Abstract
In Parkinson’s disease (PD) alpha-synuclein oligomers are thought to be pathogenic, and 3,4-dihydroxyphenylacetaldehyde (DOPAL), an obligate aldehyde intermediate in neuronal dopamine metabolism, potently oligomerizes alpha-synuclein. PD involves alpha-synuclein deposition in brainstem raphe nuclei; however, whether 5-hydroxyindoleacetaldehyde (5-HIAL), the aldehyde of serotonin, oligomerizes alpha-synuclein has been unknown. In this study we tested whether 5-HIAL oligomerizes alpha-synuclein in vitro and in PC12 cells conditionally over-expressing alpha-synuclein. Alpha-synuclein oligomers were quantified by western blotting after incubation of alpha-synuclein with serotonin and monoamine oxidase-A (MAO-A) to generate 5-HIAL or dopamine to generate DOPAL. Oligomerization of alpha-synuclein in PC12 cells over-expressing the protein was compared between vehicle-treated cells and cells incubated with levodopa to generate DOPAL or 5-hydroxytryptophan to generate 5-HIAL. Monoamine aldehyde mediation of the oligomerization was assessed using the MAO inhibitor, pargyline. Dopamine and serotonin incubated with MAO-A both strongly oligomerized alpha-synuclein (more than 10 times control); pargyline blocked the oligomerization. In synuclein overexpressing PC12 cells, levodopa and 5-hydroxytryptophan elicited pargyline-sensitive alpha-synuclein oligomerization. 5-HIAL oligomerizes alpha-synuclein both in vitro and in synuclein-overexpressing PC12 cells, in a manner similar to DOPAL. The findings may help explain loss of serotonergic neurons in PD.
Keywords: Serotonin; Dopamine; 5-Hydroxyindolealdehyde; 3,4-Dihydroxyphenylacetaldehyde; Parkinson’ disease
1. Introduction
Profound depletion of dopamine (DA) in the striatum—especially the putamen—constitutes a neurochemical hallmark of Parkinson disease (PD). Researchers have long noted less severe but more widespread decreases of tissue serotonin contents in PD [4,7–9]. Bases for the susceptibility of the monoaminergic neurons in PD have been mysterious. Lewy bodies, intra-cytoplasmic inclusions that contain abundant precipitated alpha-synuclein, constitute a neuropathologic hallmark of PD. According to Braak’s schema for the sequence of alpha-synucleinopathy in PD [1], synucleinopathy in medullary serotonergic raphe nuclei precedes involvement of midbrain dopaminergic substantia nigra neurons. Bases for the tendency of alpha-synuclein to become deposited in monoaminergic neurons have also been mysterious.
A potential pathogenetic link between monoamine depletion and alpha-synuclein deposition in PD is oligomerization of alpha-synuclein by monoamine aldehydes. It is widely suspected that alpha-synuclein oligomers, which are intermediates between the monomer in solution and the fibrillar polymer in Lewy bodies, constitute the main pathogenic form of the protein [11]. Meanwhile, neuronal metabolism of DA generates the catecholaldehyde, 3,4-dihydroxyphenylacetaldehyde (DOPAL), which potently oligomerizes alpha-synuclein [2]. Analogously, neuronal metabolism of serotonin (5HT) generates the aldehyde, 5-hydroxyindolealdehyde (5-HIAL). Whether 5-HIAL oligomerizes alpha-synuclein, in vitro or in cells, has been unknown. Filling this gap in knowledge was the main purpose of the present study. In in vitro experiments and in rat pheochromocytoma PC12 cells conditionally over-expressing alpha-synuclein we compared 5-HIAL with DOPAL in terms of the ability to oligomerize alpha-synuclein.
In the in vitro experiments, we incubated DA or 5HT with monoamine oxidase-A (MAO-A), the MAO isoform within neurons. In experiments involving synuclein-overexpressing PC12 cells, we added levodopa or 5-hydroxytryptophan (5-HTP) to the medium, because all cells express the neutral amino acid transporter and PC12 cells contain l-aromatic-amino-acid decarboxylase, which decarboxylates the amino acids to the monoamines. We used the non-selective MAO inhibitor pargyline to confirm dependence of the oligomerization on formation of the aldehydes.
2. Methods
2.1. Chemicals and reagents
Human recombinant alpha-synuclein was from Calbiochem (La Jolla, CA, USA), 5HT, DA, and MAO-A from Sigma Chemical Company (St. Louis, MO, USA) and mouse anti-alpha-synuclein from Invitrogen (Camarillo, CA, USA). All the reagents were dissolved in Type 1 water.
2.2. Experimental procedures
5-HIAL was prepared by complete conversion of 5HT (100 µM) with MAO-A at 37 °C in 50 mM phosphate buffer (pH 7.4). DOPAL was prepared by complete conversion of DA (100 µM) under the same conditions.
To assess effects of the aldehydes on alpha-synuclein oligomerization, alpha-synuclein (1.6 µM) was incubated with 5-HIAL or DOPAL (100 µM) in 50 mM phosphate buffer (pH 7.4) at 37 °C for 60 min. The reaction was stopped by heating the sample in sample buffer with 20 mM DDT at 70 °C for 5 min.
PC12 cells conditionally over-expressing alpha-synuclein were provided by Drs. Ito and Nakaso, of Tottori University, Japan. Cells were cultured in 5% fetal calf serum containing DMEM/F12 media with doxycycline. Over-expression of alpha-synuclein was achieved by removing doxycycline. Alpha-synuclein expression was confirmed by western blotting. To produce 5-HIAL and DOPAL intracellularly, PC12 cells were incubated with l-DOPA (500 µM) and 5-HTP (500 µM). Cells with no doxycycline for 7–10 days were exposed to l-DOPA or 5HTP for 6 h in medium without FCS, with or without pargyline (10 µM) to block MAO. Cell lysis was achieved using a lysis buffer of 20 mM Tris at pH 7.4, 25 mM KCl, 5 mM MgCl2, 0.25 mM sucrose, 1% Triton X-100, and protease inhibitors.
Oligomerization of alpha-synuclein was analyzed by western blotting. Each reaction mixture was electrophoresed on NuPAGE 4–12% of Bis–Tris gels and transferred to a nitrocellulose membrane using an iBlot Dry Blotting system (Invitrogen, Carlsbad, CA, USA). Protein detection was performed with mouse anti-alpha-synuclein (1:200) antibody and the secondary antibody goat anti-mouse IRDye 800CW (1:10,000). The detector was an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA). Band intensity was quantified using LI-COR Odyssey software.
For catechol assays, PC12 cells were collected in 400 µL of 20:80 (0.04 M H2PO4:0.2 M acetic acid) and disrupted by freezing and thawing. The catechols were extracted by alumina adsorption and quantified by HPLC with electrochemical detection [5]. Cellular catechol concentrations were expressed per mL of assayed sample.
2.3. Data analysis and statistics
The in vitro and cell studies were done at least in quadruplicate. Statistical analyses of western blot bands and catechol contents were analyzed by one-way analyses of variance using GraphPad Prism Software 5.0. A p value less than 0.05 defined statistical significance.
3. Results
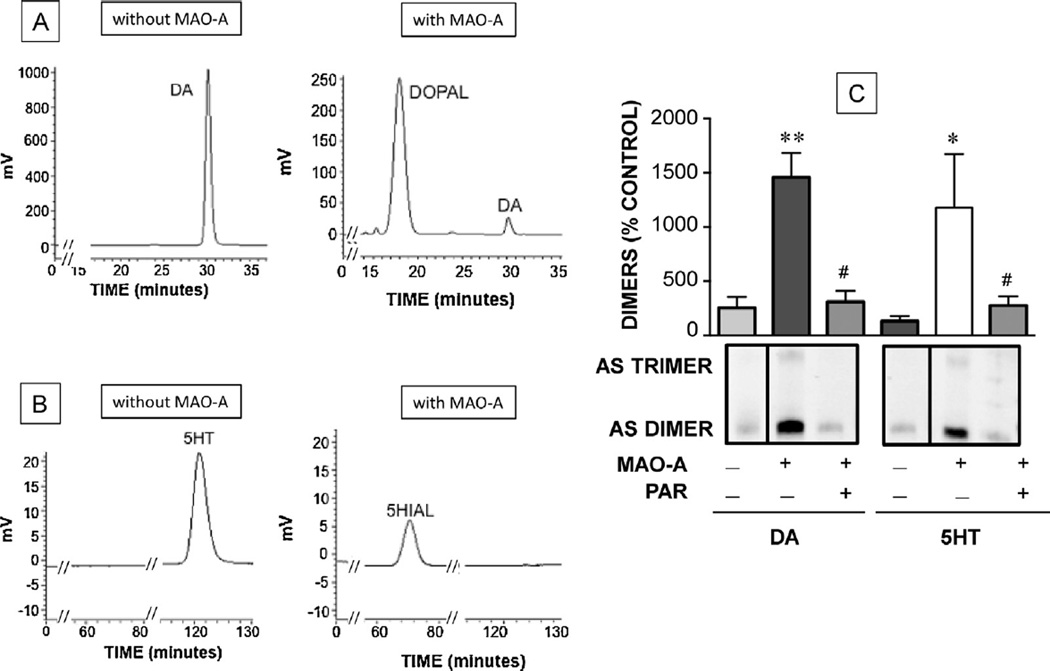
Incubation of 5HT with MAO-A rapidly generated 5-HIAL in the reaction mixture (Fig. 1). After 1 h of incubation, 100% of 5-HTP was metabolized, and a broad chromatographic peak characteristic of aldehydes appeared. After 1 h of incubation of DA with MAO-A, 96% of the DA was metabolized, and a chromatographic peak was seen that had the same retention time as DOPAL standard. Incubation of 5HT or DA alone did not produce the additional chromatographic peaks (Fig. 1A and B), and pargyline prevented the appearance of both peaks (data not shown).
Fig. 1.
Chromatographic confirmation of monoamine aldehyde production from monoamines by MAO-A (A, B) and alpha-synuclein (AS) oligomerization evoked by the aldehydes (C). (A) Conversion of dopamine (DA) to 3,4-dihydroxyphenylacetaldehyde (DOPAL) upon incubation of DA with MAO-A, (B) Conversion of serotonin (5HT) to 5-hydroxyindolealdehyde (5-HIAL) upon incubation of the 5HT with MAO-A. (C) Western blotting of alpha-synuclein oligomers produced by incubating DA or 5HT with MAO-A. The oligomerization was prevented by pargyline (PAR). Histogram shows mean (±SEM) values. (*) indicates p < 0.01 vs. control, (**) indicates p < 0.001 vs. control) and (#) indicates p < 0.001 compared to no PAR.
Incubation of the 5HT/MAO-A and DA/MAO-A reaction mixtures with 1.6 µM alpha-synuclein for 1 h at 37 °C resulted in similar extents of alpha-synuclein oligomerization by the two reaction mixtures. Pargyline prevented the oligomerization (Fig. 1C). 5-Hydroxytryptamine (5HT) and DA alone did not produce any AS aggregation (data not shown).
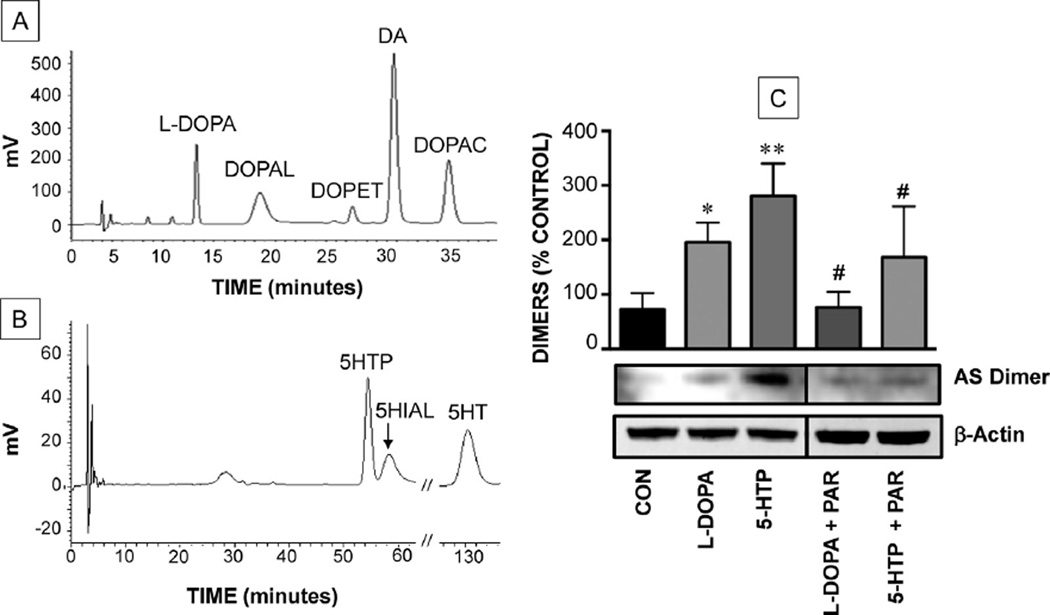
When PC12 cells conditionally over-expressing alpha-synuclein were incubated with l-DOPA or 5-HTP (500 µM) for 6 h, assays of the cell contents confirmed intracellular synthesis of DOPAL from l-DOPA and 5-HIAL from 5-HTP (Fig. 2A and B). Western blotting revealed alpha-synuclein dimers in both the 5-HTP-and l-DOPA-treated cells, with pargyline attenuating this effect (Fig. 2C).
Fig. 2.
Chromatographic confirmation of intracellular production of monoamine aldehydes from amino acid precursors (A, B) and alpha-synuclein (AS) dimerization evoked by monoamine aldehydes (C) in PC12 cells. (A) Production of DOPAL and DA from l-DOPA; (B) production of 5-HIAL and 5HT from 5-hydroxytryptophan (5-HTP). (C) Alphasynuclein oligomerization by 5-HIAL and DOPAL and effects of pargyline (PAR) (*) indicates p < 0.05 vs. control, (**) indicates p < 0.01 vs. control and (#) indicates p < 0.05 compared to no PAR). Abbreviations: DOPET – 3,4-dihydroxyphenylethanol; DOPAC – 3,4-dihydroxyphenylacetic acid; CON – control.
4. Discussion
This study shows that 5-HIAL, the aldehyde produced upon enzymatic oxidative deamination of 5HT, oligomerizes alpha-synuclein, in both in vitro experiments and in PC12 cells. Whereas incubation of alpha-synuclein with 5HT or DA alone exerted no effects, incubation of alpha-synuclein with the monoamines in the setting of MAO-A resulted in oligomerization of the alpha-synuclein. Prevention of the oligomerization by pargyline to block MAO confirmed that the monoamine aldehydes evoked the oligomerization. Enzymatic deamination of the monoamines by MAO also generates hydrogen peroxide (equimolar with the monoamine aldehydes), but hydrogen peroxide oligomerizes alpha-synuclein only at far higher concentrations than does 5-HIAL [6].
The aldehyde of DA, DOPAL, potently oligomerizes alpha-synuclein [2,6]; however, until this study the ability of 5-HIAL to oligomerize alpha-synuclein had not been examined, and the abilities of 5-HIAL and DOPAL to oligomerize alpha-synuclein had not been compared. We found about the extent of alpha-synuclein oligomerization by the two aldehydes, same both in vitro and in synuclein-overexpressing PC12 cells.
Oligomerized alpha-synuclein is thought to be pathogenic in PD [11]. The finding of oligomerization of alpha-synuclein by the aldehydes of both 5HT and DA may help explain the prominent, relatively selective deposition of alpha-synuclein in and loss of neurons producing these monoamines in PD.
Mice with genetically determined very low activity of the type 2 vesicular monoamine transporter have prominent central dopaminergic and serotonergic lesions [3,10]. The present results seem to justify future studies of monoamine aldehyde-induced oligomerization of alpha-synuclein in animal PD models.
HIGHLIGHTS.
Serotonin aldehyde strongly oligomerizes alpha-synuclein in vitro.
The effect is more than 10 times control.
Serotonin aldehyde oligomerizes alpha-synuclein in PC12 cells.
The effect is blocked by pargyline, a monoamine oxidase inhibitor.
Serotonin aldehyde has about the same potency effect as dopamine aldehyde.
Acknowledgments
The research reported here was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, NIH.
Abbreviations
- 5HT
serotonin
- 5-HTP
5-hydroxytryptophan
- 5-HIAL
5-hydroxyindolealdehyde
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- DOPAL
3,4-dihydroxyphenylacetaldehyde
- MAO
monoamine oxidase
- PD
Parkinson’s disease
Footnotes
Conflicts of interest
The Authors have no conflicts of interest to disclose.
References
- 1.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 2.Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O’Dell M, Li SW, Pan Y, Chung HD, Galvin JE. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 3.Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J. Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahn S, Libsch LR, Cutler RW. Monoamines in the human neostriatum: topographic distribution in normals and in Parkinson’s disease and their role in akinesia, rigidity, chorea, and tremor. J. Neurol. Sci. 1971;14:427–455. doi: 10.1016/0022-510x(71)90178-x. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Sullivan R, Gross DJ, Holmes C, Kopin IJ, Sharabi Y. Vesicular uptake blockade generates the toxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 cells: relevance to the pathogenesis of Parkinson disease. J. Neurochem. 2012;123:932–943. doi: 10.1111/j.1471-4159.2012.07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jinsmaa Y, Sullivan P, Gross D, Cooney A, Sharabi Y, Goldstein DS. Divalent metal ions enhance DOPAL-induced oligomerization of alpha-synuclein. Neurosci. Lett. 2014;569:27–32. doi: 10.1016/j.neulet.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- 8.Seidel K, Mahlke J, Siswanto S, Kruger R, Heinsen H, Auburger G, Bouzrou M, Grinberg LT, Wicht H, Korf HW, den Dunnen W, Rub U. The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Pathol. 2014 doi: 10.1111/bpa.12168. http://dx.doi.org/10.1111/bpa.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shannak K, Rajput A, Rozdilsky B, Kish S, Gilbert J, Hornykiewicz O. Noradrenaline, dopamine and serotonin levels and metabolism in the human hypothalamus: observations in Parkinson’s disease and normal subjects. Brain Res. 1994;639:33–41. doi: 10.1016/0006-8993(94)91761-2. [DOI] [PubMed] [Google Scholar]
- 10.Taylor TN, Alter SP, Wang M, Goldstein DS, Miller GW. Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology. 2014;76(Pt A):97–105. doi: 10.1016/j.neuropharm.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R. In vivo demonstration that {alpha}-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]