Abstract
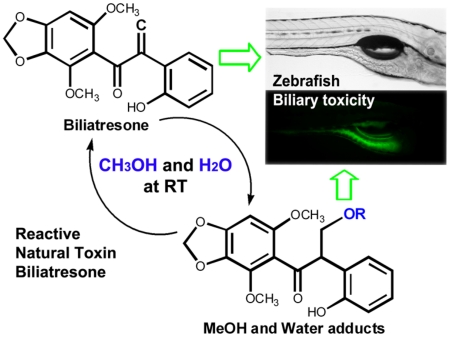
We identified a reactive natural toxin, biliatresone, from Dysphania glomulifera and D. littoralis collected in Australia that produces extrahepatic biliary atresia in a zebrafish model. Three additional isoflavonoids, including the known isoflavone betavulgarin, were also isolated. Biliatresone is in the very rare 1,2-diaryl-2-propenone class of isoflavonoids. The α-methylene of the 1,2-diaryl-2-propenone of biliatresone spontaneously reacts via Michael addition in the formation of water and methanol adducts. The lethal dose of biliatresone in a zebrafish assay was 1 μg/mL, while the lethal dose of synthetic 1,2-diaryl-2-propen-1-one was 5 μg/mL, suggesting 1,2-diaryl-2-propenone as the toxic Michael acceptor.
Outbreaks of biliary atresia in neonatal Australian livestock (1964, 1988, 2007, and 2013) affected 14–100% of newborn animals (primarily sheep but in one case cows) but no adults.1 Biliary atresia is an obliterative fibrosing disorder that affects the extrahepatic biliary tree and, possibly as a consequence of obstruction, leads to liver fibrosis and cirrhosis. The years had in common (i) severe drought; (ii) grazing of pregnant animals on land normally covered by water; and (iii) the presence of Dysphania glomulifera ssp. glomulifera (Nees) Paul G. Wilson and, in some cases, D. littoralis R. Br. in the flora of these pastures.2 In order to identify a causative toxin, we harvested a mixture of these two species from the pasture associated with the 2007 outbreak (Figure S1, Supporting Information). Members of the genus Dysphania are perennial plants; D. glomulifera and D. littoralis are endemic to Australia.3 An epidemiologic study implicated D. glomulifera as a risk factor in a case of sudden death of 40 cows by acute cyanide poisoning.4 The assessment of cyanide poisoning was established from the cyanogenic potential of the residual plant in the rumen fluid, although HCN and cyanogenic compounds were not directly detected.
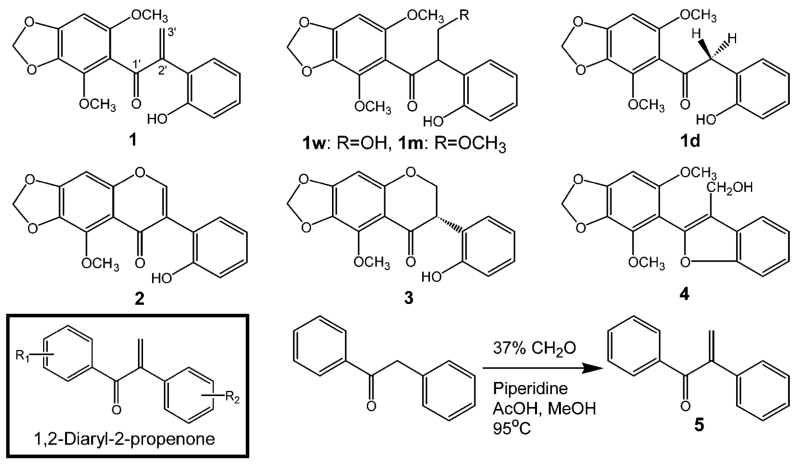
The biology of the biliary toxicity of biliatresone (1) has been reported elsewhere.5 We report herein a thorough characterization of an unusual, toxic, and reactive isoflavonoid, biliatresone (1), along with three related isoflavonoids (Figure 1). To screen for toxicity, we used larval zebrafish, commonly used for whole-organism in vivo screening in pharmaceutical and toxicological studies.6 The zebrafish larvae were exposed to crude extracts, fractions, and purified compounds in different concentrations for 24–48 h. Toxicity was evaluated by the determination of the lethal dose and microscopic examination of the fate of a fluorescent lipid reporter, Bodipy-C16, added to the medium.7 The zebrafish ingest the lipid, with fluorescence observed within 6 h in the intestine and gallbladder of the control zebrafish.8 Fluorescence is not detected in zebrafish with biliary damage.
Figure 1.
Structures of toxic isoflavonoid 1, its derivatives (1w, 1m, and 1d), an additional three isoflavonoids (2–4), and the synthetic route of 1,2-diaryl-2-propen-1-one (5).
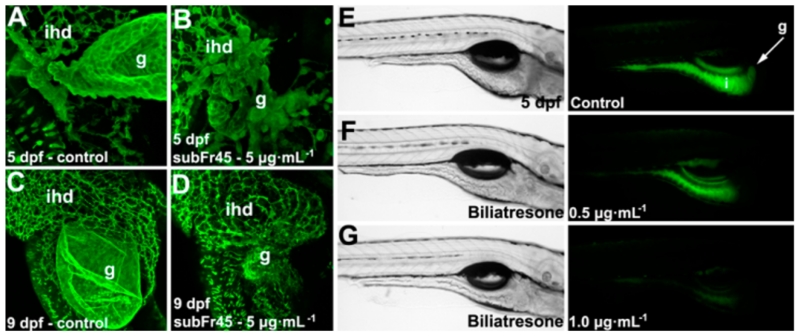
We identified toxic fractions and compounds in a sequential isolation of Fr1 → CH2Cl2 Fr → subFr45 → compounds (1–4) from the crude extract in the toxicity-guided screen (Figure S2, Supporting Information). Treatment with the toxic subFr45 led to significant defects of the gallbladder in 5 and 9 days postfertilization (dpf) zebrafish (Figure 2A–D). Four compounds (1–4) were isolated from the subFr45, and their toxicities were investigated. Biliatresone (1) exhibited toxicity at doses of 0.065–1.0 μg/mL with a marked reduction compared to the control of the fluorescent metabolites in the gallbladder and intestinal lumen (Figure 2E–G); full details of the biological studies are published.5 Compounds 2–4 had no apparent toxicity in this assay.
Figure 2.
Toxicity of subFr45 and biliatresone in the zebrafish larvae. (A–D) Fluorescent images of a control larva (A,C) and a larva treated with subFr45 (B,D). Treatment with subFr45 caused destruction of the gallbladder with preservation of the intrahepatic ducts. (E–G) (Left) Brightfield lateral images of control and biliatresone-treated zebrafish larvae. (Right) Fluorescent images of the same larvae as shown in the brightfield images showing the fate of the ingested lipid. Fluorescent lipid metabolites are present in the gallbladder and intestine of the control larvae (E) but were reduced (F; 0.5 μg/mL) or not detected (G; 1.0 μg/mL) in the treated larvae. [g, gallbladder; ihd, intrahepatic duct; i, intestine].
Compound 1 was isolated as a yellowish gum; a molecular formula of C18H16O6 was established by HRMS from the mass of m/z 329.1022 [M + H]+. NMR analysis showed that 1 is a 1,2-diaryl-2-propenone structure, an α-methylene ketone bridge between two phenyls, with methoxyl, hydroxyl, and dioxymethylene functional groups (Table S1 and Figures S3–S8, Supporting Information). This skeleton may arise by C-ring cleavage of betavulgarin to form seco-betavulgarin and subsequent methylation at C4. Similar 1,2-diaryl-2-propenone metabolites were produced by intestinal clostridia in humans who ingested the dietary isoflavonoids daidzein and genistein.9 The isolation of 1 suggests the possibility of a biochemical route for C-ring cleavage similar to that found in the human intestinal bacteria, which would be a novel biosynthetic pathway in the plants. The data collectively enabled 1 to be identified as 4-methoxy-seco-betavulgarin, to which we gave the trivial name of biliatresone in recognition of the biliary atresia-like phenotype it caused in the zebrafish assay. Compound 2 was identified as betavulgarin, a known antimicrobial phytoalexin, by comparing our NMR data with the literature.10 Compound 3 has the S-stereoisomer configuration, with a negative Cotton effect between 290 and 340 nm, and we identified 3 as (3S)-2′-hydroxy-5-methoxy-6,7-methylenedioxy isoflavanone, not previously reported.11 Compound 4 was identified as a novel 1,2-methylenedioxy-4-methoxy-seco-pterocarpan. We have given 4 the trivial name of humeone in recognition of plant collection along the Hume Weir. Details are in the Chemical Structure Elucidations section, Figures S3–S22, and Tables S1–S2 in the Supporting Information.
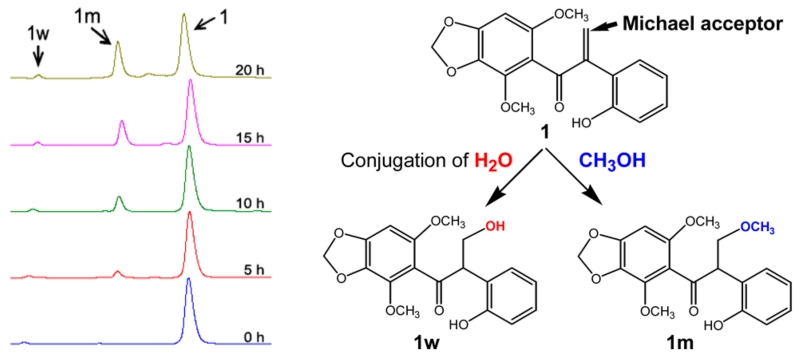
In the course of identifying 1, we found that 1 divided into four peaks (1w, 1m, 1d, and 1) in the HPLC (Figure S23, Supporting Information). We first suspected a case of tautomerism of 1, but the LC-MS analysis revealed that the four compounds had different molecular masses, indicating separate compounds rather than tautomers. The LC-MS data of 1w showed a molecular ion at m/z 347 [M + H]+, while the molecular ion of 1m was m/z 361 [M + H]+, suggesting the addition of 18 amu, a water molecule, or 32 amu, a MeOH molecule, to 1, respectively. Purification of each of 1, 1w, and 1m led to spontaneous formation of the same products in the water/MeOH/ACN solvent (Figure S24, Supporting Information). The formations of 1w and 1m from 1 were reversible reactions with an equilibrium peak area ratio of 2:3 (1m/1) in solution (Figure S24B and C, Supporting Information). Use of a water/EtOH/ACN solvent, instead of MeOH, stopped the transformation to 1m from the purified 1 and 1w (Figure S24C, Supporting Information). A time-course HPLC analysis of the addition of MeOH to 1 showed that 1m increased over a reaction time of 20 h (Figure 3), indicating that 1m was the MeOH adduct of 1. The structure of 1w corresponds to 3′-hydroxy-biliatresone, a water adduct of 1 on the basis of the elucidation of 1m. NMR spectra of 1m were measured from a mixture of 1m and 1 because 1m could not be completely purified without conversion to 1 (Figures S25–S30, Supporting Information). The chemical structure of 1m was completed with peaks selected by the elimination of all peaks arising from the 1H NMR data of 1 and identified as 3′-methoxy-biliatresone, generated by the oxidative cleavage of the α-methylene (C-2′ and C-3′) caused by electron attack of nucleophilic MeOH via a Michael addition.12
Figure 3.
HPLC analysis of the time-course study of the formation of the 1w and 1m adducts from 1.
We noticed the presence of another tiny peak 1d, which is marked by a red open circle in the HPLC chromatograms (Figures S23, S24, and S31, Supporting Information). The peak of 1d was found in all chromatograms during the characterization of the solvent adducts. The molecular mass of 1d was m/z 317 [M + H]+, corresponding to a molecular formula of C17H16O6, representing the loss of one carbon from 1 (Figure S31, Supporting Information). A very small quantity of 1d was purified, and 1H NMR and HMBC data were acquired (Figure S32, Supporting Information). Comparison of the NMR data showed that 1d lacked the olefinic protons (3′-H) of 1. Instead, a new methylene peak, (2′-H, 2H) was present, indicating a 1,2-diaryl-ethanone with an ethanone bridge (−CH2–). We named 1d demethylene biliatresone. Although isoflavonoids are frequently isolated from various plants, and diaryl-ethanone (benzoins) and -ethene (stilbenes) compounds are not infrequent, the 1,2-diaryl-propenone isoflavonoids are extremely rare. Besides the intestinal metabolites of soy isoflavonoids, the only other 1,2-diaryl-propenone compounds have been reported as products of fungal degradation of plant lignin.13
To summarize, in the zebrafish toxicity assay with 1, all of 25 zebrafish larvae were killed at a concentration of 1 μg/mL (3.05 μM) with marked changes in the gallbladder and extrahepatic biliary tree structures (Figure 2).5 The water and MeOH adducts (1w and 1m) showed toxicities similar to those of 1. We feel that the toxicity of the solvent adducts reflects the reversible reaction to 1 in vivo. We thus focused on the 1,2-diaryl-2-propenone moiety as a core moiety and/or a reactive toxic Michael acceptor contributing to the extrahepatic biliary toxicity that led to the death of the zebrafish larvae. In order to determine whether 1,2-diaryl-2-propenone was a toxic moiety, 1,2-diaryl-2-propen-1-one (5) was synthesized from 1,2-diaryl-ethanone with a slight modification (Figure S33, Supporting Information).14 The synthetic 5 also spontaneously but very slowly conjugated with MeOH in the same way as 1 (Figure S34, Supporting Information) and killed the zebrafish larvae at a higher concentration (5 μg/mL; 24.03 μM) than 1. By comparison of the structures of 1 and 5, we suggest that the functional groups of 1 contribute to the biliary tree-specific and higher toxicity of biliatresone. Although the extrahepatic biliary toxicity of 1 needs further investigation, the high content of biliatresone (~1.84% of the dry weight) supports the hypothesis that 1 is responsible for biliary toxicity in livestock (Figure S2B, Supporting Information).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of Rodney Reece, Penny Farrell, and Michael Linton in investigating the 2007 outbreak, Remy Witherspoon and Allan Scammell for assistance in harvesting the plants, and Janet Wilkins and the late Surrey Jacobs for plant identification. We are grateful to Drs. Jeff Cave, Mark Hawes, Richard Sanders, and David Hall for information about the 2013 biliary atresia outbreak in lambs in Australia. We thank Mani Muthumani for expert technical assistance with zebrafish husbandry and assistance with the zebrafish screening assay, Amber Gunderwala for assistance with synthesis, Guillermo Moyna and Cristina Tettamanzi de Sproveiro for assistance with the NMR studies, Charles McEwen for assistance with the HRMS, the late Fred Biesecker and Drug Plastics, Inc. for plant importation, and the MPIC of the Center for Molecular Studies of Digestive and Liver Diseases for imaging support. We thank John Beutler for critical review of the manuscript.
Funding
This work was supported by grants from the NIDDK (R01-DK-092111), the Commonwealth of Pennsylvania (KIZ KISK C000043713), the Fred and Suzanne Biesecker Center for Pediatric Liver Diseases at The Children’s Hospital of Philadelphia, the College of Graduate Studies at the University of the Sciences in Philadelphia, and a pilot grant from the University of Pennsylvania NIDDK Center for Molecular Studies of Digestive and Liver Diseases (P30-DK-05036).
ABBREVIATIONS
- ACN
acetonitrile
- dpf
days postfertilization
- EtOH
ethanol
- Fr
fraction
- HMBC
heteronuclear multiple-bond correlation spectroscopy
- HPLC
high performance liquid chromatography
- HRMS
high resolution mass spectrometry
- LC-MS
liquid chromatography–mass spectrometry
- MeOH
methanol
- NMR
nuclear magnetic resonance
- subFr
subfraction
Footnotes
ASSOCIATED CONTENT
NMR, CD, IR, HRMS, HPLC, and LC-MS analysis for chemical structure elucidation. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrestox.5b00227.
The authors declare no competing financial interest.
REFERENCES
- (1).Robson S. Congenital biliary atresia and jaundice in lambs. NSW Anim. Hlth. Surveill. 2007;2:2–4. [Google Scholar]
- (2).Harper P, Plant JW, Unger DB. Congenital biliary atresia and jaundice in lambs and calves. Aust. Vet. J. 1990;67:18–22. doi: 10.1111/j.1751-0813.1990.tb07385.x. [DOI] [PubMed] [Google Scholar]
- (3).Western Australian Herbarium . FloraBase: The Western Australian Flora. Department of Parks and Wildlife; 1998. https://florabase.dpaw.wa.gov.au/ [Google Scholar]
- (4).McKenzie RA, Burren BG, Noble JW, Thomas MB. Cyanide poisoning in cattle from Dysphania glomulifera (red crumbweed): using the internet for rapid plant identification and diagnostic advice. Aust. Vet. J. 2007;85:505–509. doi: 10.1111/j.1751-0813.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- (5).Lorent K, Gong W, Koo KA, Waisbourd-Zinman O, Karjoo S, Zhao X, Sealy I, Kettleborough RN, Stemple DL, Windsor PA, Whittaker SJ, Porter JR, Wells RG, Pack M. Identification of a plant isoflavonoid that causes biliary atresia. Sci. Transl. Med. 2015;7:286ra67. doi: 10.1126/scitranslmed.aaa1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Zon LI, Peterson RT. Nat. Rev. Drug Discovery. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- (7).Farber SA, Pack M, Ho SY, Johnson ID, Wagner DS, Dosch R, Mullins MC, Hendrickson HS, Hendrickson EK, Halpern ME. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science. 2001;292:1385–1388. doi: 10.1126/science.1060418. [DOI] [PubMed] [Google Scholar]
- (8).Lorent K, Moore JC, Siekmann AF, Lawson N, Pack M. Reiterative use of the notch signal during zebrafish intrahepatic biliary development. Dev. Dyn. 2010;239:855–864. doi: 10.1002/dvdy.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Joannou GE, Kelly GE, Reeder AY, Waring M, Nelson C. A urinary profile study of dietary phytoestrogens. The identification and mode of metabolism of new isoflavonoids. J. Steroid Biochem. Mol. Biol. 1995;54:167–184. doi: 10.1016/0960-0760(95)00131-i. [DOI] [PubMed] [Google Scholar]
- (10).Elliger CA, Halloin JM. Phenolics induced in Beta vulgaris by Rhizoctonia solani infection. Phytochemistry. 1994;37:691–693. doi: 10.1016/s0031-9422(00)90340-6. [DOI] [PubMed] [Google Scholar]
- (11).Slade D, Ferreira D, Marais JPJ. Circular dichroism, a powerful tool for the assessment of absolute configuration of flavonoids. Phytochemistry. 2005;66:2177–2215. doi: 10.1016/j.phytochem.2005.02.002. [DOI] [PubMed] [Google Scholar]
- (12).Mather BD, Viswanathan K, Miller KM, Long TE. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006;31:487–531. [Google Scholar]
- (13).Umezawa T, Nakatsubo F, Higuchi T. Lignin degradation by Phanerochaete chrysosporium: metabolism of a phenolic phenylcoumaran substructure model compound. Arch. Microbiol. 1982;131:124–128. [Google Scholar]
- (14).Tramontini M. Advances in the chemistry of Mannich bases. Synthesis. 1973;12:703–775. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.