RNA interference (RNAi) harnesses short interfering RNA (siRNA) to inhibit gene expression post-transcriptionally by targeted destruction of specific messenger RNA (mRNA) molecules. This promising direction in disease therapeutics and regenerative medicine is problematic due to ionic, hydrophilic and macromolecular nature of naked siRNA.[1] These properties prevent siRNA from entering cells, and make it susceptible to degradation by serum ribonucleases (RNases).[2] Many approaches have been developed to deliver siRNA to target tissue, including direct injection of siRNA solutions,[3] incorporation of siRNA into liposomes,[3c,4] complexation of siRNA with cationic polymers and lipids to form nanoparticles[1a,1d,3c,5] or encapsulation of siRNA into microparticles.[6]
Nano- and microparticles can protect siRNA from RNase degradation, but siRNA delivered by these particles disperses rapidly in vivo owing to their small size, leading to poor retention at targeted tissue sites.[1f,1g,2a-c] Prolonged presentation of siRNA at desired target tissue, however, would increase its bioavailability and thus therapeutic efficacy.[1g,2a,2b] In efforts to extend siRNA-mediated gene silencing, macroscopic hydrogels,[1g-j,2a,7] nanofibers[8] and porous scaffolds[9] have recently been reported for localized and/or sustained delivery of siRNA. The delivery of siRNA from these systems has been achieved through the regulation of siRNA diffusion, hydrolytic degradation of the carrier biomaterial and/or affinity of interactions between siRNA and hydrogels.[1d-h,2a,2b,7a,8-9]
Externally applied stimuli for triggering siRNA release would provide an alternative mechanism for physician- or patient-controlled delivery, permitting the release of desired defined doses at specific times. Among external stimuli such as light[1g], ultrasound[10], and magnetic[11] and electric[12] fields, UV light has been widely used for control over the release of low molecular weight compounds[13] and cells[14], and its application may be spatially and temporally[15] regulated with high precision. For example, externally applied UV light has been reported to regulate the photodegradation of the polymer backbone of nanoparticles[13a-d] or linkages between polymers and bioactive agents[13e-h,14,15b,15d] to release payloads, such as 5-fluorouracil,[13f] doxorubicin[13h] and cells.[14] Similarly, methyl-ether-PEG-b-poly(5-(3-(amino) propoxy)-2-nitrobenzyl methacrylate,[16] a cationic photodegradable polymer, or lipid-derived photolabile-bridged-diethylenetriamine,[17] an amphiphilic photodegradable molecule, have also been synthesized to photo-activate the gene silencing capacity of siRNA. Recently, 3D macroscopic photodegradable PEG hydrogels were engineered to control cell morphology and proliferation[15b] and regulate the release of encapsulated cells via UV application.[18] To date, however, the capacity to deliver genetic material from 3D macroscopic hydrogels locally and in a sustained manner, and to regulate the release profile via on-demand triggering by light stimulation has not been reported. In this work, photodegradable poly(ethylene glycol)-di(photolabile acrylate) (PEG-DPA) hydrogels chemically modified with a cationic molecule (i.e., 2-amino ethyl methacrylate (AEMA)) were engineered for photo-triggering siRNA release at target sites, a potentially valuable tool for on-demand regulation of cell gene expression with applications in disease therapeutics and tissue regenerative medicine.
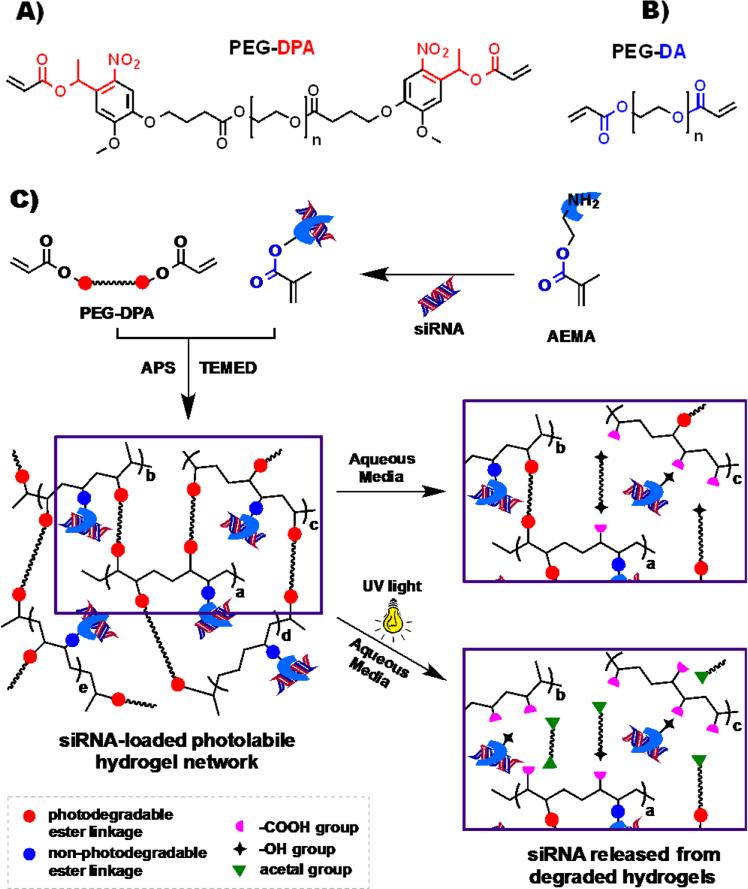
A photodegradable PEG-DPA macromer (Figure 1A) was synthesized using modifications of previous reports[15b-d,18-19] to fabricate a hydrogel system that can activate the release of siRNA by UV light application. The PEG-DPA contains two ortho-nitrobenzyl (ONB) photolabile groups with the photocleavable ester groups linked directly to ONB groups. These ester moieties are known to cleave into acetal and acid groups upon exposure to UV light.[15b-d,18] The photodegradable hydrogels were fabricated via free radical polymerization of PEG-DPA and AEMA in the presence of ammonium persulfate (APS) and tetramethylethylenediamine (TEMED) as redox initiator and catalyst, respectively. The gelation time of the fabricated photolabile hydrogels ranges from 4.8 to 12.6 min as a function of hydrogel concentrations (Table S1, Supporting Information). This gelation time range is sufficient for injecting the hydrogel precursor solutions with the redox catalysts into the body using a syringe and needle or a long micro-catheter before the gelation. AEMA bearing a primary amine group was employed to electrostatically interact with and retain negatively charged siRNA within the hydrogel network following the crosslinking of the methacrylate moiety with the acrylates of the PEG-DPA. Upon exposure to low-intensity UV irradiation (365 nm), the resulting siRNA-loaded photodegradable PEG hydrogels can degrade via the photolysis of ONB-ester units and thus release incorporated siRNA. The capacity of the photolabile hydrogels to release siRNA on demand in response to UV light was compared to non-photodegradable hydrogels synthesized from PEG-diacrylate (PEG-DA) (Figure 1B), which was synthesized using modifications of previous reports,[7a,20] and AEMA. A schematic illustrating siRNA loading, hydrogel formation and siRNA release from the hydrogels upon their hydrolytic and/or UV light exposure-based degradation is depicted in Figure 1C.
Figure 1.
Structure of the (A) photolabile PEG-DPA and (B) non-photolabile PEG-DA macromers used in this study. (C) Schematic of photolabile hydrogel formation and subsequent siRNA release upon the degradation of hydrogel network in aqueous media in the absence and presence of an external UV light source.
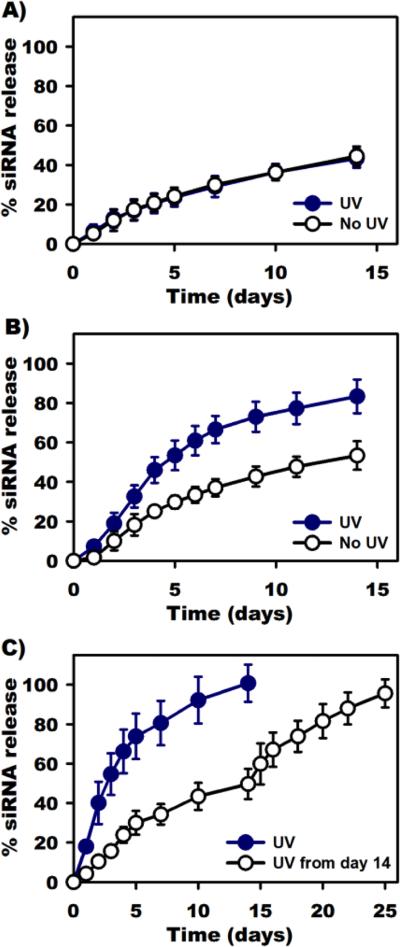
The release of siRNA (siRNA against luciferase, siLuc) from non-photodegradable and photodegradable PEG hydrogels with and without UV light exposure into nuclease-free phosphate buffered saline (PBS) solution was performed to demonstrate the capacity of the hydrogel system to release siRNA in response to UV light application. UV light with an intensity of 10 mW/cm2 applied to the hydrogels for 20 min (denoted as “UV 10-20”) at each release time point. 20 minutes at this irradiation intensity was previously reported to be benign to human mesenchymal stem cells encapsulated within the similar PEG hydrogels.[15b] First, siRNA release from non-photodegradable hydrogels (15% w/w) was investigated to demonstrate that UV light did not accelerate siRNA release using this hydrogel system. As shown in Figure 2A, siRNA was released from non-photolabile hydrogels with and without UV exposure at a similar release rate, with 43 and 44% cumulative siRNA released after 14 days, respectively, likely due to the hydrolytic degradation of ester linkages within the hydrogel networks.[1g] These similar siRNA release profiles from the non-photodegradable hydrogels support a UV light exposure-independent mechanism. siRNA release from 15% (w/w) photolabile hydrogels in the absence and presence of “UV 10-20” was then examined to demonstrate that UV light can regulate siRNA release from the photodegradable hydrogels. As shown in Figure 2B, the photolabile hydrogels without UV application (“No UV”) released 53% of siRNA after 14 days. In contrast, the release rate of siRNA increased upon the application of UV light (“UV”), with a total siRNA release of 83% after 14 days, likely as a result of photodegradation of the hydrogel network,[15b] indicating light-stimulated siRNA release (illustrated in Figure 1C). A similar trend was also observed with the 7.5 and 10 % (w/w) hydrogels (Figure S1, Supporting Information).
Figure 2.
Release profiles of siRNA from 15% (w/w) hydrogels in the absence and presence of UV light exposure. (A) Non-photolabile (the 2 curves overlap), (B) photolabile hydrogels exposed to UV light at an intensity of 10 mW/cm2 for 20 min (“UV 10-20”) at each time point and (C) photolabile hydrogels exposed to the same UV light intensity of 10 mW/cm2 for 60 min (“UV 10-60”) at each time point. UV light exposure triggered the release of siRNA from photolabile hydrogels due to the photolytic degradation of the crosslinked networks, which did not occur in non-photolabile hydrogels.
After establishing that UV application could accelerate siRNA release from the hydrogels, photolabile hydrogels (15% w/w) were exposed to UV light with an intensity of 10 mW/cm2 for an increased time of 60 min (denoted as “UV 10-60”) at each release time point to quantify the effect of UV irradiation duration on hydrogel degradation and thus siRNA release. As shown in Figure 2C, while the hydrogels subjected to UV released all encapsulated siRNA within 14 days as the hydrogels underwent complete degradation, the hydrogels without UV exposure for the first 14 days (denoted as “UV from day 14”) cumulatively released 50% of siRNA and remained intact. The “UV from day 14” hydrogels in Figure 2C were then exposed to UV for 60 min starting at day 14 and at every subsequent release time point. siRNA release was then accelerated until the hydrogels completely degraded at day 25 with 96% siRNA was recovered. These results indicate that varying UV exposure time while keeping the UV intensity constant can regulate siRNA release kinetics by regulating hydrogel degradation rate.[15b]
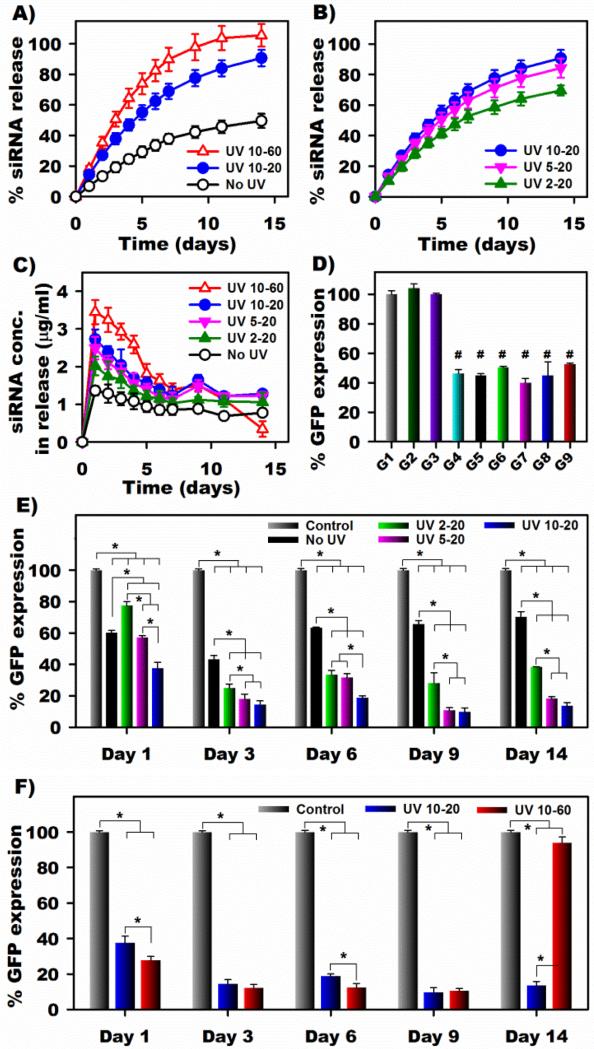
To examine the ability of released siRNA to inhibit green fluorescent protein (GFP) expression via flow cytometry, photodegradable hydrogels were loaded with a siRNA targeting GFP (siGFP). 15% (w/w) photodegradable hydrogels were exposed to either no UV or UV light at defined intensities and exposure durations at specific time points, and release of siGFP into phenol red free Dulbecco's Modified Eagle Medium High Glucose (DMEM-HG) at 37°C was measured. For hydrogels subjected to UV light, an intensity of 10 mW/cm2 was used for exposure durations of either 20 or 60 min. As shown in Figure 3A, the UV-exposed hydrogels exhibited higher release rates than those without UV treatment (“No UV”). siRNA was gradually released from “No UV” hydrogels with only 50% of encapsulated siRNA released over 2 weeks due to the hydrolytic degradation of ester linkages within the hydrogel network.[1g] These hydrogels released 98% siRNA after 39 days upon complete degradation (Figure S2A, Supporting Information). In contrast, the “UV 10-20” hydrogels released 91% siRNA after 2 weeks and completely degraded by day 27 with all incorporated siRNA recovered (Figure S2A, Supporting Information). Furthermore, the “UV 10-60” hydrogels fully degraded by day 14 with all encapsulated siRNA released (Figure 3A). The faster release rate from the “UV 10-60” hydrogels compared to the “UV10-20” hydrogels was likely due to faster degradation of the polymer backbone resulting from longer UV exposure time at the same UV intensity. In addition, at the same hydrogel concentration (15%, w/w) and UV light conditions (“UV 10-20”), the release profile of non-targeting control siLuc was similar to that of siGFP (Figure S2A, Supporting Information). When hydrogels were irradiated with UV at different UV intensities (2, 5 and 10 mW/cm2), but for a constant UV exposure time (20 min), the release of siRNA increased with increasing UV intensity (Figure 3B and S2B). When the hydrogels were exposed to UV light with different exposure times and intensities, siRNA release concentrations in DMEM-HG (μg/mL) over time were greater with increases in either or both of these variables (Figure 3C). Taken together, these results indicate that siRNA release can be controlled by regulating UV exposure time and/or intensity.
Figure 3.
Release profiles of siRNA into DMEM-HG from 15% (w/w) photolabile hydrogels exposed to no UV and UV light at (A) an intensity of 10 mW/cm2 for 20 (“UV 10-20”) and 60 min (“UV 10-60”) or (B) different intensities of 2, 5 and 10 mW/cm2 for 20 min (“UV 2-20”, “UV 5-20” and “UV 10-20”, respectively). (C) Concentration of siRNA in the releasates. (D) % GFP expression of deGFP-expressing HeLa cells after 2 days of culture with the same concentration (0.5 mL 0.075 μM siRNA) of fresh siGFP and released siRNA collected at day 4 of the release experiment. G1) Control; G2) Gel only, UV 10-20; G3) siLuc, Gel UV 10-20; G4) fresh siGFP; G5) siGFP, Gel No UV; G6) siGFP, Gel UV 2-20; G7) siGFP, Gel UV 5-20; G8) siGFP, Gel UV 10-20; G9) siGFP, Gel UV 10-60. The released siGFP from photolabile hydrogels treated with different UV doses (G5-G9) silenced GFP expression at a similar degree compared to fresh siGFP. Percentage GFP expression of deGFP-expressing HeLa cells after 2 days of culture with the same volume (0.5 mL) of releasates from hydrogels exposed to different UV (E) intensities or (F) exposure durations collected at different time points. # p<0.01 compared to “Control” (G1), * p<0.05.
The effect of UV on the bioactivity of released siRNA was evaluated and compared to that of fresh siRNA and siRNA released from hydrogels without UV light application as UV light exposure may compromise the bioactivity of released siRNA. The same amount of released siGFP and released siLuc, and fresh siGFP (0.5 mL of 0.075 μM) in DMEM-HG solutions were cultured with HeLa cells constitutively expressing destabilized GFP (deGFP) seeded in monolayer for 48h. Cells were then harvested for flow cytometry to quantify the degree of GFP silencing. The cells cultured with media only served as a control with 100% GFP expression (G1) and the other experimental conditions were normalized to G1. As shown in Figure 3D, the cells treated with the samples from “UV 10-20” hydrogels alone (G2) or with released siLuc from “UV 10-20” hydrogels (G3) exhibited 100% GFP expression. In contrast, when the same siRNA concentration (0.075 μM) was used, siGFP released from the hydrogels with no UV (G5) and different UV doses (G6-G9) knocked down GFP expression similarly to the fresh siGFP (G4) that inhibited 54% GFP expression (Figure 3D), indicating that the UV intensity and time utilized did not affect the bioactivity of released siRNA.
Since it was demonstrated that increasing UV irradiation intensity and duration accelerated siRNA release from the photolabile hydrogel system, it is important to examine the effect of exogenously controlling siRNA dosing by UV light on the degree of gene knockdown. Therefore, siRNA samples released from hydrogels with different UV exposure intensities and durations were cultured with deGFP-expressing HeLa cells in monolayer for 48h, and then the cells were harvested for GFP expression quantification using flow cytometry. Cells cultured with media only were used as a control with 100% GFP expression (“Control”), and all other groups were normalized to the “Control”.
As shown in Figure 3E, reduction of GFP expression was observed when the cells were cultured with releasates from “No UV” and UV-treated hydrogels. Interestingly, the released siRNA from hydrogels exposed to various intensities of UV for the same amount of time significantly silenced GFP expression more compared to that released from “No UV” hydrogels, as a result of more siRNA having been released from the UV-applied hydrogels (Figure 3C), except for the “UV 2-20” and “UV 5-20” hydrogels at day 1. In addition, the released siRNA from hydrogels exposed to greater UV intensity with the same exposure duration significantly or equally knocked down GFP expression compared to the released siRNA from hydrogels exposed to lower UV intensity (Figure 3E). This trend can be observed for the released samples from day 3 up to day 14, which correlates with the concentration of siRNA released (Figure 3C). Moreover, the released siRNA from hydrogels exposed to the same UV intensity for a longer duration significantly or equally knocked down GFP expression compared to the released siRNA from hydrogels with shorter UV exposure at all examined release time points (Figure 3F), except for day 14 due to the much lower released siRNA concentration at the last time point from the “UV 10-60” hydrogels compared earlier time points (Figure 3C). These results indicate that variations in UV dose can be used to trigger the release of siRNA from photolabile hydrogels at different rates, which permits regulation of the timing and extent of gene silencing.
In summary, a photodegradable hydrogel system has been engineered for stimulating siRNA release in response to UV light. AEMA, a cationic molecule, has been incorporated into the hydrogels to retain siRNA within the hydrogel networks. The release profiles of siRNA were regulated by UV intensity, UV exposure duration, and hydrogel concentration. The released siRNA from hydrogels with and without UV exposure could knockdown GFP expression in cells to the same extent as fresh siRNA. The releasates from hydrogels exposed to higher UV doses could inhibit GFP expression at significantly higher levels compared to that from hydrogels subjected to lower UV doses, which effectively correlates with the concentration of siRNA in releasates. To the best of our knowledge, this is the first report of on-demand delivery of genetic material from photolabile hydrogels. This hydrogel system provides an excellent platform for nucleic acid delivery using UV light as an external stimulus. By applying light directly through the skin or by using a light cable or catheter guide wire to access more distant sites, genetic material could be released at defined times and spatial locations in the body, an approach that would be valuable for tissue engineering strategies and treating diseases such as cancer.
Experimental Section
Materials
Accell siGFP and siLuc were obtained from Thermo Scientific Dharmacon (Lafayette, CO). Tetramethylethylenediamine (TEMED) and phenol red free DMEM-HG were purchased from Sigma Aldrich (St Louis, MO). 2-amino ethyl methacrylate (AEMA) was obtained from Polysciences, Inc. (Warrington, PA). Ammonium persulfate (APS) was purchased from MP Biomedicals, LLC (Solon, OH). RiboGreen RNA assay kit was obtained from Invitrogen (Carlsbad, CA). 10x nuclease-free PBS solution was obtained from Life Technologies (Carlsbad, CA). DMEM-HG with 4.5 g/L glucose, fetal bovine serum (FBS) and G-418 (50 mg/mL) were purchased from Hyclone (Logan, UT).
Synthesis of PEG-DA and PEG-DPA
PEG-DPA and PEG-DA were synthesized according to modifications of previous reports.[7a,15b-d,20] The detailed procedures for synthesis and characterization are provided in the Supporting Information.
Hydrogel preparation
The hydrogels were prepared by free radical polymerization of PEG-DA or PEG-DPA macromers and AEMA in the presence of APS and TEMED as redox initiator and catalyst, respectively. To prepare siRNA-loaded 50 μL 15% (w/w) hydrogel, siRNA (13.3 μg) was mixed with AEMA (160 μg) in a total volume of 11.3 μL PBS for 30 min prior to combining with PEG-DA or PEG-DPA solutions in PBS (33.7 μL, 22.3% w/w). APS (2.5 μL, 40% w/w) and TEMED (2.5 μL, 5% w/w) solutions in PBS were then added to the above mixture, and the mixture was vortexed and incubated at RT for 2 h for hydrogel crosslinking. Prior to release studies, the hydrogels were rinsed with PBS solution (0.5 mL, pH 7.4) at 4°C and the PBS was changed every 12 h for 3 days to remove the catalysts. The amount of siRNA in the rinse solutions was measured (7-17%) and subtracted from the total amount initially loaded to obtain the true baseline for release and bioactivity experiments.
siRNA release and quantification
The prepared hydrogels in 1.7 mL vials were exposed to a 320-500 nm UV light source using an Omnicure S1000 UV Spot Cure System (Lumen Dynamics Group, Mississauga, Ontario, Canada). Nuclease-free PBS solution (0.5 mL) at pH 7.4 was added to the vials and siRNA release was performed at 37°C. At each predetermined time point, the 0.5 mL released sample was collected, and UV light was applied to the hydrogels and then 0.5 mL fresh PBS was added to the vials. A series of known siRNA concentrations in PBS solution was used to establish the standard curve. The released siRNA was quantified with RiboGreen RNA assay on a plate reader (fmax, Molecular Devices, Inc., CA, USA) set at excitation 485/emission 538 nm. For bioactivity evaluation, Accell siGFP and siLuc were released into phenol red free DMEM-HG instead of PBS.
Bioactivity of the released siRNA
To examine the effect of UV exposure on the bioactivity of the released siRNA, the same amount of released or fresh siRNA in DMEM-HG (0.5 mL, 0.075 μM siRNA) was cultured with deGFP-expressing HeLa cells. The cells were seeded in monolayer in 24-well plate at a density of 50,000 cells/well in 0.5 mL of DMEM-HG supplemented with 5 % FBS and G-418 (500 μg/mL) and cultured at 37°C and 5% CO2 in a humidified incubator. After 1 day of culture, the growth media were aspirated and replaced with 0.5 mL media containing the same concentration of fresh or released siRNA (0.075 μM siRNA). The cells were transfected for 24h, and then FBS (10 μL) was added and the cells were cultured for an additional 24h before harvesting for flow cytometry (EPICS XL-MCL, Beckman Coulter, Fullerton, CA) to quantify the degree of GFP silencing. To examine the bioactivity of different siRNA concentrations in the releasates from photolabile hydrogels exposed to different UV doses, the releasates from 3 hydrogels at each specific time point were combined and cultured with the deGFP-expressing HeLa cells (0.5 mL/well), which were seeded as described above 1 day prior. After 24h, FBS (10 μL) was added and the cells were then cultured for an additional 24h before harvesting for flow cytometry to quantify the degree of GFP silencing. The GFP expression of control samples was normalized to 100% and the GFP expression of the other conditions was normalized to controls.
Statistical analysis
The data are expressed as mean ± standard deviation (N = 3). Statistical analysis was performed with one-way analysis of variance (ANOVA) with Tukey–Kramer Multiple Comparisons using GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA). P < 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgements
The authors acknowledge funding from the National Institutes of Health (R01AR063194, R56DE022376 (EA) and EB014277 (VR)) and the Department of Defense Congressionally Directed Medical Research Programs (OR110196 (EA)).
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Dr. Cong Truc Huynh, Department of Biomedical Engineering, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA
Dr. Minh Khanh Nguyen, Department of Biomedical Engineering, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA
Gulen Yesilbag Tonga, Department of Chemistry, University of Massachusetts, 710 North Pleasant Street, Amherst, MA 01003, USA.
Lionel Longé, Department of Chemistry, University of Massachusetts, 710 North Pleasant Street, Amherst, MA 01003, USA; Département Chimie Physique, École Nationale Supérieure de Chimie, de Biologie et de Physique 16, avenue Pey Berland 33607 PESSAC Cedex, France.
Prof. Vincent M. Rotello, Department of Chemistry, University of Massachusetts, 710 North Pleasant Street, Amherst, MA 01003, USA
Prof. Eben Alsberg, Department of Biomedical Engineering, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA; Department of Orthopaedic Surgery, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA.
References
- 1.a Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, Paz-Ares L, Cho DC, Infante JR, Alsina M, Gounder MM, Falzone R, Harrop J, White AC, Toudjarska I, Bumcrot D, Meyers RE, Hinkle G, Svrzikapa N, Hutabarat RM, Clausen VA, Cehelsky J, Nochur SV, Gamba-Vitalo C, Vaishnaw AK, Sah DW, Gollob JA, Burris HA., III Cancer Discov. 2013;3:406. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]; b Monaghan M, Pandit A. Adv. Drug Deliv. Rev. 2011;63:197. doi: 10.1016/j.addr.2011.01.006. [DOI] [PubMed] [Google Scholar]; c Oh YK, Park TG. Adv. Drug Deliv. Rev. 2009;61:850. doi: 10.1016/j.addr.2009.04.018. [DOI] [PubMed] [Google Scholar]; d Segovia N, Pont M, Oliva N, Ramos V, Borros S, Artzi N. Adv. Healthc. Mater. 2015;4:271. doi: 10.1002/adhm.201400235. [DOI] [PubMed] [Google Scholar]; e Lee H, Lytton-Jean AK, Chen Y, Love KT, Park AI, Karagiannis ED, Sehgal A, Querbes W, Zurenko CS, Jayaraman M, Peng CG, Charisse K, Borodovsky A, Manoharan M, Donahoe JS, Truelove J, Nahrendorf M, Langer R, Anderson DG. Nat. Nanotechnol. 2012;7:389. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J. Nat. Biotechnol. 2005;23:709. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]; g Nguyen K, Dang PN, Alsberg E. Acta. Biomater. 2013;9:4487. doi: 10.1016/j.actbio.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Han HD, Mora EM, Roh JW, Nishimura M, Lee SJ, Stone RL, Bar-Eli M, Lopez-Berestein G, Sood AK. Cancer Biol. Ther. 2011;11:839. doi: 10.4161/cbt.11.9.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Kim YM, Park MR, Song SC. Biomaterials. 2013;34:4493. doi: 10.1016/j.biomaterials.2013.02.050. [DOI] [PubMed] [Google Scholar]; j Yin L, Zhao X, Ji S, He C, Wang G, Tang C, Gu S, Yin C. Biomaterials. 2014;35:2488. doi: 10.1016/j.biomaterials.2013.12.015. [DOI] [PubMed] [Google Scholar]; k Kim WJ, Kim SW. Pharm. Res. 2009;26:657. doi: 10.1007/s11095-008-9774-1. [DOI] [PubMed] [Google Scholar]; l Bumcrot D, Manoharan M, Koteliansky V, Sah DWY. Nat. Chem. Biol. 2006;2:711. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Dykxhoorn DM, Palliser D, Lieberman J. Gene Ther. 2006;13:541. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]; n Nguyen MK, Alsberg E. Prog. Polym. Sci. 2014;39:1235. doi: 10.1016/j.progpolymsci.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Krebs MD, Jeon O, Alsberg E. J. Am. Chem. Soc. 2009;131:9204. doi: 10.1021/ja9037615. [DOI] [PubMed] [Google Scholar]; b Krebs MD, Alsberg E. Chemistry. 2011;17:3054. doi: 10.1002/chem.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jiang Y, Tang R, Duncan B, Jiang Z, Yan B, Mout R, Rotello VM. Angew. Chem. Int. Ed. 2015;54:506. doi: 10.1002/anie.201409161. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Whitehead KA, Langer R, Anderson DG. Nat. Rev. Drug Discov. 2009;8:129. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Wang Q, Ilves H, Chu P, Contag CH, Leake D, Johnston BH, Kaspar RL. J. Invest. Dermatol. 2007;127:2577. doi: 10.1038/sj.jid.5700891. [DOI] [PubMed] [Google Scholar]; b Bitko V, Musiyenko A, Shulyayeva O, Barik S. Nat. Med. 2005;11:50. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]; c de Fougerolles AR. Hum. Gene Ther. 2008;19:125. doi: 10.1089/hum.2008.928. [DOI] [PubMed] [Google Scholar]
- 4.a Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T, Takayama T, Niitsu Y. Nat. Biotechnol. 2008;26:431. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]; b Sato A, Takagi M, Shimamoto A, Kawakami S, Hashida M. Biomaterials. 2007;28:1434. doi: 10.1016/j.biomaterials.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 5.a Dunn SS, Tian S, Blake S, Wang J, Galloway AL, Murphy A, Pohlhaus PD, Rolland JP, Napier ME, DeSimone JM. J. Am. Chem. Soc. 2012;134:7423. doi: 10.1021/ja300174v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Segovia N, Dosta P, Cascante A, Ramos V, Borros S. Acta Biomater. 2014;10:2147. doi: 10.1016/j.actbio.2013.12.054. [DOI] [PubMed] [Google Scholar]
- 6.a Mountziaris PM, Tzouanas SN, Sing DC, Kramer PR, Kasper FK, Mikos AG. Acta Biomater. 2012;8:3552. doi: 10.1016/j.actbio.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Murata N, Takashima Y, Toyoshima K, Yamamoto M, Okada H. J. Control. Release. 2008;126:246. doi: 10.1016/j.jconrel.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 7.a Nguyen MK, Jeon O, Krebs MD, Schapira D, Alsberg E. Biomaterials. 2014;35:6278. doi: 10.1016/j.biomaterials.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hill MC, Nguyen MK, Jeon O, Alsberg E. Adv. Healthc. Mater. 2015;4:714. doi: 10.1002/adhm.201400458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a Cao H, Jiang X, Chai C, Chew SY. J. Control. Release. 2010;144:203. doi: 10.1016/j.jconrel.2010.02.003. [DOI] [PubMed] [Google Scholar]; b Rujitanaroj P.-o., Jao B, Yang J, Wang F, Anderson JM, Wang J, Chew SY. Acta Biomater. 2013;9:4513. doi: 10.1016/j.actbio.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Andersen MO, Nygaard JV, Burns JS, Raarup MK, Nyengaard JR, Bunger C, Besenbacher F, Howard KA, Kassem M, Kjems J. Mol. Ther. 2010;18:2018. doi: 10.1038/mt.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Nelson CE, Gupta MK, Adolph EJ, Shannon JM, Guelcher SA, Duvall CL. Biomaterials. 2012;33:1154. doi: 10.1016/j.biomaterials.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu F, Finley TD, Turkaydin M, Sung Y, Gurkan UA, Yavuz AS, Guldiken RO, Demirci U. Biomaterials. 2011;32:7847. doi: 10.1016/j.biomaterials.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tasoglu S, Kavaz D, Gurkan UA, Guven S, Chen P, Zheng R, Demirci U. Adv. Mater. 2013;25:1137. doi: 10.1002/adma.201200285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SJ, Yoon SG, Lee SM, Lee SH, Kim SI. J. Appl. Polym. Sci. 2004;91:3613. [Google Scholar]
- 13.a Yu L, Lv C, Wu L, Tung C, Lv W, Li Z, Tang X. Photochem. Photobiol. 2011;87:646. doi: 10.1111/j.1751-1097.2011.00894.x. [DOI] [PubMed] [Google Scholar]; b Yesilyurt V, Ramireddy R, Thayumanavan S. Angew. Chem. Int. Ed. 2011;50:3038. doi: 10.1002/anie.201006193. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. J. Am. Chem. Soc. 2010;132:9540. doi: 10.1021/ja102595j. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lv C, Wang Z, Wang P, Tang X. Langmuir. 2012;28:9387. doi: 10.1021/la301534h. [DOI] [PubMed] [Google Scholar]; e Griffin DR, Kasko AM. ACS Macro Lett. 2012;1:1330. doi: 10.1021/mz300366s. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Agasti SS, Chompoosor A, You CC, Ghosh P, Kim CK, Rotello VM. J. Am. Chem. Soc. 2009;131:5728. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Ki Choi S, Thomas T, Li M-H, Kotlyar A, Desai A, Baker JJR. Chem. Commun. 2010;46:2632. doi: 10.1039/b927215c. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Johnson JA, Lu YY, Burts AO, Lim Y-H, Finn MG, Koberstein JT, Turro NJ, Tirrell DA, Grubbs RH. J. Am. Chem. Soc. 2011;133:559. doi: 10.1021/ja108441d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wirkner M, Alonso JM, Maus V, Salierno M, Lee TT, Garcia AJ, del Campo A. Adv. Mater. 2011;23:3907. doi: 10.1002/adma.201100925. [DOI] [PubMed] [Google Scholar]
- 15.a Jain PK, Shah S, Friedman SH. J. Am. Chem. Soc. 2011;133:440. doi: 10.1021/ja107226e. [DOI] [PubMed] [Google Scholar]; b Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Science. 2009;324:59. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wong DY, Griffin DR, Reed J, Kasko AM. Macromolecules. 2010;43:2824. [Google Scholar]; d Kloxin AM, Tibbitt MW, Anseth KS. Nat. Protoc. 2010;5:1867. doi: 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster AA, Greco CT, Green MD, Epps TH, 3rd, Sullivan MO. Adv. Healthc. Mater. 2015;4:760. doi: 10.1002/adhm.201400671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H-J, Wang H-X, Sun C-Y, Du J-Z, Wang J. RSC Adv. 2014;4:1961. [Google Scholar]
- 18.Griffin DR, Kasko AM. J. Am. Chem. Soc. 2012;134:13103. doi: 10.1021/ja305280w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruise GM, Scharp DS, Hubbell JA. Biomaterials. 1998;19:1287. doi: 10.1016/s0142-9612(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 20.a Huynh CT, Nguyen MK, Huynh DP, Lee DS. Colloid Polym. Sci. 2011;289:301. [Google Scholar]; b Huynh CT, Kang SW, Li Y, Kim BS, Lee DS. Soft Matter. 2011;7:8984. [Google Scholar]; c Huynh CT, Lee DS. Colloid Polym. Sci. 2012;290:1077. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.