Abstract
Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and persistent pesticides have been measured in pooled samples representative of the general noninstitutionalized population of the United States. The pools were made from individual sera from the National Health and Nutrition Examination Survey (NHANES) during 2005/06 and 2007/08. The pooled concentrations have been contrasted to NHANES 2003/04 individual measurements to evaluate changes in concentration over time and within survey period differences among age groups, race/ethnicity groups (Mexican American, non-Hispanic Black, non-Hispanic White), and sex. The arithmetic mean serum concentrations of several PCB congeners decreased from NHANES 2003/04 through 2007/08. Larger percentage reductions were seen for younger subjects (12–19 years) compared with older subjects (≥60 years). For example, the arithmetic mean concentration of 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB-153) was 36% lower in 12–19 year old adolescents when comparing NHANES 2007/08 with 2003/04; while for subjects over the age of 60 a 14% lower concentration was seen, although, the 95% confidence intervals overlapped. Similarly, the arithmetic mean serum concentrations of tri- to hexaBDEs were lower in NHANES 2007/08 than in 2003/04; however, most confidence intervals of the arithmetic means overlapped. These findings suggest that a reduction in PBDE serum concentrations cannot yet be detected following the discontinuation of pentaBDE in 2004.
Graphical abstract
INTRODUCTION
The Centers for Disease Control and Prevention (CDC) through the National Health and Nutrition Examination Survey (NHANES) provides an ongoing assessment of the general noninstitutionalized U.S. population’s exposure to environmental chemicals by demographic characteristics such as age, race/ethnicity, and sex.
NHANES biomonitoring results, presented in CDC’s National Reports on Human Exposure to Environmental Chemicals,1 include data on persistent organic pollutants (POPs) like polybrominated diphenyl ethers (PBDEs), found in technical PentaBDE, containing tri- through hexabrominated congeners, and OctaBDE, containing hexa- and heptabrominated congeners, which were produced as flame retardants until their withdrawal from the U.S. market in 2004. Commercial DecaBDE, containing almost exclusively the perbrominated congener (PBDE209), has been scheduled to be phased-out by the end of 2013 for all applications by the two U.S. producers and the largest U.S. importer.2 PentaBDE was added to polyurethane foam used in multiple applications, including furniture seat cushions and in pads under wall-to-wall carpets. Technical Octa- and DecaBDE were added to hard plastics used in the casing for electrical appliances among other applications. Exposure routes to PBDEs include ingestion of residential indoor dust3 and dietary intake including fatty fish and other food stuffs, such as red meat and poultry.4 Diet is the predominant source of human exposure for other POPs evaluated in NHANES, such as polychlorinated biphenyls (PCBs), and pesticides hexachlorobenzene (HCB), 2,2-bis-(2-chloro-phenyl)-1,1-dichloroethene (p,p′-DDE) and 2,2-bis-(2-chloro-phenyl)-1,1,1-trichloroethane (p,p′-DDT),1 due to biomagnification through the food chain. PCBs and persistent pesticides have been withdrawn from the market for over four decades.
One important objective of NHANES biomonitoring program is to capture changes in exposures to environmental chemicals resulting from regulatory intervention, changes in chemical usage pattern, and/or human behavior. Historically, exposures have been categorized from concentrations of environmental compounds and/or their metabolites in individual serum/urine measurements from a representative subsample of the population. Compared to individual samples, pooling of samples allows for larger sample volumes which can result in improved analytical detection limits and reduced analysis cost.
We report the concentrations of PCBs, persistent pesticides and PBDEs—some for the first time (i.e., PBDE209)—in serum pools created from NHANES 2005/06 and 2007/09. We also compare these pooled data with individual measurement data from NHANES 2003/04.
MATERIALS AND METHODS
Since 1999, CDC has annually conducted NHANES to assess the health and nutritional status of the civilian noninstitutionalized U.S. population.1 NHANES includes household interviews, standardized physical examinations, and collection of medical histories and biologic specimens. Some of these specimens are used to assess exposure to environmental chemicals.1 For this study, serum specimens were collected from a one-third subset of NHANES 2005/06 and 2007/08 participants over the age of 12 and published data were used for NHANES 2003/04.1 The representative design of the survey was maintained because the subset was random. The National Centers for Health Statistics Institutional Review Board reviewed and approved the study protocol. All participants gave informed written consent; parents or guardians provided consent for participants <18 years of age.
NHANES Sampling Weights
NHANES is a multistage, probability sampling survey. Oversampling of certain population subgroups is performed to increase the reliability and precision of estimates for those groups. Sample weighting adjusts for unequal selection probability and is used to produce correct population estimates of means, percentiles, and other descriptive statistics. The determination of sample weights is conducted at the National Center for Health Statistics (NCHS) as follows:5 Nonpublic “Base sample weights” are assigned to all persons solicited to participate in the survey and later adjusted for (1) nonresponse for interview and/or examination (2) nonresponse for biological sampling. Subsample weights are assigned to all persons who are both selected into the approximately one-third subsample for the laboratory tests and who have sufficient serum for laboratory testing. The 42 demographic groups used in adjusting subsample weights are formed by the cross classification of (1) sex (male, female); (2) age group (6–11, 12–19, 20–29, 30–39, 40–49, 50–59, ≥60 years); and (3) race/ethnicity: non-Hispanic Black (NHB), non-Hispanic White (NHW), Mexican American, and other Hispanic, other non-Hispanic multiracial, and all others (OTHER).
Pooling Strategy
Each NHANES 2005/06 and 2007/08 aliquot was identified as belonging to one of 32 demographic groups based on two sexes, four age (12–19, 20–39, 40–59, and ≥60 years) and four race/ethnicity (MA, NHW, NHB, OTHER) categories. Of the 2201 NHANES 2005/06 individual samples, 1973 samples were available to create 247 pools. Similarly, 2070 samples of a total of 2098 were available to create 264 pools for NHANES 2007/08. The number of pools created for each demographic group varied depending on the available number of individual samples (Supporting Information, SI, Table S1).
The individual NHANES samples were assigned to pools using a SAS program (SAS 9.3; SAS Institute Inc., Cary, NC) to merge: (1) NCHS data file of subsample weights; (2) NCHS file containing demographic information; and (3) Division of Laboratory Sciences file containing the available serum volume for each sample. The resulting merged data file was sorted by demographic group and by the inverse subsample weight. A target of eight samples per pool was chosen based on the results of simulation experiments,6 but some pools contained fewer samples (SI Table S1). Then for each demographic group, the program assigned the first eight samples to the first pool, the next eight samples to the second pool, and so on until all samples were assigned to pools or until an insufficient number of serum samples were available to create additional pools. Thereafter, the program created a new variable, equal to the subsample weight of an individual sample divided by the sum of subsample weights of all samples in the corresponding pool, to calculate the proportional amount of each sample to be added to a pool so that the added volume was directly proportional to the subsample weight. The desired volume of each pool was 40 mL; hence the program flagged any samples with insufficient available volume to meet the proportional volume requirement. The process was repeated after removal of flagged samples until no sample included in a pool had an insufficient volume. Measurements of pools created in this way are mathematically equal to weighted arithmetic means of the samples in the pools in keeping with the NHANES weighted sampling design so that the estimates are representative of the general noninstitutionalized U.S. population.
Pooling of the Serum
The individual serum samples were thawed and mixed before the desired serum amounts were weighed into 2-oz precleaned KaptClean Qorpak borosilicate glass bottles with Teflon PTFE lined polypropylene screw caps. Serum was transferred using a new glass pipet for each sample. The total weight of each pool was determined as the difference in weight between the empty bottle and the bottle plus the serum on a AX105 (Mettler Toledo, Columbus, OH) balance. Separate aliquots of the serum pools were made for (1) nonortho-PCBs, polychlorinated dibenzo-p-dioxins and furans, polybrominated dibenzo-p-dioxins and furans, and polychlorinated naphthalenes (34 mL); (2) PBDEs, PCBs, and persistent pesticides (4 mL); (4) phenolic compounds including metabolites of PCBs and halogenated phenols (2 mL); and (5) serum lipids (total cholesterol and triglycerides, 0.5 mL). Each aliquot was labeled with the study and serum pool identification number and target analytes.
Analytical method for the quantification of target analytes
The sample preparation method is based on the original work by Hovander et al.7 after modification and automation conducted in our laboratory;8 the Standard Operating Procedure documentation is publicly available at: http://www.cdc.gov/nchs/nhanes.htm. Two grams of serum were used for the measurements of PBDEs, PCBs, and persistent pesticides and the pools were randomly assigned to 24 sample batches for analysis; each analytical batch contained three quality control and three blank samples prepared with bovine serum (Gibco Inc. Grand Island, NY) diluted 1:40 with water. All analytical data were subtracted using the median blank. The limits of detection (LODs) were determined as the higher of (1) three times the standard deviation of the amount present in blanks or (2) the instrumental LOD defined as the injected amount known to produce a gas chromatography/isotope dilution–high resolution mass spectrometry signal/noise ratio >3. The median amount in pg/g serum detectable in blank samples analyzed in parallel with unknown samples as well as the LOD for each analyte measured is given in SI Table S2. The CV of quality control samples made from the standard reference material (SRM) 19589 was below 10%.
Statistical Methods
Statistical analysis was performed using SAS 9.3 or SAS Enterprise Guide 5.1 (SAS Institute Inc., Cary, NC). Concentrations below the LOD were substituted with LOD/√ 2. Weighted arithmetic means by demographic group and analyte were calculated using the SAS procedure “Proc Univariate.” The 95% confidence intervals (95%CI) of the weighted arithmetic means were calculated and adjusted by the design effects determined for NHANES 2003/04 and applied to the 2005/06 and 2007/08 NHANES cycles. The formula for calculating the NHANES 2005/06 and 2007/08 adjusted 95% CIs was as follows:
where meanWA is the weighted arithmetic means, t(1-α/2,DF) is a Students t-distributed critical value, α is equal to 0.025, and DF is equal to the number of pools minus one.
Weighted arithmetic means and adjusted standard errors were calculated for the NHANES 2003/04 individual sample data to produce results comparable to the pooled data for NHANES 2005/06 and 2007/08.
To increase the statistical power for studying potential concentration changes during 2003–2008, the sex and race/ethnicity variables were collapsed to increase the number of samples per remaining age group and a new set of weighted arithmetic means and adjusted 95% CIs were calculated. Univariate comparisons of 95% CIs of the arithmetic means were performed to assess if differences between groups were statistically significant. Statistical significance was indicated if the 95% CIs did not overlap.
RESULTS
A total of 58 POPs, including PBDEs, PCBs, and persistent pesticides, were measured in NHANES 2005/06 and 2007/08 pooled sera (SI Table S2). For further statistical evaluation, only analytes with detection frequencies ≥50% in all demographic groups and all survey periods were considered (SI Table S3). PBDE-209, the main component of DecaBDE, had relatively low detection frequencies in NHANES 2005/06 (12%) and 2007/08 (11%). p,p′-DDE had the highest concentration in all demographic groups compared with the sum of PCBs and PBDE congeners with detection frequencies ≥50% (Table 1 and 2).
Table 1.
Arithmetic Mean Concentration (ng/g lipid) and 95% CI of Select Polybrominated Diphenyl Ethers (PBDEs) in NHANES 2003/04 (Individual Samples) and NHANES 2005/06 and 2007/08 (Pooled Samples) by Survey Period and Age Groupa
| survey period | concentration (ng/g lipid) with 95% CI by age Group
|
|||
|---|---|---|---|---|
| 12–19 years | 20–39 years | 40–59 years | ≥60 years | |
| 2,4,4′-tribromodiphenyl ether (PBDE28) | ||||
| 2003–04 | 1.9 ± 0.3 | 1.9 ± 0.4 | 2.1 ± 0.7 | 3.0 ± 0.7 |
| 2005–06 | 2.1 ± 0.5 | 1.9 ± 0.5 | 1.4 ± 0.4 | 2.7 ± 0.7 |
| 2007–08 | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.6 ± 0.5 | 2.4 ± 0.5 |
| 2,2′,4,4′-tetrabromodiphenyl ether (PBDE47) | ||||
| 2003–04 | 49.4 ± 8.8 | 44.9 ± 17.4 | 42.2 ± 13.2 | 52.4 ± 16.3 |
| 2005–06 | 61.3 ± 14.0 | 45.1 ± 17.1 | 25.4 ± 8.9 | 61.4 ± 27.4 |
| 2007–08 | 35.9 ± 8.0 | 31.0 ± 7.4 | 28.1 ± 8.9 | 39.9 ± 10.6 |
| 2,2′,3,4,4′-pentabromodiphenyl ether (PBDE85) | ||||
| 2003–04 | 1.6 ± 0.2 | 1.4 ± 0.5 | 1.5 ± 0.5 | 1.5 ± 0.4 |
| 2005–06 | 1.5 ± 0.3 | 1.0 ± 0.4 | 0.5 ± 0.3* | 1.6 ± 0.8 |
| 2007–08 | 0.8 ± 0.2* | 0.7 ± 0.2 | 0.7 ± 0.3 | 0.9 ± 0.2* |
| 2,2′,4,4′,5-pentabromodiphenyl ether (PBDE99) | ||||
| 2003–04 | 12.6 ± 2.3 | 13.3 ± 7.7 | 11.4 ± 3.2 | 12.3 ± 4.5 |
| 2005–06 | 15.7 ± 3.8 | 11.3 ± 5.7 | 5.5 ± 2.0* | 13.6 ± 6.5 |
| 2007–08 | 8.5 ± 2.5 | 6.8 ± 2.2 | 6.6 ± 2.0 | 8.7 ± 2.7 |
| 2,2′,4,4′,6-pentabromodiphenyl ether (PBDE100) | ||||
| 2003–04 | 10.0 ± 2.4 | 9.1 ± 2.9 | 9.3 ± 3.7 | 10.9 ± 3.4 |
| 2005–06 | 12.4 ± 3.2 | 8.9 ± 3.1 | 4.9 ± 1.8 | 12.8 ± 6.1 |
| 2007–08 | 6.7 ± 1.6 | 6.8 ± 1.4 | 6.2 ± 2.1 | 8.1 ± 2.3 |
| 2,2′,4,4′,5,5′-hexabromodiphenyl ether (PBDE153) | ||||
| 2003–04 | 16.1 ± 5.7 | 15.4 ± 3.7 | 15.8 ± 7.6 | 17.6 ± 4.7 |
| 2005–06 | 15.8 ± 4.4 | 11.7 ± 2.9 | 9.0 ± 2.2 | 13.3 ± 4.4 |
| 2007–08 | 12.0 ± 3.3 | 13.8 ± 2.8 | 11.5 ± 2.8 | 13.4 ± 3.3 |
Significantly different 95% CI of the arithmetic mean compared with NHANES 2003/04 is indicated with an asterisk.
Table 2.
Arithmetic Mean Concentration (ng/g Lipid) and 95% CI of Select Chlorinated Persistent Organic Pollutants in NHANES 2003/04 (Individual Samples) and NHANES 2005/06 and 2007/08 (Pooled Samples) by Survey Period and Age Groupa
| survey period | concentration (ng/g lipid) with 95% CI by age Group
|
|||
|---|---|---|---|---|
| 12–19 | 20–39 | 40–59 | ≥60 | |
| 2,4,4′,5-tetrachlorobiphenyl (PCB74) | ||||
| 2003–04 | 2.5 ± 0.3 | 3.3 ± 0.2 | 7.5 ± 0.7 | 17.9 ± 1.7 |
| 2005–06 | 1.8 ± 0.2* | 2.6 ± 0.3* | 6.0 ± 0.6* | 15.8 ± 2.4 |
| 2007–08 | 1.5 ± 0.3* | 2.2 ± 0.2* | 5.2 ± 0.5* | 13.2 ± 1.2* |
| 2,2′,4,4′,5-pentachlorobiphenyl (PCB99) | ||||
| 2003–04 | 2.8 ± 0.4 | 3.5 ± 0.3 | 6.7 ± 1.1 | 12.4 ± 2.2 |
| 2005–06 | 1.4 ± 0.3* | 2.4 ± 0.4* | 4.6 ± 0.8* | 9.8 ± 1.4 |
| 2007–08 | 1.2 ± 0.4* | 2.2 ± 0.3* | 4.2 ± 0.6* | 8.9 ± 1.0* |
| 2,3′,4,4′,5-pentachlorobiphenyl (PCB118) | ||||
| 2003–04 | 3.7 ± 0.5 | 4.9 ± 0.6 | 10.2 ± 1.8 | 23.3 ± 3.5 |
| 2005–06 | 2.2 ± 0.4* | 3.5 ± 0.8 | 7.9 ± 1.5 | 19.3 ± 3.6 |
| 2007–08 | 2.2 ± 0.5* | 3.7 ± 0.6* | 6.9 ± 0.9* | 18.8 ± 2.8 |
| 2,2′,3,4,4′,5′- and 2,3,3′,4,4′,6-hexachlorobiphenyl (PCB138–158) | ||||
| 2003–04 | 6.2 ± 0.8 | 11.8 ± 1.3 | 28.7 ± 3.9 | 53.0 ± 5.9 |
| 2005–06 | 3.6 ± 0.6* | 6.9 ± 1.1* | 17.8 ± 2.9* | 35.8 ± 5.8* |
| 2007–08 | 4.0 ± 1.0* | 8.3 ± 1.3* | 21.9 ± 2.4* | 46.5 ± 4.8 |
| 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) | ||||
| 2003–04 | 7.7 ± 1.0 | 15.1 ± 1.8 | 38.4 ± 4.7 | 70.6 ± 7.3 |
| 2005–06 | 5.4 ± 0.9* | 11.5 ± 2.0 | 31.4 ± 5.1 | 63.3 ± 9.4 |
| 2007–08 | 4.9 ± 1.1* | 10.3 ± 1.6* | 29.3 ± 2.7* | 60.8 ± 6.2 |
| 2,2′,3,4,4′,5,5′-heptachlorobiphenyl (PCB180) | ||||
| 2003–04 | 4.9 ± 0.7 | 12.4 ± 3.5 | 32.4 ± 3.1 | 60.2 ± 6.8 |
| 2005–06 | 3.3 ± 0.5* | 8.4 ± 1.9 | 28.1 ± 5.7 | 54.9 ± 8.7 |
| 2007–08 | 3.0 ± 0.6* | 7.5 ± 1.4 | 24.6 ± 1.8* | 51.3 ± 6.0 |
| 2,2′,3,3′,4,4′,5,5′-octachlorobiphenyl (PCB194) | ||||
| 2003–04 | 1.0 ± 0.3 | 2.5 ± 0.7 | 7.3 ± 0.6 | 14.4 ± 2.1 |
| 2005–06 | 0.5 ± 0.1* | 1.7 ± 0.5 | 6.1 ± 0.9 | 13.2 ± 2.2 |
| 2007–08 | 0.6 ± 0.2 | 1.7 ± 0.3 | 6.2 ± 0.5* | 13.3 ± 1.5 |
| hexachlorobiphenyl (HCB) | ||||
| 2003–04 | 14.6 ± 0.9 | 14.4 ± 0.9 | 18.0 ± 1.9 | 20.5 ± 1.2 |
| 2005–06 | 6.5 ± 0.8* | 7.4 ± 0.7* | 9.2 ± 0.8* | 12.9 ± 1.3* |
| 2007–08 | 9.4 ± 1.1* | 8.7 ± 0.5* | 11.5 ± 1.6* | 13.7 ± 1.0* |
| 2,2-bis(4-chorophenyl)-1,1-dichloroethene (p,p′-DDE) | ||||
| 2003–04 | 198.8 ± 75.4 | 265.4 ± 76.5 | 573.1 ± 174.4 | 1062.9 ± 237.7 |
| 2005–06 | 267.5 ± 349.5 | 507.0 ± 212.9 | 436.6 ± 232.7 | 957.0 ± 420.9 |
| 2007–08 | 103.9 ± 27.9 | 261.4 ± 87.7 | 330.7 ± 126.1 | 746.5 ± 288.9 |
Significantly different 95% CI of the arithmetic mean compared with NHANES 2003/04 is indicated with an asterisk.
Changes in POP Serum Concentration by Survey Period
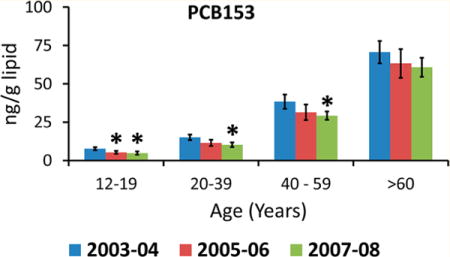
The weighted arithmetic mean concentration and 95% CI by age group and survey period are given for PBDE-47, PCB-153, and p,p′-DDE (Figure 1) and for POPs with detection frequencies ≥50% in all demographic groups and all survey periods (Tables 1 and 2). PBDE-47, PCB-153, and p,p′-DDE were the dominant POPs among PBDEs, PCBs, and persistent pesticides, respectively. The general trend among all POPs investigated were lower arithmetic mean concentrations in more recent survey periods. For example, the arithmetic mean concentration of PCB-153 in 12–19 year old adolescents was 30% lower in NHANES 2005/06 compared with NHANES 2003/04. Similarly, for the age groups 12–19, 20–39, and 40–59 years, PCB-153 arithmetic means in NHANES 2007/08 were 37%, 32%, and 24% lower, respectively, than in NHANES 2003/04. The 95% CI of the arithmetic means overlapped for all age groups and survey periods for p,p′-DDE (Figure 1 and Table 2). Arithmetic mean concentrations of PBDE congeners were lower in NHANES 2005/06 and 2007/08 than in NHANES 2003/04 (Figure 1 and Table 1), although the 95% CI overlapped for all comparisons except PBDE85 (40–59 year olds in NHANES 2005/06 and 12–19 and ≥60 year olds in NHANES 2007/08) and PBDE99 (40–59 year olds in NHANES 2005/06).
Figure 1.
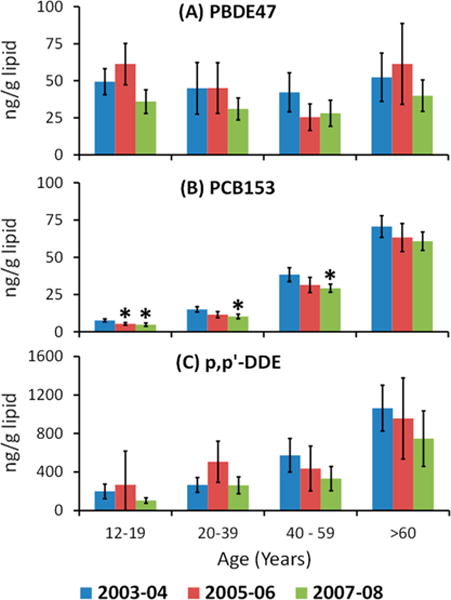
Age distribution of arithmetic mean concentrations (ng/g lipid) by survey period (NHANES 2003/04, individual samples; NHANES 2005/06 and 2007/08, pooled samples) for (A) 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), (B) 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB-153), and (C) 2,2-bis(4-chlorophenyl)-1,1-dichloroethene (p,p′-DDE). Error bars indicate 95% confidence interval (95% CI). Significantly different 95% CI of the arithmetic mean compared with NHANES 2003/04 is indicated with an asterisk.
Association between POP Serum Concentration, Age, Sex, and Race/Ethnicity
Weighted arithmetic mean concentrations of selected POPs by age group, race/ethnicity, and sex for NHANES 2003/04, 2005/06 and 2007/08 are given in Figure 2 and SI Tables S4–S11.
Figure 2.
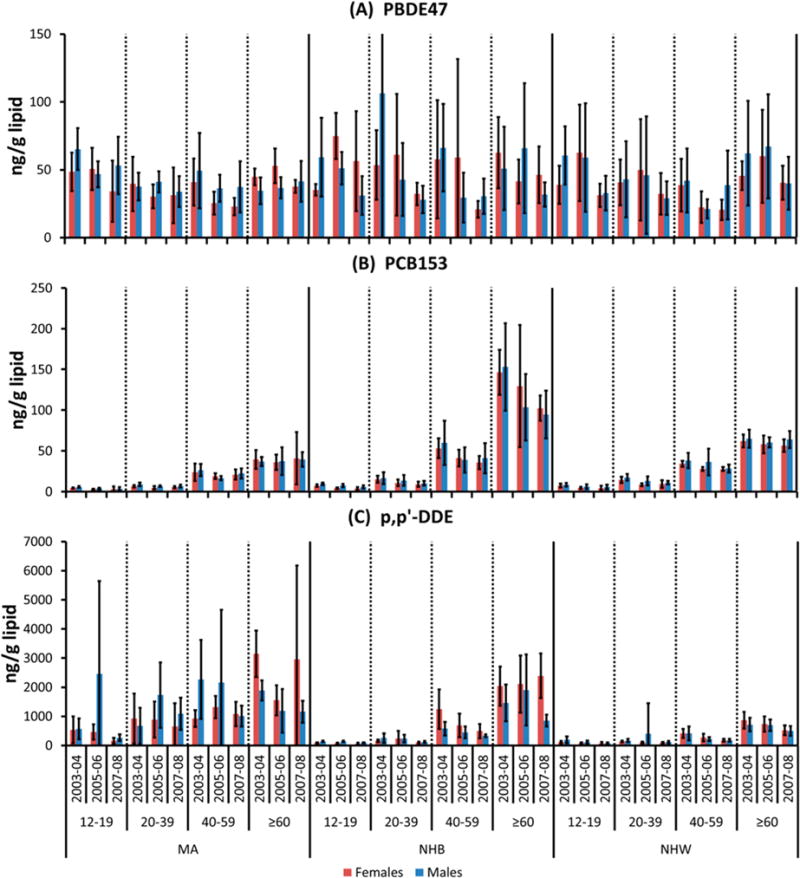
Arithmetic mean concentration (ng/g lipid) in NHANES 2003/04 (individual samples) and NHANES 2005/06 and 2007/08 (pooled samples) by survey period, race/ethnicity (MA, NHB, NHW), age group (12–19, 20–39, 40–59, ≥60 years), and sex for (A) 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), (B) 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB-153), and (C) 2,2-bis(4-chlorophenyl)-1,1-dichloroethene (p,p′-DDE). Error bars indicate 95% confidence interval (95% CI).
The concentrations of PCB-153 and p,p′-DDE were 920–1200% and 530–720%, respectively, higher in the ≥60 year old adults than in the 12–19 year old adolescents (Figure 1, Table 2). Arithmetic mean concentrations of PCBs were lower in MA than in NHB and NHW. For example, the percentage lower arithmetic mean concentration of PCB153 (averaged over three survey periods) in MA females compared with NHB females was −32, −50, −50, and −68%, for the age groups 12–19, 20–39, 40–59, and ≥60 years, respectively (Figure 2, SI Table S9). The 95% CIs of the arithmetic means did not overlap for 10 out of 12 comparisons. The absolute differences from MA subjects were comparable for NHB males (−43 to −66%), NHW females (−29 to −46%), and NHW males (−35 to −45%). No differences in arithmetic mean concentrations of PCB153 were observed for NHB and NHW for the age groups 12–19 and 20–39 years; whereas 2 out of 3 comparisons indicated significantly lower arithmetic mean concentrations in NHW females than NHB females for the age groups 40–59 and ≥60 years. The arithmetic mean concentrations over three survey periods were −44% and −113% lower in NHW females than NHB females for the age groups 40–59 and ≥60 years, respectively.
However, on the basis of comparisons of 95% CIs, the arithmetic mean concentrations of p,p′-DDEs, were significantly higher in MA subjects than in NHW persons and were significantly higher in NHB males in six or more out of twelve possible arithmetic mean comparisons per sex and race/ethnicity group (Figure 2, SI Table S11). For example, MA males had 710%, 530%, 290%, and 9% higher serum concentrations than NHB males 12–19, 20–39, 40–59, and ≥60 year olds, respectively. Only one significant difference was observed for the comparison of MA females and NHB females, although the arithmetic mean was higher in MA females. Five out of six comparisons of p,p′-DDE showed significantly higher arithmetic means for NHB females than NHW females for the age groups 40–59 and ≥60 years. The arithmetic mean concentrations over the three survey periods of p,p′-DDE were 180% and 230% higher in NHB females than in NHW females for the age groups 40–59 and ≥60 years, respectively. There were no consistent differences in arithmetic mean concentrations between NHB and NHW males and NHB and NHW females for the age groups 12–19 and 20–39 years.
In the case of HCB, no consistent differences between ethnicity and sex were observed over three survey periods (SI Table S11), although serum pools from MA females in the age group 20–39 and 40–59 years were higher than NHB females of the same age (four out of six 95%CI did not overlap).
No consistent differences were seen between demographic groups for tri- to hexaBDEs.
DISCUSSION
We detected 15 POPs in more than 50% of the pools analyzed, showing that these POPs continue to be detectable in a large proportion of the U.S. population during 2005–08, decades to years after the sale and registered use of these chemicals ended in the U.S. The main congener present in commercial DecaBDE (e.g., PBDE-209) was detected in only ~10% of samples, thus preventing statistical comparisons between demographic groups for this congener. The low detection frequency of PBDE-209 in the general population may be related to the short biological half-life of 2 weeks in humans.10
POPs Serum Concentration and Age
The concentration of all POPs excluding PBDEs increased continuously from age 12–19 years until ≥60 years, in agreement with previous studies.11 This is a reflection of older subjects being exposed continuously during their lives to contaminants with long biological half-lives and at higher levels in the past when environmental levels were higher than today.12,13 Younger individuals have experienced lower exposures in their shorter life span resulting in their body burden being lower than that of older individuals.
For PBDEs, we observed similar concentrations with increasing age from 12 to 19 years through ≥60 years, likely as a result of similar exposure to the entire population because PBDEs have been in commercial use until recently. Further, it is expected (but remains to be proven) that tetra- to hexaBDEs have biological half-lives measured in years instead of decades, as is the case for PCBs. The half-life of hepta and DecaBDEs is measured in weeks to months.10 This shorter biological half-life compared with PCBs would also result in the absence of an increasing concentration trend with age since body burden would be a reflection of exposures occurring during the past few years and not decades as in the case of PCBs.
POPs Serum Concentration by Survey Period
The higher weighted arithmetic mean concentrations in NHANES 2003/04 compared with those of 2005/06 and 2007/08 suggest declining body burdens in the U.S. population with time (Figure 1, Tables 1 and 2). It is noteworthy that for PCBs, the reduction in concentrations among older age groups was smaller on a percentage basis than the reduction observed among younger age groups (Figure 3, Table 2). This smaller relative difference may be due to several factors. First, metabolism of POPs could slow down with age because of reduced activity of some cytochrome P450 enzymes thus resulting in a slower elimination of POPs, as noted for certain drugs.14 Second, reduction in environmental levels may have proportional larger effects on the younger age groups because they had experienced exposures during a shorter period of time. In the youngest age group (12–19 years), we observed similar percentage decreases between NHANES 2003/04 and 2007/08 for all PCBs [e.g., overall reduction in concentration between −57% (PCB99) and −35% (PCB138/158)], while for the oldest age group, the decreases ranged between −28% (PCB99) and −7.6% (PCB194, nonsignificant). For PCBs, when comparing NHANES 2003/04 to 2007/08 data, we also observed an association between chlorination degree and the percentage decrease for the age groups 40–59 and ≥60 years (Figure 3). The association could be explained by the longer half-lives of more highly chlorinated PCBs.15 No similar association was found in the younger age groups or for PBDEs for any age group.
Figure 3.
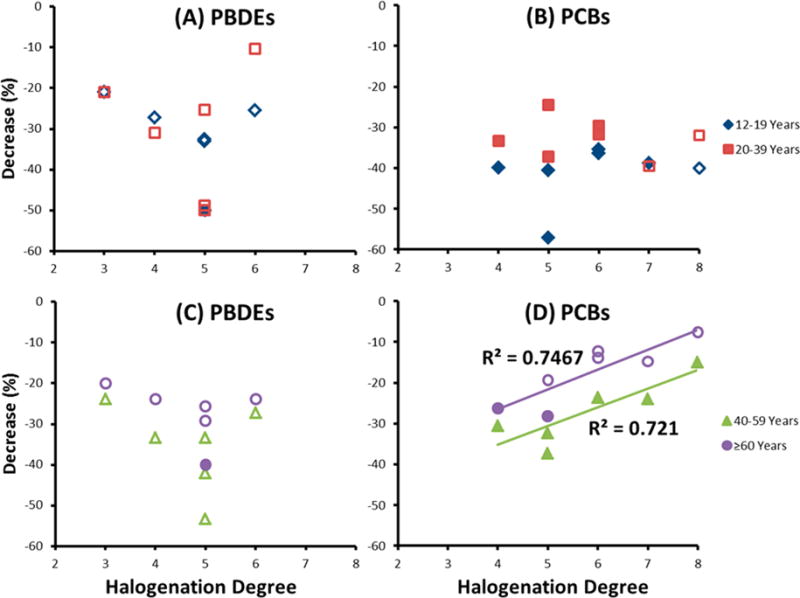
Percent decrease in arithmetic mean concentration of PCB and PBDE congeners by halogenation degree for four different age groups, from NHANES 2003/04 to 2007/08. Filed markers indicate differences in arithmetic mean for which the 95% CI did not overlap while nonfiled markers refer to comparison with overlap of 95% CI. Linear regression line has been drawn when the p-value for regression was <0.05.
For PBDEs, overall lower levels were observed in NHANES 2007/08 than in NHANES 2003/04, although most comparisons were not significantly different (Figure 1, Tables 1). The technical PentaBDE products were phased-out in 2004 in the United States; hence, sufficient time has not yet passed to observe a significant decrease in the U.S. population’s serum concentrations of most tetra- through hexaBDE congeners present in the commercial PentaBDE. It is likely that U.S. households still possess furniture and other consumer articles containing PBDEs, since the expected lifetime of such products is long (e.g., decades for upholstered furniture).
Association between POP Serum Concentration, Sex, and Race/Ethnicity
We observed that serum concentrations of p,p′-DDE were higher overall in MA than NHB and NHW participants (Figure 2). This may be due to exposure to DDT outside of the United States in the case of recent immigrants and/or dietary differences. We cannot explain at present why the serum concentration of p,p′-DDE is higher in NHB females than NHW females for the age groups 40–59 and ≥60 years (Figure 2 and SI Table S11). Higher body burdens of PCB-153 in NHB and NHW compared with MA subjects may result from exposures that are and/or have been higher within the borders of the United States or due to dietary differences. Like for p,p′-DDE, we observed higher PCB-153 concentrations in NHB females than in NHW females for the older age groups (40–59 and ≥60 years); the reason for these differences is at present unknown, however, diet-related factors cannot be excluded.
Limitations
It is important to note that the weighted arithmetic means in this report are not comparable to the geometric means as reported in the National Reports on Human Exposure to Environmental Chemicals.1 The statistical analyses performed for this paper are subject to several limitations including the following: (1) univariate methods were used involving multiple comparisons, which can lead to spurious statistically significant differences; (2) sample sizes were small for some demographic groups, which can lead to low statistical power and hence the failure to identify true statistically significant differences; (3) arithmetic means were used to compare demographic groups, whereas the NHANES 03/04 individual sample measurements and the NHANES 05/06 and 07/08 individual samples forming pools of 8 samples or less are not necessarily normally distributed and tend to be skewed to higher values, and (4) design effects could not be calculated for the NHANES 05/06 and 07/08 pooled sample results because pools were formed across the design cells of the original survey. The first limitation was mitigated to some extent by requiring consistency across survey periods before declaring statistical significance. The fourth limitation was addressed by using design effects from the closest NHANES survey (i.e., NHANES 03/04) to adjust standard error estimates for the NHANES 05/06 and 07/08 results, with the recognition that design effects from one survey may not be identical in subsequent surveys. To better address these limitations, we plan to develop and use multiple regression models based on log-transformed results.
Pooling Strategy
The use of pooled samples is of value in environmental public health. The benefits of pooling include reduced analytical costs. For the NHANES samples analyzed, the cost savings could be 1/8 of the cost of individual measurements because 8 serum samples formed one serum pool. Pooling also can increase the amount of serum available for analysis which, in turn, could improve analytical sensitivity. However, information on the distribution of individual POPs serum concentrations is lost when pools are used, as is the ability to associate individual measurements with demographic information other than sex, age, and race/ethnicity (the basis for the current NHANES pooling design) or with other relevant medical or lifestyle information collected from individual study participants. Nonetheless, we believe that NHANES pools are valuable to assess changes in the central tendency of POPs serum concentrations of the general U.S. population.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the researchers who made this work possible: Emily McDonald for preparing all samples for gas chromatography/high resolution mass spectrometry (HRMS) analysis; Sarah Anderson, Kenroy Crawford, Robert Cheek, Darlinda Harry, Carolyn Hodge, Wanda Whitfield, and Yalin Zhang for HRMS analysis; Chevine Anderson, Troy Cash, Yolanda Dalton, and Gerard Dublin for preparing the pools; Emily DiPietro, Patricia McClure, and Michelle Moore for data handling; and Pamela Olive for the serum lipids measurements. We also would like to acknowledge NCHS for serum collection and study administration.
Footnotes
Supporting Information
Pooling scheme, complete list of POPs measured, and detection frequency and arithmetic mean concentration with 95%CI by survey period and comparisons between demographic groups for select POPs. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interest.
References
- 1.Fourth National Report on Human Exposure to Environmental Chemicals: Updated Tables, March, 2013. National Center for Environmental Health; Centers for Disease Control and Prevention; Atlanta, GA: 2013. http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Mar2013.pdf. [Google Scholar]
- 2.United States Environmental Protection Agency Website. http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/deccadbe.html.
- 3.Sjödin A, Päpke O, McGahee E, Focant J-F, Jones RS, Pless-Mulloli T, Toms L-M, Herrmann T, Müller J, Needham LL, Patterson DG., Jr Concentration of polybrominated diphenyl ethers (PBDEs) in household dust from various countries. Chemosphere. 2008;73(1 Suppl):S131–S163. doi: 10.1016/j.chemosphere.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 4.Schecter A, Haffner D, Colacino J, Patel K, Päpke O, Opel M, Birnbaum L. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite U.S. food samples. Environ Health Perspect. 2010;118(3):357–362. doi: 10.1289/ehp.0901345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, Carroll MD, Hirsch R, Schober S, Johnson CL. National Health and Nutrition Examination Survey: Sample design, 1999–2006. Vital Health Stat 2. 2012;155:1–39. [PubMed] [Google Scholar]
- 6.Caudill SP. Characterizing populations of individuals using pooled samples. J Expo Sci Environ Epidemiol. 2010;20(1):29–37. doi: 10.1038/jes.2008.72. [DOI] [PubMed] [Google Scholar]
- 7.Hovander L, Malmberg T, Athanasiadou M, Athanassiadis I, Rahm S, Bergman Å, Wehler EK. Extraction and cleanup methods for analysis of phenolic and neutral organohalogens in plasma. Arch Environ Contam Toxicol. 2002;42(1):105–117. doi: 10.1007/s002440010298. [DOI] [PubMed] [Google Scholar]
- 8.Jones R, Edenfield E, Anderson S, Zhang Y, Sjödin A. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Compd. 2012;74:97–98. [Google Scholar]
- 9.Schantz MM, Eppe G, Focant J-F, Hamilton C, Heckert NA, Heltsley RM, Hoover D, Keller JM, Leigh SD, Patterson DG, Jr, Pintar AL, Sharpless KE, Sjödin A, Turner WE, Vander Pol SS, Wise SA. Milk and serum standard reference materials for monitoring organic contaminants in human samples. Anal Bioanal Chem. 2013;405(4):1203–1211. doi: 10.1007/s00216-012-6524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman Å, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114(2):176–181. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson DG, Jr, Wong LY, Turner WE, Caudill SP, Dipietro ES, McClure PC, Cash TP, Osterloh JD, Pirkle JL, Sampson EJ, Needham LL. Levels in the U.S. population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other long range transboundary air pollution agreements. Environ Sci Technol. 2009;43(4):1211–1218. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- 12.Chang F, Pagano JJ, Crimmins BS, Milligan MS, Xia X, Hopke PK, Holsen TM. Temporal trends of polychlorinated biphenyls and organochlorine pesticides in Great Lakes fish, 1999–2009. Sci Total Environ. 2012;439:284–290. doi: 10.1016/j.scitotenv.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Venier M, Hites RA. Time trend analysis of atmospheric POPs concentrations in the Great Lakes region since 1990. Environ Sci Technol. 2010;44(21):8050–8055. doi: 10.1021/es101656u. [DOI] [PubMed] [Google Scholar]
- 14.Kinirons MT, O’Mahony MS. Drug metabolism and aging. Br J Clin Pharmacol. 2004;57(5):540–544. doi: 10.1111/j.1365-2125.2004.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Environmental Health Criteria 140: Polychlorinated Biphenyls and Terphenyls. Second. International Program on Chemical Safety; Geneva, Switzerland: 1992. http://www.inchem.org/documents/ehc/ehc/ehc140.htm. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


