Abstract
Background
Inactivity and sedentary behavior are related to poorer health outcomes in breast cancer survivors. However, few studies examining these behaviors in survivors have used objective measures, considered activities other than moderate-to-vigorous intensity activity (MVPA) and/or sedentary behavior (i.e. low intensity activities) or compared survivors to healthy controls. The purpose of the present study is to compare accelerometer-measured activity of various intensities (total, light, lifestyle, MVPA) and sedentary behavior between breast cancer survivors and non-cancer controls.
Methods
An imputation-based approach of independent sample t-tests adjusting for multiple comparisons was used to compare estimates of participation in each activity and sedentary behavior between survivors [n=398; M(SD)age=56.95 (9.11)] and block-matched non-cancer controls [n=1120; M(SD)age=54.88 (16.11)]. Potential moderating effects of body mass index (BMI), age, and education were also examined.
Results
Breast cancer survivors registered less daily total (282.8 v. 346.9) light (199.1 v. 259.3) and lifestyle (62.0 v. 71.7) activity minutes and more MVPA (21.6 v. 15.9) and sedentary behavior (555.7 v. 500.6) minutes than controls (p<0.001 for all). These relationships were largely consistent across BMI, age and education. On average, survivors spent an estimated 66.4% of their waking time sedentary and 31.1% in light/lifestyle activity and 2.6% in MVPA.
Conclusions
Breast cancer survivors are more sedentary and participate in less low intensity activity than controls. Although survivors registered more MVPA, these levels were insufficient. Future research should explore these differences and potential benefits of targeting low intensity activities and reducing sedentary time in this population.
Keywords: physical activity, sedentary behavior, breast cancer survivors, exercise
INTRODUCTION
In breast cancer survivors, higher physical activity levels are associated with fewer negative treatment-related side effects, higher quality of life (QOL) and improved disease-specific outcomes including longer survival and reduced risk of recurrence and mortality [1–3]. However, self-reported population level data indicate up to 70% [4–6] of breast cancer survivors do not meet recommendations of 150 minutes per week of moderate-to-vigorous intensity physical activity (MVPA; [7, 8]; ≥ 3 METs; e.g. brisk walking, jogging, swimming). In addition to low activity levels, self-reported data indicate cancer survivors may engage in more sedentary behavior than non-cancer controls [9]. Sedentary behavior refers to any waking activity characterized by an energy expenditure ≤ 1.5 metabolic equivalents and sitting or reclining posture and is distinct from too little exercise.34 Emerging evidence indicates increased sedentary behavior in combination with low activity levels is associated with lower QOL, poorer body composition and increased mortality in breast cancer survivors and other cancer survivor group [10–14]. The combined high prevalence of inactivity and sedentary time may be particularly concerning for cancer survivors given their already heightened risk for poor health and disability [15].
Despite potential relationships between physical activity, sedentary behavior and health outcomes in breast cancer survivors, only two studies to date [12, 16] have used objective measures to estimate these behaviors. These studies found survivors spent less than 2% of their waking time in MVPA and between 66–78% of their time sedentary. Additionally, MVPA may decrease in the year following treatment while sedentary behavior may remain relatively high and stable [16]. While these studies are informative, their findings are limited because they did not compare survivors to healthy controls and consisted of relatively small sample size (<200 women). Moreover, both studies were primarily focused on MVPA and sedentary behavior and gave less consideration to lower intensity activities (1.5 to <3.0 METs; e.g. activities of daily living, slow walking) which have more recently been associated with health benefits. These include reduced disability, chronic disease prevalence, body mass index (BMI), waist circumference, depression, and fatigue and improved cardiovascular health and QOL in older adults [17–19] and individuals with Multiple Sclerosis [19] and reduced functional decline [20] and improved QOL [21] in cancer survivors. Thus, understanding breast cancer survivors’ participation in lower intensity activities may be important.
The purpose of the present study was to compare a sample of breast cancer survivors to population-based controls without a history of cancer from the National Health and Nutrition Examination Survey (NHANES; http://www.cdc.gov/nchs/nhanes.htm) block-matched on body mass index (BMI), age and education to examine whether a) objectively measured physical activity and sedentary time differ between the two groups, overall and by different activity types (sedentary, light, lifestyle, MVPA) and b) these relationships vary by demographic characteristics (BMI, age and education) associated with activity levels in the general population.
METHODS
Participants
Breast Cancer Survivors
Data for breast cancer survivors in the present study consisted of baseline data from a subset of women randomized (n=500) to wear an accelerometer as part of a larger 6-month prospective study in 2010. Full study details are provided elsewhere [22]. Briefly, survivors were recruited from the Army of Women© to participate in a study on quality of life©. Inclusionary criteria included: age ≥18 years, prior history of breast cancer, English-speaking and access to the Internet. Because of the small number of racial and ethnic minorities in this sample (n=7) and those under the age of 30 (n=2), the sample was restricted to women who were White and ≥30 years old. Finally, only women who were randomized to the accelerometer subgroup and had ≥1 valid day of accelerometer data (n=442) with complete data on all matching variables (age, BMI and education) were included in these analyses (n=398).
Non-Cancer Controls
Controls were selected from NHANES 2003–2006 which provides a nationally representative sample of the civilian, non-institutionalized U.S. population. NHANES uses a complex, multi-stage clustered probability design described in detail at: http://www.cdc.gov/nchs/nhanes.htm. For the present study, women were selected to match the characteristics of the breast cancer survivors. Thus, all women who were White, ≥30 years old, had ≥1 valid day of accelerometer data, had completed at least some high school, and had no previous history of cancer were included as potential control participants in these analyses (n=1120).
Measures
Demographics
Breast cancer survivors self-reported age, education, height and weight. BMI was estimated using the standard kg/m2 equation. NHANES controls self-reported age and education during the health interview and height and weight were measured during the examination and used to calculate BMI.
Health and cancer history
Breast cancer survivors self-reported information regarding their breast cancer (i.e. disease stage, time since diagnosis, treatment) and other cancer history. NHANES controls were asked to indicate whether they had ever been diagnosed with cancer (yes/no). All women reporting a previous history of cancer including non-melanoma skin cancer were excluded from these analyses.
Physical activity and sedentary behavior
Participants from both studies were instructed to wear Actigraph accelerometers [Model 7164 for (NHANES) and Model GT1M (survivors), Health One Technology, Fort Walton Beach, FL], a valid and reliable objective activity [23, 24] and sedentary behavior [25] measure, for 7 consecutive days on the hip attached to an elastic belt during all waking hours, except when bathing or swimming. NHANES participants were given an accelerometer during their NHANES mobile examination center appointment while survivors received their accelerometer via mail. Both groups were provided with self-addressed stamped envelopes to return accelerometers to study investigators. Only data from monitors in calibration were used in these analyses.
For both groups, activity data were collected in one-minute intervals (epochs). In accordance with methods previously used to process NHANES data [26], non-wear time was defined as intervals of ≥60 consecutive minutes of zero counts, with allowance for up to 2 minutes of observations of <100 counts/min within the non-wear interval. A day of accelerometer wear was considered valid if it registered ≥10 hours of wear time. Each minute of wear time was classified according to intensity (counts/min) using commonly accepted activity count cut-points [25, 26] as follows: sedentary (<100), light activity (100–759), lifestyle activity (760–2019), and MVPA (≥ 2020). For a valid day of accelerometer wear, the number of minutes of wear time classified as sedentary, light, lifestyle, and MVPA were taken as estimates of time spent in these activities on the reporting day. The number of minutes with intensity counts ≥100 was taken as an estimate of “total” time spent active. Raw counts from the accelerometer were summed over wear minutes to obtain “total valid counts” for the reporting day. The number of minutes in each category were divided by wear time to estimate proportions of the day spent in the respective behavior. Daily estimates of average minutes and proportion of time spent sedentary and in each classified activity were averaged across all valid days per participant to estimate mean daily minutes and proportion of time. All values were adjusted for wear time to control for potential difference between individuals.
Matching Procedure
Participants were block-matched on BMI (<25, 25 to <30, ≥30), age (30–45 years, 46–60 years, > 60 years) and education (≤High School, >High School). We matched on these variables because of their consistent relationship to physical activity participation in the general population. [26] There were no breast cancer survivors in the following 2 groups: a) BMI <25, age 30–45 years and ≤ High School education and b) BMI <25, age 46–60 years and ≤ High School education, so no NHANES matches were selected for these groups. Thus, matching was performed within16 different matching groups. For each case, there was an average of 80.7 (SD=19.6; range= 23 to 144) possible NHANES matches. We randomly selected, with replacement, two NHANES matches for each survivor. We repeated the selection process 1,000 times to ensure the comparison between survivors and NHANES controls would not be dependent upon a single set of selected NHANES participants and to account for the variability that could be attributed to unmeasured confounders.
Data Analyses
Descriptive statistics were calculated for breast cancer survivors’ demographics and disease characteristics. Group means for sedentary behavior and activity variables were calculated separately for survivors and NHANES controls, and tests for differences between the means were conducted using independent-sample t-tests. Standard point estimates of the mean and standard error were used for the breast cancer survivor sample. Means and standard errors computed from NHANES controls accounted for variability induced by donor selection using the laws of total expectation and total variance as follows. The point estimate of the mean for controls was the average of the point estimates from the 1,000 iterations. The standard error was the square root of the total variance which was calculated by adding the sample variance of the 1,000 mean estimates to the average of the sample variances from the 1,000 iterations. Activity data examined included: total valid counts, time spent sedentary and in total, light, lifestyle, and MVPA activities and percentage of wear time spent sedentary and in total, light, lifestyle, and MVPA activities. Due to the skewness in total valid counts, we log transformed each participant’s average total valid counts before testing for between-group differences on this variable. A priori, we decided to focus on potential differences in sedentary, total, light, lifestyle, and MVPA estimated minutes and proportion, overall, and within eight subgroups defined by BMI (<25, 25 to <30, ≥30), age (30–45 years, 46–60 years, > 60 years) and education (≤High School, >High School). To control for the possibility of false positives due to multiple comparisons, the Benjamini–Hochberg false discovery rate procedure (BH step-up procedure; [27]) was used for all tests (126 total). Briefly, the nominal p-values for the tests were arranged from smallest to largest, and the k-th test was judged statistically significant only if the nominal p-value was less than .05×k/n. In what follows, we report nominal p-values for all tests. Discussions of significant findings pertain only to those identified after adjustment for multiple tests. All analyses were conducted using SAS (version 9.3, SAS Institute Inc., Cary, NC).
RESULTS
Participant Characteristics
Table 1 details disease-related variables for the breast cancer survivor sample. Briefly, the majority of women (66.1%) were diagnosed with early stage (I or II) disease, were not currently receiving treatment other than hormonal therapies (97.2%) and were post-menopausal at diagnosis (85.9%). Over half of women (53.8%) were ≥ 5 years post-diagnosis. The majority of women had been treated with surgery (79.4%) and 66.6% and 54.8% had received radiation therapy and chemotherapy, respectively. There were no significant differences between survivors and non-cancer controls with regard to the mean estimates for age (57.0 v. 58.4; p=0.09) or BMI (26.3 v. 27.1; p=0.07. indicating our block matching was successful.
Table 1.
Breast cancer survivors’ demographic and disease characteristics
| Breast Cancer Survivors (n=398) n (%) |
|
|---|---|
| Age (M, SD) | 57.0 (9.11) |
| Disease Stage | |
| Stage 0 | 76 (20.1%) |
| Stage 1 | 126 (33.3%) |
| Stage 2 | 124 (32.8%) |
| Stage 3 | 42 (11.15) |
| Stage 4 | 10 (2.7%) |
| Currently Receiving Treatment | |
| Chemotherapy | 10 (2.5%) |
| Treatment Received | |
| Surgery | 395 (99.5%) |
| Chemotherapy | 224 (56.3%) |
| Radiation Therapy | 268 (67.3%) |
| Time Since Diagnosis | 7.1 (5.5) |
| <5 years | 184 (46.2%) |
| 5 to < 10 years | 121 (30.4%) |
| ≥10 years | 93 (23.4%) |
| Post-menopausal at Diagnosis | 342 (85.9%) |
Valid Wear Time
Overall, 97.0% of survivors and 86.6% of controls had ≥3 valid days of accelerometer data which is often used as the standard for evaluating accelerometer data [28]. The average log transformed total valid counts (p=0.36) and wear time (p=0.21) did not significantly differ between groups.
Physical Activity
Results regarding the estimates for the various types of activities for both groups are presented in Table 2. On average, controls registered significantly greater total daily physical activity minutes than breast cancer survivors (346.9 v. 282.8, p<0.001). Survivors registered significantly fewer daily light (199.1 v. 259.3, p<0.001) and lifestyle (62.0 v. 71.7, p=0.001) minutes and significantly more MVPA minutes (21.6 v. 15.9, p<0.001) than controls.
Table 2.
Estimates and t-tests of differences between breast cancer survivors and non-cancer NHANES matched-controls for total average daily physical activity and different intensities of activities
| Breast Cancer Survivors (n=398) |
NHANES Controls | t-statistic | nominal p-value |
|||
|---|---|---|---|---|---|---|
| Est. Mean | Est. Std Err | Est. Mean | Est. Std Err | |||
| Total Daily Activity Time (mins) | 282.75 | 3.72 | 346.88 | 6.20 | −8.87 | <0.001* |
| Age Group | ||||||
| 30–45 years (n=35) | 297.39 | 12.60 | 367.34 | 16.84 | −3.33 | <0.001* |
| 46–60 years (n=195) | 296.93 | 5.45 | 374.18 | 8.17 | −7.72 | <0.001* |
| > 60 years (n=168) | 263.25 | 5.21 | 310.92 | 9.95 | −4.25 | <0.001* |
| Body Mass Index | ||||||
| <25 (n=200) | 296.16 | 5.12 | 364.93 | 8.85 | −7.09 | <0.001* |
| 25 to 30 (n=101) | 279.16 | 7.44 | 331.99 | 11.80 | −3.79 | <0.001* |
| > 30 (n=97) | 258.85 | 7.25 | 325.15 | 11.77 | −4.80 | <0.001* |
| Education | ||||||
| ≤ High School (n=30) | 285.84 | 14.74 | 348.93 | 20.07 | −2.53 | 0.011* |
| > High School (n=368) | 282.50 | 3.84 | 346.71 | 6.51 | −8.49 | <0.001* |
| Light Intensity Activity (mins) | 199.12 | 2.40 | 259.33 | 4.12 | −12.63 | <0.001* |
| Age Group | ||||||
| 30–45 years (n=35) | 200.92 | 8.35 | 256.64 | 11.72 | −3.87 | <0.001* |
| 46–60 years (n=195) | 202.84 | 3.54 | 270.58 | 5.58 | −10.24 | <0.001* |
| > 60 years (n=168) | 194.43 | 3.50 | 246.83 | 6.66 | −6.96 | <0.001* |
| Body Mass Index | ||||||
| <25 (n=200) | 203.92 | 3.45 | 265.79 | 5.81 | −9.15 | <0.001* |
| 25 to 30 (n=101) | 198.74 | 4.64 | 254.55 | 7.86 | −6.11 | <0.001* |
| > 30 (n=97) | 189.63 | 4.67 | 250.97 | 8.18 | −6.51 | <0.001* |
| Education | ||||||
| ≤ High School (n=30) | 205.70 | 9.09 | 267.93 | 13.84 | −3.76 | <0.001* |
| > High School (n=368) | 198.58 | 2.49 | 258.63 | 4.32 | −12.04 | <0.001* |
| Lifestyle Activity (mins) | 62.02 | 1.45 | 71.65 | 2.60 | −3.23 | 0.001* |
| Age Group | ||||||
| 30–45 years (n=35) | 67.75 | 4.07 | 87.93 | 6.59 | −2.61 | 0.009* |
| 46–60 years (n=195) | 69.00 | 2.12 | 83.65 | 3.54 | −3.55 | <0.001* |
| > 60 years (n=168) | 52.72 | 2.02 | 54.34 | 4.04 | −0.36 | 0.720 |
| Body Mass Index | ||||||
| <25 (n=200) | 64.86 | 1.94 | 78.93 | 4.01 | −3.16 | 0.002* |
| 25 to 30 (n=101) | 62.29 | 3.13 | 64.10 | 4.49 | −0.33 | 0.742 |
| > 30 (n=97) | 55.88 | 2.87 | 64.51 | 4.64 | −1.58 | 0.114 |
| Education | ||||||
| ≤ High School (n=30) | 63.82 | 5.21 | 69.87 | 8.38 | −0.61 | 0.539 |
| > High School (n=368) | 61.87 | 1.51 | 71.80 | 2.73 | −3.18 | 0.001* |
| MVPA (mins) | 21.62 | 0.98 | 15.90 | 1.05 | 3.99 | <0.001* |
| Age Group | ||||||
| 30–45 years (n=35) | 28.72 | 3.43 | 22.78 | 3.22 | 1.26 | 0.206 |
| 46–60 years (n=195) | 25.10 | 1.44 | 19.95 | 1.60 | 2.39 | 0.017* |
| > 60 years (n=168) | 16.09 | 1.34 | 9.75 | 1.32 | 3.94 | <0.001* |
| Body Mass Index | ||||||
| <25 (n=200) | 27.39 | 1.48 | 20.20 | 1.74 | 3.15 | 0.002* |
| 25 to 30 (n=101) | 18.13 | 1.63 | 13.35 | 1.67 | 2.05 | 0.041 |
| > 30 (n=97) | 13.34 | 1.58 | 9.67 | 1.23 | 0.29 | 0.774 |
| Education | ||||||
| ≤ High School (n=30) | 16.32 | 3.26 | 11.12 | 3.16 | −1.15 | 0.252 |
| > High School (n=368) | 22.05 | 0.25 | 16.29 | 1.10 | −3.84 | 0.0001* |
Note: MVPA=moderate and vigorous physical activity= ≥2020; Total activity = ≥100 counts/min; Light intensity activity= 100–759 counts/min; Lifestyle activity=760–2019 counts/min; values in bold indicate significant between groups difference at p <0.05
p-value significant after Benjamini–Hochberg procedure adjustment at α =0.05
Estimated differences in total and light activity were consistent between survivors and controls across analyses stratified on age, BMI and education. Difference in lifestyle activity estimates only remained for women who were aged 30–45 years (67.75 v. 87.93, p=0.009) and 46–60 years (69.00 v. 83.65, p≤ 0.001), normal weight (64.86 v. 78.93, p=0.002) and more educated (61.87 v. 71.80, p=0.001). Between-group differences in MVPA only remained significant for middle-aged (46–60; p=0.017), older (≥60; p<0.001), normal weight (p=0.002) and more educated (p=0.001) women.
Sedentary Time
Differences between estimates of daily sedentary time for breast cancer survivors and controls are presented in Table 3. On average, survivors spent an additional 55 minutes sedentary compared to controls (555.7 v. 500.6, p<0.001). This relationship was consistent across age, BMI and education (p≤0.02 for all).
Table 3.
Estimates and t-tests of differences between breast cancer survivors and non-cancer NHANES matched-controls for total average daily sedentary time
| Breast Cancer Survivors (n=398) |
NHANES Controls | t-statistic | nominal p-value |
|||
|---|---|---|---|---|---|---|
| Est. Mean | Est. Std Err | Est. Mean | Est. Std Err | |||
| Total Sedentary Time (mins) | 555.70 | 3.56 | 500.59 | 6.62 | 7.33 | <0.001* |
| Age Group | ||||||
| 30–45 years (n=35) | 554.45 | 9.00 | 463.48 | 18.72 | 4.38 | <0.001* |
| 46–60 years (n=195) | 542.51 | 5.26 | 488.05 | 8.96 | 5.24 | <0.001* |
| > 60 years (n=168) | 571.26 | 5.31 | 522.87 | 10.99 | 3.96 | <0.001* |
| Body Mass Index | ||||||
| <25 (n=200) | 549.17 | 4.67 | 490.71 | 9.70 | 5.43 | <0.001* |
| 25 to 30 (n=101) | 564.75 | 7.11 | 512.05 | 12.31 | 3.71 | <0.001* |
| > 30 (n=97) | 559.75 | 8.08 | 509.01 | 13.68 | 3.19 | 0.001* |
| Education | ||||||
| ≤ High School (n=30) | 545.81 | 15.23 | 479.82 | 22.82 | 2.41 | 0.016* |
| > High School (n=368) | 556.51 | 3.65 | 502.28 | 6.95 | 6.91 | <0.001* |
Note: Sedentary behaviors <100 counts/min; values in bold indicate significant between groups difference at p <0.05
p-value significant after Benjamini–Hochberg procedure adjustment at α =0.05
Proportion of Time Spent Active and Sedentary
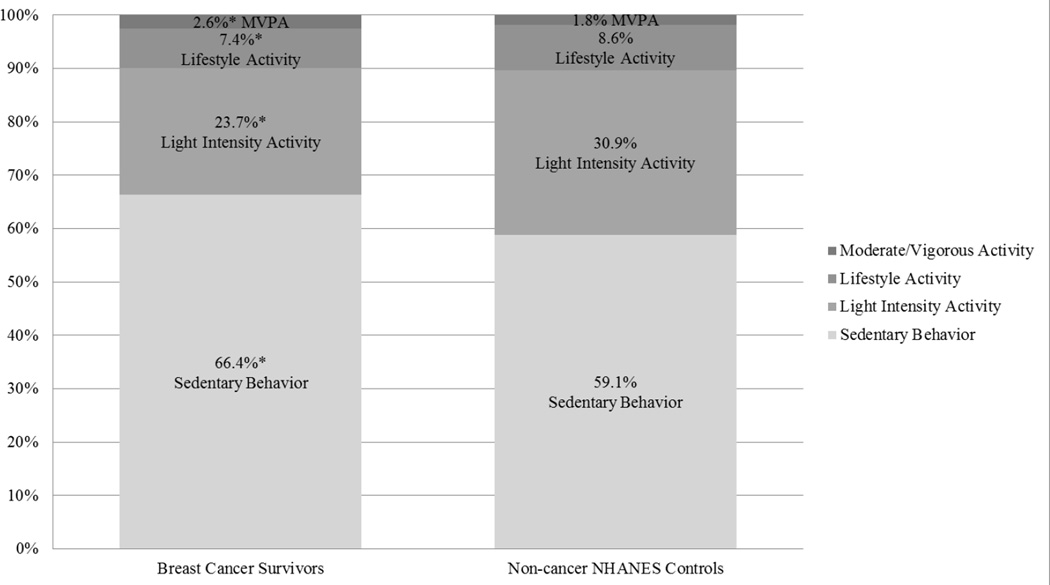
On average, compared to controls, the proportion of time registered as sedentary was higher in breast cancer survivors (66.4% v. 59.1%, p<0.001; see Figure 1). The proportion of time registered in light and lifestyle activities were significantly lower for survivors compared to controls (23.7% v. 30.9%, p<0.001 and 7.4% v. 8.4%,p=0.002, respectively). However, survivors registered a significantly greater proportion of time in MVPA (2.6% v. 1.8%, p=<0.001). Differences persisted across all age groups, BMI categories and education for the proportion of time spent sedentary (p≤ 0.02 for all) and in light intensity activity (p≤ 0.02 for all). Differences in the proportion of time registered in lifestyle activity and MVPA remained significant for most groups. Lifestyle activity did not differ for women who were ≥60 (p=0.77), overweight (p=0.74) and obese (p = 0.15) and less educated (p=0.51). MVPA did not differ for younger (30 to 45; p=0.28) and less educated women (p=0.27).
Figure 1.
Estimated proportions of daily time spent in sedentary behavior and each activity type for breast cancer survivors and non-cancer NHANES matched-controls.
*All proportion were significant at p<0.05 even after correcting for multiple tests using Benjamini–Hochberg procedure (BH step-up procedure) at α =0.05
DISCUSSION
By using accelerometers to objectively compare breast cancer survivors to healthy controls on a full range of activity intensities (sedentary, light, lifestyle, MVPA), this study expands on current knowledge regarding participation in sedentary and activity behaviors among survivors. Our findings suggest survivors registered significantly more time sedentary (about 1 hour/day more) and less time active (about 1 hour/day less light/lifestyle activity) than healthy controls. However, breast cancer survivors registered significantly more time in MVPA than controls, although the magnitude of this difference was small (about 6 minutes), and these levels were not adequate to meet physical activity recommendations [8] or result in significant differences in total daily energy expenditure. Generally, findings were consistent across age, BMI and education. Between-group differences were not observed for older (≥60 years), overweight/obese, or less educated women for lifestyle activity and younger (≤45 years), obese, or less educated women for MVPA.
Overall, our estimates of survivors’ activity and sedentary behavior are similar to other published studies using accelerometry [12, 16]. However, our sample registered more MVPA than those previous studies by Lynch and colleagues (21.6 v. 3.7 minutes; [12]) and Sabiston and colleagues (21.6 v. 14.2 to 16.3 minutes; [16]) and slightly less sedentary behavior than Sabiston et al. (555.7 v. 641.4 to 647.5; [16]). Our sedentary estimates were identical to those of Lynch et al.[12] These differences could potentially be explained by sample recruitment sources. Higher levels MVPA in our sample may result from recruitment of a more educated, motivated, and healthier population via Army of Women.© Additionally, our sample was restricted to White women, recruited nationally and consisted of longer-term survivors. The higher sedentary behavior levels observed by Sabiston et al. could be due to recruitment of participants from regional clinics and hospitals immediately post-treatment whereas our study and Lynch et al.’s were more heterogeneous in terms of time post-treatment and geography.
Despite the large number of MVPA interventions and large body of evidence indicating increased MVPA is beneficial for breast cancer survivors [3, 29], the current study provides further evidence that very few survivors engage in enough MVPA to fully realize these benefits even if they are engaging in more MVPA than similar non-cancer controls. This paradox of substantial benefits, yet lack of participation, is not unique to survivors [19], but represents a significant challenge cancer survivorship research [30]. Our findings indicating breast cancer survivors spend significantly less time participating in lower intensity activities than healthy controls add an additional dimension to this paradox. Survivors may not only engage in insufficient MVPA, but also insufficient lower intensity activity. As emerging evidence suggests lower intensity activities are associated with health benefits [17, 18, 20, 21] after controlling for MVPA, increasing these behaviors in survivors may have important health implications. Furthermore, lower intensity activities may contribute to the baseline fitness and functioning necessary to perform MVPA so limited participation in low intensity activities may be related to lower MVPA [11]. Finally, our findings could have implications for weight management. For example, in our sample, the energy expenditure from an extra 5.7 minutes of MVPA (i.e. brisk walking= 3.3 METs; ~21 calories for a 140 pound survivor; [31]) would not compensate for the missed energy expenditure from 9.7 minutes of lifestyle activity (i.e. light cleaning =2.5 METs; ~27 calories; [31]) and 60.2 minutes of light intensity activity (i.e. household walking=2.0 METs; ~134 calories; [31]).
These data suggest future research is warranted in cancer survivors to: a) examine potential health benefits of lower intensity activity, b) explore the relationship between lower intensity activities and MVPA and c) identify the minimal dosages of activity necessary to achieve clinically meaningful health benefits. This is not to discount the importance of identifying the dosage to maximize health benefits, but rather to identify a minimal activity level that may be easier for survivors to achieve and incorporate into their daily lives. Finally, future research should examine how activity patterns change pre- and post-diagnosis using objective measures and investigate multi-level factors including treatment-related side effects, physical environment and behavioral factors influencing breast cancer survivors’ participation in different activity types to identity potential intervention targets.
Our results indicate breast cancer survivors not only spend less time active than controls but also more time sedentary. Given emerging evidence supporting the negative health consequence of sedentary behavior in the general population [32, 33] and cancer survivors [10, 12, 34, 35], these findings are particularly concerning. While it is biologically plausible increased sedentary behavior could be related to poorer health outcomes in survivors through increased adiposity, hormone dysregulation, metabolic dysfunction and inflammation [34], additional research is needed to understand the relationship between sedentary behavior, health outcomes and survival in breast cancer survivors and other survivor populations. This research should include examining dose-response relationships, identifying clinically-meaningful cut-points and investigating the effects of breaking up sedentary time [11]. Additionally, it is important to develop a better understanding of the multi-level determinants of sedentary behavior to identify potential intervention targets in survivors. Finally, future research should examine the relationship between different activity intensities and sedentary behavior in cancer survivors.
Collectively, findings from this study highlight breast cancer survivors’ low physical activity and high sedentary behavior compared to controls using objective measures. While many important benefits may result from increasing cancer survivors’ MVPA, it may be equally important, and potentially more feasible at a population-level, to increase lower intensity activities and reduce sedentary behavior. Future research is warranted to understand the potential benefits and risks of increasing lower intensity activities and reducing sedentary behavior and the feasibility and effectiveness of these interventions in comparison to, or in conjunction with, interventions to increase MVPA in breast cancer survivors and other survivor populations.
There are several limitations to the current study worth noting. First, we used a convenience sample of breast cancer survivors who were predominately well-educated, were relatively young and had early stage disease. Additionally, because of the low number of minority participants, we had to limit our sample to White women. Therefore, our sample may not be representative of the larger breast cancer survivor population. In addition, we did not use a true case control-design, but rather gathered our controls from a population-based sample and used a block-matching design. Thus, study methods and participant recruitment were different. We were unable to perfectly match patients on specific characteristics and could have neglected a key matching characteristic (i.e. comorbidity) or mismatched on our criteria (i.e. self-reported v. measured BMI) which could have influenced estimates of between-group differences. However, we conducted simulation analyses of 1,000 iterations to correct for potential errors resulting from our matching-scheme, and cases and controls did not differ on matching variables suggesting our matching scheme was successful. Additionally, we are not able to infer why differences in time spent active and sedentary might exist from our data. As mentioned above, future studies should explore determinants of changes in survivors’ activity and sedentary behavior patterns to identify intervention targets. Finally, while accelerometers are not subject to self-report bias, they do not capture accurate workloads pertaining to certain activities (e.g. stationary biking, swimming, weight training) or data relative to body positioning (e.g. sitting, standing, lying down). Despite these limitations, this study improves on past studies examining objective activity and sedentary behavior in breast cancer survivors by comparing activity levels to controls and incorporating a large group of relatively heterogeneous survivors in terms of disease characteristics (e.g. time since treatment, age, stage).
In conclusion, the current study suggests breast cancer survivors spend less time active and more time sedentary than healthy controls despite the fact that they engage in more MVPA. It is critical to further understand the health implications of these behavioral patterns and better understand their determinants at various time points post-treatment and diagnosis so effective interventions can be developed to increase physical activity, reduce sedentary time and, ultimately, enhance health and disease outcomes in breast cancer survivors and other survivor populations.
Highlights.
Breast cancer survivors registered more moderate-to-vigorous physical activity than controls but still did not participate meet public health recommendations.
Breast cancer survivors are engaged in more sedentary behavior and less low intensity activity than matched non-cancer controls.
Sedentary behavior and light intensity activity may be viable intervention targets in addition to moderate-to-vigorous physical activity.
Acknowledgments
Funding Source: This work was supported by: grant #F31AG034025 from The National Institute on Aging (SP); a National Cancer Institute Cancer Prevention Fellowship Program Cancer Research Training Award (SP); Shahid and Ann Carlson Khan endowed professorship (EM) and grant #AG020118 from the National Institute on Aging (EM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: Data collection and analyses for this project was completed at the University of Illinois Urbana-Champaign and the National Cancer Institute. Dr. Alfano is now with the American Cancer Society.
Disclosures: The authors have no financial disclosures to report.
REFERENCES
- 1.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–765. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 2.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 4.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 5.Courneya KS, Katzmarzyk PT, Bacon E. Physical activity and obesity in Canadian cancer survivors. Cancer. 2008;112:2475–2482. doi: 10.1002/cncr.23455. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. 2008 [Google Scholar]
- 8.Schmitz KH, Courneya KS, Matthews CE, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sport Exer. 2010 doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 9.Kim RB, Phillips A, Herrick K, Helou M, Rafie C, Anscher MS, et al. Physical activity and sedentary behavior of cancer survivors and non-cancer individuals: results from a national survey. PLoS ONE. 2013;8:e57598. doi: 10.1371/journal.pone.0057598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch BM, Cerin E, Owen N, Hawkes AL, Aitken JF. Television viewing time of colorectal cancer survivors is associated prospectively with quality of life. Cancer Causes Control. 2011;22:1111–1120. doi: 10.1007/s10552-011-9786-8. [DOI] [PubMed] [Google Scholar]
- 11.Lynch BM, Dunstan DW, Vallance JK, Owen N. Don't take cancer sitting down. Cancer. 2013;119:1928–1935. doi: 10.1002/cncr.28028. [DOI] [PubMed] [Google Scholar]
- 12.Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003–2006) Cancer Causes Control. 2010;21:283–288. doi: 10.1007/s10552-009-9460-6. [DOI] [PubMed] [Google Scholar]
- 13.Matthews CE, George SM, Moore SC, Bowles HR, Blair A, Park Y, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. The American journal of clinical nutrition. 2012;95:437–445. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31:876–885. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58:M82–M91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 16.Sabiston CM, Brunet J, Vallance JK, Meterissian S. Prospective examination of objectively-assessed physical activity and sedentary time after breast cancer treatment: Sitting on the crest of the teachable moment. Cancer Epidemiology Biomarkers & Prevention. 2014 doi: 10.1158/1055-9965.EPI-13-1179. cebp. 1179.2013. [DOI] [PubMed] [Google Scholar]
- 17.Dunlop DD, Song J, Semanik PA, Sharma L, Bathon JM, Eaton CB, et al. Relation of physical activity time to incident disability in community dwelling adults with or at risk of knee arthritis: prospective cohort study. BMJ. 2014;348 doi: 10.1136/bmj.g2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loprinzi PD, Lee H, Cardinal BJ. Evidence to Support Including Lifestyle Light-Intensity Recommendations in Physical Activity Guidelines for Older Adults. Am J Health Promot. 2014 doi: 10.4278/ajhp.130709-QUAN-354. [DOI] [PubMed] [Google Scholar]
- 19.Motl RW. Lifestyle physical activity in persons with multiple sclerosis: the new kid on the MS block. Multiple Sclerosis Journal. 2014:1352458514525873. doi: 10.1177/1352458514525873. [DOI] [PubMed] [Google Scholar]
- 20.Blair CK, Morey MC, Desmond RA, Cohen HJ, Sloane R, Snyder DC, et al. Light-Intensity Activity Attenuates Functional Decline in Older Cancer Survivors. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thraen-Borowski KM, Trentham-Dietz A, Edwards DF, Koltyn KF, Colbert LH. Dose–response relationships between physical activity, social participation, and health-related quality of life in colorectal cancer survivors. J Cancer Surviv. 2013;7:369–378. doi: 10.1007/s11764-013-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psycho-Oncology. 2012 doi: 10.1002/pon.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassett DR. Validity of four motion senso moderate intensity physical activity. Med Sci Sports Exerc. 2000;32 doi: 10.1097/00005768-200009001-00006. S471.rs in measuring. [DOI] [PubMed] [Google Scholar]
- 24.Tudor-Locke C, Ainsworth B, Thompson R, Matthews C. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Sci Sports Exerc. 2002;34:2045. doi: 10.1097/00005768-200212000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995:289–300. [Google Scholar]
- 28.Trost SG, Mciver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37:S531. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 30.Courneya KS, Friedenreich CM. Semin Oncol Nurs. Elsevier; 2007. Physical activity and cancer control; pp. 242–252. [DOI] [PubMed] [Google Scholar]
- 31.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 32.Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS ONE. 2012;7:e34916. doi: 10.1371/journal.pone.0034916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katzmarzyk PT, Lee I-M. Sedentary behaviour and life expectancy in the USA: a cause-deleted life table analysis. BMJ open. 2012;2 doi: 10.1136/bmjopen-2012-000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiology Biomarkers & Prevention. 2010;19:2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 35.Wijndaele K, Lynch BM, Owen N, Dunstan DW, Sharp S, Aitken JF. Television viewing time and weight gain in colorectal cancer survivors: a prospective population-based study. Cancer Causes Control. 2009;20:1355–1362. doi: 10.1007/s10552-009-9356-5. [DOI] [PubMed] [Google Scholar]