Introduction
Chemotherapeutics have been reported to produce, equally in males and females, a peripheral polyneuropathy that can persist for months to years beyond cessation of treatment [Dougherty et al., 2007; Tofthagen et al., 2010; Mols et al., 2014] and has been reported to occur in 35–100% of people receiving treatment [Han and Smith, 2013]. Unfortunately, the severe pain associated with the polyneuropathy not only decreases quality of life, but also affects patient compliance and can limit clinical use. Understanding the mechanisms underlying the development of this pain state is important to further efforts to develop targets for therapeutic intervention.
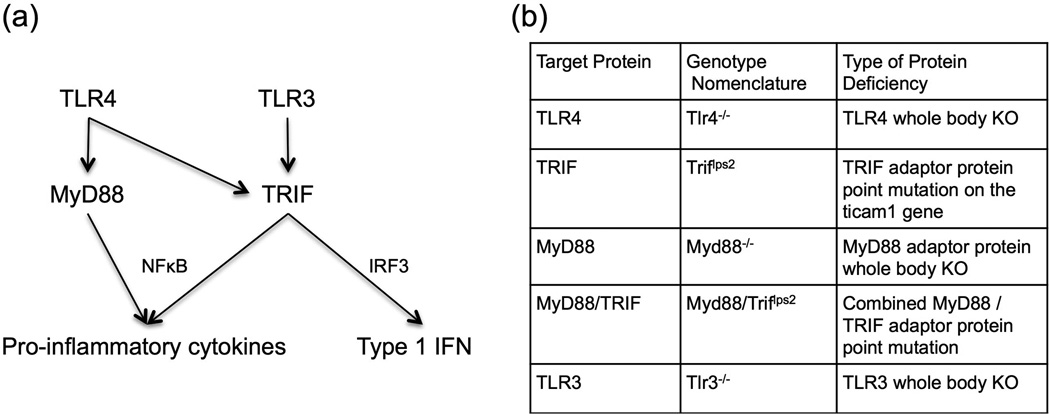
While the mechanisms underlying the polyneuropathy are complex, it is appreciated that the engagement of Toll-like receptors (TLRs) may facilitate pain development by inducing pro-inflammatory cytokine expression [Li et al., 2014; Liu et al., 2014; Park et al., 2014]. This induction occurs via different signaling cascades (Figure 1A). Activation of the Myeloid differentiation primary response gene 88 (MyD88) pathway leads to the activation of NFκB and the production of pro-inflammatory cytokines. Alternatively, activation of the TIR-domain-containing adaptor-inducing interferon-β (TRIF) adaptor protein results in production of type 1 interferon (IFN) and a delayed production of pro-inflammatory cytokines [O’Neill and Bowie, 2007]. All TLR activation leads to signaling through one of these two pathways and may play a role in cisplatin-induced pain.
Figure 1.
(a) Toll-like receptors (TLRs) signal through a MyD88-dependent and independent (TRIF) pathway. The MyD88-dependent pathway leads to the activation of NFκB and the induction of pro-inflammatory cytokines, such as TNF and IL-1β. (b) In the present experiments, we used several strains of knockout mice. The target protein, genotype nomenclature, and the type of protein deficiency are outlined.
A study by Park et al. showed that male mice deficient in both MyD88 and TRIF signaling do not develop tactile allodynia following cisplatin treatment [Park et al., 2014]. In addition, the group examined Myd88−/− and Triflps2 mice, revealing distinct roles of each pathway. For example, Triflps2 mice showed a delayed, but persistent tactile allodynia. In contrast, Myd8−/− mice showed a less severe allodynia. Furthermore, Li et al. [2014] found that paclitaxel-induced allodynia in rats was prevented by intrathecal administration of the TLR4 antagonist LPS-RS, and was transiently reversed by both LPS-RS and, to a lesser extent, by MIP, a MyD88 antagonist. Together, these results demonstrate a complex role of TLR signaling pathways in chemotherapeutic-induced polyneuropathy.
It is well recognized that many chronic pain conditions affect females at a higher rate than males [Mogil, 2012], yet many experimental protocols only examine male mice. In addition, cisplatin is used for the treatment of a variety of female-specific cancers and females equally experience chemotherapeutic-induced polyneuropathies. This is important to note because recent work has made the case that spinal TLR4 signaling mediates pain associated with mononeuropathy in male, but not female, mice [Sorge et al., 2011]. Examining whether innate immune signaling plays an equal role in both sexes may point to important mechanistic differences underlying the development of persistent pain following administration of chemotherapeutic agents, and, potentially, the necessity to develop different therapeutic interventions for each sex. Accordingly, the current study examined the development of cisplatin-induced polyneuropathy in male and female wild type mice, and whether innate immune signaling is necessary for the induction and maintenance of pain in this model.
Method
Animals
The protocol was approved by the Institutional Animal Care and Use Committee at the University of California, San Diego. Mice were housed up to four per standard cage while maintained on a 12:12-hr light/dark cycle, with food and water available ad libitum. All of the procedures and testing were conducted during the light portion of the cycle. Male and female wild type C57Bl/6 (n=7 and n=8, respectively) mice were purchased from Harlan (Indianapolis, IN). TheTlr4−/− (n=4 male, n=5 female), Tlr3−/− (n=6 male, n=5 female), and Myd88−/− (n=7 male, n=8 female) mice were a gift from Dr. S. Akira (Osaka University, Japan) and were backcrossed for 10 generations onto the C57Bl/6 background. Triflps2 (n=8 male, n=7 female) mice were a gift from Dr. B. Beutler (University of Texas Southwestern, Tx) and were directly generated on the C57Bl/6 background. Myd88−/− mice and Triflps2 mice were intercrossed to generate Triflps2/Myd88−/− mice (n=8 each male and female). These mice are described in Figure 1B.
Drug Treatment
In the current experiments, male and female WT, Tlr3−/− Tlr4−/−, Myd88−/−, Triflps2, and Triflps2/Myd88−/− mice received 6 intraperitoneal (i.p.) injections of cisplatin (2.3 mg/kg/day; Spectrum Chemical MFG, Gardena, CA, USA) every other day over the course of two weeks, a dose previously shown to induce tactile allodynia, but not impair motor function or result in morbidity (Park et al., 2013; Park et al., 2014). Between cisplatin injection days, lactated Ringer’s solution (0.25 ml) was injected to maintain hydration and to protect the kidney and liver. During the period of cisplatin administration, gross behavioral observations were made and animals were assessed for general health, including changes in body weight. In case of dehydration, additional lactated Ringer’s solution was administered. In this study, the criteria for euthanasia was weight loss in excess of 20%, however, no animals required euthanasia.
On day 18 following initiation of cisplatin treatment, baseline thresholds were measured. Then, mice received an i.p. injection of gabapentin (100mg/kg; Toronto Research Chemicals) diluted in 0.9% sterile saline. To test the efficacy of gabapentin in reversing established neuropathic pain, behavioral testing was conducted again 45-min later.
Behavioral Tests
Tactile allodynia testing was conducted prior to each injection of cisplatin (days 0, 2, 4, 7, 9, 11) and at days 15, 18, 21, and 23 following initiation of treatment. Animals were placed in clear, plastic, wire mesh-bottomed cages for 45-min prior to the initiation of testing. Tactile thresholds were measured with a series of von Frey filaments (Seemes Weinstein von Frey anesthesiometer; Stoelting Co., Wood Dale, IL) ranging from 2.44 to 4.31 (0.02–2.00 g) using the up-down method [Chaplan et al., 1994]. In light of reports of the possible contribution of the gender of the experimenter [Sorge et al., 2014], we note that a female performed mouse behavioral testing.
Statistical Analysis
Results are represented as mean ± SEM. Statistical analysis was performed using GraphPad Prism (version 6; GraphPad Software, San Diego, CA). For comparison of weight and mechanical hyperalgesia, 2-way analysis of variance (ANOVA) was used to determine main effects, depending on sex and time. In addition, Bonferroni multiple comparisons tests for post hoc analyses of specific differences were used. In all cases p < .05 was considered significant.
Results
Baseline reactivity is similar across sex and strain of mouse
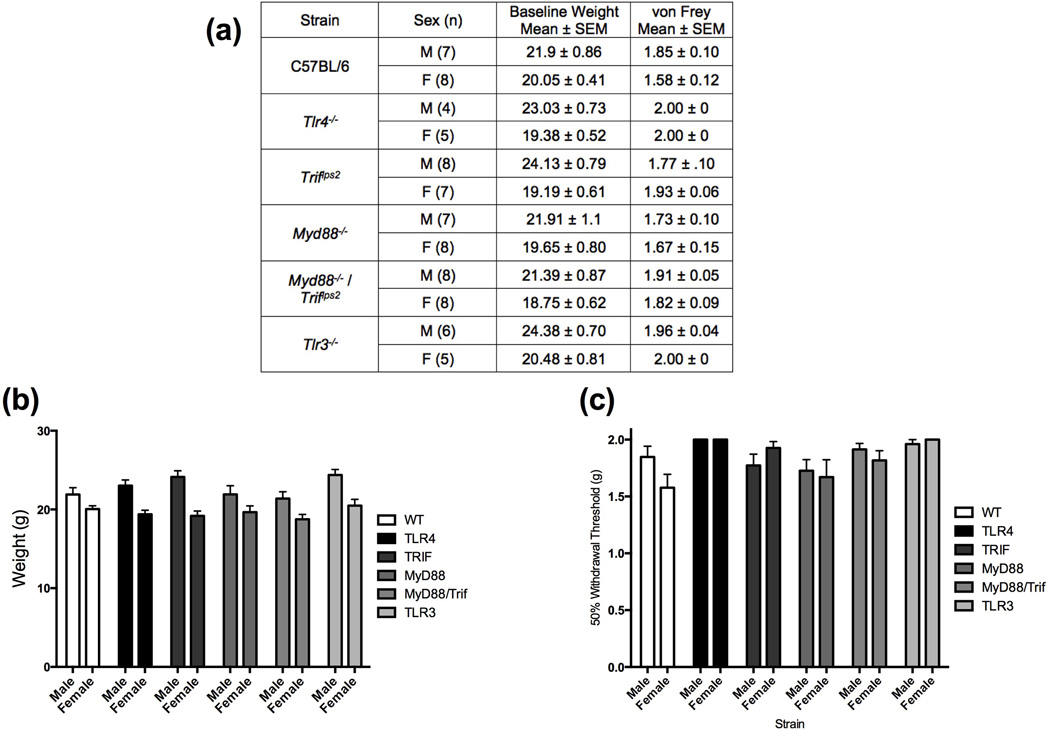
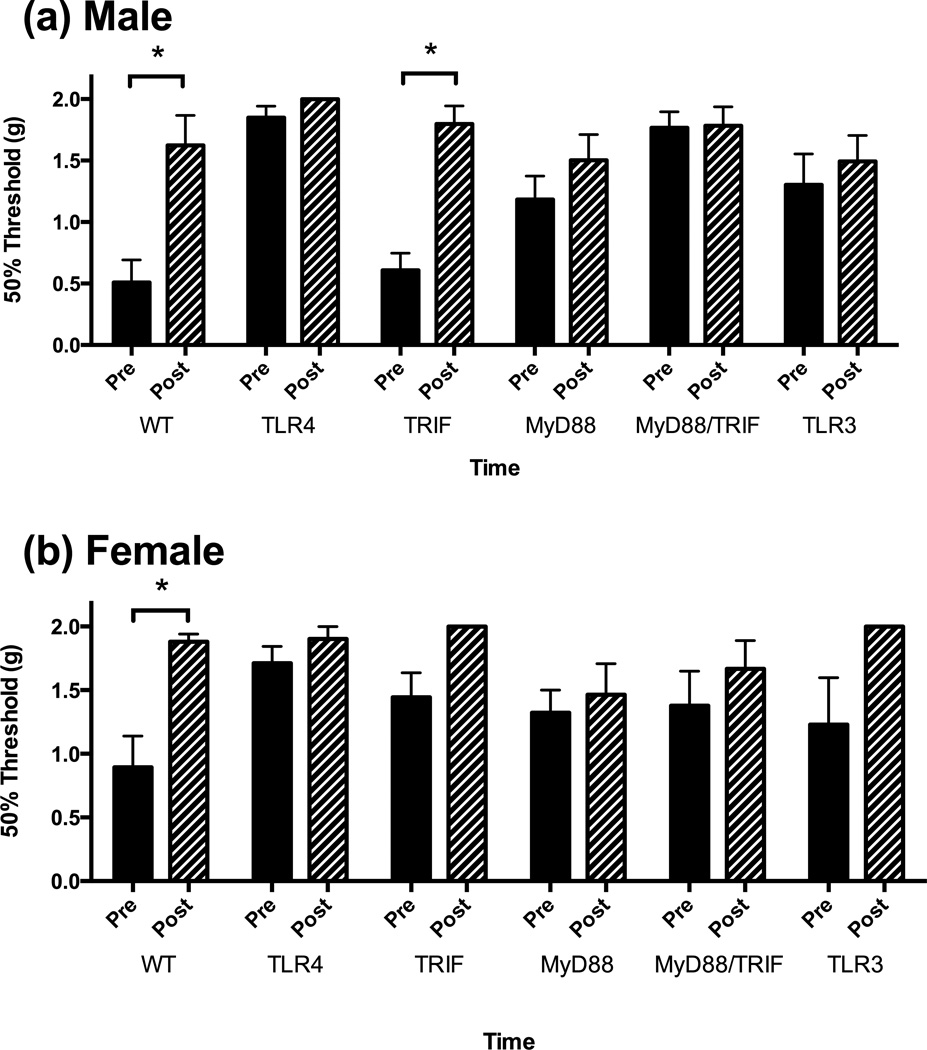
We first examined whether there were baseline differences in tactile reactivity between male and female mice, and across strains (Figure/Table 2 A or B). In comparing baseline values, an ANOVA revealed a significant main effect of strain of mouse [F (5, 69) = 3.314, p = 0.0097] however, post hoc analyses do not indicate any specific differences between groups (p > .05). Additionally, there were no differences between the sexes at baseline [F (1, 69) = 0.469, p > .05]. All mice had tactile thresholds above 1.5 g, which is considered to be normal reactivity for this mouse strain (range 1.578 ± 0.117 – 2.0 ± 0.0).
Figure 2.
A summary of the mice used and the sample size are presented in (a) along with the baseline weights and tactile reactivity for each strain. All female mice were smaller than their male counterparts at baseline (p < .05), however there were no differences between the strains (b). Baseline reactivity between the strains of mice varied (p < .05), but no specific differences between the groups (c). Furthermore, all baseline values were above 1.5 g, indicative of normal reactivity (a, c).
Cisplatin treatment induces persistent tactile allodynia in male, but not female mice
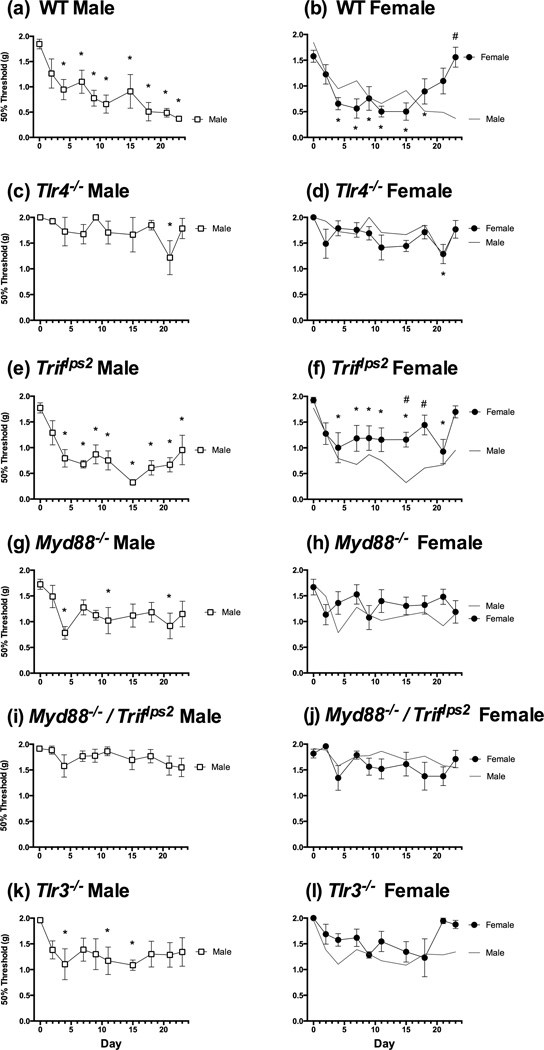
We first examined whether male mice developed a persistent tactile allodynia in response to cisplatin treatment. Because baseline reactivity varied significantly by strain of mouse, the development of tactile allodynia, over time, was assessed by comparing each score to the baseline threshold in that group. In the present study, we replicated previous results, demonstrating that male WT mice showed a significant decrease in mechanical threshold from Day 4-Day 23 (Figure 3A; p < .05) following cisplatin treatment. Surprisingly, we found that female animals did not show the same pattern of tactile allodynia after cisplatin treatment (Figure 3B). Instead, we found female WT animals exhibit a decrease in mechanical threshold from Day4-Day 18 (p < .05), and then showed a return to baseline by Day 23 remaining significantly higher in threshold than male WT mice (p < .05). These changes in tactile threshold over time were confirmed using a repeated measures (RM) ANOVA revealing a significant main effect of time [F(9,117) = 8.78, p<.0001] and a significant Time × Sex interaction [F(9,117) = 5.57, p< .0001]. Due to the similarities in the initial development of tactile allodynia, there was not a main effect of sex (p> .05).
Figure 3.
Tactile reactivity was assessed over a period of 23 days, both during and following cisplatin treatment. Male WT mice (a) develop a persistent tactile allodynia in response to cisplatin treatment. Unlike male mice, female WT mice develop an initial tactile allodynia, but return to baseline threshold by the end of testing, remaining significantly higher in threshold than their male counterparts. As shown in previous studies, a deficiency in TLR signaling protects male mice (C) from cisplatin-induced polyneuropathy. We found female Tlr4−/− mice (d) show the same response. Examining the role of the TRIF adaptor protein signaling pathway, we found males develop a persistent tactile allodynia (e), while females (f) show a reduction in threshold, but return to baseline by the end of testing. Male Myd88−/− mice (g) show a slight reduction in threshold relative to baseline, but do not differ from their female counterparts (h). Blocking all TLR-related signaling in the Triflps2/ Myd88−/− mice results in complete protection from cisplatin-induced tactile allodynia in male (i) and female (j) mice. To further examine the role of TRIF signaling, we examined the response of Tlr3−/− mice to cisplatin and found a slight reduction in threshold in male (k), but not female (l), mice relative to their own baselines. There was no difference in threshold between the sexes.
* = p < .05 relative to baseline; # = p < .05 comparing male to female animals
Both male and female TLR4 deficient mice fail to develop cisplatin-induced allodynia
TLR4 signaling has been shown to play a role in a number of pain states including chemotherapy-induced polyneuropathies [Li et al., 2014;Park et al., 2014]. However, it is, again, important to note that TLR4 signaling has been reported to differ in male and female mice in mononeuropathies [Sorge et al., 2011; Stokes et al., 2013]. Thus, we examined whether male and female Tlr4−/− mice respond similarly to the polyneuropathic pain state initiated by cisplatin treatment. As shown previously in male mice, we found that there was no development of allodynia in male Tlr4−/− mice (Figure 3C). There was only one testing point (Day 21) when thresholds were significantly different from baseline (p < .05). Similarly, and in contrast to the previous work with a mononeuropathy, female mice did not develop tactile allodynia (Figure 3D), but do show one testing point (Day 21) that is lower than baseline. In a repeated measures ANOVA, there was a significant main effect of time [F (9, 63) = 2.873, p < .05]. No other results approached significance.
TRIF signaling plays a distinct role in male and female animals
All TLRs signal through either a TRIF-dependent or MyD88-dependent pathway, with the exception of TLR4, which uses both pathways (Figure 1A). Given a deficiency in TLR4completely prevents the development of cisplatin-induced polyneuropathy in either sex, we examined the role of each adaptor protein. First, we assessed the role of TRIF signaling. Tactile allodynia in male Triflps2 mice began to develop as soon as 48-hours after cisplatin treatment (Figure 3E). Thresholds continued to fall over the course of treatment and remained significantly lowered through D23, indicating the development of a persistent tactile allodynia (p < .05).
Female Triflps2 mice showed a significant decrease in threshold from Day 4 of treatment through Day 21 of testing (Figure 3F; p < .05). However, they remained significantly higher than male Triflps2 mice. These results were confirmed with an ANOVA yielding a significant main effect of sex [F (1,13) = 7.34, p < .05], and a significant main effect of time [F (9, 117) = 8.33, p < .05].
MyD88 deficient mice show reduced development of cisplatin-induced tactile allodynia
When looking solely at the role of MyD88-dependent signaling, Park et al. (2014) found male mice deficient in MyD88 signaling exhibit slightly increased mechanical reactivity during cisplatin treatment, returning to baseline by day 30. In the present study, we found male Myd88−/− mice develop a slightly reduced tactile allodynia during the period of cisplatin administration, which does not change after cessation of treatment (Figure 3G). Similarly, female Myd88−/− mice do not show a significantly different threshold from their male counterparts (Figure 3H; p >.05), nor any difference from their baseline reactivity (p > .05).
Male and female Myd88−/−/Triflps2 mice are protected from the development of tactile allodynia following cisplatin treatment
As noted previously, all TLRs signal through MyD88 or TRIF. Male Myd88−/−/Triflps2 mice treated with cisplatin do not differ from Myd88−/−/Triflps2 mice treated with saline in mechanical reactivity (Park et al., 2014). We replicated this result in male and female mice, showing no development of tactile allodynia in response to cisplatin treatment (p >.05), and no significant sex differences (p >.05), indicating a crucial role of TLRs in the development of this pain (Figure 3I and J).
Male and female Tlr3−/− mice are protected from the development of tactile allodynia following cisplatin treatment
The TRIF pathway plays a distinct role in the development of cisplatin-induced neuropathy as demonstrated above. Both TLR4 and TLR3 utilize the TRIF pathway. We examined the contribution that TLR3 might play in TRIF signaling in this model. Mice deficient in TLR3 signaling are protected from the development of tactile allodynia (Figure 3K and L). Neither group showed any difference from their baseline reactivity (p >.05).
Weights
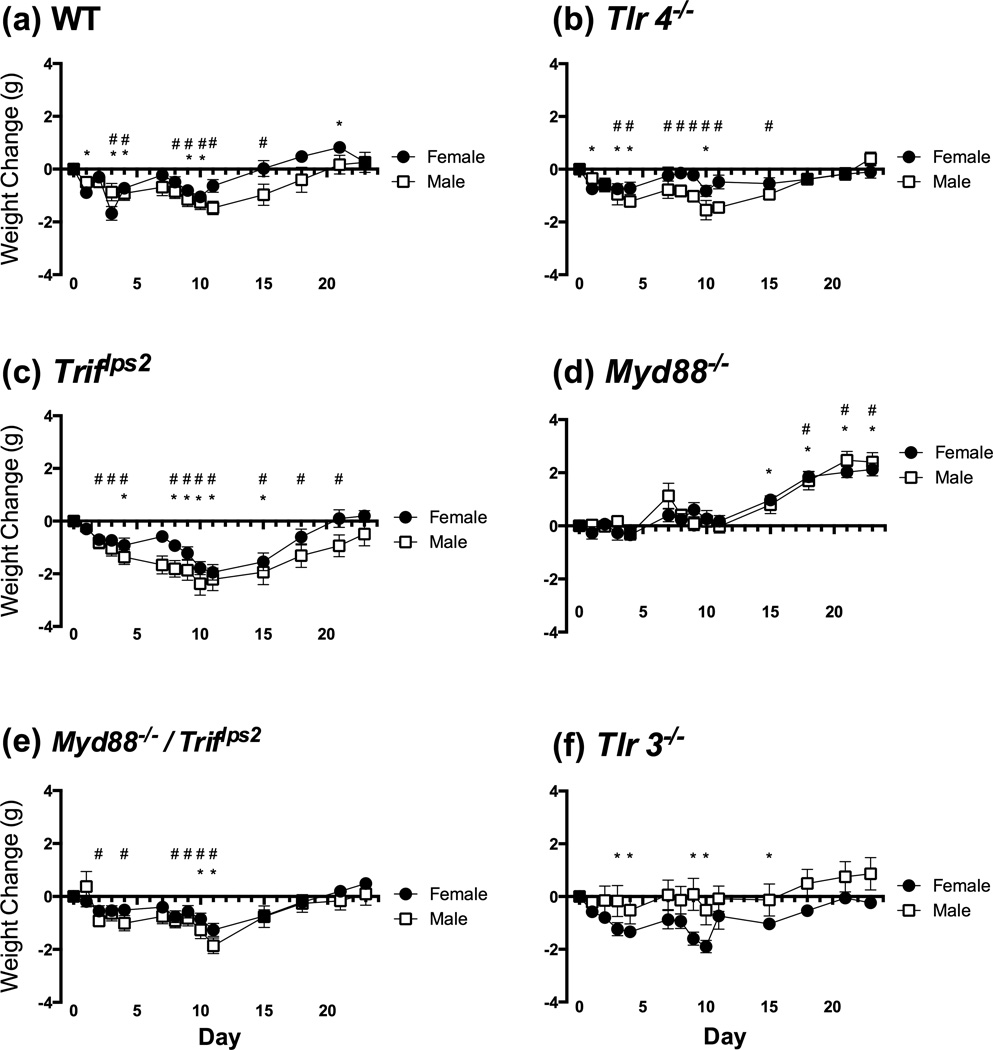
Despite a lack of tactile allodynia in some of the groups, lack of normal weight gain or a frank weight loss indicated biological activity of the drug (Figure 4A–F). Weight was monitored throughout the 23-day testing. As indicated above there were differences in baseline weights between the males and the females, where the males were larger (Figure 2A). However, there were no sex-related differences in weight change (p >.05). Both male and female WT mice (a) lose weight relative to baseline during the period of cisplatin treatment, with females recovering and even gaining weight. Tlr4−/− (b), Triflps2 (c), Triflps2/Myd88−/− (e), and Tlr3−/− (f) mice all lost weight during treatment, and returned to baseline weight by the end of testing. Myd88−/− (d) mice were the only group that did not lose weight and, instead, gained a significant amount of weight (p < .05).
Figure 4.
Changes in weight have been used to assess the biological activity and general state of health of rodents following drug treatments. We found that mice of both sexes (a–c, e–f) lost weight as a result of cisplatin treatment, except Myd88−/− mice (d). * = p < .05 in female mice, relative to their baseline; # = p <.05 in male mice, relative to their baseline
Gabapentin transiently reverses established tactile allodynia
Tactile allodynia, in the time post cisplatin treatment, has been shown to have characteristics that resemble neuropathic pain [Park et al., 2013]. In the present study, we examined whether gabapentin, a drug effective in treating neuropathic pain, was effective in reversing established allodynia on Day 18. In those groups exhibiting signs of tactile allodynia gabapentin was effective in transiently reversing the allodynia (Figure 5A–B). This was confirmed by a main effect of time [Male: F (1, 26) = 31.27; Female: F (1, 27) = 14.98, p’s<.05] and Bonferroni post hocs (p<.05).
Figure 5.
Gabapentin has been shown to be effective in transiently reducing cisplatin-induced tactile allodynia. In the present experiment, mice were given a single injection (i.p.) of gabapentin (100 mg/kg) on Day 18 of testing. In those animals, both male (a) and female (b), experiencing tactile allodynia, gabapentin was effective in transiently reversing established tactile allodynia. * = p < .05
Discussion
In the clinical population, a subset of males and females report prominent dysesthesias and hypersensitivities following treatment with chemotherapeutics. The present work sought to define mechanisms related to the polyneuropathy in males and females.
Sex differences in development of cisplatin induced polyneuropathy in mice
In preclinical work, it has previously been reported that male rodents develop a persistent tactile allodynia [Zhang et al., 2012; Park et al., 2013; Park et al., 2014; Robinson et al., 2014; Yoon et al., 2014], and an associated aversive state as measured by the place preference paradigm [Park et al., 2013] following treatment with a variety of chemotherapeutics, but there is little data in female animals. Here, we replicated previous results showing a persistent allodynia in male WT mice treated with cisplatin. In female WT mice, we found that these mice developed an allodynia, which unexpectedly showed reversal over time. This result was particularly interesting and unexpected as, such sex differences have not been reported in humans, and, clinically, females have a higher incidence of chronic pain conditions[LeResche, 1999]. Often, the discrepancy in chronic pain conditions between sexes is attributed to differential pain threshold and sex hormones, among others [Mogil, 2012]. The present experiments do not provide evidence as to the mechanisms underlying these sex differences in the propensity to develop a persistent allodynia after cisplatin. It cannot be excluded that the differences in allodynia between male and female mice was associated with the lower absolute dose of cisplatin that the females received because they were lighter. However, as the baseline pain measures of mechanical reactivity between wild type male and female mice did not differ, and females showed a decrease in threshold during the period of cisplatin treatment commensurate with males, these results suggest that the female mice are not transitioning to a chronic pain state. This anomalous difference between the sexes provides an avenue to gain further insight into why a subset of the human population develops chronic pain.
Role of TLR4 in persistent pain
We have previously shown that TLR4 signaling mediates development of a persistent allodynia in the face of a transient tissue inflammation, e.g. a transition from an acute to a chronic pain state [Christianson et al., 2011]. In a mononeuropathy, TLR4 KO significantly attenuated the nerve injury induced allodynia in males, but not females [Sorge et al., 2011; Stokes etal., 2013]. These results raised the question of whether this sex difference could be generalized to the chemotherapy-induced polyneuropathy. In addition to assessing sex differences in the persistence of the cisplatin polyneuropathic pain state in the WT mice, we examined the role of TLR4 and innate immune signaling in the development of pain following cisplatin treatment. In the present work with cisplatin, we observed, in this model, that both male and female mice deficient in TLR4 signaling robustly fail to develop any change in sensory reactivity in response to cisplatin treatment. This demonstrates TLR4 activation is necessary for the development of cisplatin-induced tactile allodynia in both male and female mice, and is not just affecting a transition to chronic pain.
TLR signaling
TLR4 is unique in that it uses both MyD88- and Trif-dependent pathways in signaling. MyD88-dependent signaling results in the activation of NFκB and the increased release of pro-inflammatory cytokines and chemokines [Palsson-McDermott and O’Neill, 2004; O’Neill and Bowie, 2007], while Trif-dependent signaling results in the production of IFNβand a delayed activation of NFκB resulting in induction of pro-inflammatory cytokines. To gain further insight into potential mechanistic differences between male and female mice, we examined the development of pain in both male and female mice lacking these adaptor proteins. In MyD88 deficient animals, we found a significantly reduced tactile allodynia in female, but not male mice. We found a similar pattern in the TRIF deficient animals – the development of allodynia was significantly reduced in female, but not male mice. However, when animals are deficient in both MyD88 and TRIF signaling, neither group develops pain behavior. Together, these results suggest that both MyD88 and TRIF signaling contribute to the development of tactile allodynia in female mice, and to the persistent pain seen in male mice.
TRIF and MyD88 signaling pathways have been the focus of recent chemotherapeutic-induced polyneuropathy research, yielding similar results [Li et al., 2014; Liu et al., 2014]. Other data suggest that the specific deletion of MyD88 in nociceptors expressing Nav1.8 causes a decrease in the pain phenotype [Liu et al., 2014]. However, MyD88 deletion does not completely prevent the pain, suggesting an alternate pathway, such as TRIF signaling, may also be contributing. This is confirmed by our data showing TLR3 KO mice also do not develop mechanical allodynia in response to cisplatin administration. Importantly, in previous work we showed that TLRKO reduces the hyperpathia associated with mononeuropathy. The specific involvement of each of these pathways requires further examination.
Concluding comments
The role of the TLR4 and the downstream signaling cascades in the chemotherapeutic induced polyneuropathy has three interesting implications. 1) The effects of the TLR4 mutation on the development of pain emphasize the potential role of endogenous TLR4 ligands. We note that chemotherapeutics can initiate the release of damage associated molecular pattern (DAMP) protein, such as HSP90 or HMGB1 that can activate TLR4 [Garg et al., 2010; Tesniere et al., 2010; Fang et al., 2014] and these agents have been implicated in the development of neuropathic states [Shibasaki et al., 2010; Feldman et al., 2012; Maeda et al., 2013; Agalave et al., 2014; Agalave and Svensson, 2014]. 2) These studies raise the likelihood that targeting these cascades may have therapeutic utility. Administration of LPS-RS (i.t.) was shown to partially block the development of, and transiently reverse, CIPN in rats [Li et al., 2014] and the transition of the acute to chronic pain states in persistent inflammatory models in mice [Christianson et al., 2011]. Treatment with a MyD88 inhibitor also ameliorated the chemotherapeutic pain state [Li et al., 2014]. The utility of these approaches may depend upon the role of these components of innate immunity in suppressing the immune response to tumor and limit their utility in persistent pain states present after dealing with the cancer. 3) These results emphasize that male and female mice should be examined in various models of chronic pain for potential differences in pain phenotype and further emphasizes the mechanistic heterogeneity of these nerve injury pain states. Thus, TLR4 displays sex differences in mono, but not polyneuropathies. In this regard, the difference between wild type male and female mice noted here provides an avenue to examine differences in signaling that protect the female mice from the development of persistent pain. This may lead to new insight into therapeutic interventions for the clinical population.
What is already known about this topic?
Male mice develop a persistent tactile allodynia after treatment with various chemotherapeutics, including cisplatin.
Toll-like receptor signaling differentially regulates this pain.
What does this study add?
In the present study, we examined the development of cisplatin-induced polyneuropathy in both male and female mice, finding that they do not show the same pattern of tactile allodynia.
Signaling downstream of TLRs differentially regulates pain in male and female mice.
TLR4 signaling differentially affects male and female animals in a mononeuropathy, but not in this polyneuropathy model, suggesting that sex differences should be further examined in multiple models.
Acknowledgments
Funding Sources: This work was supported by NS16541 (TLY), DA02110 (TLY), T32 AR 64194-2 (SAW) and AR062236-02 (MC).
Footnotes
Conflict of Interest: None
Author Contributions:
Sarah A. Woller contributed to the design of the study, conducted the experiments, analyzed the data, and wrote the manuscript.
Maripat Corr helped with designing the study, analyzing the data, and writing the manuscript.
Tony L. Yaksh helped with designing the study, analyzing the data, and writing the manuscript.
All authors have seen the original data, reviewed analysis of the data, and approved the final manuscript.
References
- Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundbäck P, Palmblad K, Andersson U, Harris H, Svensson CI. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain. 2014;155(9):1802–1813. doi: 10.1016/j.pain.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Agalave NM, Svensson CI. Extracellular HMGB1 as a mediator of persistent pain. Mol Med. 2014 doi: 10.2119/molmed.2014.00176. [Epub Ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 2011;152(12):2881–2891. doi: 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugaard GK, Petrera J, Trojaborg W. Electrophysiological study of the peripheral and central neurotoxic effect of cisplatin. Acta Neurol Scand. 1987;76(2):86–93. doi: 10.1111/j.1600-0404.1987.tb03551.x. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Symptom Manage. 2007;33(2):166–179. doi: 10.1016/j.jpainsymman.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Fang H, Ang B, Xu X, Huang X, Wu Y, Sun Y, Wang W, Li N, Cao X, Wan T. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell Mol Immunol. 2014;11(2):150–159. doi: 10.1038/cmi.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman P, Due MR, Ripsch MS, Khanna R, White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation. 2012;9:180. doi: 10.1186/1742-2094-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Imunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Han Y, Smith MT. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN) Front Pharmacol. 2013;4:156. doi: 10.3389/fphar.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeResche L. Gender considerations in the epidemiology of chronic pain. In: Crombie IK, editor. Epidemology of Pain. Seattle: IASP Press; 1999. pp. 43–52. Print. [Google Scholar]
- Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to paclitaxel-induced peripheral neuropathy. J Pain. 2014 doi: 10.1016/j.jpain.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, Zhang Y, Liu T, Xu ZZ, Park cK, Berta T, Jiang D, Ji RR. Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 2014 doi: 10.1038/cr.2014.106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Ozaki M, Kobayashi Y, Kiguchi N, Kishioka S. HMGB1 as a potential therapeutic target for neuropathic pain. J Pharmacol Sci. 2013;123(4):301–305. doi: 10.1254/jphs.13r08cp. [DOI] [PubMed] [Google Scholar]
- Markman M. Intraperitoneal chemotherapy in the treatment of ovarian cancer. Ann Med. 1996;28(4):293–296. doi: 10.3109/07853899608999082. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. 2014;22(8):2261–2269. doi: 10.1007/s00520-014-2255-7. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Stokes JA, Corr M, Yaksh TL. Toll-like receptor signaling regulates cisplatin-induced mechanical allodynia in mice. Cancer Chemother Pharmacol. 2014;73(1):25–34. doi: 10.1007/s00280-013-2304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth Analg. 2013;116(1):224–231. doi: 10.1213/ANE.0b013e31826e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CR, Zhang J, Dougherty PM. Altered discharges of spinal neurons parallel the behavioral phenotype shown by rats with bortezomib related chemotherapy induced peripheral neuropathy. Brain Res. 2014;1574:6–13. doi: 10.1016/j.brainres.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M, Sasaki M, Miura M, Mizukoshi K, Ueno H, Hashimoto S, Tanaka Y, Amaya F. Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain. 2010;149(3):514–521. doi: 10.1016/j.pain.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31(43):15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11(6):629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL. Toll-like receptor signaling adaper proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J Neuroinflammation. 2013;10:148. doi: 10.1186/1742-2094-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesniere A, Schiemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Varault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- Tofthagen C, Donovan KA, Morgan MA, Shibata D, Yeh Y. Oxaliplatin-induced peripheral neuropathy’s effects on health-related quality of life of colorectal cancer survivors. Support Care Cancer. 2013;21(12):3307–3313. doi: 10.1007/s00520-013-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S-Y, Robinson CR, Zhang H, Dougherty PM. Spinal astrocyte gap junctions contribute to oxaliplatin-induced mechanical hypersensitivity. J Pain. 2013;14(2):205–214. doi: 10.1016/j.jpain.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yoon S-Y, Zhang H, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of paclitaxel-induced painful neuropathy. J Pain. 2012;13(3):293–303. doi: 10.1016/j.jpain.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]