Abstract
A rapid expansion of HFMD with enterovirus 71 infection outbreaks has occurred and caused deaths in recent years in China, but no vaccine or antiviral drug is currently available for EV71 infection. This study aims to provide treatment programs for HFMD patients. We conducted a randomized, double-blind, controlled trial and evaluated clinical efficacy of therapy with rHuIFN-α1b in HFMD patients with EV71 infection. There were statistical differences in outcomes including the fever clearance time, healing time of typical skin or oral mucosa lesions, and EV71 viral load of the HFMD patients among ultrasonic aerosol inhalation group, intramuscular injection group and control group. rHuIFN-α1b therapy reduced the fever clearance time, healing time of typical skin or oral mucosa lesions, and EV71 viral load in children with HFMD.
Trial Registration: Chinese Clinical Trial Registry ChiCTR-TRC-14005153
Introduction
Hand, foot, and mouth disease (HFMD) is an important infectious disease in young children, particularly in those less than 5 years old. HFMD epidemics have affected various countries in the past 40 years [1]. Large-scale outbreaks have been a serious public health issue in China since 2008 [2]. Human Enterovirus 71 (EV71) and Coxsackievirus A16 (CVA16) are common etiological agents of HFMD in children, but the former can cause severe complications, such as aseptic meningitis, acute flaccid paralysis (AFP), meningoencephalitis and cerebellitis, with mortality rates ranging from 10 to 25.7% [3–5]. A cyclical epidemic of HFMD has been ongoing for 2 to 3 years in the region, EV71 is an important pathogen with increasing health threat to humans [6].
Interferons (IFNs) are natural glycoproteins belonging to the cytokine superfamily, and are produced in response to foreign agents such as viruses, parasites and tumor cells. They are important modulators of the immune response, particularly in inhibiting viral replication within host cells, activating natural killer cells and macrophages, increasing antigen presentation to lymphocytes, and inducing the resistance of host cells to viral infection [7, 8]. Interferon alpha (IFN-α) is a type I interferon; at least 23 variants of IFN-α are known. The individual proteins have molecular masses between 19–26 kDa and consist of proteins with lengths of 156–166 and 172 amino acids. All IFN-α subtypes possess a common conserved sequence between amino acid positions 115–151 while the amino-terminal ends are variable. Recombinant human interferon α1b (rHuIFN-α1b) can be expressed in E. coli to yield an approximately 19 kDa single non-glycosylated polypeptide chain containing 166 amino acids [9]. rHuIFN-α1b has been used for the treatment of chronic viral hepatitis B, hepatitis C virus (HCV), viral pneumonia and adenovirus infections [10–13].
We undertook a clinical trial in HFMD patients with EV71 infection to examine clinical efficacy of therapy with rHuIFN-α1b. Our studies intended to provide treatment programs for HFMD patients.
Materials and Methods
Ethics Statement
The protocol for this clinical trial and supporting CONSORT checklist are available as supporting information; see S1 CONSORT Checklist and S1 Protocol. The study protocol was approved by Chinese Ethics Committee of Registering Clinical Trials (ChiECRCT) on 21 August 2014 and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The study was registered as a clinical trial at Chinese Clinical Trial Registry (ChiCTR) [Registration number: ChiCTR-TRC-14005153; URL http://www.chictr.org.cn/showproj.aspx?proj=4422]. All the participating children’s parents provided informed consent and signed the informed consent prior to enrolment of their child into the trial, see S1 Consent.
Recombinant Human Interferon α1b
Recombinant human interferon α1b (rHuIFN-α1b) for injection (Shenzhen Kexing Biotech Co., Ltd, Shenzhen, China) was expressed in E. coli, purified and processed into a freeze-dried powder including human serum albumin, sodium chloride. The freeze-dried powder is stable when stored and transported at 2–8°C from light. 20 μg of the freeze-dried powder per vial was used for this study (Authorization number: Guo Yao zhun Zi S20033034).
Patients
Sample Size
The incidence of severe cases is about 15% among hospitalized patients with HFMD in Henan province [14]. To base this difference in proportion of responders between control group and experimental groups (nebulization group and injection group), with 80% power and two tailed 5% level of significance, the minimal sample size was calculated to be 72 for each group.
Clinical Diagnostic Criteria
HFMD was clinically diagnosed based on the guidelines for control and prevention of HFMD issued by the National Health and Family Planning Commission of the People’s Republic of China (2010). Briefly this includes patients from HFMD endemic regions presenting during the epidemic season; fever; and, skin eruptions on the hands, feet, and mouth, or only in the oral cavity.
Inclusion and Exclusion Criteria
Criteria for inclusion were: age between 0.5 to 5 years; according to the clinical diagnostic criteria of HFMD, subject patients with one of following clinical manifestations: 1) fever (axillary temperature ≥ 38.5C); 2) neurological complications including mental fatigue, irritability, headache, vomiting, convulsions, limb weakness; and 3) respiratory frequency (RF) ≥30 under resting conditions, heart rate (HR) ≥ 140; and stool specimen was positive for EV71 by real-time RT-PCR.
Exclusions from participation included: 1) critically ill HFMD patients whose treatment could be complicated by central respiratory failure or who had brain-stem encephalitis, acute flaccid paralysis, neurogenic pulmonary edema, neurogenic shock or other severe symptoms, or required endotracheal intubation and mechanical ventilation; 2) allergic constitution, especially allergy to multiple antibiotics or interferon products; 3) congenital heart disease; and 4) epilepsy and other central nervous system dysfunction.
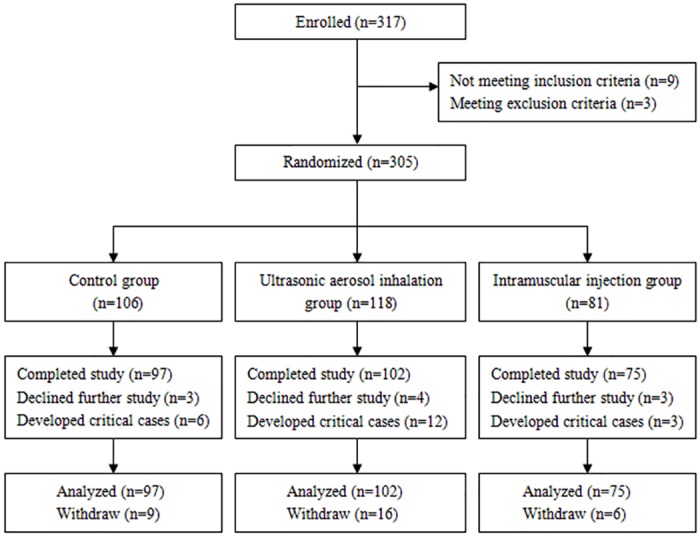
Between August 2014 and July 2015, 317 patients with HFMD were registered at Children’s Hospital of Zhengzhou and Children’s Hospital of Kaifeng. The patients were randomized into a control group, an ultrasonic aerosol inhalation group, and an intramuscular injection group. The randomization codes were prepared by the Department of Epidemiology and Statistics, College of Public Health, Zhengzhou University with the use of STATA 8.0 software. The study was conducted in an inpatient setting according to a randomized, double-blind, comparative study design, in which the purpose was to evaluate efficacy of rHuIFN-α1b in patients with EV71 infection (Fig 1).
Fig 1. Consort statement flow chart.
Treatment Plan
The patients who met the eligibility criteria were randomized to an ultrasonic aerosol inhalation group, an intramuscular injection group and a control group. All HFMD patients received supportive and symptomatic treatment. Patients randomized to the ultrasonic aerosol inhalation group were administered inhaled ultrasonically-nebulized rHuIFN-α1b (20 μg freeze-dried powder of rHuIFN-α1b dissolved in 4 mL physiological saline) once daily over 15–20 minutes for 5 days. The intramuscular injection group received rHuIFN-α1b (20 μg freeze-dried powder of rHuIFN-α1b dissolved in 1 mL physiological saline) by intramuscular injection for 5 days. The control group only received supportive and symptomatic treatment as per current standard of care. Stool specimens for viral load measurements were collected on days 0 and 6.
Efficacy and Safety Assessments
The patients were observed from 0 to 5 days and data was recorded in case report forms (CRF) by the investigators, including the following: temperature, skin rashes, oral ulcers, heart rate, respiratory rate, and nervous system signs/symptoms (apathetic, irritability, headache, vomiting, muscular spasms, limb weakness, neck rigidity). During the treatment period, safety was assessed by clinical examination and laboratory investigations, and adverse events were recorded by asking the HFMD subjects’ parents. Adverse events were graded according to the degree of correlation between those events and rHuIFN-α1b.
Termination Criteria
Termination criteria for the study or for individual patients included: 1) development of critical illness in HFMD subjects; 2) development of intolerable side effects from rHuIFN-α1b in the HFMD subjects; 3) requests from parents for withdrawal from the trial; 4) protocol treatment discontinuation after physician evaluation.
RNA Extraction and Real-time Quantitative RT-PCR
Total RNA was extracted from clinical samples using the QIAamp viral RNA mini Kit (Qiagen, Germany), following the manufacturer’s instructions. The RNA was eluted in a final volume of 60 μL of elution buffer and used immediately or stored at -80°C.
In order to establish copy number as a unit of viral load, a 226 bp fragment of the VP1 gene of EV71 was amplified by RT-PCR then cloned into vector pGEM-T (Promega, Madison, WI, USA). Next, the recombinant plasmids extracted were linearized by the SpeI digestion (TaKaRa, Dalian, China). The digestion products were both transcribed in vitro into RNAs and purified by a RioMAX large-scale RNA production system-T7 (Promega). Finally, the RNA panels were serially diluted from 2.5 × 106 to 2.5 copies per μL. Since the primers and probe were designed based to amplify the 226 bp cloned fragment, the RNA panels were used as a standard for real-time quantitative RT-PCR and EV71 viral load testing as in our previous studies [15, 16]. The real-time quantitative RT-PCR assay was performed using primers (P1, CCATAAGAACTACACTATGAATATGT, P2, AGTCCACTRCTCACGCTATC), probe (CAGACGTGCACCATCCAGTGAG) and the QIAGEN QuantiTectTM Probe RT-PCR Kit (Qiagen, Germany). The conditions for real-time RT-PCR reaction included 50°C for 30 min, 95°C for 15 min, 45 cycles of 95°C for 20 s, 55°C for 40 s, 68°C for 30 s. Data were analyzed using the software supplied by the manufacturer. The quantitative real-time PCR assay was performed to detect copy number of viral load in stool specimens of the HFMD patients.
Statistical Analysis
All data was double-entered with the EPIDATA 3.1 software to prevent transcription errors. All statistical analyses were performed using SAS v9.13 (SAS Institute Inc., Cary, NC). Measurement data was analyzed by single factor variance analysis or Student’s t-test. Count data was analyzed by χ2 test or Fisher’s exact test. A significant difference was considered with a P value less than 0.05.
Results
Patient Characteristics
A total of 317 clinically diagnosed HFMD subjects were enrolled in our study; 9 did not meet inclusion criteria and 3 met exclusion criteria. 305 HFMD subjects were then enrolled into the rHuIFN-α1b treatment trial after obtaining written informed consent. The patients were randomized, 106 cases to control group, 118 cases to ultrasonic aerosol inhalation group and 81 cases to intramuscular injection group. 10 HFMD patients withdrew from the treatment trial because of parental request, and 21 HFMD patients withdrew because they developed critical illness requiring transfer to the intensive care unit (ICU). 274 HFMD subjects completed the rHuIFN-α1b treatment trial (Fig 1).
Of the 274 subjects who completed treatment trial patients, 172 (62.77%) were male and 102 (37.23%) were female. The mean age of the HFMD patients was 1.98 years (range, 0.66–5.00). Baseline demographics and clinical characteristics were well matched among the randomized groups (Table 1).
Table 1. Demographics and clinical characteristics for participants with HFMD in the randomized groups.
| Characteristics | Control group (n = 97) | Ultrasonic aerosol inhalation group (n = 102) | Intramuscular injection group (n = 75) | χ2/F Value | P Value |
|---|---|---|---|---|---|
| Gender (male/female) | 56/41 | 66/36 | 50/25 | χ2 = 1.704 | 0.426 |
| Age (mean ± SD) years | 1.93±0.98 | 1.89±0.83 | 2.17±0.74 | F = 2.509 | 0.083 |
| Weight (mean ± SD) kg | 12.34±2.30 | 12.20±2.24 | 12.68±2.66 | F = 0.888 | 0.413 |
| Temperature (mean ± SD) °C | 38.72±0.64 | 38.71±0.77 | 38.68±0.68 | F = 0.075 | 0.928 |
| Respiratory rate (mean ± SD) times/min | 32.93±3.17 | 32.10±6.62 | 32.38±6.67 | F = 0.258 | 0.772 |
| Heart rate (mean ± SD) times/min | 130.40±11.47 | 131.55±12.43 | 128.87±6.97 | F = 1.392 | 0.250 |
SD: Standard deviation
Clinical Efficacy
There were statistical differences in outcomes including the fever clearance time, and the healing time for typical skin or oral mucosal lesions. The 3 groups had similar mean scores and no statistical differences for initial body temperature, but the improvement was greater for the ultrasonic aerosol inhalation and intramuscular injection groups compared with the control group at follow up. The mean time to fever clearance was 80.64 hours (95% confidence interval, 76.80 to 84.48) for the control group, 66.00 hours (95% confidence interval, 61.44 to 70.80) for the ultrasonic aerosol inhalation group, and 65.52 hours (95% confidence interval, 60.72 to 70.32) for the intramuscular injection group, respectively. There were statistically significantly differences between the intramuscular injection and the control group (t = 6.275, P<0.001), and between the ultrasonic aerosol inhalation and the control groups (t = 6.059, P<0.001), but no significant differences between the ultrasonic aerosol inhalation and the intramuscular injection groups (t = 0.216, P = 0.873).
The healing time for typical skin or oral mucosal lesions was significantly different between the intramuscular injection group (mean: 61.44 h, 95% CI 57.36 to 65.28 h) and the control group (mean 75.60 h, 95% CI 71.28 to 79.92 h) (t = 5.946, P<0.001), and between the ultrasonic aerosol inhalation group (mean 62.88 h, 95% CI, 60.72 to 65.28h) and the control group (t = 5.272, P<0.001), but no significant differences were observed between the ultrasonic aerosol inhalation group and the intramuscular injection group (t = 0.675, P = 0.536).
The respiratory rates and heart rates were measured every day during clinical trials. The mean respiratory rates were significantly different in the groups on second day, and heart rates were significantly different on third day; there were no significant differences on other days by variance analysis (S1 Table). Generalized estimating equations (GEE) analysis was conducted to examine associations between interact time and groups. The respiratory rates were statistical difference among the groups (Waldχ2 = 33.32, P<0.001) and interact time (Waldχ2 = 202.91, P<0.001). When compared by pairwise comparison methods, there was a statistical difference between the control group and other groups or between the ultrasonic aerosol inhalation group and the intramuscular injection group. In interact time, the respiratory rates of first day and second day were not statistical difference, the fourth day and fifth day were also not statistical difference, but the first day and second day were statistically significant difference between the third day, fourth day or fifth day, respectively (P<0.05). The mean respiratory rates decreased gradually from first day to fifth day. The heart rates were not statistical difference among the groups (Waldχ2 = 2.563, P = 0.278) and interact time (Waldχ2 = 9.137, P = 0.058) by GEE. There were a statistical difference between the first day and third day (P<0.05). The mean heart rates increased on second day, but began to decrease from third day (Table 2). No adverse events were observed in 3 groups.
Table 2. Generalized estimating equations (GEE) evaluating associations of interact time and groups with respiratory rates and heart rates of the HFMD patient.
| Variables | Mean (95% CI) | P1 value | P2 value | P3 value | P4 value |
|---|---|---|---|---|---|
| Respiratory rates (times/min) | |||||
| Groups | |||||
| Control | 29.73 (29.01, 30.46) | Reference | |||
| Ultrasonic aerosol inhalation | 28.69 (28012, 29.25) | 0.029 | Reference | ||
| Intramuscular injection | 31.08 (30.49, 31.67) | 0.005 | <0.001 | ||
| Time | |||||
| First day | 32.60 (31.83, 33.36) | Reference | |||
| Second day | 32.46 (31.63, 33.30) | 0.820 | Reference | ||
| Third day | 30.08 (29.23, 30.92) | <0.001 | <0.001 | Reference | |
| Fourth day | 27.52 (26.68, 28.37) | <0.001 | <0.001 | <0.001 | Reference |
| Fifth day | 26.51 (25.65, 27.36) | <0.001 | <0.001 | <0.001 | 0.098 |
| Heart rates (times/min) | |||||
| Groups | |||||
| Control | 129.86 (120.33, 139.38) | Reference | |||
| Ultrasonic aerosol inhalation | 126.31 (121.08, 131.55) | 0.528 | Reference | ||
| Intramuscular injection | 132.82 (126.77, 138.87) | 0.608 | 0.111 | ||
| Time | |||||
| First day | 129.73 (128.23, 131.22) | Reference | |||
| Second day | 140.19 (125.12, 155.26) | 0.171 | Reference | ||
| Third day | 127.18 (125.80, 128.57) | 0.014 | 0.090 | Reference | |
| Fourth day | 127.08 (117.17, 137.00) | 0.608 | 0.152 | 0.985 | Reference |
| Fifth day | 124.13 (115.20,133.06) | 0.228 | 0.072 | 0.513 | 0.665 |
Laboratory Findings
Stool specimens of the HFMD patients were collected on days 0 and 6, and EV71 viral load was detected by the quantitative real-time PCR assay. The mean EV71 viral load was not significantly different at day 0 among groups (F = 439, P = 0.645), but was significantly different at day 6 (F = 7.863, P<0.001). The mean viral load decrease (the viral load from the second time measurement minus the viral load from the first time measurement) were significantly different among groups (F = 10.076, P<0.001), but not significantly different between the ultrasonic aerosol inhalation and the intramuscular injection group (t = 0.8646, P = 0.122) (Table 3).
Table 3. The results of EV71 viral load testing in Stool specimens of the HFMD patients.
| Groups | Day 0 (log10 copies/mL) | Day 6 (log10 copies/mL) | Viral load decrease (log10 copies/mL) |
|---|---|---|---|
| Control group (n = 97) | 4.76±3.60 | 3.82±3.19 | 0.94±3.48 |
| Ultrasonic aerosol inhalation group (n = 102) | 5.21±4.14 | 2.67±3.10 | 2.53±3.43 |
| Intramuscular injection group (n = 75) | 5.27±4.19 | 1.87±3.46 | 3.39±4.17 |
| F value | 0.439 | 7.863 | 10.076 |
| P value | 0.645 | <0.001 | <0.001 |
Discussion
EV71 is a small, non-enveloped, positive-stranded RNA virus with a genome of approximately 7,400 bases, and it is a member of the genus Enterovirus in the family Picornaviridae [1]. Since its first isolation in the United States in 1969, EV71 has been identified worldwide as a common cause of HFMD in young children and infants [6]. Large EV71-associated HFMD outbreaks have been reported in the United States, Europe, Australia, and Asia [17]. In China, a large-scale HFMD outbreak gave rise to 2,203,597 reported cases that caused 559 deaths in 2012. Currently, there is neither an approved vaccine nor any effective antiviral drugs for EV71 infection [18].
This randomized, double-blind, controlled trial demonstrates that rHuIFN-α1b combined with supportive and symptomatic therapy greatly decreases viral load, significantly accelerates fever clearance time and the healing time for shin or oral mucosal lesions. No deaths or severe adverse events were observed in rHuIFN-α1b therapy group. Some previous studies showed that ribavirin, several kinds of herbal injections (such as Reduning, Yanhuning, Tanreqing and Qingkailing, etc.) could reduce the time of healing, time of fever clearance, and time for rash to subside. However, the evidence supporting the use of herbal medicine for HMFD is of insufficient quality, and the available data indicate that herbal medicine, especially herbal injections or combinations of herbal and Western medications might improve symptoms of HMFD [19].
When used in the systemic therapy, IFNs are mostly administered by an intramuscular injection. The most frequent adverse effects are flu-like symptoms, especially increased body temperature. EV71 infection causes HFMD, a common exanthema of young children that is characterized by a fever, rashes on the palms and the bottoms of the feet, and ulcers in the oral cavity [20]. As a result of fever as an enrollment criterion, increased body temperature that could have potentially resulted from rHuIFN-α1b treatment could not separately discern in this study.
We observed that the mean respiratory rates and heart rates were significantly different in the groups on the second and third days, respectively. The mean respiratory rates for the intramuscular injection group was higher than for other groups on day 2, and the mean heart rates of intramuscular injection group was higher than that of other groups on third day. This phenomenon could be considered a potential adverse effect of intramuscular rHuIFN-α1b.
There were a number of limitations related to this study, including: lack of long-term follow up after the treatment; and outcome measures during rHuIFN-α1b treatment were only collected for HFMD patients with EV71 infection. Thus, it is premature to conclude that treatment with rHuIFN-α1b for HFMD is safe owing to the lack of long-term follow up. Furthermore, we only investigated hospitalized patients, and these results might not be applicable to the primary care setting.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We especially want to thank Dr. Xiangguo Qiu for critical reading and review of this manuscript. We are grateful to the students for participating in the study. We thank Hongxia Ma and Yuling Xu for their assistance in the laboratory and in preparing this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from Henan Province Health Department and National Health and Family Planning Commission of the People’s Republic of China Co-build Project (201001015), and Science and Technology Bureau of Henan Province (122102310268).
References
- 1.Xu M, Su L, Cao L, Zhong H, Dong N, Xu J. Enterovirus genotypes causing hand foot and mouth disease in Shanghai, China: a molecular epidemiological analysis. BMC Infect Dis. 2013;13: 489 10.1186/1471-2334-13-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan X, Huang X, Zhu S, Chen H, Yu Q, Wang H, et al. The persistent circulation of enterovirus 71 in People's Republic of China: causing emerging nationwide epidemics since 2008. PLoS One. 2011; 6(9): e25662 10.1371/journal.pone.0025662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X, Wei H, Wu S, Du Y, Liu L, Su J, et al. Epidemiological and etiological characteristics of hand, foot, and mouth disease in Henan, China, 2008–2013. Sci Rep. 2015;5:8904 10.1038/srep08904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadurugamuwa JL, Shaheen E. Inactivation of human enterovirus 71 and coxsackie virus A16 and hand, foot, and mouth disease. Am J Infect Control. 2011;39(9):788–789. 10.1016/j.ajic.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Tan X, Cui A, Mao N, Xu S, Zhu Z, et al. Complete genome analysis of the C4 subgenotype strains of enterovirus 71: predominant recombination C4 viruses persistently circulating in China for 14 years. PLoS One. 2013; 8(2):e56341 10.1371/journal.pone.0056341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tee KK, Lam TT, Chan YF, Bible JM, Kamarulzaman A, Tong CY, et al. Evolutionary genetics of human enterovirus 71: origin, population dynamics, natural selection, and seasonal periodicity of the VP1 gene. J Virol. 2010;84(7):3339–3350. 10.1128/JVI.01019-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urin V, Levin D, Sharma N, Harari D, Schreiber G. Fine Tuning of a Type 1 Interferon Antagonist. PLoS One. 2015;10(7):e0130797 10.1371/journal.pone.0130797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannidis I, Ye F, McNally B, Willette M, Flano E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J Virol. 2013;87(6):3261–3270. 10.1128/JVI.01956-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masci P, Olencki T, Wood L, Rybicki L, Jacobs B, Williams B, et al. Gene modulatory effects, pharmacokinetics, and clinical tolerance of interferon-alpha1b: a second member of the interferon-alpha family. Clin Pharmacol Ther. 2007;81(3):354–361. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Zhao L, Wu T, Guo S, Chen Y, Zhou T. Efficacy of consensus interferon in treatment of HbeAg-positive chronic hepatitis B: a multicentre, randomized controlled trial. Virol J. 2009;6:99 10.1186/1743-422X-6-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stegmann KA, Björkström NK, Ciesek S, Lunemann S, Jaroszewicz J, Wiegand J, et al. Interferon α-stimulated natural killer cells from patients with acute hepatitis C virus (HCV) infection recognize HCV-infected and uninfected hepatoma cells via DNAX accessory molecule-1. J Infect Dis. 2012;205(9):1351–1362. 10.1093/infdis/jis210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun W, Yang L, Jia YY, Qiao Y, Wen AD. Tolerance and Safety Study of Aerosol Inhalation of Recombinant Human Interferon α1b in Children with Viral Pneumonia. China Pharmacy. 2011;22(14): 1277–1280. [Google Scholar]
- 13.Liu X, Lu J, He ML, Li Z, Zhang B, Zhou LH, et al. Antitumor effects of interferon-alpha on cell growth and metastasis in human nasopharyngeal carcinoma. Curr Cancer Drug Targets. 2012;12(5):561–570. [DOI] [PubMed] [Google Scholar]
- 14.Huang XY, Kang K, Xu YL, Wei HY, Li XL, Ma H, et al. Etiology surveillance of hand-foot-mouth disease in Henan province between 2008 and 2011. Chin J Prev Med. 2012;46(10):883–887. [PubMed] [Google Scholar]
- 15.Yu HJ, Xu BL, Huang XY, Wei HY, Li XL, Du YH. Construction and primary application on a TaqMan PCR detection method for detection of enterovirus 71. Modern Preventive Medicine. 2010; 37(8): 1517–1523. [Google Scholar]
- 16.Ding X, Nie K, Shi L, Zhang Y, Guan L, Zhang D, et al. Improved detection limit in rapid detection of human enterovirus 71 and coxsackievirus A16 by a novel reverse transcription-isothermal multiple-self-matching-initiated amplification assay. J Clin Microbiol. 2014;52(6):1862–1870. 10.1128/JCM.03298-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bible JM, Pantelidis P, Chan PK, Tong CY. Genetic evolution of enterovirus 71: epidemiological and pathological implications. Rev Med Virol. 2007;17(6):371–379. [DOI] [PubMed] [Google Scholar]
- 18.Xu LJ, Jiang T, Zhang FJ, Han JF, Liu J, Zhao H, et al. Global transcriptomic analysis of human neuroblastoma cells in response to enterovirus type 71 infection. PLoS One. 2013;8(7):e65948 10.1371/journal.pone.0065948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao HJ, Liu ZL, Steinmann P, Mu YJ, Luo H, Liu JP. Chinese herbal medicines for treatment of hand, foot and mouth disease: A systematic review of randomized clinical trials. European Journal of Integrative Medicine. 2012; 4: 85–111. [Google Scholar]
- 20.Ryu WS, Kang B, Hong J, Hwang S, Kim J, Cheon DS. Clinical and etiological characteristics of enterovirus 71-related diseases during a recent 2-year period in Korea. J Clin Microbiol. 2010;48(7):2490–2494. 10.1128/JCM.02369-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


