Abstract
Objective
SSI rates after gynecologic oncology surgery vary from 5% to 35%, but are up to 45% in patients with diabetes mellitus (DM). Strict postoperative glucose control by insulin infusion has been shown to lower morbidity, but not specifically SSI rates. Our project studied continuous postoperative insulin infusion for 24 h for gynecologic oncology patients with DM and hyperglycemia with a target blood glucose of <139 mL/dL and a primary outcome of the protocol's impact on SSI rates.
Methods
We compared SSI rates retrospectively among three groups. Group 1 was composed of patients with DM whose blood glucose was controlled with intermittent subcutaneous insulin injections. Group 2 was composed of patients with DM and postoperative hyperglycemia whose blood glucose was controlled by insulin infusion. Group 3 was composed of patients with neither DM nor hyperglycemia. We controlled for all relevant factors associated with SSI.
Results
We studied a total of 372 patients. Patients in Group 2 had an SSI rate of 26/135 (19%), similar to patients in Group 3 whose rate was 19/89 (21%). Both were significantly lower than the SSI rate (43/148, 29%) of patients in Group 1. This reduction of 35% is significant (p = 0.02). Multivariate analysis showed an odd ratio = 0.5 (0.28–0.91) in reducing SSI rates after instituting this protocol.
Conclusions
Initiating intensive glycemic control for 24 h after gynecologic oncology surgery in patients with DM and postoperative hyperglycemia lowers the SSI rate by 35% (OR = 0.5) compared to patients receiving intermittent sliding scale insulin and to a rate equivalent to non-diabetics.
Keywords: Surgical site infection, Gynecologic oncology surgery, Diabetes mellitus, Intensive glycemic control, Post operative morbidity, Surgical outcome
Introduction
Surgical site infection (SSI), a surgical complication, is defined as infection(s) occurring after surgical procedures. It is the third most common (17%) [1] of all nosocomial infections in hospitalized patients, and is a significant cause of postoperative morbidity, mortality, and healthcare costs [2–4].
Data from the National Healthcare Safety Network show that SSI rates vary by type of surgical procedure [5,6]. SSI rates are estimated to be 1.7% for abdominal hysterectomy and 0.9% for vaginal hysterectomy [6]. However, in gynecologic oncology this rate ranges from 5% to 35% [2]. This variation depends on numerous factors including: high body mass index (BMI), low socio-economic status, poor nutritional status, high intraoperative blood loss, prolonged operative time, performance of bowel resection, perioperative blood transfusion, and patients' other medical co-morbidities [2].
Preventing SSI is vitally important and several interventions have been shown to reduce infection rates. The most important intervention is using perioperative prophylactic antibiotics [6,7]; others include improving antiseptic techniques and maintaining perioperative normal body temperature. Despite the [2] above measures, SSI remains a challenge due to the propensity of patients' medical co-morbidities, including the perioperative management of diabetes mellitus (DM).
Diabetes mellitus has been recognized as a risk factor for SSI in many surgical specialties, including cardiothoracic, hepato-biliary, and colorectal [4,8–12]. A recent study reported that the rate of SSI was an alarming 44.8% in gynecologic oncology patients with DM [2]. Fortunately, multiple studies have indicated that lowering postoperative blood glucose can reduce SSI rates [4,10–12]. Most of these studies support keeping a target postoperative blood glucose <200 mg/dL in patients with DM [13,14]. Van den Berghe et al. showed a better outcome (decreased morbidity and mortality) in surgical patients in an intensive care unit with more intensive postoperative glucose control (<139 mg/dL) [15]. A recently published study, NICE-SUGAR, had a contradictory outcome, showing that intensive glucose control among adults in the ICU to a blood glucose target of <180 mg/dL resulted in lower mortality than did a target of 81 to 108 mg/dL [16]. It is important to note that this study looked only at death rates. Nonetheless, SSI rates were not the primary outcome of both of these studies.
Considering the above studies, we hypothesize that a strict postoperative glucose control (<139 mg/dL) will lower SSI rates in gynecologic oncology patients. On March 1, 2008 our group adopted a quality improvement (QI) project at our institution. This entailed a strict postoperative blood glucose control (<139 mg/dL) by continuous intravenous insulin infusion for 24 h after surgery for patients with DM and postoperative hyperglycemia (PH) on the general surgical floor, as well as in the ICU. We utilized this QI project not only to improve morbidity and mortality outcomes, but also to hopefully lower surgical site infection rates.
Two years after adopting the QI project, we evaluated the impact of our protocol on SSI rates. To achieve that, we compared the SSI rates of our patients in three groups: Group 1, patients with DM who were managed by subcutaneous intermittent insulin injections before the QI project adoption; Group 2, patients with DM and PH managed with continuous intravenous insulin infusion after the QI project adoption; and Group 3, patients who had neither DM nor PH after the QI project adoption.
The objective of this study is to examine the effects of our protocol of an intensive postoperative glucose control regimenon SSI rates in gynecologic oncology patients.
Methods
Prior to the beginning of our QI project on March 1, 2008, patients with gynecologic malignancies at our institution with known DM had their blood glucose controlled postoperatively by intermittent subcutaneous (SC) insulin injections (traditionally known as a sliding scale). This was accomplished by using short acting regular insulin given SC every 6 h according to a set algorithm. The target blood glucose was <200 mg/dL.
After March 1, 2008, as part of our QI project, we instituted an intense postoperative blood glucose control. This strict control was achieved by initiating a continuous intravenous (IV) insulin infusion for 24 h after surgery, with a target finger stick glucose range of 90–139 mg/dL. The targeted patients were those with known DM and patients who were not diabetic but had postoperative blood glucose levels >=150 mg/dL (or >=200 mg/dL if they received steroids during surgery), who were labeled patients with postoperative hyperglycemia (PH).
Insulin was infused in 250 mL of normal saline at a concentration of 1 Unit/mL. The dose of insulin varied from 1 to 12 Units/h according to the patient's starting blood glucose. Once infusion began, blood glucose was checked every hour (using AccuCheck® machines) and the insulin infusion rate changed according to a defined algorithm adopted by the American College of Endocrinology [17].
After 24 h of insulin infusion, patients with pre-existing DM were restarted on their preoperative regimens of either subcutaneous (SC) insulin or oral hypoglycemic agent (OHA) when they resumed bowel function. Patients with PH without preexisting DM had no further treatment unless their blood glucose continued >150 mg/dL; at that point, they were managed by SC insulin until discharge. Since all PH patients are at risk of having undiagnosed DM, they were also advised to follow up with their primary care physicians for further management and/or diagnosis of DM.
Our QI project protocol allowed us to study our primary outcome, SSI rates, in three different groups. Group 1 included patients with DM who were managed by subcutaneous intermittent insulin injections before the QI project adoption prior to March 2008. Group 2 included patients with DM and PH managed with continuous intravenous insulin infusion after the QI project adoption from March 2008 through March 2010. Group 3 included consecutive patients who had neither DM nor PH after the QI project adoption; we collected data from these patients from January 1, 2009 through December 31, 2009 for simplicity and convenience reasons. These groups can be seen in Fig. 1.
Fig. 1.
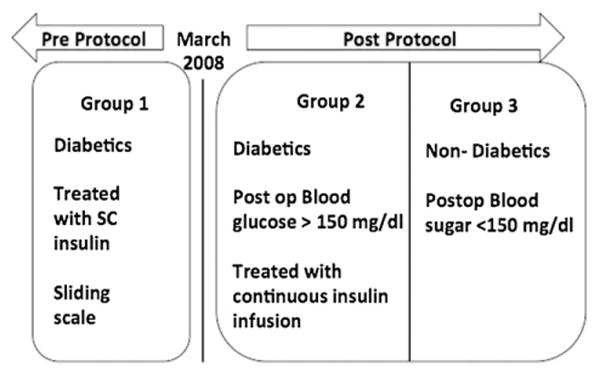
The three groups of patients.
After we obtained Institution Review Board approval, we retrospectively collected data. The inclusion criteria were: women undergoing major surgery for gynecologic malignancies and above 18 years of age. We excluded patients with incomplete medical records (those with missing or inconsistent data) and incarcerated patients. We also excluded those who underwent minimally invasive surgery (MIS), recognizing the lower rates of SSI with this surgical approach.
Demographic and surgical data collected from the electronic medical records included: age, body mass index (BMI), American Society of Anesthesiology scoring system (ASA), medical co-morbidities including DM (both types), hypertension, current smoking status, chronic renal or hepatic disease, immune deficiency, perioperative antibiotic use, length of surgery (LOS: defined from surgical incision to closure) and estimated operative blood loss (EBL). SSI was defined according to NHSN/CDC criteria [1]. The follow up time for each patient also followed the CDC criteria of 30 days after surgery.
Comparisons of patient characteristics among the three groups were performed using Fisher's exact test for dichotomous variables or t-tests for continuous variables. Univariate logistic regression was used to determine the effect of each variable on SSI. Multivariate logistic regression was conducted to examine the association between SSI in the three groups by blood glucose control methods after controlling for covariates. Results were quantified in terms of odds ratios (ORs) with corresponding 95% confidence intervals (CIs). All statistical analyses were performed using SAS 9.1 software (SAS Institute Inc. Cary, NC). Statistical significance was defined as a two-tailed p-value <0.05.
Results
The three patient groups evaluated in this study can be seen in Fig. 2. Of the 200 women with known DM in Group 1, 52 were excluded secondary to either incomplete medical records (n = 36) or MIS approach (n = 16), resulting in a study group of 148 patients. Out of 207 patients with DM or PH in Group 2, 72 were excluded secondary to either incomplete medical records (n = 20) or MIS approach (n = 52), resulting in a group of 135 patients. Lastly, out of 169 non-diabetic, non-PH patients in Group 3, 80 were excluded secondary to either incomplete medical records (n= 23) or MIS approach (n= 57), resulting in a study group of 89 patients.
Fig. 2.
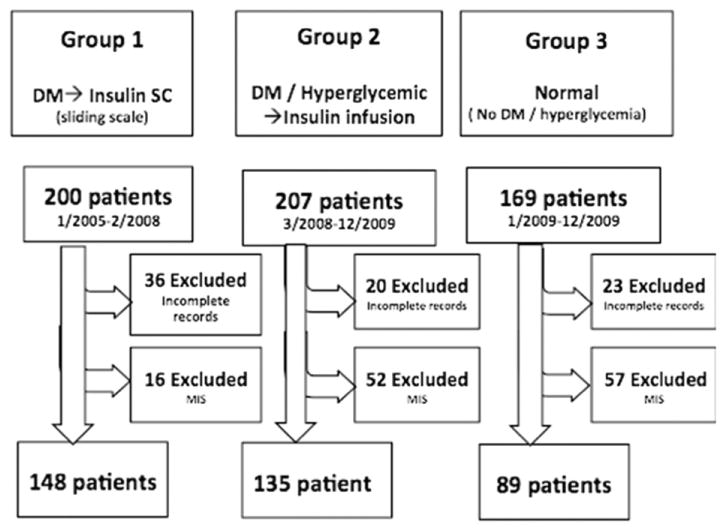
The patients in each group.
Patient characteristics and surgical data are shown in Table 1. Analysis comparing Groups 1 and 2 with Group 3 showed no difference in age, smoking status, preoperative serum albumin level, preoperative Hb level, preoperative bowel preparation, or in the use of perioperative antibiotics (the average time between infusion and incision time is 38 min). Analysis comparing the three groups also showed no difference among patients in the type of cancer (ovarian, uterine, or cervical), debulking stage (primary or interval), type of surgical incision (vertical midline or horizontal), or intraoperative core body temperature. The presence of DM in Groups 1 and 2 was correlated with higher ASA classification, higher body mass index, longer surgery, and more estimated blood loss, compared to those in Group 3. Comparisons between Groups 1 and 2 revealed the groups to be similar in characteristics with the exception of greater intraoperative blood loss in Group 2.
Table 1.
Patient characteristics.
| Variable | Group 1 (Historic) →Insulin SC n = 148 |
Group 2 (DM/PH) →Insulin infusion n = 135 (DM = 46, PH = 92) |
Group 3 Normal No insulin n = 89 |
p value | |
|---|---|---|---|---|---|
| Patients' data | |||||
| Age (mean) | 60.6 | 57.4 | 57.6 | 0.1 | |
| ASA | 1–2 | 79 (53%) | 80 (59%) | 66 (74%)a | <0.01 |
| 3–4 | 69 (46%) | 55 (41%) | 23 (26%)a | <0.01 | |
| BMI (mean) | 37.8 | 37.2 | 31.5a | <0.01 | |
| Smoking | 27 (18%) | 12 (9%) | 14 (16%) | 0.07 | |
| HTN | 106 (72%) | 79 (59%) | 35 (39%)a | <0.01 | |
| Preoperative serum albumin level | 2.8 g/dL | 2.9 g/dL | 3.0 g/dL | 0.34 | |
| Preoperative Hb level | 11.2 g/dL | 10.8 g/dL | 10.7 g/dL | 0.26 | |
| Preoperative bowel preparation | 55 (37%) | 57 (42%) | |||
| Surgery data | |||||
| Ovarian cancer | 59 (40%) | 58 (43%) | 45 (51%) | 0.06 | |
| Primary debulking | 48 (81%) | 50 (86%) | 38 (84%) | 0.54 | |
| Interval debulking | 11 (19%) | 8 (14%) | 7 (16%) | 0.35 | |
| (Post NAT) | |||||
| Uterine cancer | 87 (58.7)% | 75 (55.6%) | 43 (48%) | 0.76 | |
| Cervical cancer | 2 (1.3%) | 2 (1.4%) | 1 (1.1%) | 0.54 | |
| Type of surgical incision | |||||
| Vertical midline | 144 (97.3%) | 130 (96.3%) | 84 (94.4%) | 0.43 | |
| Horizontal | 4 (2.7%) | 5 (3.7%) | 5 (5.6%) | 0.07 | |
| Length of surgery (min) | 245.5 | 287.7 | 197.2a | <0.01 | |
| Antibiotics | 99% | 99% | 98% | 0.73 | |
| EBL (mL) | 415.7 | 542.5 | 285.3a | <0.01 | |
| Intraoperative core body temperature | 36.9 | 36.7 | 36.5 | 0.34 | |
Statistically different from the other two groups.
Patients who received the protocol's strict IV insulin infusion (Group 2) had a statistically significant lower SSI rate compared to that of patients who received historic subcutaneous insulin (Group 1), 19% (26/135) vs. 29% (43/148) (p = 0.001). This SSI rate is similar to that of patients who had neither DM nor PH (Group 3), 21% (19/89) (p = 0.53), shown in Fig. 3.
Fig. 3.
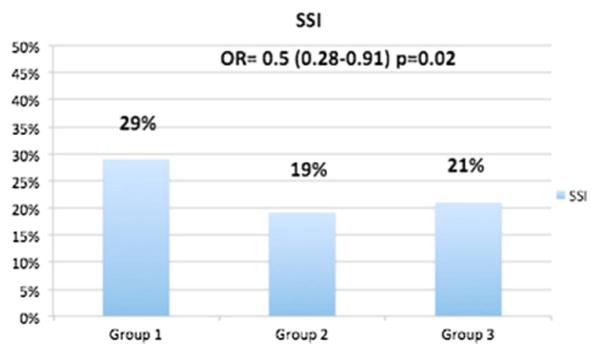
SSI rate in each group.
Multivariate analysis (Table 2) showed that Group 2 (post-protocol DM and PH) patients had 35% lower SSI rates with an odd ratio of 0.5 (0.28–0.91, p = 0.02) compared with the patients in Group 1 (patients who received SC intermittent insulin) prior to the management change. This represents a 50% reduction in the odds of having SSI in diabetic patients after implementing the insulin infusion, bringing the incidence to the same level as that of the non-diabetic patients in Group 3.
Table 2.
Multivariate analysis after adjusting for all covariates.
| Parameter | OR | CL | p-value | |
|---|---|---|---|---|
| Group | Group 2 vs. Group 3 | 0.67 | 0.32–1.41 | 0.29 |
| Group 2 vs. Group 1 | 0.5 | 0.28–0.9 | 0.02 | |
| ASA | (3–4 vs. 1–2) | 0.98 | 0.58–1.66 | 0.93 |
| Smoking | Yes vs. no | 1.38 | 0.71–2.69 | 0.34 |
| Antibiotics | Yes vs. no | 1.11 | 0.12–10.62 | 0.93 |
| Hypertension | Yes vs. no | 0.86 | 0.49–1.51 | 0.59 |
| Age (years) | 0.99 | 0.97–1.01 | 0.52 | |
| BMI | 1.01 | 0.98–1.03 | 0.49 | |
| Length of surgery (h) | 1.18 | 1.00–1.36 | 0.05 | |
| EBL (mL) | 1010 | 610–1670 | 0.97 | |
The odds of having SSI in the sliding group (Group 1) was about two times higher than the drip group (Group 2) after adjusting for other covariates (p = 0.02) while statistical significance was not found for the comparison of Group 3 vs. Group 2.
As seen in Fig. 3, implementing the insulin infusion protocol produced a statistically significant lower 24-hour average blood glucose of 110 mg/dL in Group 2 compared to the historic average of 162 mg/dL (p < 0.01) in the intermittent SC insulin group (Group 1). The rate of hypoglycemia in Group 2 (the insulin infusion group) was significantly lower than that of Group 1 (0.7% vs. 5.4%, p < 0.05).
Within Group 2, we also separated patients with DM from those with PH and analyzed SSI rates in each subgroup (Supplemental Fig. 1). In the DM sub-group, the SSI rate was 17.3% (8/46), while in the PH sub-group, the SSI rate was 20.6% (19/92). Out of the 92 patients diagnosed with postoperative hyperglycemia, 62 patients followed up after the surgery to check for the presence of DM. At that time, 28 patients were diagnosed with DM; out of this subset, 21.4% (6/28) had had SSI at the time of surgery. Therefore, the SSI rate of all patients with DM (including those diagnosed prior to surgery and those who had PH and were later diagnosed) was 18.9%, or 14/74; this was comparable to the overall SSI rate of all DM and PH patients, which was 19.5%.
Discussion
This study shows that utilizing insulin infusion to control blood glucose after surgery virtually eliminated the effect of diabetes on surgical site infection.
Surgical site infections have been and continue to be a cause of significant postoperative morbidity and mortality in the gynecologic oncology patient population. After controlling for perioperative antibiotic use, other studies have identified other independent risk factors for SSI, including high BMI, perioperative blood transfusion, low socio-economic status, and prolonged operative time [6], along with the presence of diabetes mellitus (DM).
Many studies in a variety of specialties [5,9,10,18] have shown that DM remains a significant factor contributing to SSI. Cardiothoracic patients with DM compared to non-diabetics are two to five times more likely to have SSI, [4,8,9], twice as likely to be re-hospitalized, and 1.38 times more likely to die [4,10]. Diabetic patients undergoing colorectal surgery are also at a higher risk for developing SSI when compared with non-diabetic patients, with an odds ratio of 1.32 (1.11–1.57) [4]. Several studies have shown that stricter postoperative glycemic control lowers the risk of SSI in diabetic patients. Talbot et al. showed that not only is DM an independent risk factor for developing SSI, but a higher postoperative blood glucose (>250 mg/dL) was associated with a higher SSI rate (3.7%) compared to a rate of 0.6% in patients with a blood glucose <150 mg/dL [19]. McConnell et al. reported that in colorectal surgery, the SSI rate dropped from 29% to 14% in favor of better postoperative blood glucose control [18]. Similarly, in hepato-biliary surgery, the rate of SSI was 20% when the average postoperative blood glucose was <200 mg/dL compared to 52% for patients when it was >200 mg/dL (p < 0.01) [13]. The aforementioned studies underscore the importance of postoperative blood glucose control in lowering SSI rates. Although those studies had SSI as a primary outcome, most of them aimed for a postoperative blood glucose of less than 200 mg/dL, utilized intermittent subcutaneous insulin, and none were randomized trials.
Several randomized clinical trials have been published on intensive glycemic control. Van den Berghe et al. randomized nearly 1500 patients and demonstrated that strict insulin infusion (maintaining blood glucose at or below 110 mg/dL) reduced morbidity and mortality among critically ill patients in the intensive care unit. However, SSI was not the primary outcome in this study, and not all patients were surgical patients. A Cochrane [14] publication reviewed five randomized controlled trials with SSI as a primary outcome in relation to perioperative glycemic control. This review concluded that a final recommendation is difficult to determine due to the significant heterogeneity among patient characteristics, surgical procedures, and measured outcomes. Another recent Cochrane analysis [20] did not find any significant differences in most of the outcomes when targeting intensive perioperative glycemic control compared with conventional glycemic control in patients with diabetes mellitus. It is important to point out there that none of the studies included in this Cochrane have SSI as a primary outcome and more importantly, the wide heterogeneity of the modality of the postoperative glycemic control makes a unified conclusion difficult. Due to the aforementioned limitations, it is clear there was a need for a study that had a specific patient population, postoperative blood glucose target and modality, and outcome.
Our study included a specific patient population, gynecologic oncology patients at high risk for SSI who had known DM or postoperative hyperglycemia. We followed a specific postoperative blood glucose control both in terms of modality and target blood glucose. Additionally, we measured a specific outcome, surgical site infection, in three different groups: those with DM controlled by SC insulin injection (Group 1, historic and pre-protocol), those with DM and postoperative hyperglycemia (PH) controlled by insulin infusion (Group 2, post-protocol), and those without DM (Group 3, post-protocol). In our analysis, we excluded patients who had minimally invasive surgery (MIS) because of the low risk of SSI associated with such procedures. As a matter of fact, after we completed the current study, we limited postoperative insulin infusion to patients who underwent laparotomy only. However, to minimize medical morbidities of diabetes, we continue to do insulin infusion with all patients who have MIS if they already have poorly controlled DM.
The outcome showed that strict blood glucose control by insulin infusion outside the intensive care unit for 24 h after gynecologic oncology surgery reduces SSI rates. This positive effect is shown both in patients with known DM and those with PH. The findings reveal that implementing 24 h of strict continuous insulin infusion (target blood glucose <139 mg/dL) in patients with DM and PH lowers SSI rates by 35% compared to those DM patients managed with intermittent SC insulin injections. Remarkably, this SSI rate is equivalent to that of patients without DM or PH.
Implementing the insulin infusion protocol also produced a statistically significant lower 24 hour average blood glucose of 110 mg/dL in Group 2 compared to the historic average of 162 mg/dL (p < 0.01) in the intermittent SC insulin group (Group 1). Although other studies demonstrated higher hypoglycemia rates with insulin infusion, we showed significantly lower hypoglycemia rates after adopting the continuous insulin infusion compared with intermittent insulin use (0.7% vs. 5.4%, p < 0.05). This can be explained by more frequent blood glucose checks (i.e. every 1 h) as opposed to every 6 h in the intermittent protocol.
The strengths of our study include having a large number of patients in each of the three groups. Additionally, all patients in the cohort had a postoperative clinic visit between four to six weeks after surgery. Infections that were diagnosed at an outside facility were also documented at the time of this postoperative clinic visit. This lowers the chance of under-reporting SSI. Patients who did not have that postoperative visit were excluded from our analysis. Also, we implemented a strict glucose control (target of 90–139 mg/dL) compared to existing surgical literature, where the goal of glycemic control was generally less than 200 mg/dL. Our study looked at surgical site infection as a primary outcome, as well. Lastly, we implemented a multivariate analysis that allowed us to control for many obvious clinical factors that affect SSI.
The major limitations of our study include those typically associated with any retrospective analyses, including the possible lack of collection of key confounding factors that might have affected the outcome. We tried to address this limitation by collecting data on all clinical factors that have historically affected SSI. Additionally, SSI reporting after 2008 may have increased, given heightened awareness of infection control and the introduction of other methods to reduce infection rates at our institution. It is worrisome that as time changes and pattern of practice evolved, there might have been an adoption of clinical practice pattern that might have also lowered the SSI along with the adoption of our protocol. However, our group has not adopted any new protocol that could have lowered SSI rates during this time; moreover, we controlled for all the potential factors, and so our analysis showed that glycemic control was indeed affecting SSI rates. In Group 2, we also included patients with postoperative hyperglycemia with the intent of identifying women with undiagnosed DM who might be at increased risk of surgical and medical complications. Although that inclusion increased the heterogeneity of this group, a subset analysis of the SSI rate in women who had postoperative hyperglycemia showed that it was similar to those who had been diagnosed with DM. This obviates the question of whether we under-reported the incidence of SSI in Group 2 by including this patient population. Furthermore, with longitudinal follow-up data (Supplemental Table 1), 30% of patients with postoperative hyperglycemia was subsequently diagnosed with DM. Additional studies should explore this further.
In conclusion, initiating intensive postoperative glucose control through insulin infusion for 24 h after major gynecologic oncology surgery in patients with DM and postoperative hyperglycemia lowers the SSI rate by 35% compared to patients controlled with intermittent sliding scale insulin, bringing the rate equivalent to that of non-diabetics. This study has significant future implications on costs and survival. Because of the burden of SSI on healthcare costs, we are currently quantifying the cost-effectiveness of this protocol. The significant decrease in the SSI rate will not only decrease surgical morbidity and mortality, but also might be associated with less delay in the initiation of important adjuvant chemotherapy or radiation, thus improving overall survival, as well. However, randomized clinical trials are necessary to fully assess the impact of this protocol on survival outcomes.
Supplementary Material
Highlights.
Patients with DM in gynecologic oncology can have an SSI rate up to 45%.
We adopted a quality improvement protocol to start postoperative insulin infusion for target blood glucose <139 mg/dL.
SSI was lowered by 35%.
Acknowledgments
This work was supported by Haley Madden, M.S., Department of Life Sciences Communication, University of Wisconsin-Madison.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2014.09.013.
Conflict of interest statement: The authors have no conflict of interest.
References
- 1.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132. quiz 3–4; discussion 96. [PubMed] [Google Scholar]
- 2.Nugent EK, Hoff JT, Gao F, Massad LS, Case A, Zighelboim I, et al. Wound complications after gynecologic cancer surgery. Gynecol Oncol. 2011;121:347–52. doi: 10.1016/j.ygyno.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 3.de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387–97. doi: 10.1016/j.ajic.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Ata A, Valerian BT, Lee EC, Bestle SL, Elmendorf SL, Stain SC. The effect of diabetes mellitus on surgical site infections after colorectal and noncolorectal general surgical operations. Am Surg. 2010;76:697–702. [PubMed] [Google Scholar]
- 5.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen MA, Higham-Kessler J, Yokoe DS, Butler AM, Vostok J, Stevenson KB, et al. Developing a risk stratification model for surgical site infection after abdominal hysterectomy. Infect Control Hosp Epidemiol. 2009;30:1077–83. doi: 10.1086/606166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannien J, van Kasteren ME, Nagelkerke NJ, Gyssens IC, Kullberg BJ, Wille JC, et al. Effect of optimized antibiotic prophylaxis on the incidence of surgical site infection. Infect Control Hosp Epidemiol. 2006;27:1340–6. doi: 10.1086/509842. [DOI] [PubMed] [Google Scholar]
- 8.Hruska LA, Smith JM, Hendy MP, Fritz VL, McAdams S. Continuous insulin infusion reduces infectious complications in diabetics following coronary surgery. J Card Surg. 2005;20:403–7. doi: 10.1111/j.1540-8191.2005.200472.x. [DOI] [PubMed] [Google Scholar]
- 9.Shilling AM, Raphael J. Diabetes, hyperglycemia, and infections. Best Pract Res Clin Anaesthesiol. 2008;22:519–35. doi: 10.1016/j.bpa.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–3. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 11.Guvener M, Pasaoglu I, Demircin M, Oc M. Perioperative hyperglycemia is a strong correlate of postoperative infection in type II diabetic patients after coronary artery bypass grafting. Endocr J. 2002;49:531–7. doi: 10.1507/endocrj.49.531. [DOI] [PubMed] [Google Scholar]
- 12.Tang R, Chen HH, Wang YL, Changchien CR, Chen JS, Hsu KC, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single-center prospective study of 2,809 consecutive patients. Ann Surg. 2001;234:181–9. doi: 10.1097/00000658-200108000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambiru S, Kato A, Kimura F, Shimizu H, Yoshidome H, Otsuka M, et al. Poor postoperative blood glucose control increases surgical site infections after surgery for hepato-biliary-pancreatic cancer: a prospective study in a high-volume institute in Japan. J Hosp Infect. 2008;68:230–3. doi: 10.1016/j.jhin.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Kao LS, Meeks D, Moyer VA, Lally KP. Peri-operative glycaemic control regimens for preventing surgical site infections in adults. Cochrane Database Syst Rev. 2009:CD006806. doi: 10.1002/14651858.CD006806.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 16.NICE-SUGAR study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 17.Garber AJ, Seidel J, Armbruster M. Current standards of care for inpatient glycemic management and metabolic control: is it time for definite standards and targets? Endocr Pract. 2004;10(Suppl. 2):10–2. doi: 10.4158/EP.10.S2.10. [DOI] [PubMed] [Google Scholar]
- 18.McConnell YJ, Johnson PM, Porter GA. Surgical site infections following colorectal surgery in patients with diabetes: association with postoperative hyperglycemia. J Gastrointest Surg. 2009;13:508–15. doi: 10.1007/s11605-008-0734-1. [DOI] [PubMed] [Google Scholar]
- 19.Talbot TR. Diabetes mellitus and cardiothoracic surgical site infections. Am J Infect Control. 2005;33:353–9. doi: 10.1016/j.ajic.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Buchleitner AM, Martinez-Alonso M, Hernandez M, Sola I, Mauricio D. Perioperative glycaemic control for diabetic patients undergoing surgery. Cochrane Database Syst Rev. 2012;9:CD007315. doi: 10.1002/14651858.CD007315.pub2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


