Abstract
Type-2 diabetes and obesity-related metabolic abnormalities are major risk factors for the development of colon cancer. In the present study, we examined the effects of polyol pathway enzyme aldose reductase (AR) inhibitor, fidarestat, on the development of azoxymethane (AOM)-induced colonic premalignant lesions in C57BL/KsJ-db/db obese mice. Our results indicate that fidarestat given in the drinking water caused a significant reduction in the total number of colonic premalignant lesions in the AOM-treated obese mice. Further, the expression levels of PKC-β2, AKT, COX-2 and iNOS in the colonic mucosa of AOM-treated mice were significantly decreased by fidarestat. The serum levels of IL-1α, IP-10, MIG, TNF-α and VEGF are significantly suppressed in AOM + fidarestat treated obese mice. Fidarestat also decreased the expression of COX-2, iNOS, XIAP, survivin, β-catenin and NF-κB in high glucose-treated HT29 colon cancer cells. In conclusion, our results indicate that fidarestat inhibits the development of colonic premalignant lesions in an obesity-related colon cancer and is chemopreventive to colorectal carcinogenesis in obese individuals.
Keywords: Aldose reductase, Colon cancer, Obesity, Oxidative stress and inflammation
Introduction
Colorectal cancer (CRC) is a key health problem worldwide. Several evidences indicate that the risk of CRC is increased in patients with metabolic syndrome, also called insulin resistance syndrome, which is usually associated with obesity and related metabolic defects [1,2]. Obesity is the leading cause of insulin resistance and hyperinsulinemia, which is also a likely risk factor for CRC [3]. Several hypotheses have emerged to explain the influence of obesity on the development of CRC, including insulin resistance, alterations in the insulin-like growth factor (IGF)/IGF-1 receptor axis and adipocytokine imbalance, such as increased leptin levels and decreased adiponectin levels [4–7]. ROS-mediated inflammation induced by excessive production of storage lipids, high circulating glucose levels and pro-inflammatory cytokines produced by adipose tissue plays a critical role in obesity- and diabetes-related colorectal carcinogenesis [4,8–11]. These reports suggest that targeting ROS-induced inflammation may provide an effective strategy for preventing the development of CRC.
Our recent studies in human colon cancer cells, human lens epithelial cells, vascular endothelial cells and vascular smooth muscle cells suggest that the polyol pathway enzyme, aldose reductase (AR), a member of aldo–keto reductase super family, is a regulator of ROS signals induced by growth factors, cytokines, chemokines, lipo-polysaccharide and high glucose (HG) [12–19]. Overexpression of AR has been shown in the tissues of diabetic and obese subjects [20,21]. Further, AR has been shown to be overexpressed in a number of human tumors [22]. In human colon cancer Caco-2 cells, we have shown that inhibition of AR prevents the cytokines- and growth factor-induced COX-2 expression, activation of NF-κB and PGE2 production [12]. Further, our studies have also shown that inhibition of AR prevents tumor growth in nude mice xenografts, and CRC metastasis in mice [16–18]. An early event in the development of colon cancer is the formation of preneoplastic lesions called aberrant crypt foci (ACF).We have also shown that AR inhibition prevents ACF formation in azoxymethane-treated BALB/c mice [19]. Since, obesity and hyperglycemia are the main risk factors for the development of colon cancer, we investigated the role of AR in azoxymethane (AOM)-induced ACF in C57BL/KsJ-db/db (db/db) mice, one of the most widely used animal models for obesity and type 2 diabetes. Our results suggest that AR inhibition prevents AOM-induced ACF formation by decreasing various inflammatory markers. Further, we also investigated in vitro the role of AR inhibition on various inflammatory markers in HG-induced HT29 human colon cancer cells. Our in vitro results also suggest that inhibition of AR prevents the expression of inflammatory markers in HT29 colon cancer cells.
Materials and methods
Materials
4-week-old male C57BL/KsJ-db/db mice purchased from Jackson Laboratories (Bar Harbor, ME) were housed in pathogen-free conditions at the institutional animal care facility with free access to food and water. The animals were maintained in accordance to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and in accordance with the Institution’s ‘Guideline of the Animal Care and Use Committee’. Mice were kept in suspended cages ~10 cm above bedding trays with a 12 h light–dark cycle in the animal facility. Temperature and relative humidity were controlled at 21 °C and 55% respectively. AOM was bought from the Sigma-Aldrich Chemical Company (St. Louis, MO), and fidarestat was obtained as a gift chemical from Livwel Therapeutics, Inc. (USA). Antibodies against COX-2, iNOS, cyclin D1, survivin, XIAP, β-catenin and protein kinase C (PKC) β2, phospho-AKT, total and phospho-NF-κB P65, and GAPDH were obtained from Cell Signal, Inc. All other reagents used were of analytical grade.
AOM-induced colon carcinogenesis and ACF analysis
Approximately 6-week-old db/db mice were divided into three groups with six mice in each group. Mice in groups 2 and 3 were given AOM in sterile saline, at a dose of 10 mg/kg body wt intraperitoneally once a week, for 3 weeks. In group 3, mice were given AR inhibitor, fidarestat (50 mg/kg body wt, in drinking water) after 24 h of first AOM injection and continued for the entire period (10 weeks). Mice in group 1 received equal volume of sterile saline. All mice were euthanized by exposure to CO2 followed by cervical dislocation. The colons were removed, flushed with saline and opened from anus to cecum and fixed flat between two pieces of filter paper in 10% buffered formalin for 24 h. Colons were stained with 0.2% methylene blue dissolved in saline, and the numbers of ACF were counted under the microscope.
Determination of cytokines/chemokines
The levels of cytokines and chemokines in the mice sera were determined by the Milliplex MAG mouse cytokine/chemokine magnetic bead array panel along with Luminex xMAP detection method as per manufacturer’s protocol using a Millipore Multiplex system. The results are expressed as picograms per milliliter.
Immunohistochemistry
For subsequent microscopic evaluation of ACF, the colons were Swiss-rolled and embedded in paraffin. For immunohistochemical (IHC) analyses, serial sections (5 µm) of colon were cut as described before [19]. Briefly, slides were warmed at 60 °C for 1 h and deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Antigen retrieval was performed by boiling slides in 10 mM sodium citrate (pH 6.0) for 10 min followed by blocking peroxidase reaction with 3% H2O2. Subsequently, the sections were rinsed in phosphate-buffered saline twice and incubated with blocking buffer (2% bovine serum albumin, 0.1% Triton X-100 and 2% normal goat serum) overnight at 4 °C. The sections were incubated with primary antibodies against proliferating cell nuclear antigen (PCNA), COX-2, AR, iNOS, cyclin D1, and phospho-NF-κB P65 for 1 h at room temperature. Antigen–antibody binding was detected by using DakoCytomation LSAB System-HRP kit. Sections were examined by bright-field light microscopy (EPI-800 microscope; Nikon, Tokyo, Japan) and photographed with a camera (Nikon) fitted to the microscope. Photomicrographs of the stained sections were acquired using an EPI-800 microscope (bright-field) connected to a Nikon camera.
Western blot analysis
Colon extracts were prepared in radio immunoprecipitation assay (RIPA) cell lysis buffer and an equal amount of protein was separated on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, electroblotted on nitrocellulose membranes and probed with specific antibodies against COX-2, iNOS, cyclin D1, survivin, β-catenin and protein kinase C (PKC) β2, phospho-AKT, total and phospho-NF-κB P65, and GAPDH. Antibody binding was detected by enhanced pico chemiluminescence (Pierce, Rockford, IL).
In vitro studies
HT29 human colon cancer cells were obtained from the American Type Culture Collection. Cells were maintained and grown in McCoy’s 5A medium supplemented with 10% FBS and 1% penicillin/streptomycin. Non-malignant primary human aortic endothelial cells (HAEC) were obtained from Cell Systems, Kirkland, WA. For treatment, cells were growth-arrested in 0.1% FBS in the presence or absence of AR inhibitor, fidarestat (10 µM) for 24 h, followed by stimulation with high glucose (HG) 25 mM for another 48 h. For HG stimulation, the medium was replaced with fresh medium containing 25 mM glucose (added 19.5 mM glucose to McCoy’s 5A medium that already contained 5.5 mM glucose) in the absence and presence of fidarestat (10 µM).
Statistical analysis
Data presented as mean ± SE and P-values were determined by unpaired Student’s t test using Microsoft Office Excel 2007 software. P < 0.05 was considered as statistically significant.
Results
Inhibition of AR prevents AOM-induced ACF formation in db/db mice
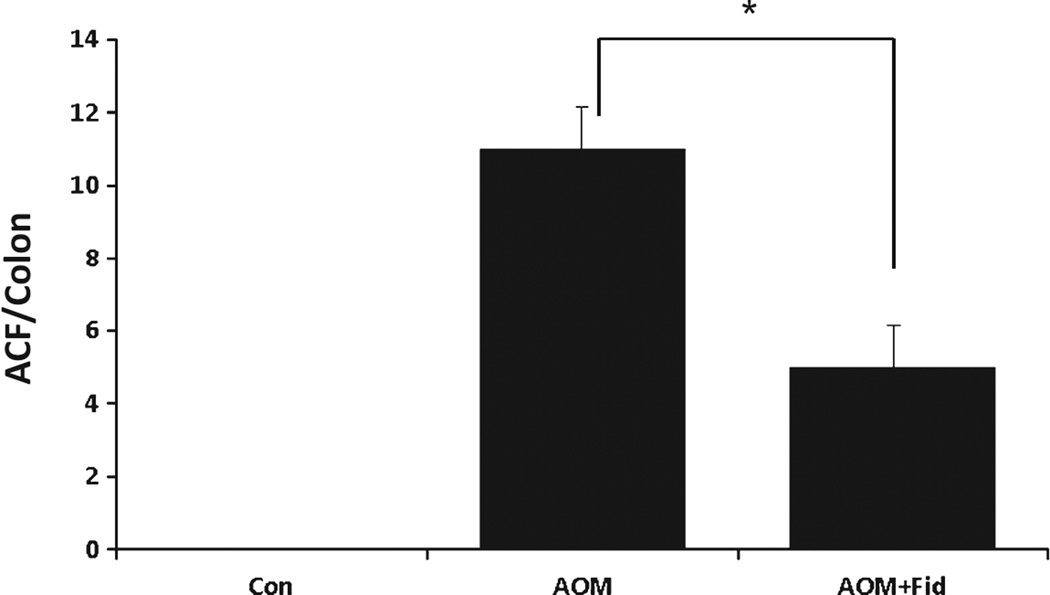
Since obesity and diabetes are major risk factors for colon cancer and AR is overexpressed during diabetic conditions [20,21], we investigated the effect of an AR inhibitor, fidarestat on AOM-induced ACF formation in db/db mice. The body weights of AOM and vehicle or AR inhibitor-treated groups were comparable and no significant changes were observed throughout the study (data not shown). At the early preneoplastic stage (10 weeks after first AOM injection), mice were killed and their colons were removed and analyzed microscopically for the presence of ACF. ACF were distinguished from the surrounding normal crypts by increased thickening of the crypt walls and aberrant change in the shape of the crypt lumen as discussed earlier [19]. In db/db mice, AOM treatment significantly induced the formation of ACF (mean number ± SD of ACF per colon; 11 ± 1.1) (Fig. 1). No evidence of ACF was observed in the vehicle-treated control animals. Administration of fidarestat to AOM mice significantly (P = 0.0026) suppressed the formation of ACF (5 ± 1.1), indicating that inhibition of AR prevents preneoplastic lesion formation in db/db mice.
Fig. 1.
Inhibition of AR prevents AOM-induced ACF formation in db/db mice. Mice were divided into three groups: (i) control; (ii) AOM (10 mg/kg body wt, weekly for 3 weeks) and (iii) received fidarestat (50 mg/kg body wt, in drinking water) throughout the study after 24 h of first AOM injection. Mice were euthanized 10 weeks after first AOM injection and colons were stained and examined for ACF formation. Data represent means ± SD (n = 6) from the various experimental groups. *P = 0.0026, AOM alone vs AOM + Fid. Con, control; AOM, azoxymethane; AOM + Fid, azoxymethane + fidarestat.
Inhibition of AR decreases the levels of inflammatory cytokines, chemokines, and growth actors in AOM-treated db/db mice
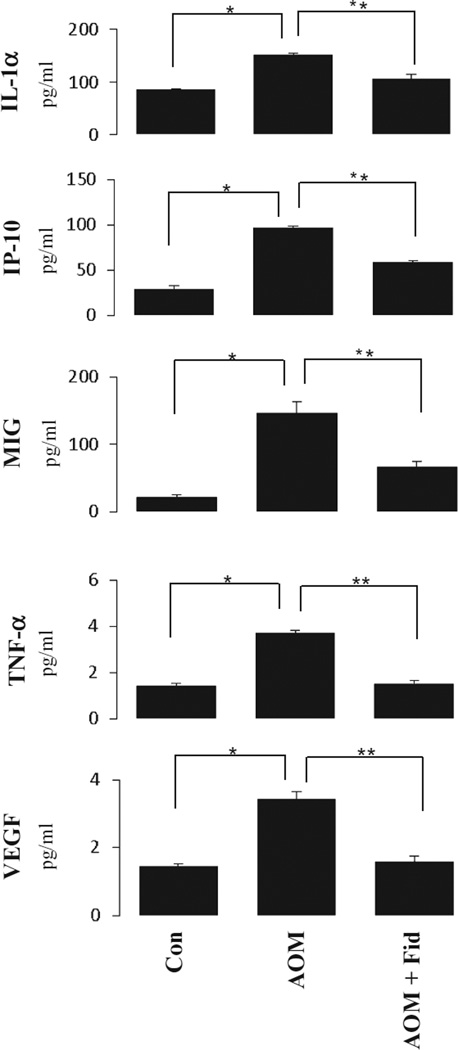
Various cytokines, chemokines, and growth factors play an important role in various inflammatory diseases such as obesity, diabetes and cancer. Therefore, we next determined the levels of various cytokines, chemokines, and growth factors in db/db mice by using a magnetic bead array-based mouse-specific inflammation kit (Millipore, Billerica, MA) that measures 25 inflammatory marker proteins from a single sample. The results were analyzed by Luminex xPONENT software (Luminex, Austin, TX) and expressed as pg/ml. As shown in Fig. 2, a significant increase in the levels of inflammatory proteins, such as IL-1a, IP-10, MIG, TNF-α, and VEGF, was observed in the AOM-treated group (*P < 0.05). However, administration of AR inhibitor significantly (P < 0.05) suppressed the levels of cytokines, chemokines and growth factors in the sera of AOM-treated db/db mice.
Fig. 2.
Inhibition of AR suppressed AOM-induced increase in cytokines and chemokines in sera of db/db mice. Milliplex MAG mouse cytokine/chemokine magnetic bead panel along with Luminex xMAP detection method was used to determine cytokines and chemokines. Results are expressed as the mean ± SD (n = 6); *P < 0.05, Con vs AOM alone; **P < 0.05, AOM alone vs AOM + Fid. Con, control; AOM, azoxymethane; AOM + Fid, azoxymethane + fidarestat.
Inhibition of AR prevents AOM-induced inflammatory markers expression in db/db mice
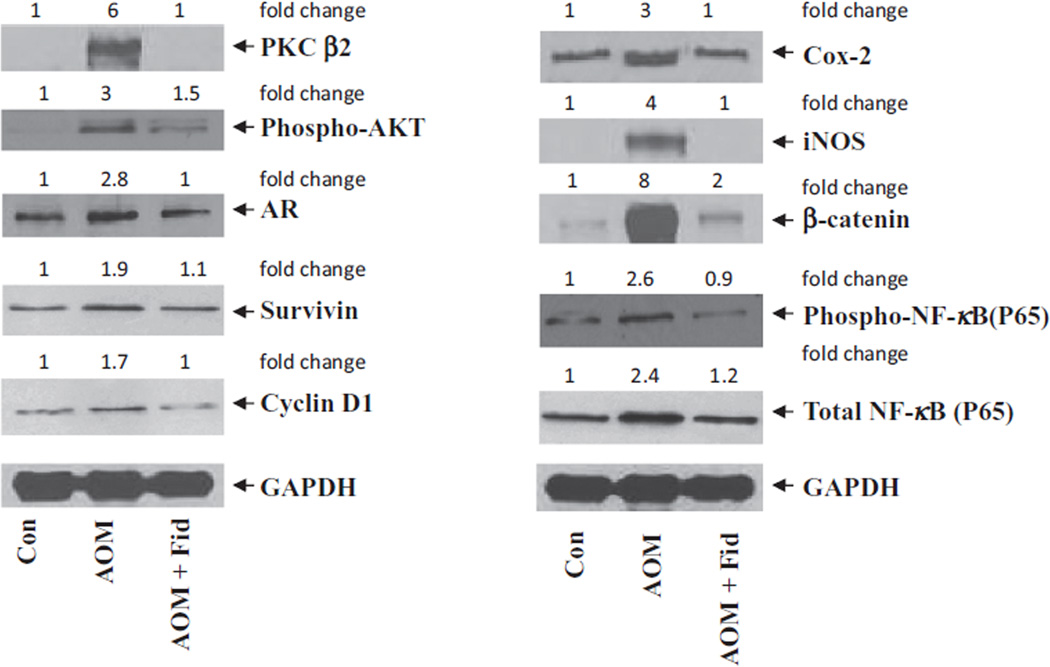
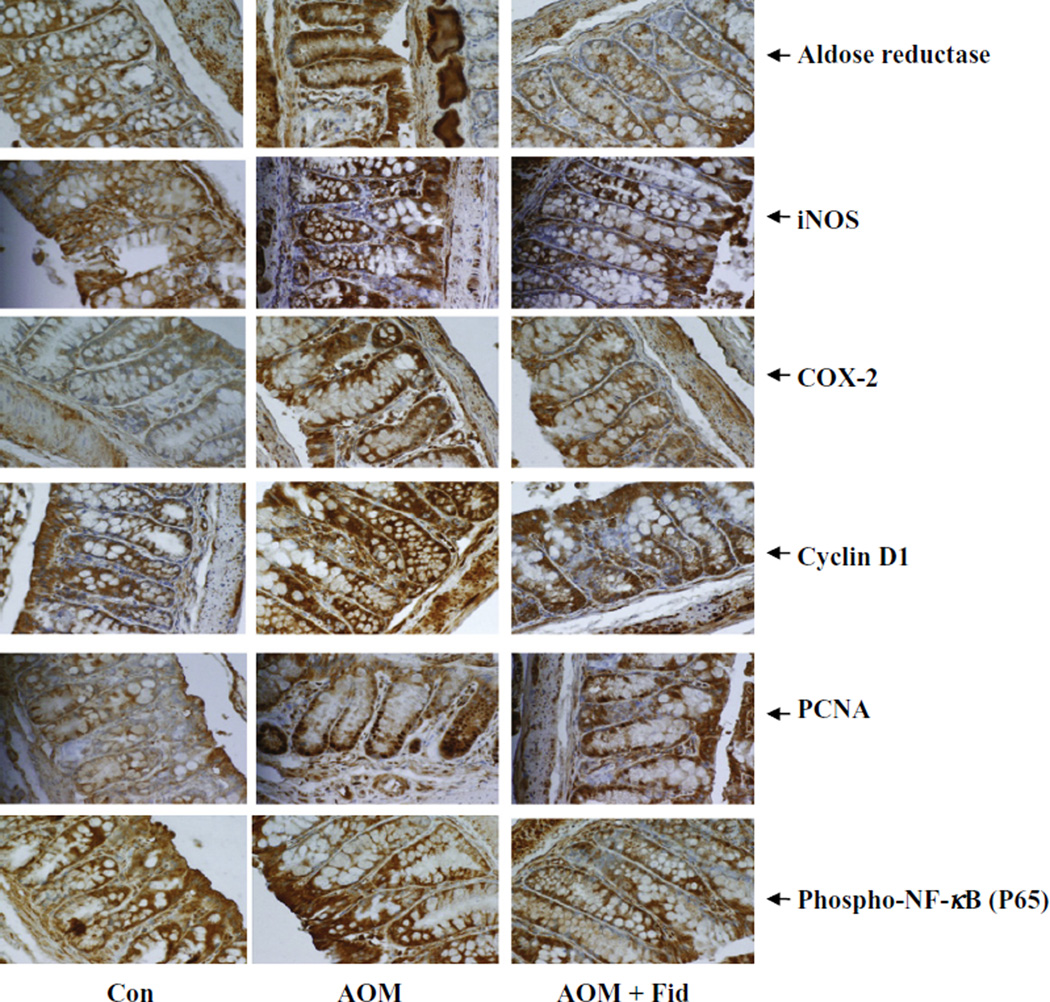
Both COX-2 and iNOS are known to be involved in chronic inflammation, which creates a microenvironment contributing to the development of pre-neoplastic lesions in the colon carcinogenesis, and inhibitors of COX-2 and iNOS have been shown to reduce ACF formation in rodents [23–25]. Therefore, we next investigated if inhibition of AR could prevent AOM-induced COX-2 and iNOS expression in db/db mice colons. As shown in Fig. 3, AOM-induced expression of COX-2 and iNOS was significantly prevented by AR inhibition. These results were further confirmed by immunohistochemistry using antibodies against COX-2 and iNOS (Fig. 4). The colon from AOM-treated mice showed a significant intensity of antibody staining, whereas treatment of mice with fidarestat showed a marked decrease in antibody staining, suggesting that inhibition of AR prevented COX-2 and iNOS expression.
Fig. 3.
Inhibition of AR prevents expression of inflammatory markers in AOM-treated db/db mice. Ten weeks after first AOM injection, colons were removed and homogenized. Equal amounts of cell lysates were subjected to Western blot analysis using antibodies against AR, survivin, cyclin D1, COX-2, iNOS, β-catenin, PKC β2, phospho-AKT, total and phospho-NF-κB and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The same blots were stripped and reprobed with GAPDH antibody to verify equal protein loading. Western blot analysis was performed using equal amounts of proteins from each group and representative blots are shown (n = 3). The fold-changes were calculated after densitometry and normalized with the loading control. *Con, control; AOM, azoxymethane; AOM + Fid, azoxymethane + fidarestat.
Fig. 4.
Inhibition of AR prevents the expression of inflammatory and preneoplastic markers in AOM-treated db/db mice. Colon sections (n = 6) were stained with antibodies against COX-2, iNOS, AR, cyclin D1, PCNA and phospho-NF-κB. Immunoreactivity of the antibody was assessed by quantifying the dark brown stain in the cells, whereas the non-reactive areas displayed only the background color. Photomicrographs of the stained sections were acquired using an EPI-800 microscope (bright-field) connected to a Nikon camera (×400 magnification). Con, control; AOM, azoxymethane; AOM + Fid, azoxymethane + fidarestat. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Inhibition of AR prevents AOM-induced expression of AKT and PKC β2, and expression of AR in db/db mice
Carcinogen-induced pre-neoplastic lesions in the colonic epithelium are known to be associated with the overexpression of PKC β2 and AKT that could cause hyperproliferation [26–28]. We therefore examined the effect of AR inhibition on the expression of PKC β2 and AKT in AOM-induced db/db mice colons. A dramatic increase in PKC β2 expression and AKT phosphorylation was observed in the AOM-treated mice, whereas inhibition of AR in AOM-treated mice showed a significant decrease in the expression of PKC β2 and phosphorylation of AKT (Fig. 3). Since AR is an oxidative stress response protein that is elevated in various pathological conditions including cancer [20,21,29,30], we next examined the expression of AR in AOM-treated mice colons. As shown in Fig. 4, IHC staining of colon sections with anti-AR antibodies confirmed the increased expression of AR in AOM-treated mice as evidenced by a strong staining of AR in colon epithelium compared with colon from untreated controls. These results suggest that inhibition of AR prevents AOM-induced expression of PKC β2, AKT, COX-2 and iNOS, which could be considered as major factors in the development of ACF.
Inhibition of AR prevents AOM-induced expression of cyclin D1, survivin, NF-κB p65 and β-catenin in db/db mice
We next measured the expression of key colon cancer-related markers such as cyclin D1, survivin, β-catenin, total and phospho-NF-κB P65 in mice colon. Cyclin D1 is an important cell cycle-regulated protein involved in G1–S phase transition. This cyclin is overexpressed in the presence of growth factors and carcinogens and causes epithelial cell proliferation in various malignancies, including colon cancer [31]. Survivin and PCNA proteins serve as marker for survival and proliferation and are overexpressed in various cancers [32–36]. β-Catenin is involved in Wnt-signaling pathway. During normal conditions, β-Catenin binds to axin–GSK-3–APC complex and undergoes proteolytic degradation, whereas during carcinogenesis β-catenin stabilizes in the cytoplasm by deregulated Wnt-signaling pathway and translocates into the nucleus and interacts with T-cell factor/lymphoid enhancer factor family of transcription factors to promote the expression of various oncogenes [37,38]. A significant increase in the expression of AR, cyclin D1, survivin, β-catenin, total and phospho-NF-κB P65 was observed in the AOM-treated mice, whereas inhibition of AR in AOM-treated mice showed a significant decrease in the expression of these proteins (Figs. 3 and 4). IHC examination of PCNA, cyclin D1 and phospho-NF-κB P65 indicates that the abundance of these proteins was markedly elevated when mice were treated with AOM alone as evidenced by dark brown staining using antibodies (Fig. 4). Inhibition of AR by fidarestat resulted in lower levels of AOM-induced expression of cyclin D1, survivin, PCNA, β-catenin, and NF-κB P65, indicating that AR regulates the expression of these proteins.
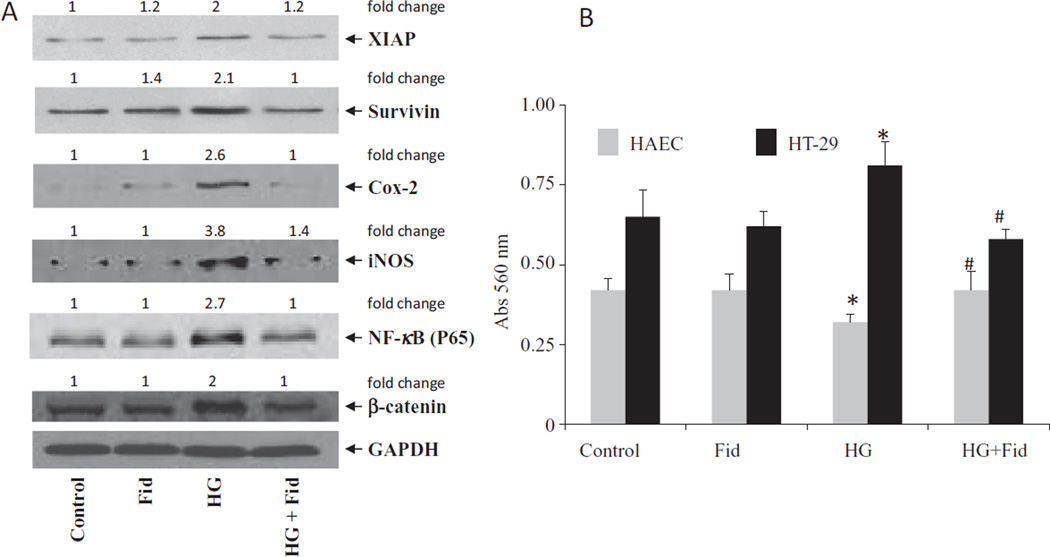
Inhibition of AR prevents the expression of COX-2, iNOS, XIAP, survivin, beta-catenin and NF-κB in high glucose-induced HT 29 human colon cancer cells
To further confirm our in vivo findings, we examined the effect of inhibition of AR on the expression of COX-2, iNOS, XIAP, survivin, β-catenin and NF-κB in HG-induced HT29 human colon cancer cells. Results shown in Fig. 5 indicate that HG caused increased expression of COX-2, iNOS, XIAP, survivin, beta-catenin and NF-κB in HT29 colon cancer cells and AR inhibitor, fidarestat, significantly prevented it (Fig. 5A). Further, our results shown in Fig. 5B indicate that fidarestat did not cause any cytotoxicity in the non-malignant HAECs when treated alone. However, fidarestat prevents HG-induced variations in the cell viability of both malignant and non-malignant cells. Thus, our results from in vivo and in vitro experiments suggest that AR inhibition prevents diabetes-induced colon cancer by decreasing the expression of various inflammatory markers.
Fig. 5.
(A) Inhibition of AR prevents the expression of inflammatory and preneoplastic markers in HG-induced HT29 human colon cancer cells. HT29 cells were growth-arrested in 0.1% FBS in the presence or absence of AR inhibitor, fidarestat (10 µM) for 24 h, followed by stimulation with HG for another 48 h. For HG stimulation, the medium was replaced with fresh medium containing 25 mM glucose (added 19.5 mM glucose to McCoy’s 5A medium that already contained 5.5 mM glucose) in the absence and presence of fidarestat. Expressions of XIAP, survivin, COX-2, iNOS, β-catenin, NF-κB and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were determined by using Western blot. Western blot analysis was performed using equal amounts of proteins from each group and representative blots are shown (n = 3). The fold-changes were calculated after densitometry and normalized with the loading control. (B) HAEC and HT29 cells were treated with fidarestat ± HG for 24 and cell viability was determined by MTT assay. Data are mean ± SD (n = 6) *P < 0.001 vs control and #p < 0.001 Vs HG. Con, control; Fid, fidarestat; AOM, azoxymethane; AOM+ Fid, azoxymethane + fidarestat.
Discussion
We have shown earlier that AR inhibition prevents the formation of ACF, preneoplatic lesions of colon cancer in AOM-induced BALB/c mice [19]. Since obesity is a known cause of metabolic syndrome which includes type 2 diabetes, hypertension, and dyslipidemia and obesity-induced diabetes which could result in chronic inflammation which are known to influence the risk of CRC development [1–6,9,10], we have investigated the effect of AR inhibition on ACF formation in AOM-induced db/db mice model. Our demonstration that AR inhibition prevents the formation of ACF in db/db mice is comparable with other studies in which curcumin, renin–angiotensin system inhibitors, supplementation with branched-chain amino acids, dietary flavonoids show the decreased ACF formation in db/db mice [8,11,39–41].
Chronic inflammation, which could lead to preneoplastic lesion and then colon cancer, is mainly mediated by increased inflammatory proteins such as cytokines, chemokines and growth factors [1–7,9–16]. Therefore, we measured the inflammatory proteins in the sera of AOM-induced db/db mice. Our results show that AR inhibition significantly decreased the expression of IL-1a, IP-10, MIG, TNF-α, and VEGF in mice. The expression levels of COX-2 and iNOS, which represent an early response to proinflammatory mediators and a critical target for CRC chemoprevention, are also inhibited in the colonic mucosa of mice treated with AR inhibitor. Inhibition of these inflammatory markers could be the reason for decreased ACF formation in AR inhibitor-treated mice.
Further, increase in the levels of inflammatory markers such as COX-2 and iNOS is mediated by NF-κB in various diseases including cancer. We therefore investigated the effect of AR inhibition on the activation and expression of NF-κB. Our results indicate that activation and expression of NF-κB is significantly prevented by AR inhibition in db/db mice. In addition, AR inhibition also decreased the expression of cyclin D1, survivin, and β-catenin in the colonic mucosa of AOM-treated db/db mice. These findings are consistent with the previous study which showed that curcumin inhibited the induction of COX-2 by TNF-α stimulus via the inhibition of NF-κB activation in human colon epithelial cells [42]. Therefore, overexpression of inflammatory mediators, especially IL-1a, IP-10, MIG, TNF-α, VEGF and COX-2, which are relevant to excessive storage of lipids, could represent critical targets of AR inhibition to prevent the development of obesity-related CRC.
Since the activation of transcription factor, NF-κB depends on its upstream targets such as PKC β2 and AKT, we determined the effect of AR inhibition on the expression of PKC β2 and AKT phosphorylation colonic mucosa. Our findings indicated that AR inhibition prevented the increased expression of PKC β2 and AKT phosphorylation in the colonic mucosa of AOM-treated db/db mice. Similarly, AR inhibition also decreased the expression of survivin, cyclin D1, COX-2, iNOS, β-catenin, and NF-κB, thereby preventing ACF formation in db/db mice.
To further confirm our in vivo findings, we investigated the effect of AR inhibition on HG-induced HT29 human colon cancer cells. Our results showed that HG increased the levels of XIAP, COX-2, iNOS, β-catenin, and NF-κB, and treatment of HT29 cells with AR inhibitor significantly prevented the expression of these proteins. Thus our results with in vivo and in vitro studies suggest that AR inhibition prevents diabetes-induced colon cancer by decreasing various inflammatory markers.
In conclusion, obesity and obesity-related metabolic abnormalities represent serious global health care problems and CRC is one of the representative malignancies associated with excessive body weight and related metabolic defects. Therefore, the prevention of CRC by targeting the dysregulation of energy homeostasis, which includes chronic inflammation, could be a promising strategy for treating obese individuals who are at an increased risk of developing CRC. AR inhibitor, fidarestat which has been shown to express various chemopreventive and anticancer properties, appears to be a potentially effective candidate for prevention of the development of colonic precancerous lesions in db/db mice. Since fidarestat has already undergone Phase-II (in the USA) and Phase-III (in Japan) clinical trials and has been found to be safe for human use without any major side effects [43], this drug could be readily developed for the prevention of colon cancer.
Acknowledgments
Supported by NIH grant CA129383 to SKS.
Footnotes
Conflict of interest
All the authors declare no conflict of interest.
References
- 1.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 2.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55:285–291. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang CK, Ulrich CM. Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologia. 2003;46:595–607. doi: 10.1007/s00125-003-1109-5. [DOI] [PubMed] [Google Scholar]
- 4.Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J. Nutr. Biochem. 2006;17:145–156. doi: 10.1016/j.jnutbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes. Rev. 2009;10:610–616. doi: 10.1111/j.1467-789X.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 6.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J. Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 7.Otake S, Takeda H, Suzuki Y, Fukui T, Watanabe S, Ishihama K, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin. Cancer Res. 2005;11:3642–3646. doi: 10.1158/1078-0432.CCR-04-1868. [DOI] [PubMed] [Google Scholar]
- 8.Kubota M, Shimizu M, Sakai H, Yasuda Y, Ohno T, Kochi T, et al. Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem. Biophys. Res. Commun. 2011;410:108–113. doi: 10.1016/j.bbrc.2011.05.115. [DOI] [PubMed] [Google Scholar]
- 9.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda Y, Shimizu M, Shirakami Y, Sakai H, Kubota M, Hata K, et al. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci. 2010;101:1701–1707. doi: 10.1111/j.1349-7006.2010.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–9713. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- 13.Tammali R, Saxena A, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents hypoxia-induced increase in hypoxia-inducible factor-1alpha (HIF-1alpha) and vascular endothelial growth factor (VEGF) by regulating 26 S proteasome-mediated protein degradation in human colon cancer cells. J. Biol. Chem. 2011;286:24089–24100. doi: 10.1074/jbc.M111.219733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramana KV, Srivastava SK. Aldose reductase: a novel therapeutic target for inflammatory pathologies. Int. J. Biochem. Cell Biol. 2010;42:17–20. doi: 10.1016/j.biocel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramana KV, Tammali R, Srivastava SK. Inhibition of aldose reductase prevents growth factor-induced G1-S phase transition through the AKT/phosphoinositide 3-kinase/E2F-1 pathway in human colon cancer cells. Mol. Cancer Ther. 2010;9:813–824. doi: 10.1158/1535-7163.MCT-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena A, Tammali R, Ramana KV, Srivastava SK. Aldose reductase inhibition prevents colon cancer growth by restoring phosphatase and tensin homolog through modulation of miR-21 and FOXO3a. Antioxid. Redox Signal. 2013;18:1249–1262. doi: 10.1089/ars.2012.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena A, Shoeb M, Ramana KV, Srivastava SK. Aldose reductase inhibition suppresses colon cancer cell viability by modulating miR-21 mediated PDCD4 expression. Eur. J. Cancer. 2013;49:3311–3319. doi: 10.1016/j.ejca.2013.05.031. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tammali R, Reddy AB, Saxena A, Rychahou PG, Evers BM, Qiu S, et al. Inhibition of aldose reductase prevents colon cancer metastasis. Carcinogenesis. 2011;32:1259–1267. doi: 10.1093/carcin/bgr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tammali R, Reddy AB, Ramana KV, Petrash JM, Srivastava SK. Aldose reductase deficiency in mice prevents azoxymethane-induced colonic preneoplastic aberrant crypt foci formation. Carcinogenesis. 2009;30:799–807. doi: 10.1093/carcin/bgn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr. Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 21.Kador PF. The role of aldose reductase in the development of diabetic complications. Med. Res. Rev. 1988;8:325–352. doi: 10.1002/med.2610080302. [DOI] [PubMed] [Google Scholar]
- 22.Laffin B, Petrash JM. Expression of the aldo-ketoreductases AKR1B1 and AKR1B10 in human cancers. Front. Pharmacol. 2012;3:104. doi: 10.3389/fphar.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pretlow TP, Barrow BJ, Ashton WS, O’Riordan MA, Pretlow TG, Jurcisek JA, et al. Aberrant crypts: putative preneoplastic foci in human colonic mucosa. Cancer Res. 1991;51:1564–1567. [PubMed] [Google Scholar]
- 24.Jackson L, Evers BM. Chronic inflammation and pathogenesis of GI and pancreatic cancers. Cancer Treat. Res. 2006;130:39–65. doi: 10.1007/0-387-26283-0_2. [DOI] [PubMed] [Google Scholar]
- 25.Rao CV, Indranie C, Simi B, Manning PT, Connor JR, Reddy BS. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–170. [PubMed] [Google Scholar]
- 26.Murray NR, Davidson LA, Chapkin RS, Clay Gustafson W, Schattenberg DG, Fields AP. Overexpression of protein kinase C betaII induces colonic hyperproliferation and increased sensitivity to colon carcinogenesis. J. Cell Biol. 1999;145:699–711. doi: 10.1083/jcb.145.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gökmen-Polar Y, Murray NR, Velasco MA, Gatalica Z, Fields AP. Elevated protein kinase C betaII is an early promotive event in colon carcinogenesis. Cancer Res. 2001;61:1375–1381. [PubMed] [Google Scholar]
- 28.Rajamanickam S, Kaur M, Velmurugan B, Singh RP, Agarwal R. Silibinin suppresses spontaneous tumorigenesis in APC min/+ mouse model by modulating beta-catenin pathway. Pharm. Res. 2009;26:2558–2567. doi: 10.1007/s11095-009-9968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J. Biol. Chem. 2002;277:32063–32070. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- 30.Saraswat M, Mrudula T, Kumar PU, Suneetha A, Rao TS, Srinivasulu M, et al. Overexpression of aldose reductase in human cancer tissues. Med. Sci. Monit. 2006;12:CR525–CR529. [PubMed] [Google Scholar]
- 31.Arber N, Hibshoosh H, Moss SF, Sutter T, Zhang Y, Begg M, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–674. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- 32.Chen WC, Liu Q, Fu JX, Kang SY. Expression of survivin and its significance in colorectal cancer. World J. Gastroenterol. 2004;10:2886–2889. doi: 10.3748/wjg.v10.i19.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Luo H, Wang A. Expression of survivin and correlation with PCNA in osteosarcoma. J. Surg. Oncol. 2006;93:578–584. doi: 10.1002/jso.20507. [DOI] [PubMed] [Google Scholar]
- 34.Erkanli S, Kayaselcuk F, Kuscu E, Bagis T, Bolat F, Haberal A, et al. Expression of survivin, PTEN and p27 in normal, hyperplastic, and carcinomatous endometrium. Int. J. Gynecol. Cancer. 2006;16:1412–1418. doi: 10.1111/j.1525-1438.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- 35.Ou JM, Qui MK, Dai YX, Dong Q, Shen J, Dong P, et al. Combined blockade of AKT/mTOR pathway inhibits growth of human hemangioma via downregulation of proliferating cell nuclear antigen. Int. J. Immunopathol. Pharmacol. 2012;25:945–953. doi: 10.1177/039463201202500412. [DOI] [PubMed] [Google Scholar]
- 36.Tan Z, Wortman M, Dillehay KL, Seibel WL, Evelyn CR, Smith SJ, et al. Small-molecule targeting of proliferating cell nuclear antigen chromatin association inhibits tumor cell growth. Mol. Pharmacol. 2012;81:811–819. doi: 10.1124/mol.112.077735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21:1319–1327. [PubMed] [Google Scholar]
- 38.Nath N, Kashfi K, Chen J, Rigas B. Nitric oxide-donating aspirin inhibits beta-catenin/T cell factor (TCF) signaling in SW480 colon cancer cells by disrupting the nuclear beta-catenin-TCF association. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12584–12589. doi: 10.1073/pnas.2134840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubota M, Shimizu M, Sakai H, Yasuda Y, Terakura D, Baba A, et al. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr. Cancer. 2012;64:72–79. doi: 10.1080/01635581.2012.630554. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu M, Shirakami Y, Iwasa J, Shiraki M, Yasuda Y, Hata K, et al. Supplementation with branched-chain amino acids inhibits azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Clin. Cancer Res. 2009;15:3068–3075. doi: 10.1158/1078-0432.CCR-08-2093. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto S, Yasui Y, Ohigashi H, Tanaka T, Murakami A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem. Biol. Interact. 2010;183:276–283. doi: 10.1016/j.cbi.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 43.Hotta N, Toyota T, Matsuoka K, Shigeta Y, Kikkawa R, Kaneko T, et al. SNK-860 Diabetic Neuropathy Study Group. Clinical efficacy of fidarestat, a novel aldose reductase inhibitor, for diabetic peripheral neuropathy: a 52-week multicenter placebo-controlled double-blind parallel group study. Diabetes Care. 2001;24:1776–1782. doi: 10.2337/diacare.24.10.1776. [DOI] [PubMed] [Google Scholar]