Abstract
To identify a novel biopesticide controlling rice blast disease caused by Magnaporthe oryzae, 700 plant extracts were evaluated for their inhibitory effects on mycelial growth of M. oryzae. The L. foenum-graecum Herba extract showed the lowest inhibition concentration (IC50) of 39.28 μg/ml, which is lower than the IC50 of blasticidin S (63.06 μg/ml), a conventional fungicide for rice blast disease. When treatments were combined, the IC50 of blasticidin S was dramatically reduced to 10.67 μg/ml. Since ABC transporter genes are involved in fungicide resistance of many organisms, we performed RT-PCR to investigate the transcriptional changes of 40 ABC transporter family genes of M. oryzae treated with the plant extract, blasticidin S, and tetrandrine, a recognized ABC transporter inhibitor. Four ABC transporter genes were prominently activated by blasticidin S treatment, but were suppressed by combinational treatment of blasticidin S with the plant extract, or with tetrandrine that didn’t show cellular toxicity by itself in this study. Mycelial death was detected via confocal microscopy at 24 h after plant extract treatment. Finally, subsequent rice field study revealed that the plant extract had high control efficacy of 63.3% and should be considered a biopesticide for rice blast disease. These results showed that extract of L. foenum graecum Herba suppresses M. oryzae ABC transporter genes inducing mycelial death and therefore may be a potent novel biopesticide.
Keywords: ABC transporter gene, biopesticide, blasticidin S, fungicide resistance, Lysimachia foenum-graecum Herba, Magnaporthe oryzae, plant extract, rice blast disease
Rice blast disease is caused by M. oryzae, a filamentous pathogenic fungus. Many kinds of chemical pesticides are used to control rice plant infection caused by M. oryzae and cause drastic environmental problems including pesticide resistance. Microbial pathogens resist various natural toxicants (Dixon and Lamb, 1990; Dixon et al., 1994), and emerging resistances against chemical pesticides have been observed in various pathogenic fungi of rice plant. Molecular mechanisms of pesticide resistance include mutation, overproduction of target proteins and pesticide detoxification (Gulshan and Moy-Rowley, 2007). Another pesticide resistance mechanism that ATP binding cassette (ABC) transporter gene family is involved has been reported in M. oryzae (Lee et al., 2005).
ABC transporter family genes are involved in protection of human cancer cells from accumulating anticancer drugs, which have chemically different structures, in the cytoplasm (Sharom, 2008). ABC transporter family genes is one of the largest gene families and members of this gene family are present in almost all organisms (prokaryotes and eukaryotes), mainly in the membranes of cells or cellular organelles in the form of transmembrane (TM) proteins. They participate in metabolic activities and transport of diverse substrates such as metabolites, lipids, sterols, and medicines by hydrolyzing ATP (Jones and George, 2004; Ponte-Sucre, 2009). They have two sets of TM domains trespassing the cellular membrane six times, and one set of nucleotide binding domains (NBDs) to bind ATP nucleotides and phosphorylate each TM domain, respectively. The nucleotide sequences or amino acids of ABC cassettes are highly conserved in many organisms (Li and Wang, 2006; Zolnerciks, 2011).
About fifty ABC transporter genes have been found in the genome of M. oryzae, which has 11,109 genes (Dean et al., 2005). Since M. oryzae is the first plant pathogen genome sequenced (Shalabh et al., 2009), this fungus is used frequently as a model for plant pathogen molecular studies. The loci of its genes have been annotated (Kim et al., 2010) and genome wide analysis on putative ABC transporter genes has revealed 50 genes and divided them into 11 subfamily groups: ABCA, ABCB, ABCC-1, ABCC-2, ABCD, ABCE, ABCF, ABCG-1, ABCG-2, ABCI, and YDR061W (Kim et al., 2013).
M. oryzae ABC transporters are involved in multidrug resistance (MDR) (Lee et al., 2005) through the efflux of different chemical pesticides out of the cells. Therefore, the inhibition of ABC transporter genes in this model would result in a decrease of pesticide efflux leading to decreased MDR (Osbourn, 1996; Riordan and Ling, 1979; Stavrovskaya, 2000). Also, ABC transporters are known to be involved in fungicide resistance in M. oryzae (Lee et al., 2005) and in Colletotrichum acutatum (Kim et al., 2014). In addition to ABC transporter drug efflux, MDR can be caused by other mechanisms including cell death evasion (Colabufo et al., 2010), target deviation (Abouzeed et al., 2008), cell cycle, and membrane permeation (Mohandas et al., 1992; Szakács et al., 2006). These mechanisms are present in human cancer (Szakács et al., 2006), malaria (Cowman et al., 1991) and tuberculosis (Rodrigues et al., 2012). It is necessarily to overcome MDR by substituting the chemical pesticides.
We noticed that a variety of plant extracts has a possibility of overcoming pesticide resistance of plant pathogens like M. oryzae. Up to 700 kinds of plant extracts were screened for their mycelial growth inhibitory effects on M. oryzae. Antifungal activity of L. foenum graecum was reported to inhibit the mycelial growth of M. oryzae (Park et al., 2008) and Botrytis cinerea (Sesan et al., 2015).
Based on previous reports and our preliminary experimental result of plant extracts, we hypothesized that unknown constituents in L. foenum-graecum Herba extract are involved in functional inhibition of ABC transporter family genes because the genes are known to be responsible for the transport of various kinds of physiological materials, toxicants in and out of cells. To investigate the effects of L. foenum-graecum Herba extract on M. oryzae, the changes of ABC transporter family genes mRNA expression levels were investigated by reverse transcriptase polymerase chain reaction (RT-PCR), and subsequently, the conformational change of M. oryzae mycelia were observed by a confocal laser scanning microscope after extract treatment. Finally, the extract control rate on rice blast disease was tested in the field of rice crop to discover a novel biopesticide.
Materials and Methods
Strains and culture conditions
M. oryzae strain KJ201 obtained from Korea National Research Resource Center (KNRRC) at Seoul National University was maintained on potato dextrose agar (PDA) in a 28 ºC incubator [Wooshin]. Pre-culture was performed in 3 ml of PDB (Potato dextrose broth) for three days. Culture was performed in 50 ml of PDB with shaking (in 200 rpm) for 2 days in a 28 ºC incubator. All cultures were treated with pesticides and plant extracts for 3 hr prior to mycelial harvests.
Plant extracts and Chemicals (toxicants)
Approximately 700 plant extracts were obtained from the Plant Extract Bank of Korea at dry weight of 20 mg each. Iprobenfos (IBP) and edifenphos (EDDP) were purchased from the Wako Pure Chemical Industries of Japan. Blasticidin S HCL was purchased from Invitrogen. Tetrandrine was purchased from Sigma Aldrich.
Large scale screening of mycelial growth inhibition
The M. oryzae mycelial growth inhibition assay was performed in 24 well plates (SPL Life Science). Mycelia, cut by round cork borer (KOREA Ace, 5 mm), were placed on PDA supplemented with 500 μg/ml of plant extract and incubated for five days in 28 ºC. Plant extracts with mycelial growth less than 0.2 cm of the radii surrounding M. oryzae were selected for further determination of minimal inhibitory concentration (MIC) and 50 % inhibition concentration (IC50) using different concentrations of plant extract in six well plates (SPL Life science) in a 28 ºC incubator. Each experiment was repeated in triplicates.
MICs and IC50 Assays
MICs was determined using the method described by Kubo and Lee (Kubo and Lee, 1998). Mycelial radii were measured on PDA medium supplemented with different concentrations of plant extracts, pesticides or tetrandrine. When the mycelia of M. oryzae reached the edges of the no treatment (NT) well in the 6 well plate (SPL Life science), the lowest concentration without any signs of mycelial growth was selected as the MIC value. IC50 was determined based on the treatment of tetrandrine, EDDP, IBP, or blasticidin S either individually or in combination with the plant extracts on PDA solid media amended by specific concentrations of the plant extracts. PDA plates were incubated at 28 ºC for five days.
Statistical analysis
ANOVA tests using Graphpad Prism were performed for statistical analysis regarding the measured mycelial radii in comparison to each experimental control (treatment with plant extract, fungicides and other chemicals). All experiments were performed in triplicate. P <0.001 is considered statistically significant. In field study of rice plant, Duncan’s multiple range tests (DMRT) using ARM 8 software of Gylling Data Management, INC. were performed for statistical analysis regarding the infected panicle of rice plant in comparison to each experimental control (treatment with plant extract, no treatment). All experiments were performed in triplicate. P<0.05 is considered statistically significant.
Total RNA Extraction
Pre-culture of M. oryzae was performed in 3 ml of PDB to activate mycelia. Next, 1 ml of activated mycelia was added to 50 ml of PDB for main culture. Each additive was added to each culture for 3 h prior to harvesting. Cultured mycelia were harvested on filter paper (100 mm, HYUNDAI Micro) by filtrating through a vaccum pump (2522C-10 WELCH). Distilled water was added to remove residual PDB media from mycelia. After mycelia were vaccum dried, total RNA was extracted using the RNA-spin Plasmid DNA Purification Kit (iNtRon bio technology) (Lee et al., 2002). RNA concentration was quantified using the Qubit RNA assay Kit (Invitrogen) on a Qubit flourocytometer (Invitrogen).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
First stranded cDNAs were synthesized using the Power cDNA synthesis Kit (iNtRon biotechnology). Total RNA (2 μg) was used to synthesize the first cDNA strand using PCR (PTC1148C, Bio-Rad). Synthesized first stranded cDNAs were used as templates for the amplification of partial cDNA fragments of 40 ABC transporter genes of M. oryzae. Based on the full length cDNA sequences of 40 ABC transporter genes supplied by professor Choi Woo Bong (Dong-Eui university, Busan, Korea), RT-PCR primers were designed (data not shown) and synthesized by Bioneer cooperation (Korea). PCR was performed with the following constituents: 1 μl (10 pmol) of forward primer, 1 ul of reverse primer, 2 μl of first strand cDNA template, 10 μl of 2x PCR Master mix solution (iNtRon biotechnology), and 6 μl of sterilized distilled water. PCR cycles included an initial denaturation at 95 ºC for 5 min; 25 cycles of 94 ºC for 30 s for denaturation, 55 ºC for 30 s for annealing, and 72 ºC for 30 s for extension, 72 ºC for 1 min and 4 ºC for termination. PCR products were subjected to 1.5 % agarose gel electrophoresis in 0.5 % TAE (20 mM Tris [pH 7.6], 10 mM acetic acid, 0.5 mM EDTA) buffer using an electrophoresis apparatus (AD1102. Takara, Japan). Gels were stained with ethidium bromide solution (10 μg/ml) in distilled water. Amplified cDNA fragments were recorded using Digital Gel Documentation (GDS-200C, Korea Lab Tech Cooperation).
Confocal laser scanning microscopy
Mycelia of M. oryzae were exposed to MIC of L. foenum-graecum Herba extract on glass slides for 24 h to observe mycelial conformational changes. After the plant extract was dissolved in 90 % ethyl alcohol, it was used to treat the mycelia and compared to experimental controls treated only by 90 % ethyl alcohol and a non-treated normal control, respectively. Images were acquired using a confocal laser scanning microscope (CLSM) (TCS SP5/AOBS/Tandem, Leica Microsystems, Germany) at the Korea Basic Science Institute, Gwangju Center. To capture transmitted light images, we used a PMT transdetector in differential interference contrast (DIC) mode and the 488 nm laser line at low power not applying the excitation, emission and any fluorescent filter set.
Field study to determine the control rate for rice blast disease
For the field study, 500 g of L. foenum-graecum Herba extract was prepared and distributed to the Korea Plant Environmental Research (KPER) center in Korea. To determine the control rate of L. foenum-graecum Herba extract on rice blast disease, the field study was performed by Seounmyeon (Ansung, Gyeonggi-Do) using the Chucheng rice strain from August 20th to September 19th of 2012. The nursery bed test was performed using L. foenum-graecum Herba extract diluted 500 times (4 mg/ml). Foliage was treated in flood season (2012/8/27, 2012/9/3) with intervals of seven days. Phytotoxicity tests were performed on August 27th, 2012 using 250 fold and 500 fold dilutions of the standard extract. Nursery plants were transplanted by machines on the 23rd and 26th of May. Plant spacing was 30 cm × 14 cm and ear was emerged on August 20th. Compared to the conventional amount of sprayed nitrogen fertilizer (9 kg/10 a), greater than two times of it were sprayed to facilitate the occurrence of rice blast disease in this study. Plant extracts were applied using general practices banning the spray of other pesticides. The area for the control rate assay was 30 m2 and the nursery bed test plot was 10 m2. The area for the total test plot was 270 m2. Each test was repeated three times by randomized block design (RBD). Nursery bed tests were analyzed by the degree of diseased panicle after 30 days to heading from 30 rice of each plot. Phytotoxicity tests were performed 3, 5, and 7 days (August 30th, September 1st, and September 3rd) after treatment with L. foenum-graecum Herba extract.
Results and Discussion
Inhibitory effects of L. foenum-graecum Herba extract on mycelial growth
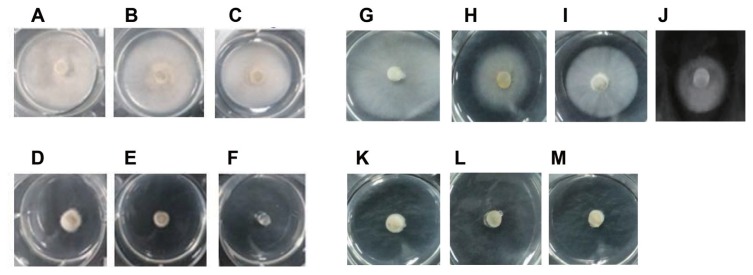
To investigate whether a plant extract could inhibit M. oryzae mycelial growth, approximately 700 kinds of plant extracts were massively screened based on measured values of mycelial radii of M. oryzae on PDA. As a result, five plant extracts were found to prominently inhibit mycelial growth. In particular, L. foenum-graecum Herba extract showed the strongest inhibitory effects (data not shown). Therefore, we determined the IC50 and MIC of M. oryzae based on the values of mycelial radii after M. oryzae were treated with different doses of the plant extract (Fig. 1, Table 1). Both L. foenum-graecum Herba extract IC50 and MIC were lower than those of blasticidin S (about two times and four times lower, respectively) (Table 1). To compare the relative inhibitory effects of L. foenum graecum Herba extract, the commercial fungicides for the control of rice blast disease, IBP, EDDP, and blasticidin S were also applied to the PDA culture media. Both IBP and EDDP showed much stronger antifungal effects than that of L. foenum-graecum Herba. However, the antifungal activity of the plant extract exceeded that of chemical fungicide blasticidin S (Table 1).
Fig. 1.
Mycelial growth inhibitory effect of L. foenum-graecum Herba extract on M. oryzae. (A) No treatment. L. foenum-graecum Herba extract was applied to (B), (C), (D), (E), and (F) at concentrations of 10 μg/ml, 20 μg/ml, 50 μg/ml, 100 μg/ml and 200 μg/ml, respectively. Combinational inhibitory effect tests on the mycelial growth of M. oryzae. (G) No treatment, (H) 63.06 μg/ml (IC50) of blasticidin S, (I) 25 μg/ml of tetrandrine, (J) combinational treatment of 63.06 μg/ml (IC50) of blasticidin S with 25 μg/ml of tetrandrine, (K) 39.28 μg/ml (IC50) of L. foenum-graecum Herba extract, (L) combinational treatment of 39.28 μg/ml (IC50) of L. foenum-graecum Herba with 63.06 μg/ml (IC50) of blasticidin S, and (M) combinational treatment of 39.28 μg/ml (IC50) of L. foenum-graecum Herba with 25 μg/ml (IC50) of tetrandrine. M. oryzae mycelia cut by cork borer and incubated at 28 ºC on PDA solid media containing the plant extract for five days.
Table 1.
IC50 and MICs of pesticides and L. foenum-graecum Herba extract
| Treatment | IC50 | MIC |
|---|---|---|
| L. foenum-graecum | 39.28 μg/ml | 100 μg/ml |
| Blasticidin S | 63.06 μg/ml | 400 μg/ml |
| Blasticidin S + L. foenum-graecum (IC50) | 10.67 μg/ml | – |
| Blasticidin S + Tetrandrine (25 mg/ml) | 42.76 μg/ml | – |
| Tetrandrine | 1.5 μg/ml | > 3 μg/ml |
Synergistic inhibitory effects of fungicides on mycelial growth
Since M. oryzae showed comparatively low sensitivity to blasticidin S compared to IBP or EDDP, we decided to examine whether such sensitivity to blasticidin S can be enhanced by combinational treatment of blasticidin S with L. foenum-graecum Herba extract by their respective IC50. Our results show that the plant extract synergistically enhanced the sensitivity of M. oryzae to blasticidin S. The IC50 value of blasticidin S (63.06 μg/ml) was significantly lowered to 10.67 μg/ml when it was treated in combination with the plant extract (Table 1). Therefore, the results suggest that the amount of blasticidin S used for the control of rice blast disease can be drastically reduced in rice crop fields by combining treatment with this plant extract. Our results meaningfully suggest that some cellular factors of M. oryzae protecting its mycelial cells from blasticidin S toxicity might be inhibited by components in L. foenum-graecum Herba extract.
Transcriptional inhibitors of ABC transporter increased sensitivity to blasticidin S
Reports show that plant fungal pathogen resistance against several toxicants may be greatly increased by toxicant efflux out of plant pathogen cells due to their intrinsic ABC transporters (Lee et al., 2005). Therefore, to test the sensitivity change of M. oryzae to blasticidin S concerned with ABC transporter genes, we combinatorially treated blasticidin S with tetrandrine, a well-known transcriptional inhibitor of eukaryotic ABC transporters. The IC50 of blasticidin S was markedly lowered to 42.76 μg/ml after combinational treatment with tetrandrine (Fig. 1J). However, it was not as low as 10.67 μg/ml that was measured from the experimental control of blasticidin S combinatorially treated with L. foenum-graecum Herba plant extract (Fig. 1L, Table 1). Although tetrandrine lowered the IC50 of blasticidin S, tetrandrine itself did not to show noticeable inhibition on M. oryzae mycelial growth (Fig. 1I, Table 1), indicating that transcriptional inhibition of ABC transporters may suppress the efflux of blasticidin S out of M. oryzae cells. Interestingly, it is noticeable that a single plant extract treatment showed similar radius to that of combinational plant extract treatment with tetrandrine. This strongly indicates that unknown constituents in the plant extract may already exist in and play a similar functional role to tetrandrine in suppressing M. oryzae ABC transporter gene expression (Fig. 1K, M).
mRNA expression of ABC transporter genes are highly induced by blasticidin S
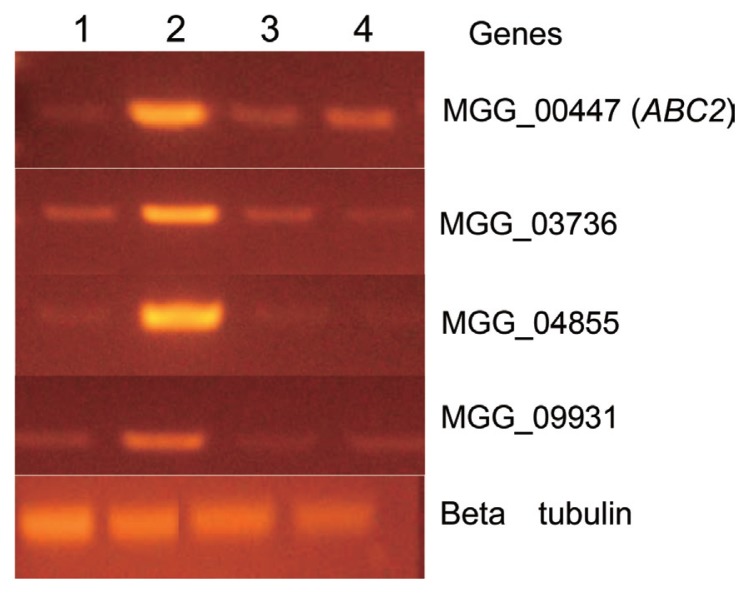
RT-PCR was performed to investigate whether mRNA expression of M. oryzae ABC transporter genes can be affected by blasticidin S, IBP, or EDDP treatment. Primers designed by putative cDNA ORFs of forty M. oryzae ABC transporter genes amplified partial cDNA fragments of those ABC transporter genes. Treatment of blasticidin S induced high transcriptional levels of four specific ABC transporters (MGG_00447 (ABC2), MGG_03736, MGG_04855, MGG_09931). However, the four genes did not show significant changes in transcriptional levels after treatment with EDDP or IBP. From these results, we suggest that at least four ABC transporters may be involved in lowering the sensitivity of M. oryzae to blasticidin S (Fig. 2).
Fig. 2.
RT-PCR of mRNA expression levels of ABC transporter genes. Commercial fungicides EDDP, IBP, and blasticidin S were used to treat the mycelia of M. oryzae on PDA for 3 h. Lane 1: No treatment, Lane 2: blasticidin S, Lane 3: EDDP (Edifenphos), Lane 4: IBP (Iprobenfos). Each mRNA expression level was normalized to the expression level of beta tubulin, a house-keeping gene.
ABC transporter inhibitors increases M. oryzae sensitivity to blasticidin S
To investigate whether suppression of the four ABC transporter genes can affect the sensitivity of M. oryzae to blasticidin S, we treated tetrandrine to suppress the expression of the forty ABC transporter genes of M. oryzae including the four genes and performed RT-PCR to detect the changes in mRNA expression levels of these genes (Fig. 3A).
Fig. 3.
RT-PCR of ABC transporter gene mRNA expression levels after combinational treatment with blasticidin S and tetrandrine or with L. foenum-graecum Herba extract. Total RNA was isolated in M. oryzae mycelia treated by single or simultaneous treatment of blasticidin S with tetrandrine or L. foenum-graecum Herba extract for 3 h. (A) Inhibition of ABC transporter gene expression by tertrandrine. + : Treatment, − : None treatment. (B) Inhibition of ABC transporter gene expression by the extract of L. foenum-graecum Herba. + : Treatment, − : None treatment.
Our results reveal that the tetrandrine treatment decreases the transcriptional levels of the four ABC transporter genes (Fig. 3A), corresponding to the decrease in mycelial radii on PDA solid media (Fig. 1J). However, tetrandrine failed to inhibit the increased mRNA expression induced by blasticidin S (Fig. 3A). This may explain why combinational treatment of blasticidin S with tetrandrine did not prominently lower the IC50 of blasticidin S as also shown by the incomplete decrease in the M. oryzae mycelia radius (Fig. 1J, Table 1). Therefore, the increased expressions of the four ABC transporter genes must play specified roles in protecting the M. oryzae mycelial cells against blasticidin S.
L. foenum graecum Herba extract inhibits ABC transporter gene mRNA expression
Because L. foenum-graecum Herba extract synergistically lowers blasticidin S IC50 and decreases the M. oryzae mycelial growth radius (Table 1, Fig. 1L), we investigated whether the extract can also suppress ABC transporter gene mRNA expression similar to tetrandrine. RT-PCR was performed using total RNA isolated from mycelia treated with L. foenum-graecum Herba extract alone or in combination with blasticidin S. Our results reveal that the transcriptional levels of the four ABC transporter genes were highly induced by blasticidin S, but suppressed by combinational treatment of blasticidin S with the plant extract (Fig. 3B). This indicates that unknown constituents in the plant extract may suppress ABC transporter gene mRNA expression. In addition to inhibiting ABC transporter genes, the plant extract may contain other constituents that affect the physiological status of M. oryzae leading to mycelial growth inhibition. Three-hour plant extract treatments to mycelia may not be long enough to induce mycelial death, however the time can induce the changes of transcriptional levels of ABC transporter genes, not affecting the stable gene expressions of beta tubulin or 18S ribosomal RNA, house keeping genes. Therefore, we conclude that the suppressed mRNA expression of ABC transporter genes must have been caused by unknown constituents in the plant extract that have similar functions to tetrandrine, not by mycelial cell death of M. oryzae during the incubation time.
Conformational changes of mycelia are induced by L. foenum-graecum Herba extract
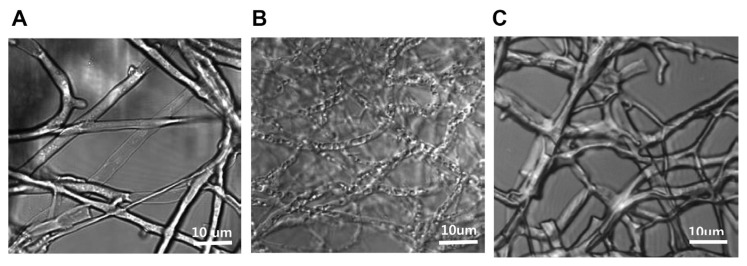
After we identified the inhibitory effects of the plant extract on mycelial growth, we investigated M. oryzae mycelial death after MIC treatment (100 μg/ml) with the plant extract through the observation of mycelia by CLSM. After M. oryzae mycelia were exposed to the plant extract MIC for 24 hrs, the mycelia showed flattened morphological changes implying that their internal content were forced to flow out, causing mycelial death (Fig. 4B). However, the normal control and 90 % ethyl alcohol treated mycelia maintained their original morphology (Fig. 4A, C). Finally, the flattened mycelial changes led to mycelial cell death (Li et al., 2014).
Fig. 4.
Confocal laser microscopy of conformational changes caused by L. foenum-graecum Herba extract treatment. (A) No treatment control, (B) Experimental control treated with L. foenum-graecum Herba extract, (C) Experimental control treated with 90 % ethyl alcohol (vehicle). The observations of all experimental controls were performed by resolution of HCX PLAPO lambda blue 63.0 x. Images were acquired using a confocal laser scanning microscope (TCS SP5/AOBS/Tandem, Leica Microsystems, Germany).
These results suggest that mycelial flattening may be caused by functional inhibition of ABC transporters which may be responsible for the transportation of water or ionic materials needed to maintain osmosis and morphology of mycelia cells. Further studies are merited to elucidate the correlation between cellular flattening and subsequent mycelial cell death.
L. foenum-graecum Herba extract shows a high control rate for rice blast disease in rice field studies
After we confirmed the inhibitory effects of L. foenum-graecum Herba extract on M. oryzae mycelial growth, we evaluated the control rate of the plant extract for rice blast disease in rice fields. For this field study, the evaluation of standard was performed by comparing the extract to untreated port with over 15 % of diseased panicle. To sensitize the rice plant to rice blast disease, a sensitive rice cultivar, Chucheong, was used, and nitrogen fertilizer was sprayed in excess of twice the conventional amount for rice plant cultivation. Compared to an untreated control plot, 63.3 % of control rate against the rice blast disease was measured (Table 2). This high control rate was statistically significant with a p-value of 0.05. Rice plant sclerophyll damage was in the standard range without drainage. From these results, we identified that the L. foenum-graecum Herba extract had a very high control rate (63.3 %) almost equal to that of chemical fungicides currently used for rice blast disease. We must have obtained a higher control rate (> 80 %) if we had planned to spray the plant extract before the rice blast disease occurred.
Table 2.
Rice field study with L. foenum-graecum Herba extract to control rice blast disease
| Materials | Rate of diseased panicle (%) | Significant Difference (DMRT) | Control value (%) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Replicated plot I | Replicated plot II | Replicated plot III | Average | |||
| L. foenum-graecum Herba extract | 8.5 | 8.6 | 9.0 | 8.7 | a | 63.3 |
| None Treatment | 23.1 | 22.2 | 25.8 | 23.7 | b | – |
We confirmed that the plant extract showed high antifungal activity against rice blast disease both in the laboratory and in crop fields. Thus, L. foenum-graecum Herba extract can be a potential candidate to substitute for conventionally used chemical fungicides in the field to control rice blast disease.
Although blasticidin S is not being frequently used for the control of rice blast disease, it is very important to understand the precise mechanisms causing the low sensitivity of M. oryzae to blasticidin S because it can provide us information to understand the mechanisms involved in the resistance or decreased sensitivities of M. oryzae to various fungicides. For better understanding the mechanism at the gene level of M. oryzae, we investigated mRNA expression changes in the ABC transporter gene family because they are involved in multidrug resistance of various organisms, including human cancer cells. To face the challenge of decreasing fungicides usage, various plant extracts were screened for their antifungal activities against M. oryzae. In the first experiment in this study, we revealed that mRNA expression levels of four ABC transporter genes were significantly decreased by L. foenum-graecum Herba extract, increasing M. oryzae sensitivity to blasticidin S. The fact that mycelial radii were similar between the experimental control of plant extract and that of plant extract with tetrandrine strongly suggests the existence of ABC transporter inhibitors are present in the plant extract. Although we proposed only a facet of fungicide resistance mechanisms concerned with blasticidin S, we suggest that unknown constituents of L. foenum-graecum Herba must contribute to mycelial death mediated by inhibition of ABC transporter genes of M. oryzae. Further research is necessary to investigate the antifungal constituents that lead to the high sensitivity of M. oryzae to blasticidin S.
As shown in the rice crop field test, L. foenum-graecum Herba extract also has a high control rate for rice blast disease. Considering the facts that we adopted a highly sensitive rice plant strain to rice blast disease and sprayed more than two times the usual amount of nitrogen fertilizer to enhance the occurrence of rice blast disease, we confidently predict that the L. foenum-graecum Herba extract is strong enough to increase the control rate of more than 63.3 % in the field test prior to rice blast disease onset. Although we already publicized the possibility of the plant extract as a novel biopesticide in a patent (data not shown), further studies are needed to verify its antifungal active constituents to develop a potent and novel biopesticide.
Supplementary Information
Acknowledgments
Mycelila confocal images were acquired using Confocal Laser Scanning Microscopic image program (TCS SP5/AOBS/Tandem, Leica Microsystems, Germany) in the Korea Basic Science Institute, Gwangju Center. This study (Grants No. 00045438) was supported by the Business for Cooperative R&D between Industry, Academy, and Research Institute funded by the Korea Small and Medium Business Administration in 2012.
References
- Abouzeed YM, Baucheron S, Cloeckaert A. ramB mutations involved in efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium. Antimicrob Agents Chemother. 2008;52:2428–2434. doi: 10.1128/AAC.00084-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colabufo NA, Berardi F, Cantore M, Contino M, Inglese C, Niso M, Perrone R. Perspectives of P-glycoprotein modulating agents in oncology and neurodegenerative diseases. pharmaceutical, biological, and diagnostic potentials. J Med Chem. 2010;53:1883–1897. doi: 10.1021/jm900743c. [DOI] [PubMed] [Google Scholar]
- Cowman AF, Karcz S, Galatis D, Culvenor JG. A P-glycoprotein homologue of plasmodium falciparum is localized on the digestive vacuole. J Cell Biol. 1991;113:1033–1042. doi: 10.1083/jcb.113.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu JR, Pan H, Read ND, Lee YH, Carbone I, Brown D, Oh YY, Donofrio N, Jeong JS, Soanes DM, Djonovic S, Kolomiets E, Rehmeyer C, Li W, Harding M, Kim S, Lebrun MH, Bohnert H, Coughlan S, Butler J, Calvo S, Ma LJ, Nicol R, Purcell S, Nusbaum C, Galagan JE, Birren BW. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434:980–986. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Lamb CJ. Molecular communication in interactions between plants and microbial pathogens. Annu Rev Plant Physiol. 1990;14:339–367. doi: 10.1146/annurev.pp.41.060190.002011. [DOI] [Google Scholar]
- Dixon RA, Harrison MJ, Lamb CJ. Early events in the activation of plant defense responses. Annu Rev Phytopathol. 1994;32:479–501. doi: 10.1146/annurev.py.32.090194.002403. [DOI] [Google Scholar]
- Gulshan K, Moye-Rowley WS. Multidrug resistance in fungi. Eukaryotic Cell. 2007;6:1933–1942. doi: 10.1128/EC.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PM, George AM. The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci. 2004;61:682–699. doi: 10.1007/s00018-003-3336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YN, Kim JS, Kim SY, Kim J-H, Lee J-H, Choi WB. Prediction and annotation of ABC transporter genes from Magnaporthe oryzae genome sequence. J Life Sci. 2010;20:176–182. doi: 10.5352/JLS.2010.20.2.176. [DOI] [Google Scholar]
- Kim SY, Park S-Y, Kim HJ, Kim DY, Lee S-W, Kim HT, Lee JH, Choi WB. A paper on ABC transporter of plant fungal pathogen should be also cited. Isolation and characterization of the Colletotrichum acutatum ABC transporter CaABC1. Plant Pathol J. 2014;30:375–383. doi: 10.5423/PPJ.OA.08.2014.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YN, Park S-Y, Kim DY, Choi JY, Lee Y-H, Lee J-H, Choi WB. Genome-scale analysis of ABC transporter genes and characterization of the ABCC type transporter genes in Magnaporthe oryzae. Genomics. 2013;101:354–361. doi: 10.1016/j.ygeno.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Kubo I, Lee SH. Potentiation of antifungal activity of sorbic acid. J Agric Food Chem. 1998;46:4052–4055. doi: 10.1021/jf980174o. [DOI] [Google Scholar]
- Lee SE, Ju EM, Kim JH. Antioxidant activity of extracts from Euryale ferox seed. Exp Mol Medicine. 2002;34:100–106. doi: 10.1038/emm.2002.15. [DOI] [PubMed] [Google Scholar]
- Lee Y-J, Yamamoto K, Hamamoto H, Nakaune R, Hibi T. A novel ABC transporter gene ABC2 involved in multidrug susceptibility but not pathogenicity in rice blast fungus, Magnaporthe grisea. Pesti Biochem Physiol. 2005;81:13–23. doi: 10.1016/j.pestbp.2004.07.007. [DOI] [Google Scholar]
- Li G, Wang YQ. Progress in the research on the ABCA gene family of vertebrates. Yi Chuan. 2006;28:1015–1022. [PubMed] [Google Scholar]
- Li RY, Wu XM, Yin XH, Liang JN, Li M. The natural product citral can cause significant damage to the hyphal cell walls of Magnaporthe grisea. Molecules. 2014;19:10279–10290. doi: 10.3390/molecules190710279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N, Winardi R, Knowles D, Leung A, Parra M, George E, Conboy J, Chasis J. Molecular basis for membrane rigidity of domain hereditary ovalocytosis. a novel mechanism involving the cytoplasmic of band 3. J Clin Invest. 1992;89:686–692. doi: 10.1172/JCI115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourn AE. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell. 1996;8:1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IK, Kim JH, Lee YS, Shin SC. In vivo fungicidal activity of medicinal plant extracts against six phytopathogenic fungi. Int J Pest Management. 2008 Jan-Mar;54:63–68. doi: 10.1080/09670870701549665. [DOI] [Google Scholar]
- Ponte-Sucre A. ABC Transporters in microorganisms: Research, Innovation and Value as Targets against Drug Resistance. Caister Academic Press; 2009. [Google Scholar]
- Riordan JR, Ling V. Purification of P-glycoprotein from plasma membrane vesicles of chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem. 1979;254:12701–12705. [PubMed] [Google Scholar]
- Rodrigues L, Machado D, Couto I, Amaral L, Viveiros M. Contribution of efflux activity to isoniazid resistance in the mycobacterium tuberculosis complex. Infect Genet Evol. 2012;12:695–700. doi: 10.1016/j.meegid.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Sesan TE, Enache E, Iacomi BM, Oprea M, Oancea F, Iacomi C. Antifungal activity of some plant extract against Botrytis cinerea Pers. In the blackcurrant crop (Ribes nigrum L.) Acta Sci Pol, Hortorum Cultus. 2015;14:29–43. [Google Scholar]
- Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–127. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- Stavrovskaya AA. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc) 2000;65:95–106. [PubMed] [Google Scholar]
- Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottsman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discovery. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Thakur S, Jha S, Roy-Barman S, Chattoo B. Genomic resources of Magnaporthe oryzae (GROMO): A comprehensive and integrated database on rice blast fungus. BMC Genomics. 2009;10:316. doi: 10.1186/1471-2164-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnerciks JK, Andress EJ. Structure of ABC transporters. Essays Biochem. 2011;50:43–61. doi: 10.1042/bse0500043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.