Abstract
In this study, antifungal activity of essential oils of Cymbopogon citratus and Ocimum basilicum and two fungicides Mancozeb and Metalaxyl-Mancozeb in six different concentrations were investigated for controlling three species of Phytophthora, including P. capsici, P. drechsleri and P. melonis on pepper, cucumber and melon under in vitro and greenhouse conditions, respectively. Under the in vitro condition, the median effective concen- tration (EC50) values (ppm) of plant essential oils and fungicides were measured. In greenhouse, soil infested with Phytophthora species was treated by adding 50 ml of essential oils and fungicides (100 ppm). Disease severity was determined after 28 days. Among two tested plant essential oils, C. citratus had the lowest EC50 values for inhibition of the mycelial growth of P. capsici (31.473), P. melonis (33.097) and P. drechsleri (69.112), respectively. The mean EC50 values for Metalaxyl-Mancozeb on these pathogens were 20.87, 20.06 and 17.70, respectively. Chemical analysis of plant essential oils by GC-MS showed that, among 42 compounds identified from C. citratus, two compounds β-geranial (α-citral) (39.16%) and z-citral (30.95%) were the most abundant. Under the greenhouse condition, Metalaxyl-Mancozeb caused the greatest reduction in disease severity, 84.2%, 86.8% and 92.1% on melon, cucumber, and pepper, respectively. The C. citratus essential oil reduced disease severity from 47.4% to 60.5% compared to the untreated control (p≤0.05). Essential oils of O. basilicum had the lowest effects on the pathogens under in vitro and greenhouse conditions. These results show that essential oils may contribute to the development of new antifungal agents to protect the crops from Phytophthora diseases.
Keywords: Cymbopogon citratus, essential oil, Ocimum basilicum, Phytophthora
Phytophthora species cause destructive diseases of a huge range of agriculturally and ornamentally important plants including those in forests and other natural ecosystems. Damping off and blight caused by the Phytophthora species, is one of the most devastating diseases affecting cucurbit and vegetables production in Iran (Etebarian, 2012). Some species such as P. capsici, P. drechsleri and P. melonis can cause strong pathogenicity on pepper, cucurbit, tomato, cantaloupe and ornamental plants that cause damping off disease and rot of crown and root (Lamour et al., 2003; Tabarrae et al., 2011). The genus Phytophthora is a soil borne pathogen and survives in the soils as oospores and mycelia for several years in plant debris (Jee et al., 2001). Management of soil borne pathogen in the field includes crop rotation, cultural practices, chemical control and use of resistant cultivars (Someya et al., 2000). The long-term survival of pathogen even in the absence of susceptible host limits the effectiveness of crop rotation (Shouan Zhang et al., 2010). Fungicide application does not always prove economic against soil borne pathogens and it has led to environmental pollution, pathogen resistance, and increased risk for human and animal health (Anna On et al., 2015; De Curtis et al., 2010; Hausbek and Lamour, 2004). In addition, excessive use of fungicides creates imbalance in the microbial community in soil (Anna On et al., 2015; Para and Ristaino, 2001). Therefore, in order to solve problems as those mentioned above, several research groups have sought cheap alternatives, of low toxicity, to control soil borne pathogens (Joyce Mendes Andrade Pinto et al., 2010; Stangarlin et al., 2008). Among the potentially useful practices, the use of plant products like essential oils and plant extracts against several phytopathogens has already been demonstrated (Behtoei et al., 2012; Carvalho et al., 2008; Dikbas et al., 2008). Plant essential oils are volatile natural complex compounds characterized by a strong odor and are formed by aromatic plants as secondary metabolites, which play an important role in the protection of the plants against plant pathogens both in vitro and in vivo (Mollaei et al., 2011; Reddy et al., 1998). These compounds not only do not leave toxic residue to the environment but also have lower toxicity against mammals (Duke, 1985; Garcia et al., 2005). Essential oils activity of C. citratus and Ocimum species against plant pathogens tests have been conducted in vitro, with a few examples of greenhouse or field studies (Helal et al., 2006; Joyce Mendes Andrade Pinto et al., 2010; Kumar et al., 2010; Mahanta et al., 2007; Prakash et al., 2011; Paret et al., 2010; Somada et al., 2007). Few researches of essential oils have been reported in Oomycetes, such as Phytophthora citrophthora (Del Rio et al., 1998), P. cactorum (Lee et al., 2008) and P. capsici (Bi et al., 2012). However, in order to increase the efficacy of essential oils in Phytophthora species management, the products need to be studied further.
The objectives of this study were to evaluate the efficacy of Cymbopogon citratus (DC.) Stapf and Ocimum basilicum L. natural essential oils to control three species of Phytophthora in compared to fungicides (Mancozeb and Metalaxyl-Mancozeb) under in vitro and greenhouse conditions.
Materials and Methods
Culture media and organisms
The pathogens including P. capsici, P. drechsleri and P. melonis were isolated from infected pepper, cucumber and melon seedling respectively in Ardabil province, Iran. Infected samples of plants after surface-disinfesting in sodium hypochlorite (2%) were cultured on corn meal agar (CMA, Merk, Germany) media amended with 100 ppm PCNB (pentachloronitrobenzene), 100 ppm ampicillin, 30 ppm rifampicin and 25 ppm hymexazol (CMA-PARPH) at 25±2ºC for 10 days. Pathogenicity of isolates was assessed on pepper, cucumber and melon seedlings at the two-three true leaf stage under greenhouse condition described by Tabarraei et al. (2011). The Phytophthora species were identified according to the morphological characteristics of their mycelia and spores as described by Ershad (1992) and Stamps et al. (1990).
Plant materials and extracting essential oils from plant
The plant species used in this research were C. citratus and O. basilicum. The leaves of C. citratus and O. basilicum were collected from growing field in their natural habit (Sanandaj, Kurdistan Province, Iran) at flowering stage on June 2013. The collected parts of plants were subsequently dried under the shade condition with proper ventilation. Approximately 300 g of air-dried leaves of both plants were subjected to hydro-distillation for 3 h using a Clevenger type apparatus at 100ºC. The oils were dried over anhydrous sodium sulphate (Na2SO4), due to sodium sulphate pentahydrate formation and then the oils were stored at 4ºC for further analysis (Amini et al., 2012; Dev et al., 2011).
Analysis of essential oils by gas chromatography-mass spectrometry (GC-MS)
The GC-MS analysis was done at 250ºC on an Agilent 7890A gas chromatograph at 70 eV. The GC column was as follows: HP-5MS; the size of fused silica capillary was 0.25×3000 μm × 0.25 μm film thickness and it was used as the carrier gas (helium) with a flow rate of 0.8 ml/min. The GC column used was programmed as follows: 50ºC (5 min), 240ºC at the rate of 3ºC/min. The injection temperature was 250ºC. The mass spectrometer was operating in E1 mode at 70 eV. The compounds of essential oil were identified tentatively by comparing their relative retention times and mass spectra with those of pure authentic samples and WILEY and NBS (Ozcan et al., 2006).
Determining antifungal activity in vitro
The antifungal assay of essential oil and two fungicides (Mancozeb and Metalaxyl-Mancozeb) were carried out in the petri dishes (8 cm in diameter) containing CMA. Essential oils were dissolved in Tween 80 (0.5% v/v) before testing for fungal toxicity. Six concentrations of essential oil and fungicides were selected by pre-test for each pathogen (Table 1) and were mixed to CMA media at 40ºC after autoclaved. A disc of 5 mm in diameter (7-days old stock cultures) of each pathogen was placed in the center of the Petri dishes. Petri dishes containing CMA media and Tween 80 were used as controls. All petri dishes were incubated at 25±2ºC. Experiments were carried out in a completely randomized design. After seven days, the colony diameter was measured and the growth inhibition percent of treatments compared to negative control was calculated by the following formula: Inhibition (%) = [(dc – dt)/dc] × 100: where dc and dt are the radial growth (mm) of pathogen in the control and treated plates, respectively (Amini et al., 2012). In order to test whether plant essential oils were fungistatic or fungicidal, fungal disc of pathogens, inhibited by essential oils in treated plates, were re-inoculated into fresh CMA media without essential oil. Pathogen growth was observed at 25±2ºC for 10 days. If renewed mycelial growth was observed, the inhibition was qualified as fungistatic. On the other hand, if the contrary was the case and pathogens did not grow, fungicidal effect was assumed.
Table 1.
Selected concentrations (ppm) of plant essential oils and fungicides based on pre-test on Phytophthora spp.
| Phytophthora spp. | C. citratus | O. basilicum | Mancozeb | Metalaxyl-Mancozeb |
|---|---|---|---|---|
| P. capsici | 15, 30, 45, 60, 75, 90 | 50, 120, 190, 260, 330, 400 | 7.5, 20.5, 33.5, 46.5, 59.5, 72.5 | 5, 14, 23, 32, 41, 50 |
| P. drechsleri | 25, 39, 53, 67, 81, 95 | 50, 120, 190, 260, 330, 400 | 7.5, 20.5, 33.5, 46.5, 59.5, 72.5 | 5, 14, 23, 32, 41, 50 |
| P. melonis | 15, 29.5, 44, 58, 5, 73, 87.5 | 50, 120, 190, 260, 330, 400 | 7.5, 20.5, 33.5, 46.5, 59.5, 72.5 | 5, 14, 23, 32, 41, 50 |
Finally, median effective inhibitory concentration (EC50) values for essential oils and fungicides (ppm) on three Phytophthora species were measured. All tests were repeated twice. The Probit analysis was used to measure EC50 with POLO-PC software (2002).
Effect of the plant essential oils and fungicides on Phytophthora diseases in greenhouse conditions
Seeds of pepper (Capsicum annum L.), cucumber (Cucumis sativus L.) and Cantaloupe (Cucumis mello L.) were disinfected with 5% sodium hypochlorite for 5 min, rinsed with sterile distilled water and sown in pots (15 cm diameter and 20 cm height) containing steam-sterilized soil (loam clay soil). After one month at the 2–3 true-leaf stage, three mycelia discs (5 mm diameter) of each pathogen were placed in the soil around the bush (at a depth of 8 cm). Plant not received mycelia discs was as control (Tabarraei et al., 2011). Then, plant essential oils or fungicide at 100 ppm were applied at 50 ml/bush and was mixed with soil around the bush. Sterile distilled water was used as control. All pots were kept in a greenhouse at 23–27ºC, 60–70% relative humidity, 16 h light, 8 h darkness. Plants were watered twice a week and fertilizer solution added to pots once a week (NPK 1:1:1) at the rate of 3 g/l. After four weeks, disease severity was determined using a scale of 0 to 5, where 0 represents healthy and symptomless of disease; 1, small brownish lesion at the base of stem; 2, stem lesions extend to cotyledons or the lesion has girdled the stem causing plant collapse; 3, plant has collapsed with all leaves wilted or turned yellow except for the young leaves; 4, plant has completely collapsed; 5, plant is dead (Shouan Zhang et al., 2010).
Statistical analysis
Experiments were designed as completely randomized design with four replicates. All data analyses were conducted using the SPSS (Statistical Package for the Social Science, Version 18). The means were compared by Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05. Experiments were repeated two times.
Results and Discussion
Chemical composition of the essential oils
The percentages and the retention indices of the identified components in two plants by the GC-MS analysis are listed in Table 2 and 3. Chemical analysis of plant essential oils by GC-MS indicated z-citral (30.95%), β-geranial (α-citral) (39.16%) and caryophyllene (3.44%) as the main components in C. citratus oils (Table 2). Gupta et al. (2011) reported that citral (77.8%), limonene (4%) and geraniol (2.7%) were the main components in C. citratus oils. Also, borenol (60.06%), caryophyllen oxide (3.98%), β-myrcene (3.57%) and fenchone (3.07%) were identified as the main chemical compounds in O. basilicum oils (Table 3). Dev et al. (2011) indicated eugenol (61.7%), isopropyl palpitate (11.3%) and 2,3-dihydroxy propyl elaidate (5.1%) as major chemical compounds among O. basilicum oils. Differences in composition of essential oils might have been derived by harvest time, as well as local, climatic and seasonal factors (Rahimi-Nasrabadi et al., 2013).
Table 2.
Components of C. citratus essential oil identified by GC-MS analysis
| Number | RT* | Compound | Percentage(%) |
|---|---|---|---|
| 1 | 6.875 | Camphene | 0.34 |
| 2 | 7.796 | Methyheptenone | 1.15 |
| 3 | 7.905 | β-Myrecene | 0.17 |
| 4 | 8.980 | Limonene | 5.83 |
| 5 | 9.158 | 1,3,6-Octatriene, 3,7-dimethyl | 0.58 |
| 6 | 9.444 | cis-β-Ocimene | 0.39 |
| 7 | 10.125 | Nonanone | 0.87 |
| 8 | 10.909 | β-Linalool | 1.38 |
| 9 | 12.047 | 1,6-Heptadiene, 2,3,6-trimethyl | 0.183 |
| 10 | 12.345 | Trans-Chrysanthemal | 0.32 |
| 11 | 12.408 | 6- Octenal, 3,7-dimethyl-, (R) | 0.75 |
| 12 | 12.728 | 2-Cyclopenten-1-one, 3,4,4-trimetnyl | 0.72 |
| 13 | 13.032 | Cyclopentanecarboxylic acid, 2-methyl-3-methylene-, methyl ester | 0.16 |
| 14 | 13.243 | Cyclohexane, ethenyl | 1.43 |
| 15 | 13.484 | Cyclohexane, ethenyl | 0.24 |
| 16 | 13.867 | Decanal | 0.25 |
| 17 | 13.959 | 2-Cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, cis | 0.17 |
| 18 | 14.725 | D-Citral | 0.58 |
| 19 | 15.172 | Z-Citral | 30.95 |
| 20 | 15.292 | Geraniol | 0.47 |
| 21 | 16.081 | β-Geranial (α-Citral) | 39.16 |
| 22 | 16.167 | 2,7-Octadiene, 4-methyl | 0.47 |
| 23 | 16.270 | Acetic acid, 1,7,7-trimethyl bicycle(2.2.1) hept-2-yl ester | 0.20 |
| 24 | 17.157 | Cyclooctanemethanol | 0.18 |
| 25 | 17.546 | n-Heptadecylcyclohexane | 0.16 |
| 26 | 18.050 | Eugenol | 0.35 |
| 27 | 18.353 | (+)-Cycloisosativene | 0.17 |
| 28 | 18.485 | Cyclobutane, (1-methylethylidene) | 0.19 |
| 29 | 18.725 | Geranyl acetate | 3.10 |
| 30 | 19.000 | β-Elemene | 0.29 |
| 31 | 19.784 | Caryophyllene | 3.44 |
| 32 | 20.081 | α-Bergamotene | 0.39 |
| 33 | 20.390 | Iso-Eugenol | 0.43 |
| 34 | 20.608 | α-Caryophyllene | 0.42 |
| 35 | 21.586 | 1h-Cyclopropa(a)naphthalene,1a,23,5,6,7,7a,7b-octahydro-1,1,7,7a-tetramethyl-) | 0.18 |
| 36 | 21.884 | Benzene,1-methyl-4-(1,2,2-trimethylcyclopentyl)-, (R) | 0.30 |
| 37 | 22.067 | Naphtalene, 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl) | 0.46 |
| 38 | 22.261 | Naphtalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl) | 0.33 |
| 39 | 22.393 | Vinyldimethyl(1,3,3-triboromoprophyl)silane | 0.22 |
| 40 | 22.879 | Cyclohexanemethanol, 4-ethenyl-α,α,4-trimethyl-3-(1-methylethenyl) | 0.17 |
| 41 | 23.772 | Caryophyllene oxide | 2.02 |
| 42 | 24.355 | 3,5-Dimethylcyclohex-1-ene-4-carboxaldehyde | 0.17 |
|
| |||
| Total | – | 99.063 | |
RT*: Retention time
Table 3.
Components of O. basilicum essential oil identified by GC-MS analysis
| Number | RT* | Compound | Percentage(%) |
|---|---|---|---|
| 1 | 7.562 | β-Pinene | 0.52 |
| 2 | 9.038 | β-Myrcene | 3.57 |
| 3 | 9.444 | Limonene | 1.26 |
| 4 | 10.617 | Eucalyptol | 1.10 |
| 5 | 11.029 | β-Ocimene | 10.51 |
| 6 | 12.271 | Fenchone | 3.07 |
| 7 | 12.820 | Linalool | 0.53 |
| 8 | 13.150 | Camphor | 0.64 |
| 9 | 14.067 | Borenol | 60.06 |
| 10 | 14.909 | Terpineol-4 | 0.57 |
| 11 | 15.698 | Estragole | 0.76 |
| 12 | 16.511 | β-Citral | 1.49 |
| 14 | 19.223 | Carvacrole | 0.93 |
| 15 | 19.738 | β-Elemene | 1.04 |
| 16 | 20.087 | Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl) | 2.06 |
| 17 | 20.539 | Isomethyl-Eugenol | 0.49 |
| 18 | 20.596 | α-Bergamotene | 0.51 |
| 19 | 21.277 | β-Farensene | 1.38 |
| 20 | 21.655 | Homulene | 0.72 |
| 21 | 21.855 | 1,6-Cyclodecadiene, 1-methyl-5-methylene-8-(1-methylethyl) | 0.63 |
| 22 | 22.067 | γ-Elemene | 1.077 |
| 23 | 23.606 | Azulene, 1,2,3,5,6,8,8a-octahydro-1,4-dimethyl-7-(1-methylethenyl) | 0.68 |
| 24 | 23.755 | Naphthalene, 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl) | 0.72 |
| 25 | 24.453 | (−)-Spathulenole | 0.58 |
| 26 | 25.065 | Caryophyllen oxide | 3.98 |
| 27 | 41.699 | (+)-Epi-bicyclosesquiphellenderene | 0.59 |
|
| |||
| Total | 99.47 | ||
RT*: Retention time
Antifungal test
Damping off and blight disease caused by the Phytophthora species can cause severe losses in cucurbit and vegetables plants in various parts of the world including Iran (Hausbek and lamour, 2004; Etebarian, 2012). In this study, we evaluated the control efficacy of two plant essential oils against three Phytophthora species under in vitro and greenhouse conditions compared to fungicides.
The antifungal activity of two plant essential oils and two fungicides (Mancozeb and Metalaxyl-Mancozeb) was tested against species of Phytophthora in vitro. The results indicated that plant essential oils and fungicides reduced mycelial growth of pathogen in the culture. Fungicides of Metalaxyl-Mancozeb and Mancozeb proved to be the first and the second most effective in inhibiting mycelial radial growth of the pathogens respectively, followed by plant essential oils of C. citratus (Figs. 1, 3, 4 and Table 4). The plant essential oils of O. basilicum showed the lowest inhibitory effects against pathogens (Fig. 2 and Table 4). Both essential oils exhibited varying degrees of antifungal activities against Phytophthora species. The present data showed that C. citratus essential oil appear to be more toxic than O. basilicum essential oil against Phytophthora species (Table 4), which is in agreement with the results of other authors (Helal et al., 2006; Shadab et al., 1992). Essential oil of C. citratus showed potent inhibitory effect on the radial growth of P. capsici (91.9%), P. drechsleri (91.2%) and P. melonis (94.6%), as shown in Fig. 1. Mycelial growth of Aspergillus spp., Alternaria alternate, Penicillum citrinum and Curvularia lunata was inhibited by C. citratus essential oil (Mahanta et al., 2007). It was effective in inhibiting fungal viability and spore germination, so morphological changes of fungal hyphae were observed under light microscope by essential oil of C. citratus (Sulaiman Ali Al Yousef, 2013). The essential oils of C. citratus and O. basilicum were found to be highly effective against A. niger, A. flavus and Saccharomyces cerevisiae, whereas C. citratus essential oil showed the strongest activity against these fungi (Helal et al., 2006).
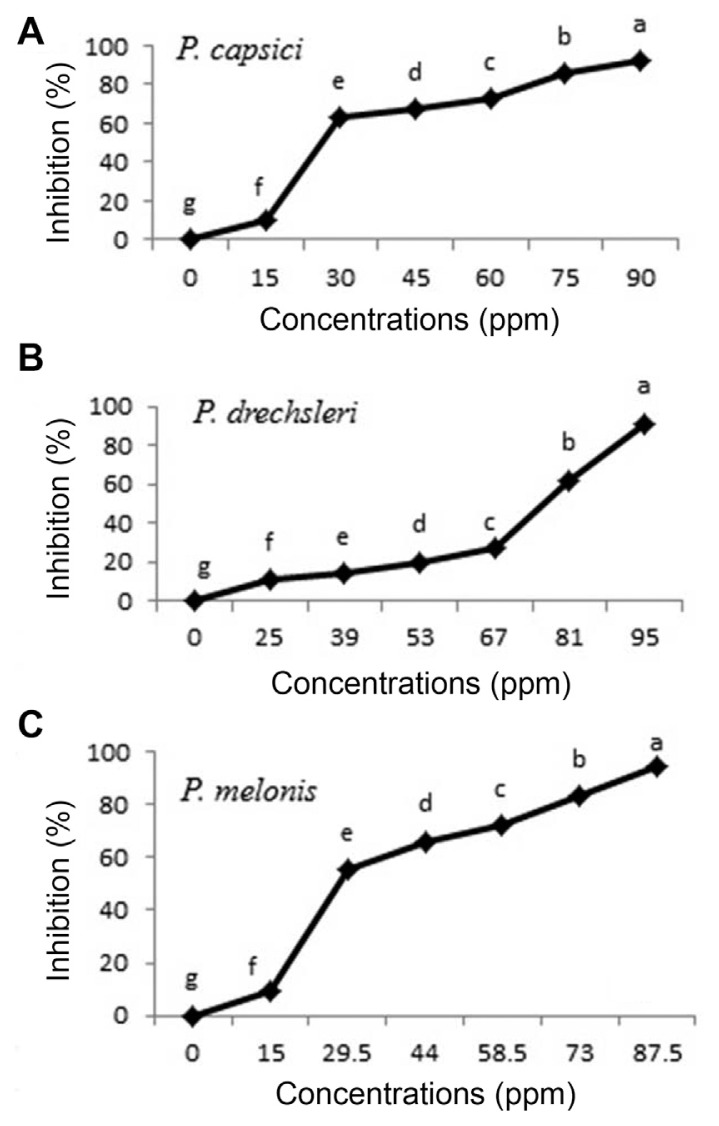
Fig. 1.
Effect of C. citratus essential oil on growth of Phytophthora species in vitro (A, P. capsici; B, P. drechsleri; C, P. melonis). Mean by different letters indicate significant differences among treatment (P ≤ 0.05) according to DMRT. Data are mean of four replicates.
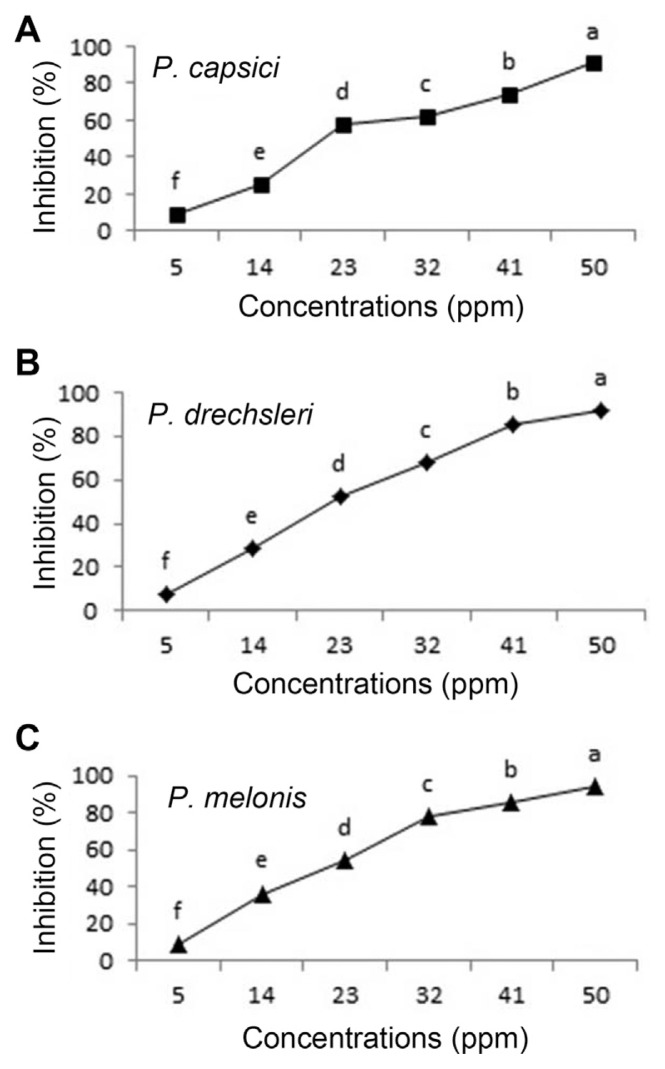
Fig. 3.
Effect of Mancozeb on growth of Phytophthora species in vitro (A, P. capsici; B, P. drechsleri; C, P. melonis). Mean by different letters indicate significant differences among treatment (P ≤ 0.05) according to DMRT. Data are mean of four replicates.
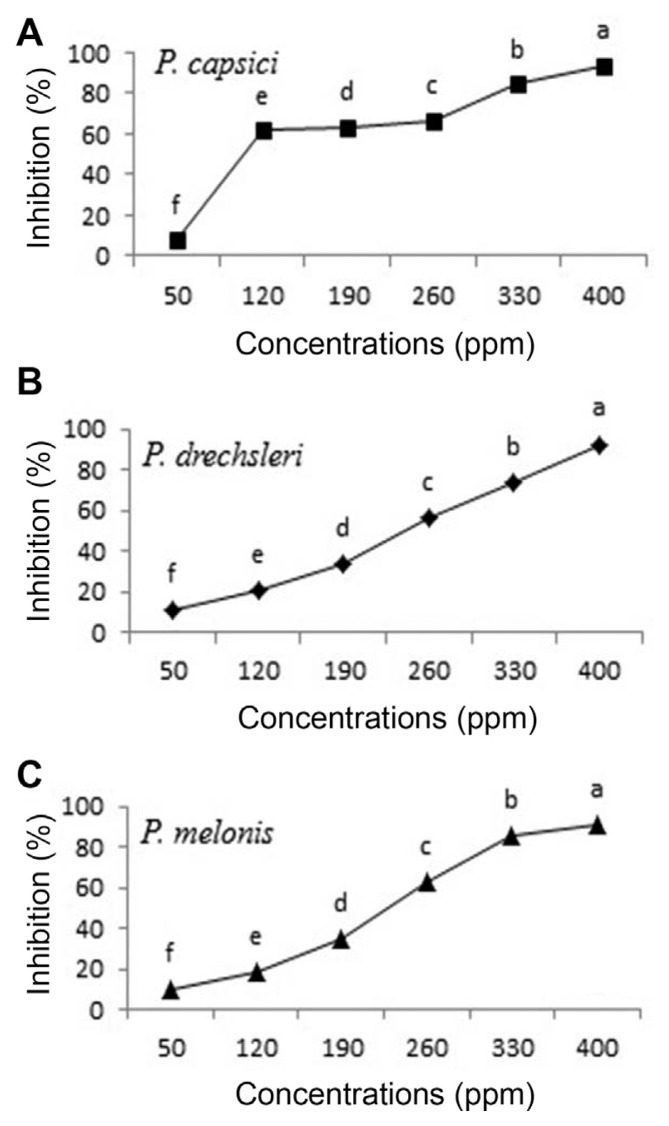
Fig. 4.
Effect of Metalaxyl - Mancozeb on growth of Phytophthora species in vitro (A, P. capsici; B, P. drechsleri; C, P. melonis). Mean by different letters indicate significant differences among treatment (P ≤ 0.05) according to DMRT. Data are mean of four replicates.
Table 4.
EC50 (Lower-Upper value) of essential oils and fungicides on Phytophthora species (at CL* 95%)
| Phytophthora spp. | Essential oils | Fungicides | ||
|---|---|---|---|---|
|
|
|
|||
| C. citratus | O. basilicum | Mancozeb | Metalaxyl + Mancozeb | |
| P. capsici | 31.473 (20.633–40.780) | 135.393 (75.892–189.535) | 28.252 (25.494–31.034) | 20.869 (15.543–26.722) |
| P. drechsleri | 69.612 (44.157–414.629) | 200.816 (124.708–313.053) | 34.737 (23.493–48.378) | 20.055 (16.244–23.957) |
| P. melonis | 33.097 (24.646–40.748) | 191.275 (115.835–288.030) | 28.242 (25.416–31.093) | 17.702 (14.180–21.219) |
Confidence limit
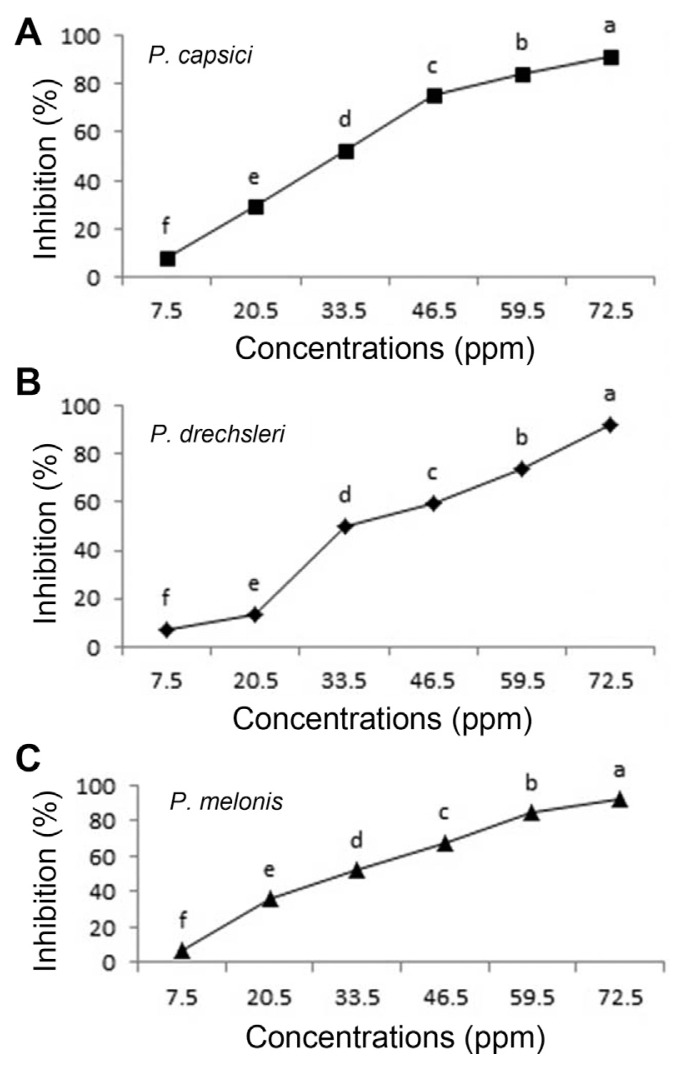
Fig. 2.
Effect of O. basilicum essential oil on growth of Phytophthora species in vitro (A, P. capsici; B, P. drechsleri; C, P. melonis). Mean by different letters indicate significant differences among treatment (P ≤ 0.05) according to DMRT. Data are mean of four replicates.
The results of EC50 are presented in Table 4. Between two tested plant essential oils on Phytophthora spp., essential oils of C. citratus had the lowest EC50 values (ppm) for inhibition the mycelial growth of P. capsici (31.473 ppm) and P. melonis (33.097 ppm), respectively. The EC50 of C. citratus seemed almost equal to EC50 of Metalaxyl+Mancozeb (17.702 to 20.869) (Table 4). Essential oils of oregano, palmarosa and red thyme had the lowest EC50 values and inhibited production and germination of sporangia, zoospores and mycelial growth of P. capsici (Bi et al., 2012). The highest EC50 value (200.816) was observed for O. basilicum against P. drechsleri (Table 4).
The results indicated that essential oils have fungistatic effect on Phytophthora species and cause inhibition of fungal growth, while essential oil of C. citratus caused the death of Phytophthora species as fungicide effect, in 10 days when used in concentration above 102 ppm.
Greenhouse assessment of plant essential oils and fungicides
The fungicides and plant essential oils applied as a soil drench significantly (p<0.05) reduced disease severity of Phytophthora diseases on pepper, cucumber and melon, compared to the nontreated control in greenhouse assays (Table 5).
Table 5.
Effect of plant essential oils and fungicides applied as soil drench on Phytophthora species after 28 days
| Treatmentsa | P. capsicib | P. drechsleri | P. melonis | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Diseasec severity | Reduction % | Disease severity | Reduction % | Disease severity | Reduction % | |
| C. citratus | 1.5c | 60.5 | 2.0b | 47.4 | 1.7b | 55.3 |
| O. basilicum | 1.9b | 50 | 2.3b | 36.8 | 2.2b | 44.7 |
| Mancozeb | 1.2d | 68.4 | 1.5c | 60.5 | 1.2c | 68.4 |
| Metalaxyl + Mancozeb | 0.6e | 84.2 | 0.5d | 86.8 | 0.3d | 92.1 |
| Nontreated control | 3.8a | – | 3.8a | – | 3.8a | – |
Fifty ml of plant essential oils and fungicide (100 ppm) were applied as a root drench into a soilless potting medium at the 2–3 true-leaf stage.
Plants were inoculated with Phytophthora spp. by applying three mycelia disc (5-mm-diameter) of each pathogen into soil per plant.
Phytophthora disease was rated based on a scale of 0–5 as described material and methods.
Mean in the column followed by different letters indicate significant differences among treatment (p<0.05) according to DMRT. Data are mean of five replicates.
Both fungicides applied as a soil drench consistently suppressed Phytophthora diseases, which is in agreement with the results of other authors (Bi et al., 2012). Metalaxyl-Mancozeb had the most significant effect against the pathogens and caused the highest decreases in the severity of the disease, which were 84.2%, 86.8%, 92.1% in relation to the negative control on melon, cucumber and pepper, respectively (Table 5). The essential oil of C. citratus had good effect in reducing the severity of the disease by P. capsici, P. drechsleri and P. melonis, which were 60.5%, 47.4% and 55.3%, respectively (Table 5). Our results are in accordance with the work by Paret et al. (2010), Mahanta et al. (2007) and Helal et al. (2006), who observed the effects of essential oils of C. citratus as bactericidal and fungicidal properties against wide range of pathogens such as Ralstonia solanacearum, Alternaria alternata, Penicilliu citrinum and Aspergillus. But essential oils of O. basilicum presented the lowest reduction on the severity of the disease, which correspond to 36.8% on P. drechsleri (Table 5). Results indicated that formulations containing clove oils, neem oil, pepper extract and mustard oil reduced the population of Phytophthora nicotians in the soil in greenhouse (Bowers and Locke, 2004). Bi et al. (2012) have reported that P. capsici population in soil was reduced by the essential oils of red thyme, oregano and palmarosa. Cucumber pepo fruits were protected against P. capsici infection when they were sprayed with red thyme essential oils (Bi et al., 2012). Application of clove and cassia extract reduced the population density of Phytophthora nicotianae 99.6 and 99.2%, respectively (Bowers and Locke, 1999).
In conclusion, our results indicated that application of two essential oils of C. citratus and O. basilicum provides significant protection against the soil-borne oomycete pathogens such as P. capsici, P. drechsleri and P. melonis in pepper, melon and cucumber plant respectively under in vitro and greenhouse conditions. Essential oils of two plants exhibited different degrees of antifungal activity against Phytophthora species, so that these essential oils may be used as alternatives for synthetic chemicals for integrated management of diseases caused by Phytophthora species in crop, vegetable and ornamental plants after the proper clinical trials. Hence, further studies are required to develop strategies for practical application in order to control the Phytophthora diseases.
Acknowledgments
The authors are thankful to the vice Chancellor for research of University of Kurdistan for the financial support (Grant No. 42/9999).
References
- Amini M, Safaie N, Salmani MJ, Shams-Bakhsh M. Antifungal activity of three medicinal plant essential oils against some Phytopathogenic fungi. Trakia J Sci. 2012;10:1–8. [Google Scholar]
- Behtoei H, Amini J, Javadi T, Sadeghi A. Composition and in vitro antifungal activity of Bunium persicum, Carum copticum and Cinnamomum zeylanicum essential oils. J Med Plant Res. 2012;6:5069–5076. [Google Scholar]
- Bi Y, Jiang H, Hausbeck MK, Hao JJ. Inhibitory effects of essential oils for controlling Phytophthora capsici. Plant Dis. 2012;96:797–803. doi: 10.1094/PDIS-11-11-0933. [DOI] [PubMed] [Google Scholar]
- Bowers JH, Locke JC. Effect of botanical extract on population of Phytophthora nicotianae and disease control in the greenhouse. Phytopathology. 1999;89:S8. [Google Scholar]
- Bowers JH, Locke JC. Effect of formulated plant extracts and oils on population density of Phytophthora nicotianae in soil and control of Phytophthora blight in the greenhouse. Plant Dis. 2004;88:11–16. doi: 10.1094/PDIS.2004.88.1.11. [DOI] [PubMed] [Google Scholar]
- Carvalho JB, Schwan-Estrada KRF, Bonaldo SM, Cruz MES, Carlos MM, Stangarlin JR. Fungitoxidade de Cymbopogon citratus e Cymbopogon martini a Colletotrichum gloeosporioides em frutos de pimentao. Br J Med Plants. 2008;10:88–93. [Google Scholar]
- De Curtis F, Lima G, Vitullo D, De Cicco V. Biocontrol of Rhizoctonia solani and Sclerotium rolfsii on tomato by delivering antagonistic bacteria through a drip irrigation system. Crop Prot. 2010;29:663–570. doi: 10.1016/j.cropro.2010.01.012. [DOI] [Google Scholar]
- Del Rio JA, Arcas MC, Benavente-Garlic O, Ortuno A. Citrus polymethoxylate flavones can confer resistance against Phytophthora citrophthora, Penicillium digitatum, and Geotrichum species. J Agric Food Chem. 1998;46:4423–4428. doi: 10.1021/jf980229m. [DOI] [Google Scholar]
- Dev N, Das AK, Hossani MA, Rahman MM. Chemical composition of different extracts of Ocimum basilicum leaves. J Sci Res. 2011;3:197–206. [Google Scholar]
- Dikbas N, Kotan R, Dadasoglu F, Sahin F. Control of Aspergillus flavus with essential oil and methanol extract of Sartureja hortensis. Int J Food Microbiol. 2008;124:179–182. doi: 10.1016/j.ijfoodmicro.2008.03.034. [DOI] [PubMed] [Google Scholar]
- Duke JA. CRC Handbook of Medicinal Herbs. CRC Press; Boca Raton, Florida: 1985. p. 677. [Google Scholar]
- Ershad J. Phytophthora species in Iran (Isolation, Purification, Identification) Agricultural Research Organization; 1992. p. 217. 1992. [Google Scholar]
- Etebarian HR. Diseases of Vegetable and Cucurbit and Their Control. University of Tehran; 2012. p. 600. [Google Scholar]
- Garcia M, Donadel OJ, Ardanaz CE, Tonn CE, Sosa ME. Toxic and repellent effect of Baccharis salicifolia essential oil on Triboliun castaneun. Pest Manage Sci. 2005;61:612–618. doi: 10.1002/ps.1028. [DOI] [PubMed] [Google Scholar]
- Gupta A, Sharma S, Naik SN. Biopesticidal value of selected essential oils against pathogenic fungus, termites, and nematodes. Int Biodeter Biodegr. 2011;65:703–707. doi: 10.1016/j.ibiod.2010.11.018. [DOI] [Google Scholar]
- Hausbek MK, Lamour KH. Phytophthpora capsici on vegetable crops: research progress and management challenges. Plant Dis. 2004;88:1292–1303. doi: 10.1094/PDIS.2004.88.12.1292. [DOI] [PubMed] [Google Scholar]
- Helal GA, Sarhan MM, Abu Shahla ANK, Abou El-khair EK. Antimicrobial activity of some essential oils against microorganism deteriorating fruit juices. Mycobiology. 2006;34:219–229. doi: 10.4489/MYCO.2006.34.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee HJ, Nam KW, Cho ED. Severe root rot on hydroponically-grown lettuce caused by Phytophthora drechsleri. Plant Pathol J. 2001;17:311–314. [Google Scholar]
- Kumar A, Shukla R, Singh P, Dubey NK. Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum L. essential oil and its safety assessment as plant based antimicrobial. Food Chem Toxicol. 2010;48:539–543. doi: 10.1016/j.fct.2009.11.028. [DOI] [PubMed] [Google Scholar]
- Lamour KH, Daughtrey ML, Benson DM, Hwang J, Hausbeck MK. Etiology of Phytophthora drechsleri and P. nicotianae (= P. parasitica) diseases affecting floriculture crop. Plant Dis. 2003;87:854–858. doi: 10.1094/PDIS.2003.87.7.854. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim J, Shin SC, Lee SG. Antifungal activity of Myrtacea essential oils and their components against three Phytopathogenic fungi. Flavour Frag J. 2008;23:23–28. doi: 10.1002/ffj.1850. [DOI] [Google Scholar]
- Mahanta JJ, Chutia M, Bordoloi M, Pathak MG, Adhikary RK, Sarma TC. Cymbopogon citratus L. essential oil as a potential antifungal agent against key weed moulds of Pleurotus spp. spawns. Flavour Frag J. 2007;22:525–530. doi: 10.1002/ffj.1835. [DOI] [Google Scholar]
- Mollaei M, Izadi H, Dashti H, Majidi A, Ranjbar KR. Bioactivity of essential oil from Satureja hortensis (Laminaceae) against three stored-product insect species. Afr J Biotechnol. 2011;10:6620–6627. [Google Scholar]
- On A, Wong F, Ko Q, Tweddell RT, Antoun H, Avis TJ. Antifungal effect of compost tea microorganism on tomato pathogens. Biol Control. 2015;80:63–69. doi: 10.1016/j.biocontrol.2014.09.017. [DOI] [Google Scholar]
- Ozcan MM, Chalchat JC, Arslan D, Ates A, Unever A. Comparative essential oil composition and antifungal effect of bitter fennel (Foenichlum vulgare ssp. Piperitum) fruit oils obtained during different vegetation. J Med Food. 2006;9:552–561. doi: 10.1089/jmf.2006.9.552. [DOI] [PubMed] [Google Scholar]
- Paret ML, Cabos R, Kratky BA, Alvarez AM. Effect of plant essential oils on Ralstonia solanacearum race 4 and bacterial wilt of edible ginger. Plant Dis. 2010;94:521–527. doi: 10.1094/PDIS-94-5-0521. [DOI] [PubMed] [Google Scholar]
- Para G, Ristaino JB. Resistance to mefanoxam and Metalaxyl among field isolates of Phytophthora capsici causing Phytophthora blight of bell pepper. Plant Dis. 2001;85:1069–1075. doi: 10.1094/PDIS.2001.85.10.1069. [DOI] [PubMed] [Google Scholar]
- Pinto JMA, Souza EA, Oliveira DF. Use of Plant extract in the control of common bean anthracnose. Crop Prot. 2010;29:838–842. doi: 10.1016/j.cropro.2010.03.006. [DOI] [Google Scholar]
- Prakash B, Shukla R, Singh P, Mishra PK, Dubey NK, Kharwar RN. Efficacy of chemically characterized Ocimum gratissimum L. essential oil as an antioxidant and a safe plant based antimicrobial against fungal and aflatoxin B1 contamination of spices. Food Res Int. 2011;44:385–390. doi: 10.1016/j.foodres.2010.10.002. [DOI] [Google Scholar]
- Rahimi-Nasrabadi M, Nazarian SH, Farahani H, Fallah-Koohbijari GR, Ahmadi F, Batooli H. Chemical composition, antioxidant, and antibacterial activities of the essential oil methanol extracts of Eucalyptus largiflorens. Int J Food Proper. 2013;16:369–381. doi: 10.1080/10942912.2010.551310. [DOI] [Google Scholar]
- Reddy MV, Angers P, Gosselin A. Characterization and use of essential oil from Thymus vvulgaris against Botrytis cinerea and Rhizopus stolonifer in strawberry fruits. Phytochemistry. 1998;47:1515–1520. doi: 10.1016/S0031-9422(97)00795-4. [DOI] [Google Scholar]
- Shadab O, Hanif M, Chaudhary FM. Antifungal activity by lemongrass essential oils. Pak J Sci Int Res. 1992;35:246–249. [Google Scholar]
- Shouan Z, Thomas LW, Miriam CM, John A, Joseph WK, Waldemar K. Evaluation of plant growth-promoting rhizobacteria for control of Phytophthora blight on squash under greenhouse conditions. Biol Cont. 2010;53:129–135. doi: 10.1016/j.biocontrol.2009.10.015. [DOI] [Google Scholar]
- Somada I, Leth V, Sereme P. Antifungal effect of Cymbopogon citratus, Eucalyptus camaldulensis and Azadirachta indica oil extracts on sorghum seed-borne fungi. Asian J Plant Sci. 2007;6:1182–1189. doi: 10.3923/ajps.2007.1182.1189. [DOI] [Google Scholar]
- Someya N, Kataoka N, Hirayae K, Hibi T, Akutsu K. Biological control of cyclamen soil borne diseases by Serratia marcescens strain B2. Plant Dis. 2000;84:334–340. doi: 10.1094/PDIS.2000.84.3.334. [DOI] [PubMed] [Google Scholar]
- Stamps DJ, Waterhouse GM, Newhook FJ, Hall GS. Revised tabular key to the species of Phytophthora. Mycol Pap. 1990;162:28. [Google Scholar]
- Stangarlin JR, Kuhn OJ, Schwan-Estrada KRF. Control de doencas de plantas por extratos de origem vegetal. Revis Anu Patol Plantas. 2008;16:265–304. [Google Scholar]
- Ali Al Yousef Sulaiman. Antifungal activity of volatiles from Lemongrass (Cymbopogon citratus) and Peppermint (Mentha piperita) oils against some respiratory pathogenic species of Aspergillus. Int J Curr Microbiol App Sci. 2013;2:261–272. [Google Scholar]
- Tabarraei M, Amini J, Harighi B. Effect of fluorescent Pseudomonads for control of damping-off disease of cantaloupe caused by Phytophthora drechsleri. Aust J Crop Sci. 2011;5:1427–1433. [Google Scholar]