Abstract
Spinach RNA aptamer contains a G-quadruplex motif that serves as a platform for binding and fluorescence activation of a GFP-like fluorophore. Here we show that Pb2+ induces formation of Spinach’s G-quadruplex and activates fluorescence with high selectivity and sensitivity. This device establishes the first example of an RNA-based sensor that provides a simple and inexpensive tool for Pb2+ detection.
Heavy metals are among the most dangerous pollutants in our environment, and lead toxicity has been a major concern in recent times.1-3 The traditional methods to detect Pb2+ such as, Atomic Absorption Spectroscopy (AAS), Atomic Emission Spectroscopy (AES) and Inductive Coupled Plasma-Mass Spectrometry (ICP-MS) involve sophisticated equipment and are not suitable for on-site detection.1 During the last decade, DNA has been used extensively as a biomolecular Pb2+sensor.3 Most DNA-based devices fall broadly into two classes based on their mechanisms of action- first, Pb2+-dependent DNAzyme-catalyzed RNA cleavage,4a-f and second, Pb2+-dependent formation of DNA G-quadruplex (Table S1†).3,5a-c,6a,b In the first class of sensors, the rates of signal enhancement need to be monitored for quantitative detection, requiring substantial data processing.4a-f The second class exploits the potential of Pb2+ to induce formation of G-quadruplexes from single or double-stranded G-rich DNA. This class of sensors usually needs covalent incorporation of fluorophores6a,b or involves chemical reactions that generate colored/chemiluminiscent products,3,5a-c which limit the use of these sensors to certain favourable conditions of pH and ionic strength. DNA based electrochemical sensors, most of which use one of the above mechanisms, have been used for sensitive Pb2+ detection. These sensors involve elaborate assemblies, usually requiring immobilization of DNA molecules to gold electrodes.2,4d,5c,6c,d Beyond these potential limitations, the high costs of these devices complicate their practical use.6b Inspired by the fact that Pb2+ could induce/stabilize G-quadruplex formation, we report the use of the Spinach RNA aptamer as the first example of an RNA-based device for detection of Pb2+ with high sensitivity and selectivity.
Spinach, an RNA aptamer that was obtained by in-vitro selection from a random sequence pool of RNAs, bind to 3,5-difluoro-4-hydroxybenzylidene imidazolinone (DFHBI), an analogue of the fluorophore in GFP, and activates its fluoresence.7 Fusion of Spinach to other aptamers8a and hybridization sequences8b,c has enabled detection of metabolites and proteins in-vivo and oligonucleotides in vitro, respectively. In these Spinach coupled sensors binding of a specific analyte induces the active conformation of Spinach, thereby triggering DFHBI fluorescence. Spinach has also been used to monitor in-vitro transcription efficiency in real time.8d Crystal structures of Spinach reveal the presence of a two-layer G-quadruplex motif that serves as a stacking platform for DFHBI binding.9a,b Formation of this quadruplex motif requires millimolar concentrations of K+, Na+, or NH4+. Micromolar concentrations of Pb2+ induce the formation of DNA G-quadruplexes even in the absence of K+ or Na+, the two most common cations associated with G-quadruplexes. Pb2+-stabilized G-quadruplexes adopt a more compact structure than those stabilized by monovalent cations due to the smaller size and high charge density of Pb2+.6a,10,11,12a,b We wondered whether these observations extend to the RNA G-quadruplex in Spinach. If Pb2+ could support formation of the RNA G-quadruplex in Spinach, the high quantum yield and low photo-bleaching of DFHBI7 would make Spinach an attractive candidate for a sensitive and selective Pb2+ sensor. We used a truncated form of Spinach (Fig. 1, Fig. S1†) that shows equivalent fluorescence to the wild-type RNA.9a In the absence of cations at concentrations sufficient to support G-quadruplex formation, no fluorescence occurred when Spinach was incubated with DFHBI. However, addition of sub-micromolar quantities of Pb2+ resulted in fluorescence, suggesting that Pb2+ can support formation of the RNA G-quadruplex in Spinach, thereby allowing the RNA to bind the fluorophore and activate its fluorescence. Even at 1 μM concentration of Pb2+ we observed a strong fluorescence signal that was visible under a hand-held UV lamp (Fig. S2†). An enhancement in the 264 nm peak in the Circular Dichroism (CD) spectrum of Spinach upon addition of 10 μM Pb2+ suggested the formation of a G-quadruplex in presence of Pb2+ (Fig. 2). The relatively low peak enhancement presumably reflects the fact that only about 14% (8 out of the total of 57 nucleotides) of the RNA folds into a G-quadruplex. Mutating the quadruplex guanines to disturb the formation of the G-quadruplex completely abrogates Pb2+-induced Spinach fluorescence, providing further evidence that Pb2+ supports formation of the G-quadruplex in Spinach (Fig. S2†). The short incubation time for this sensor (15 minutes) makes detection fast compared to most Pb2+ sensors reported in literature (Table S1†).3,4c-e,5a-c In addition, short incubation times limit any significant RNA cleavage induced by Pb2+, RNase A or pH variations that might be encountered in real life applications and thus attenuate sensitivity (Fig. S4†).
Fig.1.
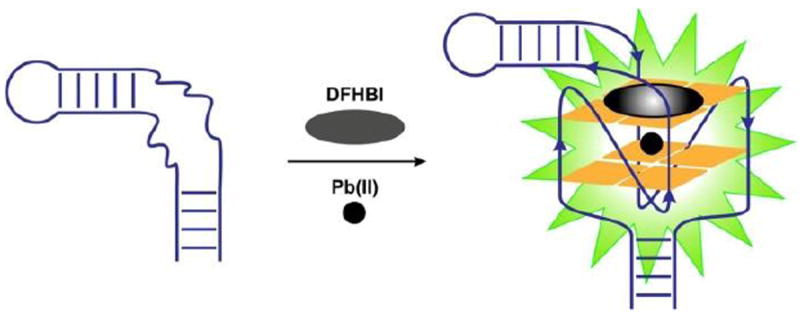
Schematic illustration of the Spinach sensor. The unstructured region (shown by curved lines) in the Spinach RNA aptamer folds into a two-layered G-quadruplex in the presence of Pb2+ and binds DFHBI fluorophore, activating fluorescence.
Fig. 2.

CD spectra of Spinach-DFHBI complex. A distinct enhancement of the positive peak at 264 nm after addition of 10 μM Pb2+ (green) indicates formation of G-quadruplex in Spinach RNA in the presence of Pb2+ at this concentration. The spectrum in blue is obtained without Pb2+. Experiments were performed at a pH 7.5 and 5 mM Mg2+. RNA and DFHBI concentrations were 5 μM and 20 μM, respectively.
For optimum performance, the sensor must be sensitive enough to detect Pb2+ at nanomolar concentrations and selective for Pb2+ over other cations. Fluorescence was measured at different concentrations of Pb2+ and signal enhancement was observed up to 10 μM (Fig. 3), consistent with increased folding of the RNA into a quadruplex with increased concentrations of Pb2+. Increasing concentrations of Pb2+ in the presence of DFHBI (without the Spinach aptamer) showed no fluorescence enhancement, underscoring the role of the RNA in fluorescence activation of DFHBI (Fig. S5†). The decrease in fluorescence after 10 μM Pb2+ might reflect non-specific quenching by Pb2+ (Fig. S6†).5d The signal response was linear in the range 5 nM-500 nM Pb2+ (inset Fig. 3) and the detection limit for Pb2+ was 6 nM (based on 3σ/slope method, where σ is the standard deviation of blank), which is well below the maximum permissible level for Pb2+ concentration in drinking water (72 nM or 15 ppb).1 The linear range and sensitive detection limit make our Spinach sensor suitable for quantitative detection of Pb2+ at low to moderate concentrations that are likely to be encountered in real life samples.
Fig.3.
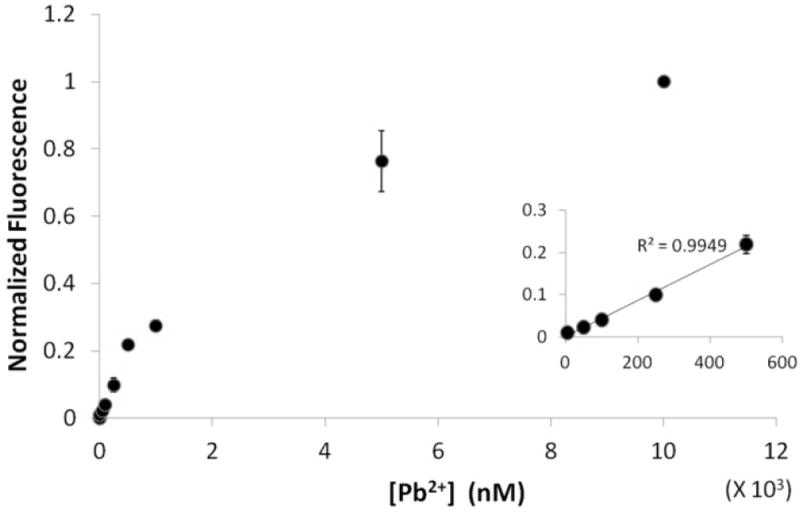
Spinach fluorescence versus the concentration of Pb2+. Inset: signal increases linearly (R2=0.9949) with Pb2+ concentration in the range of 5 nM-500 nM. Experiments were performed at a pH 7.5 and 5 mM Mg2+. RNA and DFHBI concentrations were 100 nM and 1 μM, respectively.
We tested the Spinach sensor with different environmentally relevant cations and found it to be highly selective towards Pb2+ (Fig. 4, Fig. S7†). Cations known to stabilize G-quadruplexes, Na+, K+ and Ca2+ 12a,13 showed fluorescence signals that were only a little above background even when present in 30-fold (Na+) and 3-fold (K+, Ca2+) excess over Pb2+. In fact the Spinach sensor is more than 17000-fold selective for Pb2+ compared to the most competing ion Ca2+ (inset Fig. 4), which to our knowledge makes it the most selective nucleic acid-based sensor for Pb2+(Table S1†). Spinach signal due to the presence of Pb2+ was virtually unaffected in a metal soup that contained Ag+, Ca2+, Co2+, Cr3+, Cd2+, K+, Na+, Cu2+, Fe2+, Mn2+, Ni2+, Zn2+ (Fig. 4). These observations highlight the high selectivity of the sensor and demonstrate its potential utility for analysis of Pb2+ in samples containing other metal ions. The apparent KD values for metal ion binding to Spinach RNA were 0.0013±0.0004 mM (Hill coefficient n=1.5±0.4), 2.3±1.1 mM (Hill coefficient n=0.7±0.3) and 9.6±0.4 mM (Hill coefficient n=1.6±0.1)9a for Pb2+, Ca2+ and K+, respectively, indicating that Pb2+ bound to Spinach with an affinity that was three orders of magnitude greater than did K+ or Ca2+, further emphasizing the selectivity of the sensor towards Pb2+ (Figs S8†, S9†). Consistent with these observations, at micromolar concentrations of Pb2+, K+ or Ca2+, significantly lower concentrations of DFHBI were required to show fluorescence in the presence of Pb2+ compared to K+ or Ca2+ (Figs S10†, S11†), reflecting the formation of G-quadruplex in micromolar concentrations of Pb2+ but not in micromolar concentrations of K+ or Ca2+.12a
Fig.4.
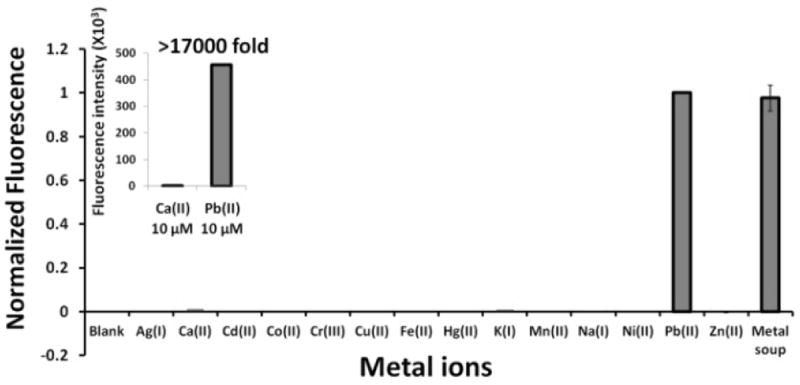
Spinach sensor detects Pb2+ with greater than 17000-fold selectivity relative to the most interfering cation Ca2+ (inset). Pb2+ was assayed at 10 μM, Ca2+ and K+ at 30 μM and the remaining metal ions at 300 μM. Experiments were performed at a pH 7.5 and 5 mM Mg2+. RNA and DFHBI concentrations were 5 μM and 20 μM, respectively.
Detection methods based on Pb2+-induced G-quadruplex formation tend to yield low signal to noise ratios due to presence of high concentrations (in the millimolar range) of metals like K+, Na+ and Ca2+ that induce G-quadruplex formation. High background signals make their use in practical samples potentially problematic.14 To assess this possible limitation in the Spinach sensor, we tested its performance using samples of tap water. First, we examined the sensor’s stability in tap water and observed no degradation over the course of the measurement including after incubation at 37°C overnight in presence of 10 μM Pb2+ (Fig. S4a†). The sensor was stable to degradation between pH 3 and pH 11 in the time frame of detection (Fig. S4b†) and fluorescence signal was optimal between pH 6 and pH 8 (Fig. S12†). ICP-MS analysis of tap water samples showed that Ca2+, Na+ and K+ were present in 568 μM (22.7 ppm), 350 μM (8.1 ppm) and 28 μM (1.1 ppm) concentrations, respectively. The absence of significant fluorescence in the presence of K+ and Na+ at these concentrations (Fig. 4) indicated that the presence of Ca2+ caused the observed background signal when the Spinach sensor was used in tap water. To identify conditions that enable improved selectivity for detection of Pb2+ over Ca2+, we analyzed fluorescence signal as a function of increasing DFHBI concentration in the presence of a constant concentration of Pb2+ or Ca2+ (Fig. S11†). We found that Spinach required significantly higher concentrations of DFHBI to give the same intensity of fluorescence signal in the presence of Ca2+ compared to the presence of Pb2+. For example, in the presence of 50 mM Ca2+, Spinach did not show substantial signal above background when the concentration of DFHBI was below 10 μM, (Fig. S11b†) whereas significant signal was obtained in the presence of 10 μM Pb2+, even at concentrations as low as 0.5 μM (Fig. S11a†). Thus, the background signal due to Ca2+ was minimized by lowering the concentration of DFHBI used from 20 μM (used with 5 μM RNA for most assays) to 1 μM (used with 100 nM RNA). These conditions rendered the sensor completely insensitive to the presence of Ca2+. Using these conditions, we then tested the performance of our sensor for Pb2+ detection in tap water. We spiked tap water with different concentrations of Pb2+. Fluorescence signal increased linearly with Pb2+ concentration in the range of 100 nM-5 μM (Fig. S13†). These results demonstrate a possible real-life application of the Spinach sensor for detecting Pb2+ in tap water. Other potential applications of our sensor could include measuring Pb2+ in paint, which contains no interfering G-quadruplex stabilizing cations.15 A DNA version of the Spinach-sensor could be potentially a cheaper and more robust alternative for these practical applications. An effective DNA-Spinach could be obtained by carrying out an in-vitro selection for DFHBI binding from a random pool of DNAs, possibly enriched in guanines to bias toward the possibility of the molecule having a G-quadruplex, which is the chemical basis for Pb2+ sensing.
In conclusion, we have reported the first example of an RNA-based sensor as a selective and sensitive tool for detection of Pb2+, thus expanding the repertoire of nucleic acid based sensors for heavy metals to include RNA (Table S1†). Our Spinach sensor offers the following distinct advantages: it does not need fluorophore-quencher pairs that require covalent attachment to the nucleic acid, does not involve expensive nanomaterials or complicated detection systems that have been used for some previous DNA-based Pb2+ sensors, 16 and has a very simple design and operation that is cost effective and easy to use for ‘on the spot’ detection of Pb2+.
We thank Hao Huang for helpful advice, Deepak Koirala for help with CD spectroscopy and data handling, Piccirilli lab members, Prof. Dipankar Sen and Urmimala Basu for their valuable comments on the manuscript, the University of Chicago Biophysics Core and the University of Chicago Mass Spectrometry facilities for their services. We also thank Carter Abney for his help with the ICP-MS experiments. This work was supported by the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust and NIH (5R01GM102489) to JAP.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: [Methods, Supplementary Figures 1-13 and Table 1]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Wang W, Jin Y, Zhao Y, Yue X, Zhang C. Biosens Bioelectron. 2013;41:137–142. doi: 10.1016/j.bios.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Li F, Feng Y, Zhao C, Tang B. Chem Commun. 2011;47:11909–11911. doi: 10.1039/c1cc15023e. [DOI] [PubMed] [Google Scholar]
- 3.Li T, Wang E, Dong S. Anal Chem. 2010;82:1515–1520. doi: 10.1021/ac902638v. [DOI] [PubMed] [Google Scholar]
- 4.(a) Lu Y, Liu J, Li J, Bruesehoff PJ, Pavot CM-B, Brown AK. Biosens Bioelectron. 2003;41:529–540. doi: 10.1016/s0956-5663(03)00013-7. [DOI] [PubMed] [Google Scholar]; (b) Lan T, Furuya K, Lu Y. Chem Commun. 2010;46:3896–3898. doi: 10.1039/b926910j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang H, Jiang B, Xiang Y, Su J, Chai Y, Yuan R. Biosens Bioelectron. 2011;41:529–540. doi: 10.1016/j.bios.2011.07.009. [DOI] [PubMed] [Google Scholar]; (d) Gao A, Tang C-X, He X-W, Yin X-B. Analyst. 2013;138:263–268. doi: 10.1039/c2an36398d. [DOI] [PubMed] [Google Scholar]; (e) Li W, Yang Y, Chen J, Zheng Q, Wang Y, Wang F, Yu C. Biosens Bioelectron. 2014;53:245–249. doi: 10.1016/j.bios.2013.09.055. [DOI] [PubMed] [Google Scholar]; (f) Li H, Zhang Q, Cai Y, Kong D-M, Shen H-X. Biosens Bioelectron. 2012;34:159–164. doi: 10.1016/j.bios.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 5.(a) Li C-L, Liu K-T, Lin Y-W, Chang H-T. Anal Chem. 2011;83:225–23. doi: 10.1021/ac1028787. [DOI] [PubMed] [Google Scholar]; (b) Li C-L, Huang C-C, Chen W-H, Chiang C-K, Chang H-T. Analyst. 2012;137:5222–5228. doi: 10.1039/c2an35599j. [DOI] [PubMed] [Google Scholar]; (c) Li F, Yang L, Chen M, Qian Y, Tang B. Biosens Bioelectron. 2013;41:903–906. doi: 10.1016/j.bios.2012.09.048. [DOI] [PubMed] [Google Scholar]; Yuen LH, Franzini RM, Tan SS, Kool ET. J Am Chem Soc. 2014;136:14576–14582. doi: 10.1021/ja507932a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Li T, Dong S, Wang E. J Am Chem Soc. 2010;132:13156–13157. doi: 10.1021/ja105849m. [DOI] [PubMed] [Google Scholar]; (b) Guo L, et al. Biosens Bioelectron. 2012;35:123–127. doi: 10.1016/j.bios.2012.02.031. [DOI] [PubMed] [Google Scholar]; (c) Liu M, et al. Environ Sci Technol. 2010;44:4241–4246. doi: 10.1021/es1003507. [DOI] [PubMed] [Google Scholar]; (d) Zhang H, Jiang B, Xiang Y, Su J, Chai Y, Yuan R. Biosens Bioelectron. 2011;28:135–138. doi: 10.1016/j.bios.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Paige JS, Wu KY, Jaffrey SR. Science. 2011;333:642–646. doi: 10.1126/science.1207339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Strack RL, Song W, Jaffrey SR. Nat Prot. 2014;9:146–155. doi: 10.1038/nprot.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bhadra S, Ellington AD. RNA. 2014;20:1183–1194. doi: 10.1261/rna.045047.114. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Akter F, Yokobayashi Y. ACS Synth Biol. 2014 doi: 10.1021/sb500314r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hofer K, Langejurgen LV, Jaschke A. J Am Chem Soc. 2013;135:13692–13694. doi: 10.1021/ja407142f. [DOI] [PubMed] [Google Scholar]
- 9.(a) Huang H, et al. Nat Chem Biol. 2014;10:686–691. doi: 10.1038/nchembio.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Deigan-Warner K, et al. Nat Struct Mol Biol. 2014;21:658–663. doi: 10.1038/nsmb.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Fu Y, Zheng B, Cheng S, Li W, Lau T-C, Liang H. J Phys B. 2011;115:13051–13056. doi: 10.1021/jp2074489. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, et al. Nucleic Acids Res. 2012;40:4229–4236. doi: 10.1093/nar/gkr1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Smirnov I, Shafer RH. J Mol Biol. 2000;296:1–5. doi: 10.1006/jmbi.1999.3441. [DOI] [PubMed] [Google Scholar]; (b) Kotch FW, Fettinger JC, Davis JT. Org Lett. 2000;2:3277–3280. doi: 10.1021/ol0065120. [DOI] [PubMed] [Google Scholar]
- 13.Lee MPH, Parkinson GN, Hazel P, Neidle S. J Am Chem Soc. 2007;129:10106–10107. doi: 10.1021/ja0740869. [DOI] [PubMed] [Google Scholar]
- 14.Zhan S, et al. RSC Adv. 2013;3:16962–16966. [Google Scholar]
- 15.Mazumdar D, Liu J, Lu G, Zhou J, Lu Y. Chem Commun. 2010;46:1416–1418. doi: 10.1039/b917772h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Pelossof G, Tel-Vered R, Willner I. Anal Chem. 2012;84:3703–3709. doi: 10.1021/ac3002269. [DOI] [PubMed] [Google Scholar]; (b) Li F, Yang L, Chen M, Li P, Tang B. Analyst. 2013;138:461–466. doi: 10.1039/c2an36227a. [DOI] [PubMed] [Google Scholar]; (c) Zhang D, Yin L, Meng Z, Yu A, Guo L, Wang H. Anal Chim Acta. 2014;812:161–167. doi: 10.1016/j.aca.2013.12.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


