Abstract
Background
Pulmonary arterial hypertension (PAH) is common disease among hemodialysis (HD) patients and is associated with increased morbidity and mortality. However, its pathogenesis has not been completely elucidated. We aimed to evaluate the frequency of PAH in HD patients, as well as the relationship between fluid status and PAH.
Material/Methods
We enrolled 77 HD patients in this study. Multifrequency bioimpedance analysis (BIA) was used to assess fluid status. BIA was performed before and 30 min after the midweek of HD. Overhydration (OH)/extracellular water (ECW)% ratio was used as an indicator of fluid status. Fluid overload was defined as OH/ECW ≥7%. Echocardiographic examinations were performed before and after the HD. Pulmonary arterial hypertension was defined as systolic pulmonary artery pressure at rest (sPAP) higher than 35 mmHg.
Results
PAH was found in 33.7% of the HD patients. OH/ECW and the frequency of fluid overload were significantly higher in HD patients with PAH than those without PAH, whereas serum albumin and hemoglobin levels were significantly lower. sPAP level was significantly higher in HD patients with fluid overload than in those without fluid overload after hemodialysis session. Furthermore, sPAP, OH/ECW levels, and the frequency of PAH were significantly reduced after HD. We also found a significant positive correlation between sPAP and OH/ECW. Multivariate logistic regression analysis demonstrated fluid overload to be an independent predictor of PAH after HD.
Conclusions
PAH is prevalent among HD patients. This study demonstrated a strong relationship between fluid overload and PAH in HD patients.
MeSH Keywords: Electric Impedance, Pulmonary Heart Disease, Renal Dialysis
Background
Cardiovascular diseases are the leading cause of morbidity and mortality in end-stage renal disease (ESRD) patients [1]. Pulmonary arterial hypertension (PAH) is one of the cardiovascular complications [2] and is characterized by sustained elevations of pulmonary artery pressure [3]. PAH is a rare disease in the general population, but the prevalence of PAH is substantially higher in HD patients, with a range between 30% and 66% [2,4–7].The importance of PAH has recently been recognized among ESRD patients receiving hemodialysis (HD) treatment.
The pathogenesis of PAH in end-stage renal disease patients remains poorly understood, and there is a need for better understanding of the pathogenesis of the condition, which would help to individualize treatment of PH in this patient population [8]. Several factors, such as fluid overload, arteriovenous fistulae, anemia, hypoalbuminemia, cardiac dysfunction, bone mineral disorder, uremic vasculopathy, and non-biocompatible dialysis membranes, have been suggested in the etiopathogenesis. Regardless of the etiology, PAH is associated with increased morbidity and mortality [9]. Therefore, early diagnosis and appropriate treatment is extremely important to avoid serious consequences [6].
Fluid overload is a common and serious problem that leads to severe complications in HD patients and has a great impact on the pathogenesis of cardiovascular disease. Furthermore, it is also suggested that fluid overload plays an important determining factor in the development of PAH [10].
In clinical practice, fluid status is most commonly evaluated based on clinical signs, such as changes in body weight, edema, and blood pressure. However, these clinical signs may lead to an erroneous evaluation. In recent years, multifrequency bioelectrical impedance analysis (BIA), which is a simple, safe, novel, rapid, noninvasive, and promising method, has been used to determine fluid status in patients on dialysis therapy [11,12]. Multifrequency BIA has been well validated and is the criterion standard method for fluid status measurement [13].
To the best of our knowledge, only a few studies have investigated the prevalence and the etiopathogenic mechanisms underlying development of PAH in HD patients [2,5,10]. In the present study, we aimed to determine if ESRD patients on maintenance hemodialysis have an increased risk in PAH development, and the possible etiologic risk factors. We particularly focussed on the relationship between fluid status and PAH in HD patients.
Material and Methods
Study population
The study design included 77 patients with ESRD receiving long-term HD therapy, 3 times per week in the Dicle University Hospital dialysis unit. The local Human Research Ethics Committee approved the study protocol, and informed consent was obtained from all patients at the time of study enrollment.
Examinations of patients on HD were performed during the middle of the week. The dialyses were carried out using Fresenius 4008S machines. All patients were dialyzed using 1.6 m2 surface area high-flux polysulfone dialyzers (Fresenius, Bad Homberg, Germany) with bicarbonate-based dialysate (Glucose 1 mmol/L, Na+ 140 mEq/L, HCO3−32 mEq/L, K+ 2.0 mEq/L, Ca2+ 1.25 mmol/L, Mg2+ 0.5 mEq/L). The prescribed duration time was 5 h with a blood flow rate of 250–350 ml/min and a dialysate flow rate of 500 ml/min. The adequacy of dialysis was assessed by using Kt/V (K – dialyzer clearance of urea, t – dialysis time, V – volume of distribution of urea) according to the single-compartment Daugirdas formula [14] and urea reduction rate [URR=100*(1-ureapost-dialysis/ureapre-dialysis)%] formula.
The exclusion criteria were: (1) chronic obstructive pulmonary disease, congenital cardiac anomalies, pulmonary emboli, left ventricular ejection fraction <50%; (2) hemodynamically unstable; (3) general poor medical status; (4) poor echocardiographic view; (5) limb amputation, pacemakers, or metallic intravascular devices; (6) any malignant disease; (7) pregnancy; and (8) receiving diuretic treatment.
Clinical and laboratory investigations
All patient demographics and baseline clinical characteristics were provided from patient registries and by the patients themselves. Body mass index (BMI) was calculated as the ratio weight/height2 (kg/m2). Systolic (SBP) and diastolic blood pressure (DBP) was measured before the beginning (pre-HD) and 30 min after the end (post-HD) of hemodialysis by mercury sphygmomanometry. Blood samples were collected from all patients for the biochemical [urea (mg/dl), creatinine (mg/dl), calcium (mg/l), phosphorus (mg/l), and albumin (g/dl)] and hematological [hemoglobin (g/dl)] serological [CRP (mg/dl)], hormonal [PTH (pg/ml)] parameters on the same day as the BIA measurements. We collected 24-h urine samples to determine urine volume.
Echocardiographic evaluation
2D-guided M-mode echocardiography (Vivid 7, GE Healthcare, Horten, Norway) with a 3.5 MHz transducer was used. An echocardiogram was performed just before (pre-HD) and 30 min after (post-HD) the midweek dialysis session, by the same cardiologist. In the presence of tricuspid valve regurgitation, systolic pulmonary artery pressure was calculated by using the modified Bernoulli equation: PAP=4×(tricuspid systolic jet)2 + 10 mmHg [15]. PAH was defined as sPAP higher than 35 mmHg at rest [5,10].
Bioelectrical impedance analysis
A multi-frequency BIA device (Body Composition Monitor, BCM, Fresenius Medical Care D GmbH), which measures 50 different frequencies from 5 to 1000 kHz, was used to assess fluid status. BIA was performed just before (pre-HD) and 30 min after (post-HD) the midweek dialysis session, by the same person. The following parameters were obtained: overhydration (OH), extracellular water (ECW), intracellular water (ICW), total body water (TBW) in liters (l), and OH/ECW ratio. Several BIA derived parameters, such as OH, ECW/TBW, and OH/ECW ratio, were used as markers of the volume status in dialysis patients. The OH/ECW ratio seems to be at the forefront of fluid overload detection. Thus, OH/ECW% ratio was used as an indicator of fluid status. Fluid overload was defined as an OH/ECW ≥7%, corresponding to the value of the 90th percentile for the reference cohort [16,17].
Statistical analysis
Data analyses were performed using Statistical Package for Social Sciences (SPSS), Version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov-Simonov test) whether or not they were normally distributed. Normally distributed variables are presented using means and standard deviations, and non-normally distributed variables using median and range (maximum and minimum). Comparisons between groups were performed using the Student’s t-test and the Mann-Whitney U-test, according to the distribution of the variables. Paired Student’s t-test was used to compare the pre-HD and post-HD measurements. The chi square test and McNemar’s chi square test were used to compare proportions in different groups.
Correlations of parameters with SPAP were studied using Pearson or Spearman correlations. A logistic regression analysis was performed to identify independent predictors of PAH. P values <0.05 were considered statistically significant.
Results
The mean age of the study population (n=77) was 51.37±15.95 years, with 51.9% males. PAH was found in 33.8% of the patients (26 out of 77). The baseline demographic and clinical characteristics, as well as relevant laboratory parameters, are presented in Table 1.
Table 1.
Demographic, clinical and biochemical characteristics of study patients.
| Parameters | |
|---|---|
| Age (years) | 51.37±15.95 |
| Gender (M/F) | 40/37 |
| Dialysis vintage (months) | 60.51±17.67 |
| Diabetes (%) | 13 (16.8%) |
| Hypertension (%) | 28 (36.3%) |
| Smoking (%) | 25 (32.4%) |
| AVF/Permanetcatheter | 61/16 |
| Body mass index (kg/m2) | 23.96±5.65 |
| Residual urine (l/day) | 0.02±0.01 |
| Ultrafiltration volume (ml) | 2400.44±417.96 |
| Duration delivered (hours) | 5.0 |
| Antihypertensive treatment (%) | 28.33% |
| PTH (pg/ml) | 377.5 (5.5–2689) |
| CRP (mg/dl) | 1.36±0.48 |
| Albumin (g/dl, pre-HD) | 3.33±0.35 |
| Haemoglobin (g/dl, pre-HD) | 11.76±1.67 |
| Calcium (mg/dl, pre-HD) | 8.14±0.95 |
| Phosphorus (mg/dl, pre-HD) | 4.84±1.28 |
| CaxP product (mg2/dl2) | 39.65±11.86 |
| Urea reduction ratio (%) | 76.52±5.59 |
| Kt/V | 1.49±0.25 |
AVF – arteriovenous fistula; PTH – parathyroid hormone; CRP – C-reactive protein; Ca – calcium; P – phosphorus.
SBP, DBP, and sPAP levels were significantly reduced after a hemodialysis session (p<0.05). The BIA parameters, OH, total body water (TBW), extracellular water (ECW), and OH/ECW were significantly lower in post-HD patients compared to pre-HD patients (p<0.05). In addition, the presence of PAH occurred more frequently in pre-HD patients compared to post-HD patients (53.2% vs. 33.8%, p<0.001). Table 2 depicts comparison of clinical, laboratory, echocardiographic, and BIA characteristics in pre-HD and post-HD patients.
Table 2.
Comparison of patient characteristics pre-HD and post HD.
| Parameters | Pre-HD | Post-HDs | p |
|---|---|---|---|
| Urea (mg/dl) | 145.93±35.66 | 35.42±13.58 | <0.001 |
| Creatinine (mg/dl) | 8.55±2.27 | 2.78±0.86 | <0.001 |
| SBP (mmHg) | 134.28±17.04 | 110.51±19.86 | <0.001 |
| DBP (mmHg) | 78.76±8.81 | 67.79±8.40 | <0.001 |
| OH (l) | 2.06±0.58 | 1.02±0.35 | <0.001 |
| TBW (l) | 31.77±7.29 | 29.27±6.33 | <0.001 |
| ECW (l) | 15.43±3.62 | 13.41±3.37 | <0.001 |
| ICW (l) | 16.34±4.03 | 15.86±3.76 | 0.065 |
| OH/ECW (%) | 12.40±2.35 | 5.13±1.72 | <0.001 |
| sPAP (mmHg) | 37.14±13.26 | 31.02±11.36 | <0.001 |
| Presence of pulmonary hypertension (%) | 53.2% | 33.8% | <0.001 |
SBP – systolic blood pressure; DBP – diastolic blood pressure; OH – overhydration; TBW – total body water; ECW – extracellular water; ICW – intracellular water; sPAP – systolic pulmonary artery pressure.
Table 3 shows demographic, clinical, laboratory, echocardiographic, and BIA parameters of the post-HD group. OH, ECW, OH/ECW, and sPAP were significantly higher in HD patients with PAH than in those without PAH (p<0.05), whereas serum albumin and hemoglobin levels were significantly lower (p<0.05). In addition, fluid overload based on OH/ECW ≥7% was more frequent in HD patients with PAH compared to HD patients without PAH (53.8% vs. 21.6%, p=0.004). Similarly, mean sPAP level was significantly higher in post-HD patients with fluid overload than in those without fluid overload (35.80±12.22 vs. 28.73±10.28, p=0.01; Figure 1).
Table 3.
Demographic, clinical, laboratory, echocardiographic and BIA parameters of post HD group.
| Parameters | HD patients with PAH (n=26) | HD patients without PAH (n=51) | p |
|---|---|---|---|
| Age (years) | 56.03±16.95 | 49.00±15.03 | 0.067 |
| Gender (M/F) | 12/14 | 28/23 | 0.467 |
| SBP (mmHg) | 116.15±18.98 | 107.64±19.85 | 0.075 |
| DBP (mmHg) | 69.23±8.08 | 67.05±8.55 | 0.287 |
| Albumin (g/dl) | 3.17±0.38 | 3.41±0.30 | 0.003 |
| CRP (mg/dl) | 1.94±0.48 | 1.05±0.32 | 0.054 |
| Haemoglobin (g/dl) | 11.19±1.83 | 12.05±1.53 | 0.035 |
| Kt/V | 1.47±0.24 | 1.49±0.26 | 0.770 |
| Urea reduction ratio (%) | 76.72±5.68 | 76.42±5.60 | 0.830 |
| CaxP product (mg2/dl2) | 40.97±10.32 | 38.96±12.63 | 0.480 |
| PTH (pg/ml) | 374.5 (80–791) | 377.5 (55–2689) | 0.460 |
| Smoking | 24% | 41.3% | 0.145 |
| Hypertension | 34.6% | 37.3% | 0.820 |
| Diabetes mellitus | 23.1% | 14% | 0.349 |
| AVF | 73.1% | 82.4% | 0.343 |
| sPAP (mmHg) | 43.84±9.21 | 24.49±4.99 | <0.001 |
| OH (l) | 2.15±0.87 | 0.45±0.15 | 0.027 |
| TBW (l) | 29.71±7.04 | 29.05±5.99 | 0.672 |
| ECW (l) | 14.80±4.23 | 12.70±2.62 | 0.009 |
| ICW (l) | 14.90±3.21 | 16.35±3.96 | 0.091 |
| OH/ECW (%) | 9.40±2.03 | 2.96±0.68 | 0.002 |
| Fluid overload | 53.8% | 21.6% | 0.004 |
SBP – systolic blood pressure; DBP – diastolic blood pressure; sPAP – systolic pulmonary artery pressure; CRP – C-reactive protein; Ca – calcium; P – phosphorus; PTH – parathyroid hormone; AVF – arteriovenous fistula; OH – overhydration; TBW – total body water; ECW – extracellular water; ICW – intracellular water.
Figure 1.
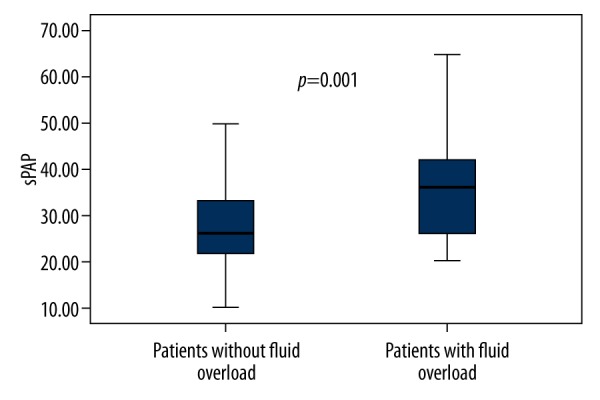
The box plots show the sPAP levels in post-HD patients with and without fluid overload.
sPAP of post-HD patients correlated positively with age, SBP, OH, ECW, and OH/ECW (Figure 2) and negatively with albumin and hemoglobin (Table 4).
Figure 2.
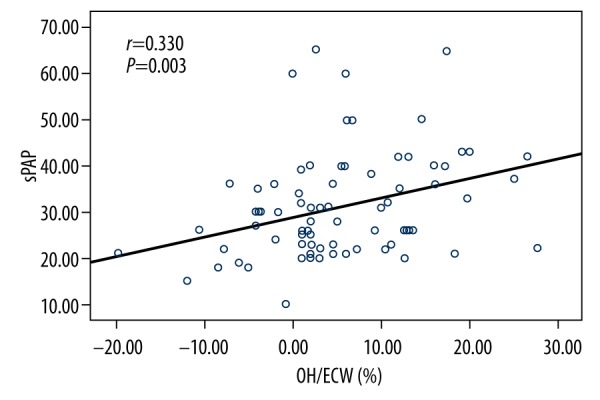
Fluid status, as defined by OH/ECW%, was positively correlated with sPAP.
Table 4.
Correlations between post-HD sPAP with post-hemodialysis satudy parameters.
| Variable | R | P |
|---|---|---|
| Age | 0.240 | 0.033 |
| Albumin | −0.359 | 0.002 |
| SBP | 0.232 | 0,042 |
| Haemoglobin | −0.292 | 0.010 |
| OH | 0.230 | 0,044 |
| ECW | 0.362 | 0.001 |
| OH/ECW | 0.330 | 0.003 |
SBP – systolic blood pressure; OH – overhydration; ECW – extracellular water.
Multivariate logistic regression analysis found fluid overload, as defined by OH/ECW≥7% [odds ratio (OR)=3.51, 95% confidence interval (CI) 1.13 to 10.93, P=0.03], to be an independent predictor of PAH after hemodialysis (Table 5).
Table 5.
Multivariate logistic regression analysis for PAH in HD patients.
| Variables | OR | %95 CI | P |
|---|---|---|---|
| Fluid overload (OH/ECW ≥7%) | 3.51 | 1.13–10.93 | 0.030 |
| Gender (Female vs. Male) | 1.63 | 0.57–4.59 | 0.355 |
| Serum albumin <3.5 g/dl | 2.20 | 0.76–6.36 | 0.145 |
| Haemoglobin <11 g/dl | 1.66 | 0.51–5.39 | 0.397 |
OR – odds ratio; CI – confidence interval.
Discussion
PAH is a progressive and symptomatic disease that leads to increase pulmonary vascular resistance and, usually, to right heart failure and death [18,19].
A high prevalence of PAH was reported among ESRD patients receiving hemodialysis treatment in previous studies. In the cross-sectional study of Agarwal et al., with 288 hemodialysis patients, PAH (defined as PAP >35 mmHg) was detected in 38% of the HD patients [5]. Etemadi et al. found that the prevalence of PAH (defined as PAP ≥35 mmHg) occurred in 41.1% of their hemodialysis patients [4]. In a study by Yigla et al., PAH (defined as PAP >35 mmHg) was observed in 39.7% of patients receiving hemodialysis [20]. Another study found that PAH (defined as PAP >35 mmHg) occurred in 52% of patients receiving hemodialysis [6]. In our study, 33.7% of the HD patients had PAH, which is consistent with previous studies. Despite the fact that PAH is a common and serious disease in HD patients, it has not been granted enough importance in clinical practice, perhaps partly due to the small number of large-scale studies [21].
Pathogenesis of PAH has not been completely elucidated and the mechanisms leading to the disease are still under investigation [22,23]. To the best of our knowledge, this is the first study to investigate the relationship between fluid overload based on OH/ECW ratio measured by using BIA and PAH in patients receiving regular HD.
A cross-sectional study by Unal et al. that including 135 peritoneal dialysis (PD) patients and 15 disease-free controls demonstrated a close association between hypervolemia and PAH by using bioimpedance analysis [10]. Agarwal et al. observed significantly higher inferior vena cava diameter, increased left atrial diameter, and increased cardiac index among HD patients with PAH than in those without PAH, and speculated that pulmonary hypertension may occur in response to chronic volume overload [5]. Interestingly, the study showed that OH/ECW and fluid overload were significantly higher in HD patients with PAH than those without PAH. Similarly, when patients were classified according to fluid status, sPAP levels were significantly higher in HD patients with fluid overload than in those without fluid overload post-HD. Also, sPAP and OH/ECW levels and the frequency of PAH were significantly reduced after HD, and a significant positive correlation was found between sPAP and OH/ECW. Importantly, this study showed that fluid overload is an independent predictor of PAH after HD. PAH is a complex disorder that manifests as abnormally high blood pressure in the vasculature of the lungs [24]. It is possible that chronic fluid overload associated with hyperdynamic circulation causes elevated right atrial pressure, elevated mean pulmonary artery pressure as a consequence of increased pulmonary blood flow in the pulmonary vasculature bed, and adversely affects left ventricular dysfunction, leading to PAH in HD patients [25,26]. Fluid overload may cause a direct lung injury from chronic pulmonary congestion, adding yet a further pathophysiological mechanism to PAH in this patient cohort [8]. Our study strongly supports to the role of hypervolemia in the development of PAH in HD patients by demonstrating the close association between fluid status and PAH.
Dry weight is a term referring to lowest tolerated post-dialysis weight achieved via gradual change in post-dialysis weight at which there are minimal signs or symptoms of either hypovolemia or hypervolemia [27]. However, in clinical practice, determination of exact dry weight is still one of the greatest challenges for the nephrologists in dialysis patients. It is known that fluid overload is a common complication among dialysis patients. Although excess fluid is removed by ultrafiltration in hemodialysis, patients still can be overhydrated. Also, significant proportions of hemodialysis patients return to the pre-dialysis period with overhydration as a consequence of sodium and water overload [28]. The high prevalence rate of PAH in HD patients may be explained by the increased frequency of fluid overload in this patient population.
Despite some conflicting results, evidence suggests that anemia can contribute to pulmonary hypertension by aggravating hypoxia and increasing cardiac output in patients with ESRD [8,9]. Interestingly, we observed significantly lower hemoglobin levels in patients with PAH than in those without PAH [20,29,30]. The low levels of hemoglobin in HD patients with PAH might be a result of hypervolemia.
Serum albumin levels were significantly lower in HD patients with PAH compared to those without PAH, which is in agreement with previously published studies [6,31]. Hypoalbuminemia was found not to be an independent predictor of PAH in the present study, but may reflect the consequence of fluid overload leading to PAH [2].
In addition, hyperparathyroidism and increased calcium-phosphate product can cause precipitation of calcium phosphate in many soft tissues, which is suggested to be an important risk factor for PAH development. Akmal et al. demonstrated that chronic renal failure in a dog model is associated with increased PTH activity and higher sPAP [32]. Furthermore, Kumbar et al. showed that pulmonary arterial pressure was positively correlated with serum levels of phosphorus, CaxP product, and PTH in 36 PD patients [33]. In contrast, our study and others did not reveal any significant association between CaxP product, PTH, and PAH in ESRD patients [4,20,34].
This study has certain limitations, including the relatively small number of patients, and it did not have long-term follow-up. After achievement of euvolemia in these patients, remeasurement of sPAP would be needed in a future prospective study.
Conclusions
Our data indicate that PAH is prevalent among HD patients. Moreover, we demonstrated a strong relationship between fluid overload and PAH in HD patients. Fluid overload is an independent predictor of PAH intervention to reduce excess volume in overhydrated patients, and could lead to decreased sPAP values and increased incidence of pulmonary hypertension in HD patients.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Source of support: Departmental sources
References
- 1.London GM, Marchais SJ, Metivier F, Guerin AP. Cardiovascular risk in end-stage renal disease: vascular aspects. Nephrol Dial Transplant. 2000;15:97–104. doi: 10.1093/ndt/15.suppl_5.97. [DOI] [PubMed] [Google Scholar]
- 2.Abedini M, Sadeghi M, Naini AE, et al. Pulmonary hypertension among patients on dialysis and kidney transplant recipients. Ren Fail. 2013;3:560–65. doi: 10.3109/0886022X.2013.766567. [DOI] [PubMed] [Google Scholar]
- 3.Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–17. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 4.Etemadi J, Zolfaghari H, Firoozi R, et al. Unexplained pulmonary hypertension in peritoneal dialysis and hemodialysis patients. Rev Port Pneumol. 2012;18:10–14. doi: 10.1016/j.rppneu.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R. Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant. 2012;27:3908–14. doi: 10.1093/ndt/gfr661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahdavi-Mazdeh M, Alijavad-Mousavi S, Yahyazadeh H, et al. Pulmonary hypertension in hemodialysis patients. Saudi J Kidney Dis Transpl. 2008;19:189–93. [PubMed] [Google Scholar]
- 7.Kiykim AA, Horoz M, Ozcan T, et al. Pulmonary hypertension in hemodialysis patients without arteriovenous fistula: the effect of dialyzer composition. Ren Fail. 2010;32:1148–52. doi: 10.3109/0886022X.2010.516854. [DOI] [PubMed] [Google Scholar]
- 8.Kawar B, Ellam T, Jackson C, Kiely DG. Pulmonary hypertension in renal disease: epidemiology, potential mechanisms and implications. Am J Nephrol. 2013;37(3):281–90. doi: 10.1159/000348804. [DOI] [PubMed] [Google Scholar]
- 9.Bozbas SS, Akcay S, Altin C, et al. Pulmonary hypertension in patients with end-stage renal disease undergoing renal transplantation. Transplant Proc. 2009;41:2753–56. doi: 10.1016/j.transproceed.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 10.Unal A, Sipahioglu M, Oguz F, et al. Pulmonary hypertension in peritoneal dialysis patients: prevalence and risk factors. Perit Dial Int. 2009;29:191–98. [PubMed] [Google Scholar]
- 11.Wizemann V, Rode C, Wabel P. Whole-body spectroscopy (BCM) in the assessment of normovolemia in hemodialysis patients. Contrib Nephrol. 2008;161:115–18. doi: 10.1159/000130423. [DOI] [PubMed] [Google Scholar]
- 12.Basile C, Vernaglione L, Di Iorio B, et al. Development and validation of bioimpedance analysis prediction equations for dry weight in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:675–80. doi: 10.2215/CJN.00240107. [DOI] [PubMed] [Google Scholar]
- 13.Moissl UM, Wabel P, Chamney PW, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27:921–33. doi: 10.1088/0967-3334/27/9/012. [DOI] [PubMed] [Google Scholar]
- 14.Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205–13. doi: 10.1681/ASN.V451205. [DOI] [PubMed] [Google Scholar]
- 15.Dabestani A, Mahan G, Gardin JM, et al. Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol. 1987;59(6):662–68. doi: 10.1016/0002-9149(87)91189-1. [DOI] [PubMed] [Google Scholar]
- 16.Hung SC, Kuo KL, Peng CH, et al. Association of fluid retention with anemia and clinical outcomes among patients with chronic kidney disease. J Am Heart Assoc. 2015;4:e001480. doi: 10.1161/JAHA.114.001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Biesen W, Williams JD, Covic AC, et al. EuroBCM Study Group. Fluid status in peritoneal dialysis patients: the European Body Composition Monitoring (EuroBCM) study cohort. PLoS One. 2011;6:e17148. doi: 10.1371/journal.pone.0017148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGoon MD, Kane GC. Pulmonary hypertension: diagnosis and management. Mayo Clin Proc. 2009;84:191–207. doi: 10.4065/84.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domenighetti GM. Idiopathic pulmonary arterial hypertension and inhaled iloprost: good night rebound effects? Respiration. 2007;74:496–97. doi: 10.1159/000105538. [DOI] [PubMed] [Google Scholar]
- 20.Yigla M, Nakhoul F, Sabag A, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123:1577–82. doi: 10.1378/chest.123.5.1577. [DOI] [PubMed] [Google Scholar]
- 21.Fruchter O, Yigla M. Underlying aetiology of pulmonary hypertension in 191 patients: a single centre experience. Respirology. 2008;13:825–31. doi: 10.1111/j.1440-1843.2008.01364.x. [DOI] [PubMed] [Google Scholar]
- 22.Fabbian F, Cantelli S, Molino C, et al. Pulmonary hypertension in dialysis patients: a cross-sectional italian study. Int J Nephrol. 2010;2011:283475. doi: 10.4061/2011/283475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domenici A, Luciani R, Principe F. Pulmonary hypertension in dialysis patients. Perit Dial Int. 2010;30:251–52. doi: 10.3747/pdi.2009.00082. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: An important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ. 2011;1(2):212–23. doi: 10.4103/2045-8932.83453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolignano D, Rastelli S, Agarwal R, et al. Pulmonary hypertension in CKD. Am J Kidney Dis. 2013;61:612–22. doi: 10.1053/j.ajkd.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 26.James KB, Stelmach K, Armstrong R, et al. Plasma volume and outcome in pulmonary hypertension. Tex Heart Inst J. 2003;30(4):305–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha AD, Agarwal R. Can chronic volume overload be recognized and prevented in hemodialysis patients? The pitfalls of the clinical examination in assessing volume status. Semin Dial. 2009;22(5):480–82. doi: 10.1111/j.1525-139X.2009.00641.x. [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz Z, Yildirim Y, Aydin FY, et al. Relationship between fluid status as assessed by bioimpedance analysis and NT-pro BNP, blood pressure and left ventricular mass index in hemodialysis patients. Clin Ter. 2014;165(1):e52–58. doi: 10.7417/CT.2014.1672. [DOI] [PubMed] [Google Scholar]
- 29.Oygar DD, Zekican G. Pulmonary hypertension in dialysis patients. Ren Fail. 2012;34:840–44. doi: 10.3109/0886022X.2012.690715. [DOI] [PubMed] [Google Scholar]
- 30.Abassi Z, Nakhoul F, Khankin E, et al. Pulmonary hypertension in chronic dialysis patients with arteriovenous fistula: pathogenesis and therapeutic prospective. Curr Opin Nephrol Hypertens. 2006;15:353–60. doi: 10.1097/01.mnh.0000232874.27846.37. [DOI] [PubMed] [Google Scholar]
- 31.Mousavi SA, Mahdavi-Mazdeh M, Yahyazadeh H, et al. Pulmonary hypertension and predisposing factors in patients receiving hemodialysis. Iran J Kidney Dis. 2008;2:29–33. [PubMed] [Google Scholar]
- 32.Akmal M, Barndt RR, Ansari AN, et al. Excess PTH in CRF induces pulmonary calcification, pulmonary hypertension and right ventricular hypertrophy. Kidney Int. 1995;47:158–63. doi: 10.1038/ki.1995.18. [DOI] [PubMed] [Google Scholar]
- 33.Kumbar L, Fein PA, Rafiq MA, et al. Pulmonary hypertension in peritoneal dialysis patients. Adv Perit Dial. 2007;23:127–31. [PubMed] [Google Scholar]
- 34.Amin M, Fawzy A, Hamid MA, Elhendy A. Pulmonary hypertension in patients with chronic renal failure: role of parathyroid hormone and pulmonary artery calcifications. Chest. 2003;124:2093–97. doi: 10.1378/chest.124.6.2093. [DOI] [PubMed] [Google Scholar]


