected with sterile saline to prevent dehydration and were given analgesics for pain relief and antibiotics. Animals were allowed to survive for up to 10 days following surgery to allow for maximal transport of retrograde tracer. Rats were perfused with fixative; the bilateral L1–S3 DRG were postfixed overnight in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4; cryoprotected in 30% sucrose for 2 days; and sectioned at 20 µm. Uterus- and colon-specific sections were processed for immunocytochemistry, mounted on glass slides, and coverslipped with Aqua PolyMount (Polysciences, Warrington, PA). The number and percentage of projection neurons to the DRG in each slice were analyzed with Olympus IX 51 microscope equipped for epifluorescence.
Inflammatory Procedures
We used a model of inflammatory uterine pain in the rat. Briefly, 10% allyl isothiocyanate (mustard oil, 0.1 cc) was injected into uterine lumen at the same time when retrograde tracers were injected into the uterus and colon for visceral DRG labeling. To verify inflammation response, we stained histological sections of both uterus and colon for apoptotic nuclei arising from CD45+ leukocytes. After the surgery, the wound was treated with topical antibiotic (polysporin), and buprenorphine was given for analgesia. Antibiotics were used for 5–7 days to minimize bacterial resistance. All procedures were in accordance with the guidance of the NIH and the Charles Drew University of Medicine and Science Policy on Humane Care and Use of Laboratory Animals.
Immunocytochemistry
Twenty-micrometer-thick sections of DRG were treated with phosphate-buffered saline (PBS) containing 1% normal goat serum (NGS) and 0.1% Triton X-100 for 1 hr, then incubated at 4°C for 2 days in a mouse primary antibody against pERK (1:200, Cell Signaling, Danvers, MA) or overnight in a rabbit primary antibody for SP (1:20,000; IncStar, Stillwater, MN) in PBS containing 1% NGS. The sections were washed in PBS and then incubated in goat biotinylated anti-mouse IgG for pERK (1:200; Vector Laboratories, Burlingame, CA) or in goat biotinylated anti-rabbit IgG for SP (1:200; Vector Laboratories) in PBS containing 1% NGS 1 hr at 48C. After several rinses, tissue sections were incubated in streptavidin-horseradish peroxidase (HRP; 1:100; Tyramide Signal Amplification kit; NEN Life Science Products, Boston, MA) for 30 min, then washed and incubated for 5 min in flouroscein-conjugated tyramide (1:50; Tyramide Signal Amplification kit; NEN Life Science Products), then washed in 0.1 M Tris buffer without saline, pH 7.5, and mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). Mounted sections were air dried and coverslipped with Aqua Poly Mount (Polisciences). In parallel, we always run absorption controls to help verify specificity for pERK and SP staining. In the some experiments, the uterus and colon were collected and stained with CD45 as a marker for immunological response to inflammation.
Quantitative and Statistical Analysis of Immunohistochemistry
For quantification of DRG neurons, three to six sections were analyzed from each ganglion from levels L1–S3 levels in each rat. The number of total and immunolabeled neurons per section was counted to determine changes in pERK and SP expression after experimentally induced inflammation of uterus. A neuron was counted only when a distinct nucleus was present (see Fig. 1). A mean of immunolabeled cells was determined for each section and averaged across all the sections studied for a particular DRG. The mean number of immunolabeled cells was expressed as a percentage of retrogradely labeled neurons per total uterus-positive, per colon-positive, and per both uterus- and colon-positive neurons obtained for each animal across the different tissue sections. The numbers of immunolabeled and retrogradely identified neurons were compared by two-way ANOVA (Prism 3.0; GraphPad, San Diego, CA). Differences at the P < 0.05 level were considered statistically significant.
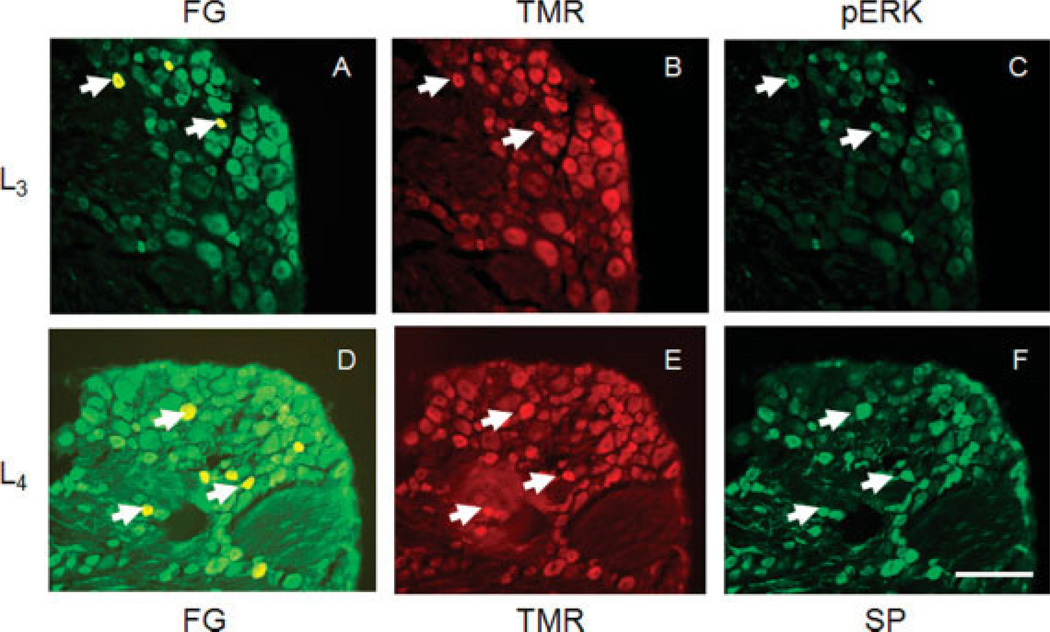
Fig. 1.
Chemically identified DRG neurons that project to the colon and uterus at levels L3 (A–C) and L4 (D–F). A,D: Images of DRGs following injection of the distal colon with Fluorogold at four sites. Arrows point to representative retrogradely labeled neurons that innervate the colon. B,E: DRGs were injected with tetramethylrhodamine (TMR) into the uterine horn. Cells containing red tracer innervate the uterus. Arrows indicate cells that are also labeled after colon injection (i.e., dichotomizing DRG neurons). C: Image of pERK immunoreactivity in the same DRG. Arrows indicate that several of the dichoromizing DRG neurons (innervating the colon and uterus) express pERK immunoreactivity. F: SP immunoreactivity in dichotomizing DRG neurons. Scale bar = 100 µm.
RESULTS
Retrograde Labeling of Visceral DRG Neurons
Neuronal profiles from DRG of seven rats were quantified for each retrograde tracer. Both colonic (identified with FG; Fig. 1A,D) and uterine (identified with TMR; Fig. 1B,E) afferents were localized in L1–S3 DRG. To identify the population of DRG neurons innervating both colon and uterus, DRG were examined from animals in which FG was injected into the colon and TMR into the uterus. Cells that project to the uterus or colon were colocalized in the same DRG, indicating a subpopulation of DRG neurons innervating both uterus and colon. At the L1 level, in total 6.3% neurons were colon specific (colonic), 4.9% were uterus specific (uterine), and 2.7% innervated both organs among a total of 9,385 neurons analyzed. For L2, 5.8% were uterine, 6.1% were colonic, and 2% neurons innervated both organs; for L3, 5.7% uterine, 6.1% colonic, 3% colocalized; for L4, 6.4% uterine, 6.1% colonic, 4.8% colocalized; for L5, 9.1% uterine, 6% colonic and 3.7% colocalizedl for L6, 11.6% uterine, 5.9% colonic, and 3.6% colocalized; for S1, 11.6% uterine, 5.9% colonic, and 2% colocalized; for S2, 14.8% uterine, 5.9% colonic, and 1.7% colocalized; and for S3, 10.9% uterine, 3.9% colonic, and 2% colocalized. The present results confirm previous observations that up to 5% of the lumbosacral DRG neurons dichotomize and innervate both the colon and the uterus (Chaban et al., 2007).
To control for potential dye leakage into the abdominal cavity, we sprayed TMR on uterine horns, FG on colon, and FG and TMR dyes on both organs in the same animal (each procedure was performed on two different rats). Less than 0.5% of cells were labeled with FG, TMR, or both tracers.
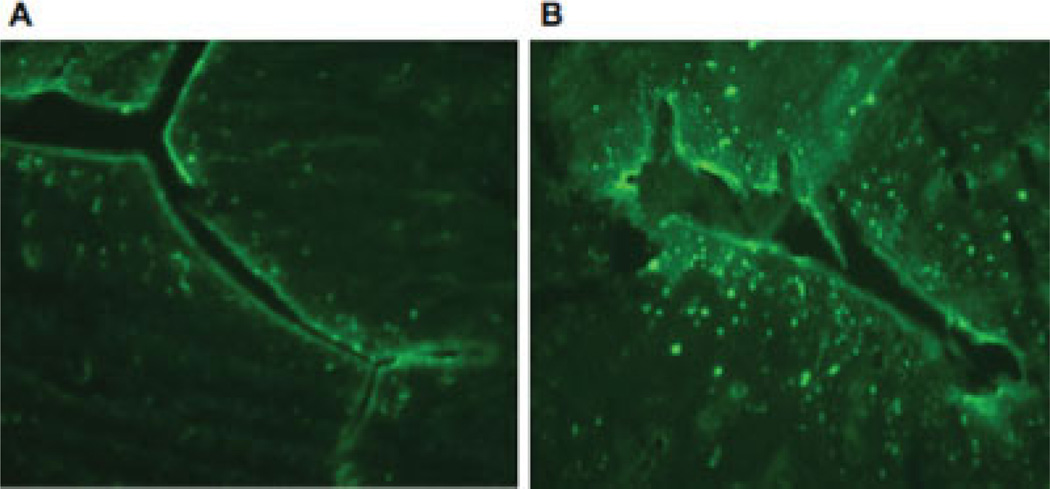
Compared with control animals, in rats after uterine inflammation induced with 10% allyl isothiocyanate, the endometrium was significantly altered, with infiltration of CD45-positive leukocytes (Fig. 2). As expected, histological sections from colon showed no signs of inflammation: there were no CD45+ cells, and the colon wall was intact.
Fig. 2.
Images of CD45 staining in the uterus in control (A) and after allyl isothiocyanate-induced inflammation (B). Animals were killed 5–7 days following the induction of inflammation. The increase in the number of CD45+ leukocytes was confirmed by visual inspection. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Expression of pERK in DRG Neurons After Induced Inflammation of the Uterus
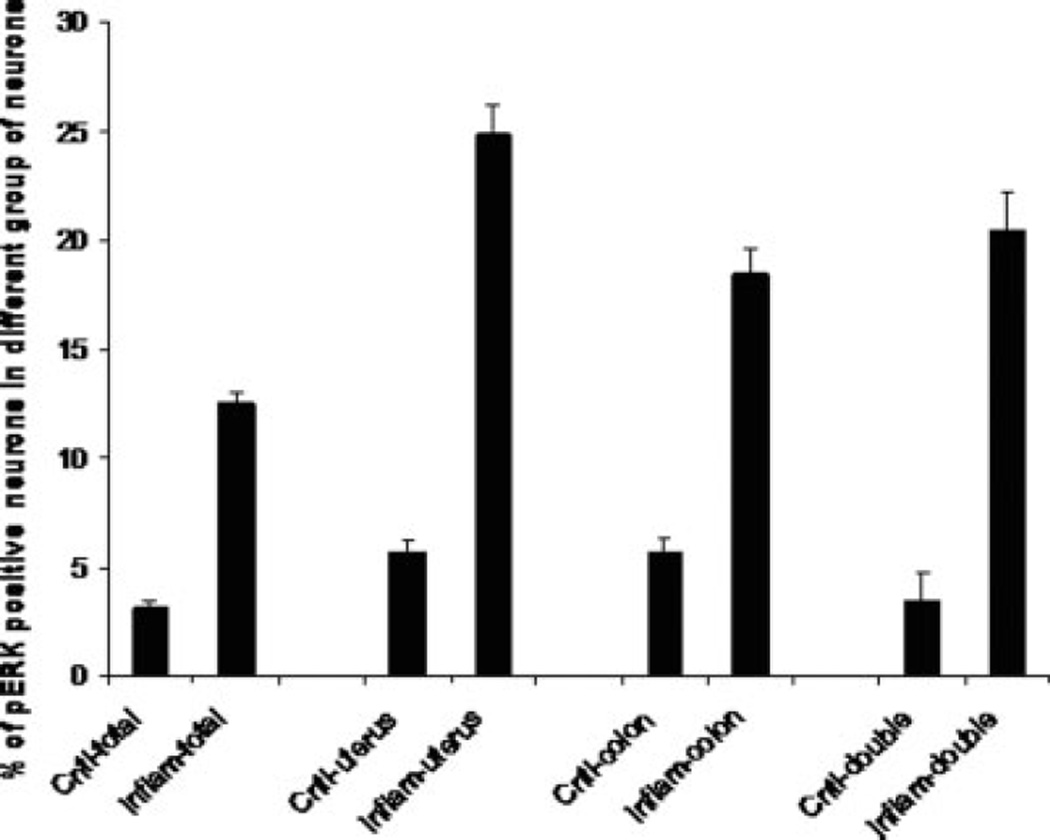
pERK immunohistochemistry was used as a marker of activated DRG neurons. This has been previously shown effectively to label primary afferent neurons that are activated by noxious stimuli (Dai et al., 2002). Under control conditions, 5.7% of DRG neurons innervating uterus, 5.7% innervating colon, and 3.4% innervating both uterus and colon showed pERK immunoreactivity (Fig. 1C). In rats with induced uterine inflammation, the number of pERK-positive DRG neurons was significantly higher (Fig. 3): 24.9% of DRG neurons innervating uterus were pERK immunoreactive, along with 18.5% of neurons innervating the colon and 20.5% of neurons innervating both uterus and colon (P < 0.01 compared with controls). Interestingly, this increase of pERK immunoreactivity was seen in DRG neurons innervating colon, uterus, and both organs. In general, the DRG population of pERK-immunoreactive neurons was not significantly increased (P > 0.05), suggesting that inflammation of uterus significantly increased pERK immunoreactivity only in viscerally labeled neurons.
Fig. 3.
Frequency histograph of pERK immunoreactivity in DRG neurons that innervate the colon or uterus or organs dichotomizing DRG neurons (double) after inflammation with allyl isothiocyanate. All values are means of seven animals ± SEM. ⋆P < 0.05.
Expression of SP in Visceral DRGs After Induced Inflammation of the Uterus
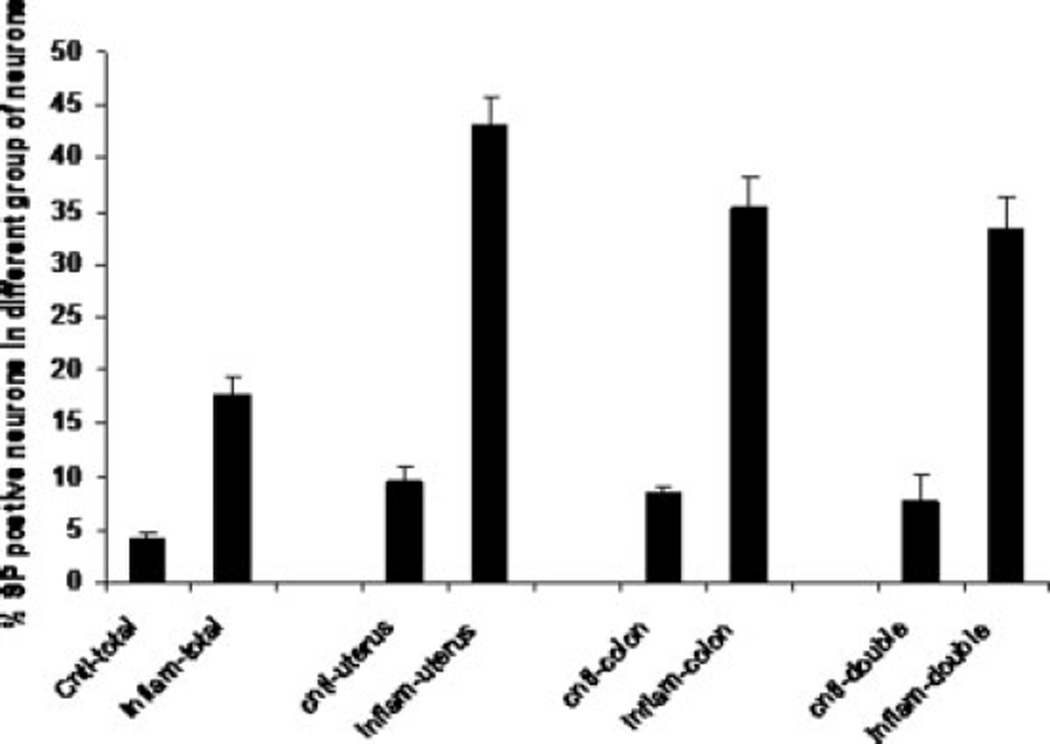
In control rats, 9.4% of neurons innervating uterus were SP immunoreactive, as were 8.4% of neurons innervating colon and 7.6% of neurons innervating both uterus and colon (Fig. 1). After uterine inflammation, expression of SP immunoreactivity in DRG neurons was significantly increased compared with control rats: 43.1% of DRG neurons innervating uterus were now SP immunoreactive, along with 35.4% of neurons innervating colon and 33.3% of neurons innervating both uterus and colon (P < 0.01). As with pERK, the greatest increase in SP immunoreactivity was observed in DRG neurons that innervate both uterus and colon (Fig. 4); however, the increase in number of SP-immunoreactive neurons of noncolonic, nonuterine origin was not significant (P > 0.05).
Fig. 4.
Frequency histograph of SP immunoreactivity in DRG neurons that innervate the colon or uterus or organs dichotomizing DRG neurons (double) after inflammation with allyl isothiocyanate. All values are means of seven animals ± SEM. ⋆P < 0.05.
DISCUSSION
The major finding of this study is that local inflammation in the uterus activates DRG neurons innervating both uterus and colon. Lumbosacral DRG neurons were classified based on their innervations of the uterus, colon, or both using retrograde labeling. Inflammation in the uterus increased the number of neurons expressing pERK or SP immunoreactivity in afferents from these viscera only. Nonretrograde labeled DRG neurons did not have a significant increase in the numbers of pERK-immunoreactive cells. These results suggest that some viscerovisceral convergence is present at the level of primary afferent cell bodies in the DRG. This convergence is not completely explained by the presence of a population of dichotomizing DRG neurons innervating both the uterus and the colon.
Our previous results indicated that sensory information to individual DRG neurons may originate in different viscera (Chaban et al., 2007). Although it is generally accepted that each primary afferent neuron is a single sensory channel transmitting information from one viscus, several studies have recently challenged that view and demonstrated that a population of DRG neurons can innervate two different visceral organs (Malykhina et al., 2006; Chaban et al., 2007; Christianson et al., 2007). These new subsets of dichotomizing neurons appeared to provide a novel pathway for sensitization of one viscus by another.
The present experiments were undertaken to understand the organization of sensory innervations of pelvic viscera that may underlie functional pain syndromes in which the seemingly affected viscus has no frank pathology (e.g., IBS). The pathophysiology of visceral hyperalgesia is less well understood than its cutaneous counterpart, and the mechanism underlying visceral hyperalgesia has been assumed to be similar to that of cutaneous hyperalgesia, which is believed to arise, in part, as a consequence of the sensitization of peripheral nociceptors resulting from long-lasting changes in the excitability of spinal neurons (Mayer and Gebhart, 1994). Peripheral sensitization can develop in response to sustained stimulation, inflammation, and nerve injury through the various nociceptive mediators such as capsaicin, prostaglandin E2, and bradykinin. Our hypothesis, based on these ideas, was that DRG neurons that innervate the uterus and the colon may be preferentially sensitized to inflammation in the uterus. The result would be hyperalgesia to normal colonic functions.
In support of this, a subset of DRG neurons was observed to respond to different nociceptive molecules, indicating that there may be cross-activation of receptors that may underlie the sensitization of visceral nociceptors. Cross-organ pelvic reflexes, as well as cross-organ alterations in physiological functioning and sensation following irritation, suggest the involvement of preexisting dichotomous afferent pathways in the pelvis (Pezzone et al., 2005; Christianson et al., 2007). The existence of convergent sensory neurons receiving afferent information from different viscera was previously found in uterus and colon (Chaban et al., 2007), urinary bladder and colon (Malykhina et al., 2006), urinary bladder and uterus (Winnard et al., 2006), and thoracic and visceral nerves (Cervero et al., 1984). In the present study, dichotomizing neurons innervating both uterus and colon were characterized immunocytochemically for SP and pERK after inducing inflammation in the uterus. These convergent colonic/uterine neurons that receive signals from both pelvic organs make direct neuronal connections between different pelvic domains and may play a key role in developing chronic pelvic pain. In that case, the inflammatory input from the uterus can cross-sensitize the colon through enhanced excitability of convergent sensory neurons.
The present results confirm the existence of dichotomizing DRG neurons and demonstrate that inflammation of the uterus activates DRG neurons that innervate uterus and the uterus and colon. DRG neurons labeled from the colon only also were activated, as evidenced by the increased number of pERK-immunoreactive neurons. These results indicate that, in addition to DRG neurons with a direct physical link to the colon and uterus, DRG neurons innervating the colon but not the uterus were also activated. Such results suggest an intraganglionic cross-talk that may be related to release of nociceptive mediators. The release of both ATP and SP has been reported, but the question of specificity has to be addressed in future experiments, because the general population of DRG neurons was not activated by uterine inflammation.
Female patients with IBS and IC often have another inflammatory pain-associated disorder in the reproductive system, such as endometriosis or dysmenorrhea (Giamberardino, 1999; Chang and Heitkemper, 2002; Heitkemper et al., 2002). Endometriosis with implanted endometrial glands in the peritoneal cavity, predominantly over the uterine serosa, visceral peritoneum, and ovaries and bowel serosa, involves various mediators of inflammation (Del Giudice and Michetti, 2004). The cross-organ effects between pelvic organs induced by inflammation might explain the clinical presentation of functional syndromes and the cooccurence of dysmenorrhea, endometriosis, IBS, and IC. Inflammation results in the production myriad inflammatory mediators, which can directly activate the nociceptors by pERK activation (Dai et al., 2002). This activity-dependent activation of the ERK signal pathway may be useful for identifying DRG neurons involved in transmission of noxious stimuli under pathological conditions associated with inflammation and may be correlated with the functional state of primary afferent neurons (Noguchi et al., 2004). In this study, we found that pERK labeling was significantly increased in DRG from rats after induced inflammation compared with control, uninflamed sensory neurons.
The cross-system viscerovisceral convergence between reproductive and gastrointestinal tracts can be modulated by ovarian hormone, possibly by estradiol during the estrous stage (Winnard et al., 2006). Visceral DRG express estrogen receptors (Papka and Mowa, 2003), and we previously reported estradiol modulation of DRG neuron excitability (Chaban et al., 2003). Moreover, some studies showed that, in the uterus, estrogen may be a proinflammatory factor (Bulun et al., 2000, 2004; Vigano et al., 2003; Lennie, 2004) and enhance pERK that is associated with hyperalgesia (Ji et al., 1999; Dai et al., 2002; Jin et al., 2003; Obata and Noguchi, 2004), but further study is needed to improve our understanding of the estrogen influence on crossorgan sensitization.
The important findings of this study are that we have seen increases of both pERK and SP activation in the convergent double-labeled sensory neurons innervating both uterus and colon after the induced inflammation. Under the conditions of inflammatory-induced reaction in the uterus, SP might be released with induction of nociceptive signaling. Because the pelvic nerve consists of visceral primary sensory fibers from lumbosacral DRG supplying the uterus and colon, the SP release from the inflamed organ during inflammation can excite convergent lumbosacral sensory neurons also innervating another uninflamed visceral organ. Although evidence consistent with the existence of such branching neurons for the different visceral organs has been presented (Malykhina et al., 2006; Winnard et al., 2006), our data provide the first evidence that uterine inflammation can directly affect the colon. Cross-organs effects can be selective, but further in vivo and in vitro studies are required to prove that a peripheral sensory fiber can indeed respond to stimulation of more than one pelvic organ.
Acknowledgments
The authors thank Dr. Y.S. Fu for technical support with histological preparation.
Contract grant sponsor: NIH; Contract grant number: DA 13185; Contract grant number: MD 00545.
REFERENCES
- Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. discussion 435–513. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86:272–280. doi: 10.1016/j.physbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Zeitoun KM, Takayama K, Sasano H. Estrogen biosynthesis in endometriosis: molecular basis and clinical relevance. J Mol Endocrinol. 2000;25:35–42. doi: 10.1677/jme.0.0250035. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Fang Z, Imir G, Gurates B, Tamura M, Yilmaz B, Langoi D, Amin S, Yang S, Deb S. Aromatase and endometriosis. Semin Reprod Med. 2004;22:45–50. doi: 10.1055/s-2004-823026. [DOI] [PubMed] [Google Scholar]
- Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- Cervero F, Connell LA, Lawson SN. Somatic and visceral primary afferents in the lower thoracic dorsal root ganglia of the cat. J Comp Neurol. 1984;228:422–431. doi: 10.1002/cne.902280309. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Chaban V, Christensen A, Wakamatsu M, McDonald M, Rapkin A, McDonald J, Micevych P. The same dorsal root ganglion neurons innervate uterus and colon in the rat. Neuroreport. 2007;18:209–212. doi: 10.1097/WNR.0b013e32801231bf. [DOI] [PubMed] [Google Scholar]
- Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO, Pezzone MA. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128:235–243. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci. 2002;22:7737–7745. doi: 10.1523/JNEUROSCI.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice G, Michetti P. Inflammation, immunity and vaccines for Helicobacter pylori. Helicobacter. 2004;9(Suppl 1):23–28. doi: 10.1111/j.1083-4389.2004.00245.x. [DOI] [PubMed] [Google Scholar]
- Doya H, Ohtori S, Takahashi K, Aoki Y, Ino H, Takahashi Y, Moriya H, Yamashita T. Extracellular signal-regulated kinase mitogen-activated protein kinase activation in the dorsal root ganglion (DRG) and spinal cord after DRG injury in rats. Spine. 2005;30:2252–2256. doi: 10.1097/01.brs.0000182091.53834.08. [DOI] [PubMed] [Google Scholar]
- Giamberardino MA. Recent and forgotten aspects of visceral pain. Eur J Pain. 1999;3:77–92. doi: 10.1053/eujp.1999.0117. [DOI] [PubMed] [Google Scholar]
- Heitkemper M, Jarrett M, Bond E. Irritable bowel syndrome: more than a gut feeling. J Wound Ostomy Continence Nurs. 2002;29:202–209. doi: 10.1067/mjw.2002.125455. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 Mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee OY, Mayer EA, Schmulson M, Chang L, Naliboff B. Gender-related differences in IBS symptoms. Am J Gastroenterol. 2001;96:2184–2193. doi: 10.1111/j.1572-0241.2001.03961.x. [DOI] [PubMed] [Google Scholar]
- Lennie TA. Sex differences in severity of inflammation-induced anorexia and weight loss. Biol Res Nurs. 2004;5:255–264. doi: 10.1177/1099800404263014. [DOI] [PubMed] [Google Scholar]
- Malykhina AP, Qin C, Greenwood-van Meerveld B, Foreman RD, Lupu F, Akbarali HI. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil. 2006;18:936–948. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- McNeill DL, Burden HW. Convergence of sensory processes from the heart and left ulnar nerve onto a single afferent perikaryon: a neuroanatomical study in the rat employing fluorescent tracers. Anat Rec. 1986;214:441–444. 396–447. doi: 10.1002/ar.1092140416. [DOI] [PubMed] [Google Scholar]
- Mowa CN, Usip S, Storey-Workley M, Amann R, Papka R. Substance P in the uterine cervix, dorsal root ganglia and spinal cord during pregnancy and the effect of estrogen on SP synthesis. Peptides. 2003;24:761–771. doi: 10.1016/s0196-9781(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Obata K, Dai Y. Changes in DRG neurons and spinal excitability in neuropathy. Novartis Found Symp. 2004;261:103–110. discussion 110–105, 149–154. [PubMed] [Google Scholar]
- Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–2653. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Papka RE, Mowa CN. Estrogen receptors in the spinal cord, sensory ganglia, and pelvic autonomic ganglia. Int Rev Cytol. 2003;231:91–127. doi: 10.1016/s0074-7696(03)31003-4. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Saban R, Simpson C, Vadigepalli R, Memet S, Dozmorov I, Saban MR. Bladder inflammatory transcriptome in response to tachykinins: neurokinin 1 receptor-dependent genes and transcription regulatory elements. BMC Urol. 2007;7:7. doi: 10.1186/1471-2490-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, de Groat WC. Neurokinins enhance excitability in capsaicin-responsive DRG neurons. Exp Neurol. 2007;205:92–100. doi: 10.1016/j.expneurol.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HB, Inoue A, Iwasa M, Hide I, Nakata Y. Substance P release evoked by capsaicin or potassium from rat cultured dorsal root ganglion neurons is conversely modulated with bradykinin. J Neurochem. 2006;97:1412–1418. doi: 10.1111/j.1471-4159.2006.03830.x. [DOI] [PubMed] [Google Scholar]
- Vigano P, Mangioni S, Odorizzi MP, Chiodini A, Rocca S, Chiodo I. Use of estrogen antagonists and aromatase inhibitors in endometriosis. Curr Opin Invest Drugs. 2003;4:1209–1212. [PubMed] [Google Scholar]
- Wang WF, Yang YS, Peng LH, Sun G. Alternation of substance P-containing neural pathways in a rat model of irritable bowel syndrome with rectal distension. Chin J Dig Dis. 2006;7:211–218. doi: 10.1111/j.1443-9573.2006.00273.x. [DOI] [PubMed] [Google Scholar]
- Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1592–R1601. doi: 10.1152/ajpregu.00455.2006. [DOI] [PubMed] [Google Scholar]