Abstract
We examine the role of dynamic susceptibility contrast (DSC) magnetic resonance imaging (MRI) perfusion in differentiating pseudoprogression from progression in 20 consecutive patients with treated glioblastoma. MRI perfusion was performed, and relative cerebral blood volume (rCBV), relative peak height (rPH), and percent signal recovery (PSR) were measured. Pseudoprogression demonstrated lower median rCBV (P=.009) and rPH (P<.001), and higher PSR (P=.039) than progression. DSC MRI perfusion successfully identified pseudoprogression in patients who did not require a change in treatment despite radiographic worsening following chemoradiotherapy.
Keywords: Glioblastoma, Pseudoprogression, Progression, MRI perfusion
1. Introduction
Glioblastomas are the most common primary malignant brain tumor in adults. Traditionally, tumor response or progression has been determined by examining changes in size of the enhancing mass lesion(s) based on the work of Macdonald et al. [1]. The diagnosis of progressive disease may be confounded by a treatment-related phenomenon termed pseudoprogression, which has become more common with the adoption of radiation therapy (RT) with concurrent and adjuvant temozolomide (TMZ, Temodar, Schering, Kenilworth, NJ, USA) as standard treatment for glioblastomas. The increased and/or new enhancing mass lesions of pseudoprogression usually occur <1–3 months after completion of RT and chemotherapy, then spontaneously stabilize or decrease on subsequent scans without additional treatment [2,3]. The worsened lesions are thought to reflect accelerated chemoradiation-induced cell killing [2,4–7]. Pseudoprogression may appear identical to progressive disease on conventional magnetic resonance imaging (MRI) [7] by fulfilling the same commonly applied response criteria that are usually based on changes in size of the enhancing mass lesion [1,8].
Accurate differentiation between pseudoprogression and progressive disease is important for making informed clinical management decisions, as the two usually require antithetical treatments. Because of the difficulty in differentiating those two entities on conventional MRI, it is common practice to continue patients on adjuvant TMZ in spite of early radiographic changes after RT. An accurate, noninvasive diagnosis of progressive disease would enable early discussions, however, on repeat surgery and/or changes in chemotherapy that may potentially improve the patient's course. On the other hand, an early diagnosis of pseudoprogression would allow increased confidence by the physician and patient to continue current effective treatment and delay or avoid potentially unnecessary surgery and/or more toxic, potentially less effective second-line treatments. This is of particular relevance when radiographic worsening due to pseudoprogression occurs or extends beyond 2–3 months following RT, when physicians often opt to change treatments. In fact, many responses in patients enrolled in clinical trials for recurrent disease soon after the end of RT may correspond to resolution of pseudoprogression rather than new treatment efficacy [9]. Thus, new tools for characterization of pseudoprogression are clearly needed.
MRI perfusion imaging is an advanced neuroimaging tool increasingly utilized in the diagnosis and management of brain tumors that can provide information on tumor blood volume and vascular permeability [10,11]. Dynamic susceptibility contrast (DSC) MRI perfusion has demonstrated utility in predicting the prognosis, grade, and time to progression in patients with gliomas [12–15]. Many groups have demonstrated the usefulness of MRI perfusion in differentiating recurrent tumor from delayed radiation necrosis [10,16–18], and less well in the related entity of early subacute radiation effects of pseudoprogression [15,19,20]. We hypothesized that MRI perfusion would be a useful technique to distinguish pseudoprogression from progressive disease in patients undergoing treatment for glioblastoma.
2. Materials and methods
2.1. Patients
This retrospective study was granted a Waiver of Informed Consent by the hospital Institutional Review Board and was compliant with Health Insurance Portability and Accountability Act regulations. We retrospectively identified 174 consecutive patients with primary treatment-naïve glioblastoma who were treated between October 2005 and April 2009 from an institutional database. All patients had a pathologic diagnosis of glioblastoma according to revised World Health Organization criteria after biopsy, subtotal resection, or gross total resection.
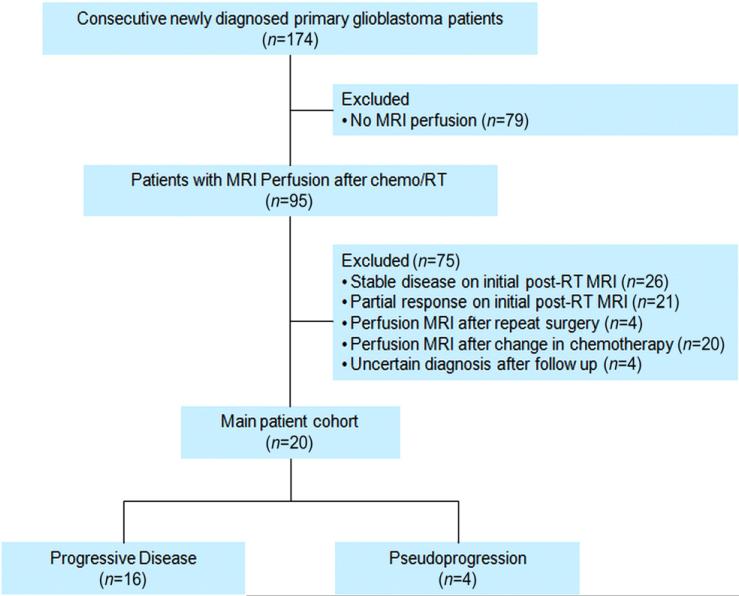
DSC MRI perfusion was performed after RT in 95 of the 174 patients. From these 95 patients, we identified 48 patients as having new or increased (≥25%) enhancing mass lesion(s) on the initial post-RT MRI (usually 2–4 weeks after completion of RT) as compared to the pre-RT MRI (usually 1–2 weeks before beginning RT). We excluded patients who had MRI perfusion after repeat surgery (n=4) or after a change in chemotherapy (n=20), or in whom the diagnosis remained uncertain after follow-up (n=4). The final study cohort consisted of 20 patients, as described in Fig. 1. If more than one perfusion scan fulfilled the above criteria, then only the earliest data set was examined.
Fig. 1.
STARD diagram.
The final analysis cohort consisted of 14 men (70%) and 6 women (30%) with a median age of 58 years (range, 9–84). Five patients (25%) had undergone gross total resection of the enhancing tumor, 10 (50%) subtotal resection, and 5 (25%) biopsy. Nineteen patients (95%) received standard RT (5940–6000 cGy given in 30–33 fractions over 6 weeks). One patient (5%) received abbreviated RT (4005 cGy given in 15 fractions over 3 weeks), an acceptable alternative for elderly or deconditioned patients unlikely to tolerate the standard 6-week course [21]. All patients received TMZ concurrent with RT at standard doses. Thirteen patients received adjuvant TMZ at standard doses, and seven received alternative TMZ schedules as part of an ongoing clinical trial: five patients received metronomic TMZ at 50 mg/m2/day and two received dose-dense TMZ at 150 mg/m2/day 1 week on/1 week off [22].
Chart review was performed by two neuro-oncologists (with 3 and 10 years of experience). O6-methylguanine–DNA methyltransferase (MGMT) methylation status was recorded when available. The status of this DNA repair enzyme promoter was determined by methylation-specific polymerase chain reaction analysis. Decisions to perform MGMT assays were based on enrollment into a clinical trial that required MGMT analysis or off-trial analysis as part of an emerging standard of practice for all glioblastoma patients in the latter part of the study.
2.2. MRI perfusion
MRI scans were obtained using 1.5-T (Signa Excite) and 3-T magnets (Discovery 750, GE Healthcare, Waukesha, WI, USA). All sequences were acquired using 5-mm slice thickness and no interslice gap. DSC MRI was obtained using gradient-echo echo-planar images (repetition time/echo time=1000–1200/40–50 ms, matrix 128×128, flip angle 60°, number of slices 12–18). The contrast bolus was injected though a peripheral Angiocath (18–21 gauge) using an MRI-compatible power injector at 2–5 ml/s and immediately followed by a 20-ml saline flush at the same rate. Multisection image data were acquired every second for a total of 90 s, with the bolus contrast injection occurring after 10 s. A standard dose (0.2 ml/kg of body weight, maximum dose 20 ml) of gadopentetate dimeglumine (Magnevist, Bayer HealthCare Pharmaceuticals, Wayne, NJ, USA) contrast was administered for both 1.5-T and 3-T scans. No preload contrast dose was administered.
DSC perfusion analysis was performed in consensus by a neuroradiology fellow and a radiology resident (with 5 and 3 years of experience in MRI perfusion, respectively) under the direct supervision of a board-certified radiologist who holds a Certificate of Added Qualification in Neuroradiology (12 years of experience). Analysis was performed while blinded to the outcome diagnosis of pseudoprogression vs. progressive disease. The axial DSC images and matching axial contrast T1-weighted images were transferred to an offline commercially available workstation (Advantage Workstation, GE Healthcare, Waukesha, WI, USA) and processed using commercially available software (FuncTools 4.3, GE Healthcare, Waukesha, WI, USA). Negative enhancement integral perfusion maps were reconstructed using standard algorithms; processing without leakage correction modeling is estimated to have 81% accuracy [23] and, in our clinical and research experience, has yielded acceptable results. Small, fixed-diameter (50–100 mm2) regions of interest (ROIs) were placed over the enhancing mass lesion and compared with control ROIs placed over the contralateral normal-appearing white matter to calculate tumoral relative cerebral blood volume (rCBV). This technique has been described as the most accurate and reproducible way to obtain ROI-based perfusion measurements [16,24,25]. Blood vessels, cystic/necrotic changes, and areas of susceptibility from hemorrhage, bone, or air were explicitly excluded from the ROIs. The rCBV measurements were recorded as CBVlesion/CBVnormal-appearing white matter. The T2* signal intensity time curve corresponding to the tumor rCBV ROI was solved for S0 (baseline signal intensity prior to contrast injection), Smin (minimal signal intensity at peak of contrast bolus), and S1 (end signal intensity at 60 s). Relative peak height (rPH) was calculated as [(S0–Smin)lesion/(S0–Smin)contralateral normal-appearing white matter], and percentage signal recovery (PSR) as [(S1–Smin)/(S0–Smin)]. rPH is the maximal change in signal intensity and correlates with rCBV and tumor blood volume [10]. PSR is influenced by leakage of contrast and size of the extravascular space [10,26], with lower PSR reflecting delayed return of the perfusion curve to baseline and being thought to indicate higher permeability.
2.3. Diagnosis
The diagnosis of pseudoprogression or progressive disease was made based on pathologic analysis after repeat biopsy or resection when available. The presence of necrotizing treatment effects and no to minimal (<10%) identifiable tumor was determined to represent pseudoprogression. The presence of residual or recurrent tumor was determined to represent progressive disease.
If repeat pathology was not available, the clinical diagnosis of pseudoprogression or progressive disease was made by consensus of a neuro-oncology fellow (with 4 years of experience) and a neuro-oncologist (with 11 years of experience) after complete chart review while blinded to the DSC perfusion results. A diagnosis of pseudoprogression was made if no change in treatment was required for a minimum of 6 months from the end of RT. Progressive disease was defined as ≥25% increase in enhancing disease and/or clinical deterioration that required a change in treatment within 6 months after the end of RT. This interpretation is based on the Response Assessment in Neuro-Oncology (RANO) criteria [8], which define first progression <12 weeks after completion of chemoradiation therapy as new enhancement outside the high-dose radiation field or histopathologic evidence of viable tumor, and any progression >12 weeks after completion of chemoradiation therapy as ≥25% increase in the sum of the products of orthogonal measurements of enhancing disease, increase in nonmeasureable disease, increase in nonenhancing disease, any new disease, or clinical deterioration considered secondary to disease.
2.4. Statistical analysis
Comparisons between the pseudoprogression and progressive disease groups were performed using two-sided Wilcoxon rank–sum tests. Statistical significance was set at P=.05. Optimal threshold values of rCBV, rPH, and PSR were obtained by area under the curve (AUC) analysis derived from ROC curves.
3. Results
Sixteen (80%) of the 20 patients were determined to have progressive disease and 4 (20%) to have pseudoprogression. Nine patients underwent repeat surgery, with subtotal resection of the worsening lesion(s) in three and gross total resection in six. Pathology in these nine patients was consistent with progressive disease, with tumor described in three and tumor plus necrotizing effects in six. The median time from completion of RT to pathology was 4.4 months (range, 1.4–9 months). For the patients without repeat pathology, the median time to treatment change was 3 months (range, 0.7–5.2 months) for progressive disease patients and 7.9 months (range, 7.2–9.4 months) for pseudoprogression patients (who were required to not have any treatment change for a minimum of 6 months).
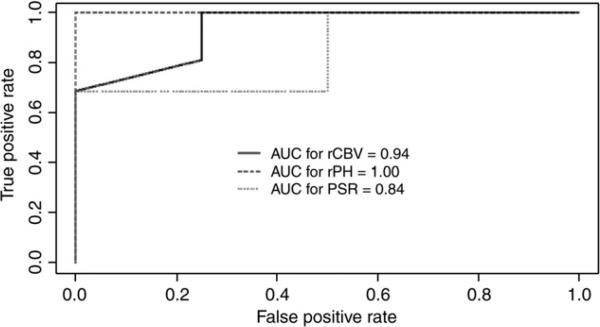
The median time from completion of RT to perform DSC MRI perfusion for all patients was 2.8 months (range, 0.5–6.3 months). As shown in Table 1, the progressive disease group demonstrated significantly higher median rCBV (P=.009), higher rPH (P<.001), and lower PSR (P=.039). The rCBV distribution is shown in Fig. 2, and representative cases are in Figs. 3 and 4. To detect pseudoprogression, ROC analysis revealed an optimal rCBV threshold ≤1.8 with 100% sensitivity and 75% specificity and AUC=.938; to maximize specificity, an rCBV threshold ≤2.4 achieved 69% sensitivity and 100% specificity. An rPH threshold ≤1.7 achieved 100% sensitivity and specificity with AUC=1.0, and a PSR threshold ≥.9 achieved 100% sensitivity and 63% specificity with AUC=.844. ROC curves are shown in Fig. 5.
Table 1.
Median MRI perfusion values in patients with pseudoprogression and progressive disease
| Pseudoprogression | Progressive disease | P value | |
|---|---|---|---|
| rCBV | 1.50 | 2.75 | .009 |
| rPH | 1.34 | 3.04 | <.001 |
| PSR | 1.01 | 0.84 | .039 |
Fig. 2.
Distribution of rCBV in pseudoprogression and progressive disease.
Fig. 3.
Patient with pseudoprogression: (A–C) contrast axial T1-weighted images. One day (A) after gross total resection, there is a blood-filled surgical cavity in the lateral posterior frontal lobe without enhancement. One month after completion of RT (not shown), there is a new enhancing mass lesion that increases at 5 months (B) before decreasing at 12 months without a change in treatment (C). rCBV map (D) at 5 months does not show hyperperfusion of the enhancing lesion (ROI2) when compared to the contralateral normal white matter (ROI1). T2* signal intensity time curve (E) with rCBV, rPH, and PSR measurements.
Fig. 4.
Patient with progressive disease: (A–C) contrast axial T1-weighted images. (A) One day after gross total resection, there is a blood-filled surgical cavity in the parietal lobe with minor peripheral enhancement. (B) One month after completion of RT, a new enhancing mass lesion is seen at the treated site that nearly triples in size in (C) at 2 months. (D) rCBV map at 2 months shows moderate heterogeneous hyperperfusion of the enhancing lesion (ROI2) compared to the contralateral normal-appearing white matter (ROI1). T2* signal intensity time curve (E) with rCBV, rPH, and PSR measurements.
Fig. 5.
ROC analysis to detect progression using the following thresholds: rCBV≥1.80, rPH≥1.72, and PSR≤0.86.
The 16 progressive disease patients received adjuvant TMZ at standard doses in 10 patients, metronomic in 4, and dose-dense in 2. The four pseudoprogression patients received adjuvant TMZ at standard doses in three patients and metronomic in one. MGMT assays revealed methylated status in two patients (one with progressive disease and one with pseudoprogression) and unmethylated status in three patients (all with progressive disease). Invalid results were returned in three patients (two with insufficient quantities of tissue and one with excessive cautery artifact). Testing was not performed in the other 12 patients.
4. Discussion
In this study, we found that DSC MRI perfusion estimates of blood volume and permeability can successfully identify a subgroup of patients presenting with radiographic worsening following chemoradiotherapy with TMZ who do not require a change in treatment, representing pseudoprogression rather than treatment failure. These pseudoprogression lesions were characterized by lower perfusion and higher permeability. The three perfusion parameters (rCBV, rPH, and PSR) were comparable, although decreases in rPH slightly outperformed decreased rCBV and increased PSR to detect pseudoprogression. We propose using rCBV, as it is already the most commonly applied metric in clinical practice. These results suggest that despite nearly identical appearances on conventional MRI [6,7], DSC MRI perfusion may play an important role in the early diagnosis of treatment effects vs. treatment failure.
The difficulty in differentiating pseudoprogression and progressive disease on conventional T1-postcontrast MRI derives from the fact that contrast enhancement reflects increased permeability and disruption of the blood–brain barrier [27]. The inflammatory/necrotic process involved in pseudoprogression and the cell proliferation/neoangiogenesis process observed in active progressive disease result in increased vascular permeability and are therefore indistinguishable on conventional MRI. MRI perfusion provides the advantage of characterizing and quantifying blood volume, in addition to flow and permeability information, thus providing a more robust and discriminating diagnostic tool [28].
A number of studies have investigated the role of MRI perfusion in the differentiation between progressive disease and radionecrosis [10,27,29,30]. Although the terms “radionecrosis” and “pseudoprogression” are occasionally used interchangeably, they likely represent distinct entities. Pseudoprogression occurs within 1–3 months after RT, is often spontaneously reversible, and may suggest higher treatment efficacy [3], while radionecrosis occurs beyond 6 months to several years afterwards, is often progressive, and is a nonbeneficial complication. While both entities may have distinct pathophysiologic mechanisms [2], they share many histologic features, particularly necrosis and reactive inflammation that should translate into similar imaging characteristics. Barajas et al. [30] examined 57 patients with glioblastoma treated with RT who developed progressive enhancement and underwent MRI perfusion. Lower rCBV and rPH and higher PSR were observed in patients with radionecrosis, with the perfusion scans performed a mean >300 days after completion of RT. We detected similar reduced perfusion and permeability metrics in our pseudoprogression patients, likely reflecting reduced or absent tumor neovascularity in comparison to active tumors, with low-grade leakiness mainly resulting from the inflammation and edema observed in both pseudoprogression and radionecrosis. Decreased PSR is thought to reflect blood–brain barrier disruption and increased capillary leakiness or vascular permeability [10]. The optimal thresholds determined in this study are similar to those proposed in the literature for distinguishing recurrent malignant gliomas from radionecrosis [15,16,31]. Hu et al. [17] proposed an rCBV threshold of .71, but they studied radionecrosis that occurred a mean 14.3 months after RT, and they used two preload contrast injections that are not standard of care at most practices performing DSC perfusion. Gasparetto et al. [32] determined that an rCBV fraction ≥20% in an enhancing mass with rCBV ≥1.8 has excellent accuracy (area under receiver operating characteristic curve=.97) and efficiency (93%) for treatment-related necrosis. Of the 30 patients they examined, however, glioblastomas represented a minority (n=14, including one secondary) in a cohort of high- and low-grade gliomas and metastases. MRI perfusion was performed a median 7 months (range, 3–109) after treatment, with only four patients imaged within 3 months. Although their time frame may potentially limit the applicability of the proposed rCBV fraction analysis to our glioblastoma patients with suspected early radiation injury or pseudoprogression, their work and others’ suggest that there is a role for MRI perfusion to help establish the correct diagnosis in these difficult cases after radiation therapy.
A few studies have investigated MRI perfusion parameters in pseudoprogression. Mangla et al. [15] showed that a percent change in rCBV at 1 month compared to the pretreatment scan could distinguish pseudoprogression from progressive disease with 77% sensitivity and 86% specificity. Despite their promising results, perfusion values obtained at a single time point and stratified by a simple threshold may be more helpful in clinical practice because it does not depend on a pre-RT MRI perfusion. Tsien et al. [19] suggested that voxel-based analysis of rCBV can outperform percent changes in rCBV. Notably, their patients received higher than usual total radiation doses with a median dose of 7200 cGy (range, 6000–7800 cGy), which may have inflated the incidence of radiation-related complications [33] such as pseudoprogression. In addition, they performed voxel-by-voxel analysis using perfusion data acquired at week 3 during chemoradiation therapy, which is not a standard time to perform MRI scans. Gahramanov et al. [34] also correlated low rCBV with pseudoprogression. Interestingly, they obtained better results with DSC perfusion using the blood pool agent ferumoxytol than gadoteridol, which is one of many gadolinium-based contrast agents that may demonstrate decreased accuracy in the setting of blood–brain barrier disruption.
Other advanced imaging techniques such as hyperperfusion fractioning, volume modeling, MRI spectroscopy, diffusion tensor imaging, and positron emission tomography/computed tomography have been used to identify radiation necrosis with promising results [35–42]. Unfortunately, however, these techniques have not been validated in large clinical trials or in comparative effectiveness research. Direct head-to-head comparisons between techniques have also been lacking. A multimodality approach incorporating multiple advanced imaging techniques may be beneficial [35,43,44], although there remains considerable uncertainty about the best course of action when the tests disagree.
One potential limitation of our study, as well as other studies seeking to radiographically or molecularly characterize pseudoprogression, is the difficulty in retrospectively defining pseudoprogression. Although pathology is the reference standard, it is an imperfect technique that is itself limited by sampling error and observer variability, given the common coexistence of viable tumor and treatment-related necrosis [45,46]. Moreover, the diagnosis and treatment decisions may be influenced by clinical status, imaging, and pathologic and molecular analyses. In our study, we defined pseudoprogression as clear lack of viable tumor at pathology, or by clinical stability or improvement that did not require additional treatment for a minimum of 6 months [47]. These clinical criteria were chosen to provide the most potentially useful information to the treating neuro-oncologist. We sought to confidently identify patients who can be safely followed without a change in treatment in spite of worsening MRI. Our results suggest that patients with low perfusion findings do not require a change in treatment. It is possible that some of the patients who were classified as progressive disease due to a change in treatment, however, could have had pseudoprogression and done well without a treatment change. On the other hand, it is possible that some patients classified as pseudoprogression instead had slowly progressive tumors. Unfortunately, these questions can only be addressed in a prospective study where all patients are kept on the same treatment for several months in spite of worsening MRI scans, which is impractical and ethically problematic.
Second, our final analysis cohort was relatively small, with a low number of pseudoprogression patients. The magnitude in the differences in MRI perfusion parameters was striking, however, and statistical significance was achieved. This suggests that such differences between the two groups are real and robust enough to prompt additional, larger studies. The limited number of patients also precludes further conclusions on the relationship between MGMT promoter methylation status and pseudoprogression or MRI perfusion data. Retrospective work has suggested that pseudoprogression is more likely in tumors with methylated MGMT promoter [4]. Although helpful when available, however, determination of MGMT methylation status is not always feasible due to insufficient tissue samples and assay limitations. Moreover, methylated MGMT status may be found in both pseudoprogression and progressive disease, and therefore cannot be solely used to distinguish between these two entities in any single patient.
Third, the patients in this study had newly diagnosed glioblastomas and underwent standard chemoradiotherapy with TMZ. We did not evaluate the potential utility of MRI perfusion in patients receiving antiangiogenic treatments such as bevacizumab, which may induce direct and profound effects on tumor enhancement, perfusion, and permeability. However, patients treated with RT, TMZ, and upfront bevacizumab rarely develop post-RT radiographic worsening [48,49], and the question of pseudoprogression is therefore less relevant. Moreover, ongoing clinical trials are still needed to define a role for bevacizumab in newly diagnosed glioblastoma, and therefore, our results remain applicable to the majority of newly diagnosed and treated glioblastoma patients.
In conclusion, we found that DSC MRI perfusion has a potential role in identifying glioblastoma patients with pseudoprogression who can be confidently followed without a change in treatment despite radiographic worsening following chemoradiotherapy. Larger studies including correlation with molecular data are needed to confirm these results.
Acknowledgments
We thank Judith A. Lampron (Department of Neurology, Memorial Sloan-Kettering Cancer Center) for sharing her editorial expertise in editing this manuscript.
References
- 1.Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 2.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–61. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82(1):81–3. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 4.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–7. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 5.Chaskis C, Neyns B, Michotte A, De Ridder M, Everaert H. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol. 2009;72(4):423–8. doi: 10.1016/j.surneu.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–10. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 7.de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–7. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 8.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 9.Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051–7. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 10.Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2009;30(2):367–72. doi: 10.3174/ajnr.A1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law M. Advanced imaging techniques in brain tumors. Cancer Imaging. 2009;9 doi: 10.1102/1470-7330.2009.9002. Spec No A:S4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha S, Johnson G, Wadghiri YZ, et al. Dynamic, contrast-enhanced perfusion MRI in mouse gliomas: correlation with histopathology. Magn Reson Med. 2003;49(5):848–55. doi: 10.1002/mrm.10446. [DOI] [PubMed] [Google Scholar]
- 13.Law M, Young RJ, Babb JS, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008;247(2):490–8. doi: 10.1148/radiol.2472070898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugahara T, Korogi Y, Shigematsu Y, et al. Value of dynamic susceptibility contrast magnetic resonance imaging in the evaluation of intracranial tumors. Top Magn Reson Imaging. 1999;10(2):114–24. doi: 10.1097/00002142-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Mangla R, Singh G, Ziegelitz D, et al. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010 doi: 10.1148/radiol.10091440. [DOI] [PubMed] [Google Scholar]
- 16.Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223(1):11–29. doi: 10.1148/radiol.2231010594. [DOI] [PubMed] [Google Scholar]
- 17.Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009;30(3):552–8. doi: 10.3174/ajnr.A1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young RJ, Knopp EA. Brain MRI: tumor evaluation. J Magn Reson Imaging. 2006;24(4):709–24. doi: 10.1002/jmri.20704. [DOI] [PubMed] [Google Scholar]
- 19.Tsien C, Galban CJ, Chenevert TL, et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol. 2010;28(13):2293–9. doi: 10.1200/JCO.2009.25.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke JL, Chang S. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009;9(3):241–6. doi: 10.1007/s11910-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 21.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–8. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 22.Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27(23):3861–7. doi: 10.1200/JCO.2008.20.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment1. Radiology. 2009;252(1):182–9. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]
- 24.Wetzel SG, Cha S, Johnson G, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraob-server reproducibility study. Radiology. 2002;224(3):797–803. doi: 10.1148/radiol.2243011014. [DOI] [PubMed] [Google Scholar]
- 25.Young R, Babb J, Law M, Pollack E, Johnson G. Comparison of region-of-interest analysis with three different histogram analysis methods in the determination of perfusion metrics in patients with brain gliomas. J Magn Reson Imaging. 2007;26(4):1053–63. doi: 10.1002/jmri.21064. [DOI] [PubMed] [Google Scholar]
- 26.Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol. 2011;32(6):1004–10. doi: 10.3174/ajnr.A2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omuro AM, Leite CC, Mokhtari K, Delattre JY. Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 2006;5(11):937–48. doi: 10.1016/S1474-4422(06)70597-X. [DOI] [PubMed] [Google Scholar]
- 28.Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9(9):906–20. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 29.Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist. 2004;9(5):528–37. doi: 10.1634/theoncologist.9-5-528. [DOI] [PubMed] [Google Scholar]
- 30.Barajas RF, Chang JS, Segal MR, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253(2):486–96. doi: 10.1148/radiol.2532090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Okaili RN, Krejza J, Woo JH, et al. Intraaxial brain masses: MR imaging-based diagnostic strategy–initial experience. Radiology. 2007;243(2):539–50. doi: 10.1148/radiol.2432060493. [DOI] [PubMed] [Google Scholar]
- 32.Gasparetto EL, Pawlak MA, Patel SH, et al. Posttreatment recurrence of malignant brain neoplasm: accuracy of relative cerebral blood volume fraction in discriminating low from high malignant histologic volume fraction. Radiology. 2009;250(3):887–96. doi: 10.1148/radiol.2502071444. [DOI] [PubMed] [Google Scholar]
- 33.Marks JE, Wong J. The risk of cerebral radionecrosis in relation to dose, time and fractionation. A follow-up study. Prog Exp Tumor Res. 1985;29:210–8. doi: 10.1159/000411642. [DOI] [PubMed] [Google Scholar]
- 34.Gahramanov S, Raslan AM, Muldoon LL, et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys. 2011;79(2):514–23. doi: 10.1016/j.ijrobp.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexiou GA, Tsiouris S, Kyritsis AP, Voulgaris S, Argyropoulou MI, Fotopoulos AD. Glioma recurrence versus radiation necrosis: accuracy of current imaging modalities. J Neuro-Oncol. 2009;95(1):1–11. doi: 10.1007/s11060-009-9897-1. [DOI] [PubMed] [Google Scholar]
- 36.Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004;25(2):201–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa T, Kanno I, Hatazawa J, et al. Methionine PET for follow-up of radiation therapy of primary lymphoma of the brain. Radiographics. 1994;14(1):101–10. doi: 10.1148/radiographics.14.1.8128041. [DOI] [PubMed] [Google Scholar]
- 38.Rock JP, Hearshen D, Scarpace L, et al. Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery. 2002;51(4):912–9. doi: 10.1097/00006123-200210000-00010. discussion 919-20. [DOI] [PubMed] [Google Scholar]
- 39.Sundgren PC. MR spectroscopy in radiation injury. AJNR Am J Neuroradiol. 2009;30(8):1469–76. doi: 10.3174/ajnr.A1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundgren PC, Fan X, Weybright P, et al. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging. 2006;24(9):1131–42. doi: 10.1016/j.mri.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Weybright P, Sundgren PC, Maly P, et al. Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. AJR Am J Roentgenol. 2005;185(6):1471–6. doi: 10.2214/AJR.04.0933. [DOI] [PubMed] [Google Scholar]
- 42.Huang J, Wang AM, Shetty A, et al. Differentiation between intra-axial metastatic tumor progression and radiation injury following fractionated radiation therapy or stereotactic radiosurgery using MR spectroscopy, perfusion MR imaging or volume progression modeling. Magn Reson Imaging. 2011;29(7):993–1001. doi: 10.1016/j.mri.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Henry RG, Vigneron DB, Fischbein NJ, et al. Comparison of relative cerebral blood volume and proton spectroscopy in patients with treated gliomas. AJNR Am J Neuroradiol. 2000;21(2):357–66. [PMC free article] [PubMed] [Google Scholar]
- 44.Galban CJ, Chenevert TL, Meyer CR, et al. Prospective analysis of parametric response map-derived MRI biomarkers: identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin Cancer Res. 2011;17(14):4751–60. doi: 10.1158/1078-0432.CCR-10-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–5. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prayson RA, Agamanolis DP, Cohen ML, et al. Interobserver reproducibility among neuropathologists and surgical pathologists in fibrillary astrocytoma grading. J Neurol Sci. 2000;175(1):33–9. doi: 10.1016/s0022-510x(00)00274-4. [DOI] [PubMed] [Google Scholar]
- 47.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–6. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omuro A, Beal K, Karimi S, et al. Phase II study of bevacizumab (BEV), temozolomide (TMZ), and hypofractionated stereotactic radiotherapy (HFSRT) for newly diagnosed glioblastoma (GBM). J Clin Oncol (Meeting Abstracts) 2010;28(15 suppl):2036. [Google Scholar]
- 49.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29(2):142–8. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]