Abstract
Acenaphthene and acenaphthylene, two known environmental polycyclic aromatic hydrocarbon (PAH) pollutants, were incubated at 50 µM concentrations in a standard reaction mixture with human P450s 2A6, 2A13, 1B1, 1A2, 2C9, and 3A4 and the oxidation products were determined using HPLC and LC-MS. HPLC analysis showed that P450 2A6 converted acenaphthene and acenaphthylene to several mono- and di-oxygenated products. LC-MS analysis of acenaphthene oxidation by P450s indicated the formation of 1-acenaphthenol as a major product, with turnover rates of 6.7, 4.5, and 3.6 nmol product formed/min/nmol P450 for P450 2A6, 2A13, and 1B1, respectively. Acenaphthylene oxidation by P450 2A6 showed the formation of 1,2-epoxyacenaphthene as a major product (4.4 nmol epoxide formed/min/nmol P450) and also several mono- and di-oxygenated products. P450 2A13, 1B1, 1A2, 2C9, and 3A4 formed 1,2-epoxyacenaphthene at rates of 0.18, 5.3 2.4, 0.16, and 3.8 nmol/min nmol P450, respectively. 1-Acenaphthenol, which induced Type I binding spectra with P450 2A13, was further oxidized by P450 2A13 but not P450 2A6. 1,2-Epoxyacenaphthene induced Type I binding spectra with P450 2A6 and 2A13 (Ks 1.8 and 0.16 µM, respectively) and was also oxidized to several oxidation products by these P450s. Molecular docking analysis suggested different orientations of acenaphthene, acenaphthylene, 1-acenaphthenol, and 1,2-epoxyacenaphthene in their interactions with P450 2A6 and 2A13. Neither these four PAHs induced umu gene expression in a Salmonella typhimurium NM tester strain. These results suggest, for the first time, that acenaphthene and acenaphthylene are oxidized by human P450s 2A6 and 2A13 and other P450s to form several mono- and di-oxygenated products. The results are of use in considering the biological and toxicological significance of these environmental PAHs in humans.
INTRODUCTION
Acenaphthene and acenaphthylene (Figure 1), two known environmental polycyclic aromatic hydrocarbon (PAH)s contaminants, have been identified in coal tar,1,2 tobacco smoke,3 organic ground water,4 air particles (PM10),5 and commercial waterpipe charcoal.6 It has been reported that among 16 PAHs determined to date, acenaphthene and acenaphthylene show daily intake in population diet studies in humans6,7 and have been detected in human milk.7,8
Figure 1.
Structures of acenaphthene and acenaphthylene
Acenaphthene and acenaphthylene (Figure 1) have been reported to be non-mutagenic in Salmonella typhimurium TA1537 and TA1538 tester strains9 and non-cytotoxic in human liver tumor cell lines and HepG2 in vitro.10 However, it has also been reported that acenaphthene and acenaphthylene are weakly mutagenic in photomutageniesis assays with S. typhimurium TA12011 and that acenaphthylene is mutagenic in a S. typhimurium TM677 strain test sytem using postmitochondrial supernatant of livers prepared from phenobarbital-treated rat .12 The International Agency for Research on Cancer13 categorizes acenaphthene in its Group 3 chemicals (”non-classifiable as to carcinogenicity to humans”).
Studies with bacterial and fungal enzymes have shown that these two compounds are oxidized by P450s and other enzymes,14,15,16 but little is known about the metabolism of acenaphthene and acenaphthylene with mammalian xenobiotic-metabolizing enzymes. Schocken and Gibson14 reported that acenaphthene and acenaphthylene were oxidized into several products by crude enzymes in Beijerinkia sp. and a mutant strain. A triple mutant (A74G/F87V/L188Q) of Bacillus megaterium P450 BM-3 (P450 102A1) oxidized acenaphthene to 1-acenaphthenol and acenaphthylene to unknown products.15 The fungus Cunninghamella elegans formed several oxidative metabolites.16
We have shown recently that acenaphthene and acenaphthylene induce Type I binding spectra with and inhibit coumarin 7-hydroxylation activities catalyzed by P450s 2A6 and 2A13.17 These results suggest possible roles of these P450s in catalyzing metabolism of acenaphthene and acenaphthylene in humans.
In this study, we studied the oxidative metabolism of acenaphthene and acenaphthylene using purified human P450s or bicistronic human P450 co-expressed with human NADPH-P450 reductase in the membranes of Escherichia coli. In our earlier studies, these chemicals were not extracted quantitatively with the organic solvents used for analysis with HPLC (i.e., CH2Cl2 and ethyl acetate),17 Accordingly, we changed our extraction procedures prior to HPLC and LC-MS. The enzymes examined in this study included human P450s 2A6, 2A13, 1B1, 1A2, 2C9, and 3A4. Spectral interactions of acenaphthene and acenaphthylene (and the metabolites 1-acenaphthenol and 1,2-epoxyacenaphthene) were determined with purified human P450 enzymes, and molecular docking simulations of interaction of acenaphthene and acenaphthylene with P450 2A6 and 2A13 are also reported.
EXPERIMENTAL PROCEDURES
Chemicals
Acenaphthene, acenaphthylene, and 1-acenaphthenol were obtained from SigmaAldrich (St. Louis, MO) or Wako Pure Chemical (Osaka).
1,2-Epoxyacenaphthene (1,2-acenaphthenyl oxide) was prepared by the stepwise reaction of acenaphthylene with two molar equivalents of 3-chloroperbenzoic acid (previously washed with base and dried to remove 3-chlorobenzoic acid) in a CH2Cl2-phosphate buffered system:18 79% yield, MS (atmospheric pressure chemical ionization, APCI+) 169.0 (MH+); UV (CH3OH) ε218 32,000 M−1 cm−1, ε288 3,650 M−1 cm−1; 1H-NMR (CDCl3, 600 MHz) δ 4.90 (s, 2H, H-1, -2), 7.47 (dd, 2H, H-4, H-7, J = 6.8, 8.3 Hz), 7.69 (d, 2H, H-3, H-8, J = 6.8 Hz), 7.75 (d, 2H, H-5, H-6, J = 6.8, 8.3 Hz); 13C-NMR (CDCl3, 125 MHz) 58.7 (C-1, C-2), 122.9 (C-5, C-6), 126.3 (C-4, C-7), 127.3 (C-3, C-8), 138.2 (C-2a, C-8a), 138.7 (C-4a, C-8b).
Other chemicals and reagents used in this study were obtained from the sources described previously or were of the highest quality commercially available.19-21
Enzymes
Expression and purification of P450 2A6 and 2A13 enzymes were described previously.17,20 Bacterial “bicistronic” P450s 2A13, 2A6, 1B1, 1A2, 2C9, and 3A4 in membranes co-expressing human NADPH-P450 reductase were prepared and the E. coli membranes were suspended in 10 mM Tris-HCl buffer (pH 7.4) containing 1.0 mM EDTA and 20% glycerol (v/v) as described.19-21 P450s 2A13, 2A6, 1B1, 1A2, 2C9, 3A4, NADPH-P450 reductase, and cytochrome b5 (b5) were purified from membranes of recombinant E. coli as described elsewhere.17,20 Anti-rat P450 2A1 was prepared in rabbits as described previously, and this antibody has previously been shown to inhibit human P450 2A6 activities.22
Spectral Binding Titrations
Purified P450 enzymes were diluted to 1.0 µM in 0.10 M potassium phosphate buffer (pH 7.4) containing 20% glycerol (v/v), and binding spectra were recorded with subsequent additions of chemicals in a JASCO V-550 (JASCO, Tokyo, Japan) or OLIS-Aminco DW2a spectrophotometer (On-Line Instrument Systems, Bogart, GA) as described previously.17.21 Spectral dissociation constants (Ks) were estimated using GraphPad Prism software (GraphPad Software, San Diego, CA), either using hyperbolic plots or quadratic fits for tight binding.
Genotoxicity Assay
Genotoxic activities of acenaphthene, acenaphthylene, 1-acenaphthenol, and 1,2-epoxyacenaphthene were determined by measuring induction of umu gene expression in the tester strain S. typhimurium NM2009 as described previously.19 Standard incubation mixtures contained these chemicals at final concentrations of 0, 2.5, 5, 10, 20, 40, 80, and 160 µM in 1 mL of bacterial suspension. The induction of umu gene expression was monitored by measuring β-galactosidase activity, using o-nitrophenyl-β-D-galactopyranoside as a substrate, and is presented as units of β-galactosidase activity mL−1.
Oxidation of Acenaphthene and Acenaphthylene
Oxidative metabolism of acenaphthene and acenaphthylene by P450 enzymes was determined in a standard incubation mixture (0.50 mL) containing 50 pmol of bicistronic P450 (in E. coli membranes co-expressing human NADPH-P450 reductase), 50 µM chemical, and an NADPH-generating system.17,20 (The chemicals were dissolved in (CH3)2SO as 10 mM stock solutions and diluted, with the final solvent concentration ≤0.5%, v/v.). In reconstitution experiments, P450 membranes were replaced by purified P450 (50 pmol), NADPH-P450 reductase (100 pmol), and b5 (100 pmol) (in cases when P450 2A6, 2C9, and 3A4 were used), and L-α-dilauroyl-syn-glycero-3-phosphocholine (50 µg) as described previously.23,24 Incubation was carried out at 37 °C, following a preincubation time of 1 min. Reactions were terminated by adding 1.0 mL of CH3OH, and the mixtures were centrifuged at 2,000 × g for 5 min. The supernatant was subjected to filtration using a Disposable Syringe Filter (Iwaki, Osaka, Japan; Cellulose Acetate Membrane, 3 mm × 0.20 µm; Code 2012-003) and the filtrates (10 µL) were used for analysis by HPLC and LC-MS.
HPLC separation was done with a JASCO system equiped with a Wakopack Navi C18-5 octadecylsilane column (2.0 mm × 150 mm) (Wako Pure Chem.), with UV detection at 254 nm and fluorometric detection using an excitation wavelength of 242 nm and emission wavelength of 380 nm. Elution of PAHs and their metabolites utilized a linear gradient from 20% CH3OH/80% H2O (v/v) to 100% CH3OH over 25 min and then at 100% CH3OH for 5 min, with a flow rate of 0.2 mL/min.
LC-MS and MS/MS Analysis for Identification of Product Formation
The separation of products was done with an LCMS IT-TOF mass spectrometer equiped with an APCI/atmospheric pressure photoionization (APPI) ion source (Shimadzu, Kyoto, Japan). The metabolite was loaded on an Inert Sustain C18 octadecylsilane column (3 µm, 2.0 mm × 100 mm; GL Science, Tokyo, Japan) equilibrated with mobile phase A (H2O containing 5 mM NH4CH3CO2 and 0.02% HCO2H, w/v) and CH3OH. The elution program was kept at 20% CH3OH for 2 min, increased to 100% B in 6 min, then kept at 100 % B for 3 min, with a flow rate of 0.2 mL/min. Both positive and negative ions were detected with the same chromatographic system, using the high-speed polarity switching function of the instrument. MS/MS analysis was done in the data-dependent mode.
Docking Simulations into Human P450 Enzymes
Crystal structures of P450s 2A625 and 2A1326 have been reported and were used for the analysis. Simulations were carried out for P450 enzymes using the MMFF94x force field described in the MOE software (ver. 2013.10, Computing Group, Montreal, Canada), as described above.17,20 Lower U values (ligand-interaction energy) are an indication of higher degree of interaction between a chemical and the enzyme.
Kinetic Analysis
Kinetic parameters were estimated by nonlinear regression analysis using the program Kaleida-Graph (Synergy Software, Reading, PA) or Graphpad Prism.
RESULTS
HPLC Analysis of Oxidation of Acenaphthene and Acenaphthylene by P450 2A6
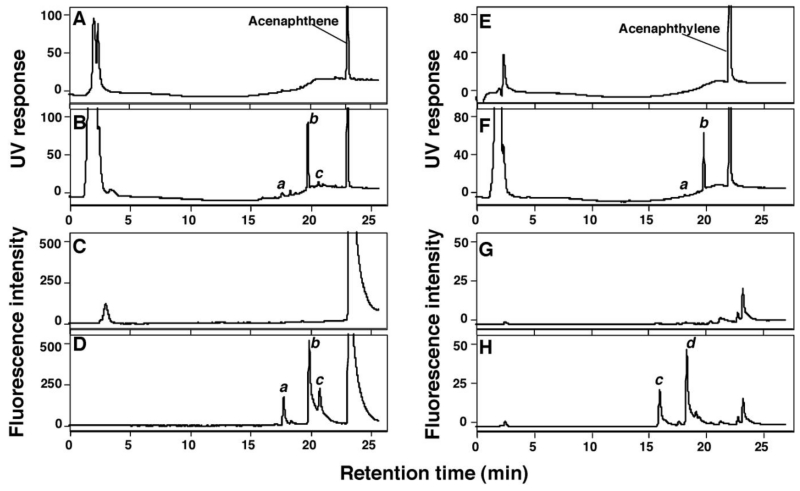
We previously showed that both acenaphthene and acenaphthylene induce Type I binding spectra with P450 2A6 and 2A13 and inhibit coumarin 7-hydroxylation activities catalyzed by P450 2A6 and 2A13.17 We first incubated acenaphthene and acenaphthylene at 50 µM substrate concentrations with 0.1 µM P450 2A6 (reconstituted with NADPH-P450 reductase) and the products formed were analyzed with HPLC (Figure 2). P450 2A6 produced three products of acenaphthene—a, b, and c—as judged by HPLC analysis (with UV and fluorescence detection) in the presence of an NADPH-generating system. Product b was later identified as 1-acenaphthenol by analysis with LC-MS (see below). Acenaphthylene was oxidized by P450 2A6 to one minor and one major product with UV detection (a and b, respectively, in Figure 2F) and two products with fluorescence detection (c and d in Figure 2H). Peak b was subsequently shown to be 1,2-epoxyacenaphthylene (vide infra).
Figure 2.
Oxidation of acenaphthene (A-D) and acenaphthylene (E-H) at 50 µM substrate concentration by purified P450 2A6 reconstituted with purified NADPH-P450 reductase and NADPH-P450 reductase in the presence (B, D, F, and H) and absence (A, C, E, and G) of an NADPH-generating system. Acenaphthene and the oxidation products, a, b, and c and acenaphthylene and the oxidative products a, b, c, and d were detected by HPLC using UV (254 nm) (A, B, E, and F) or fluorescence intensity (excitation wavelength at 242 nm and emission wavelength at 380 nm) (C, D, G, and H). Product b was identified as 1-acenaphthenol (see below).
Our preliminary data suggested that formation of oxidative products of acenaphthene and acenaphthylene by P450 2A6 increased lineally with increasing P450 concentration up to 0.2 µM.
Time course studies of the oxidation of acenaphthene (Supporting Information Figure S1A-S1F and S1G-S1L) and acenaphthylene (Supporting Information Figure S1M-S1R and S1S-S1X) were examined with purified P450 2A6 in a reconstituted monooxygenase system with incubation time. Increases in the formation of three products, a, b (1-acenaphthenol), and c, of acenaphthene (detected by UV and fluorescence) were found to be dependent on incubation time. Time course studies with the substrate acenaphthylene also showed increases in the formation of products a and b (1,2-epoxyacenaphthene) with UV and c and d with fluorescence detection (Supporting Information Figure S1). Increases in product formation with acenaphthene and acenaphthylene continued for about 30 min of incubation time (results not shown).
Role of P450 2A6 in the Oxidation of Acenaphthene and Acenaphthylene
P450 2A6 conversion of acenaphthene into three products (a, b, and c) in the presence of an NADPH-generating system showed an absolute requirement for NADPH-P450 reductase and partial requirement for b5 (Supporting Information, Figure S2). Similar results were obtained when acenaphthylene was used as a substrate (results not shown).
Formation of acenaphthene products a, b (1-acenaphthenol), and c was decreased with increasing concentrations of anti-P450 2A1 IgG22 (Supporting Information Figure S3). Anti-P450 2A1 caused accumulations of the parent compounds (substrates) in the incubation mixtures. Similar results were obtained when acenaphthylene was used (results not shown).
Oxidation of Acenaphthene and Acenaphthylene by Different Forms of Human P450 Enzymes
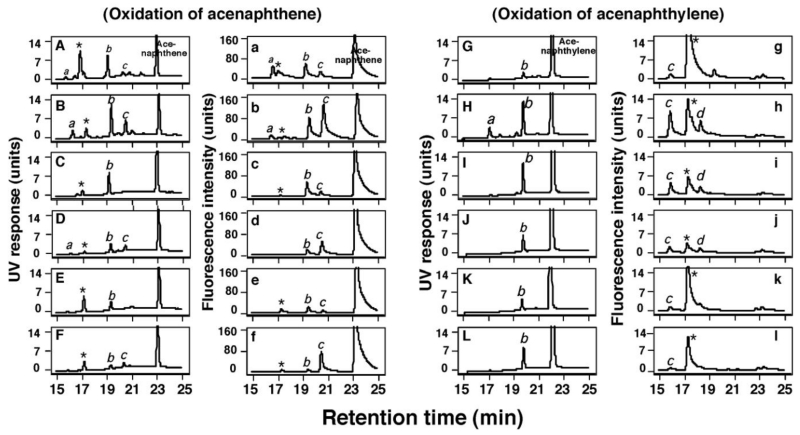
Six forms of bicistronic human P450—P450 2A13, 2A6, 1B1, 1A2, 2C9, and 3A4, expressed in bacterial membranes together with human NADPH-P450 reductase—were examined for catalytic activity in the oxidation of acenaphthene and acenaphthylene (Figure 3). In the experiments, single determination without and duplicate determinations with an NADPH-generating system were done to check the reproducibility of activities by each P450 enzyme, although only the results with an NADPH-generating system are shown in Figure 3. We also detected non-enzymatic peaks (denoted “*” in Figure 3), not seen in the experiments with a P450-containing reconstituted system (Figure 2 and Supporting Information Figure S1), but these contaminants (presumably derived from the membranes) did not interfere with measurement of product formation catalyzed by bicistronic P450s.
Figure 3.
Oxidation of acenaphthene (A-F and a-f) and acenaphthylene (G-L and g-l) by bicistronic membranes of E. coli expressing P450 2A13 (A and a and G and g), P450 2A6 (B and b and H and h), P450 1B1 (C and c and I and i), P450 1A2 (D and d and J and j), P450 2C9 (E and e and K and k), and P450 3A4 (F and f and L and l) together with NADPH-P450 reductase. Acenaphthene and acenaphthylene and their products were detected with HPLC using detection by UV (A-F and G-L) and fluorescence (a-f and g-l). The products are indicated a, b (1-acenaphthenol), and c for the oxidation of acenaphthene (A-F and a-l) and a, b (1,2-epoxyacenaphthene), c, and d for the oxidation of acenaphthylene (G-L and g-l); other peaks (indicating *) were found in the system in the absence of an NADPH-generating system.
Acenaphthene was oxidized by P450 2A6 (Figure 3B and 3b) and P450 2A13 (Figure 3A and 3a) to the products a, b (1-acenaphthenol), and c; the products a and c were less abundant with P450 2A13. P450 1B1 formed peak b (1-acenaphthenol), but was less active in forming products a and c (Figure 3C and 3c). P450s 1A2, 2C9, and 3A4 all oxidized acenaphthene at low rates to form products a, b (1-acenaphthenol), and c, as compared with P450 2A6 (Figure 3D-3F and 3d-3f).
P450 2A6 (Figure 3H and 3h) was also active in catalyzing acenaphthylene to form products a, b (1,2-epoxyacenapththene), c, and d, but P450 2A13 only slowly oxidized acenaphthylene (Figure 3G and 3g). P450s 1B1, 1A2, 2C9, and 3A4 produced 1,2-epoxyacenapththene at significant levels but formed only small amounts of products a, c, and d.
LC-MS Analysis of Oxidation of Acenaphthene by P450 2A13 and Acenaphthylene by P450 2A6
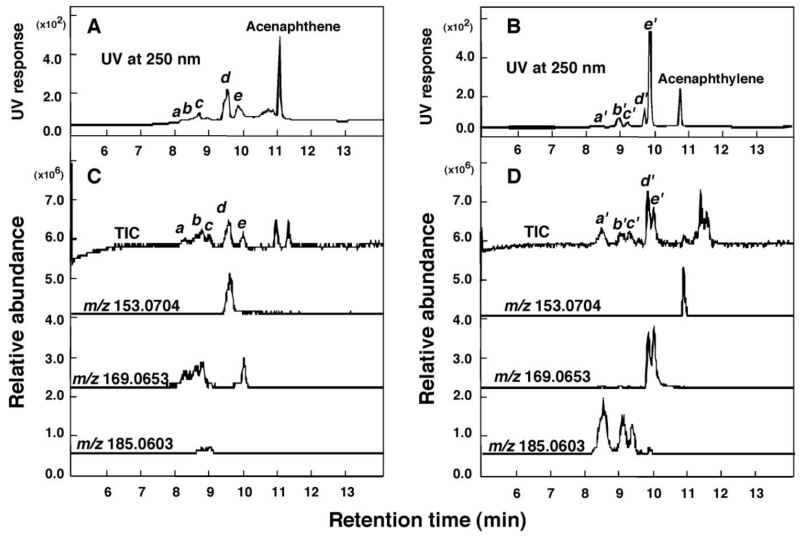
Acenaphthene and acenaphthylene were incubated with P450 2A13 and P450 2A6 (both in reconstituted monooxygenase systems), respectively, and the products formed were analyzed with LC-MS (Figure 4). At least five acenaphthene products formed with P450 2A13 were detected using UV absorbance (Figure 4A) and total ion current (TIC) (Figure 4C). Mass spectra of each of these peaks are shown in Supporting Information Figure S4. Peak d was identified by co-elution as 1-acenaphthenol (vide infra) with m/z 153.0707 (18 a.m.u. less than the expected molecular mass (cf. 171.0810 for C12H11O, 153.0704 expected for C12H9, Δ 2.0 ppm), indicating loss of H2O from the molecule during ionization (Figure 4C). Peaks a, b, and c, with peaks near the theo. m/z 169.0653 and 185.0603 for C12H9O and C12H9O2, respectively) are indicative of the formation of different mono- and di-oxygenated products (with H2O loss in the analysis) or loss of two hydrogen atoms.
Figure 4.
LC-MS analysis of oxidation of acenaphthene (A and C) and acenaphthylene (B and D) at 50µM substrate concentration by a reconstituted monooxygenase system containing P450 2A6 and NADPH-P450 reductase. Detection was by UV (250 nm), TIC, and reconstructed mass chromatograms in the positive ion mode (m/z 153.0783, 169.0653, and 185.0603). Peaks detected with UV and TIC are indicated a-e for acenaphthene products and a’-e’ for acenaphthylene products, and the mass spectra of these peaks were shown in Supporting Information Figures S4 and S5.
Peaks of P450 2A6 acenaphthylene products were observed using either UV or TIC on LC-MS analysis (Figure 4B and 4D, Supporting Information Figure S5)—peaks a’, b’, and c’ (monitoring m/z 185.0603 for C12H9O2 (di-oxygenated products) and peaks d’ and e’ (monitoring m/z 169.0653 for C12H9O (mono-oxygenated products) (Figure 4D). MS/MS analysis of m/z 169.0653 in peaks d’ and e’ showed different chemical structures of the mono-oxygenated products of acenaphthylene (Supporting Information Figure S5), and peak e’ in this series was identified as 1,2-epoxyacenaphthene (vide infra).
Identification of 1-Acenaphthenol and 1,2-Epoxyacenaphthene as Major Products of Acenaphthene and Acenaphthylene by P450s
In order to identify the oxidation products of acenaphthene and acenaphthylene, 1,2-epoxyacenaphthene was synthesized from 1-acenaphthylene, as described under Experimental Procedures, and used as a standard.
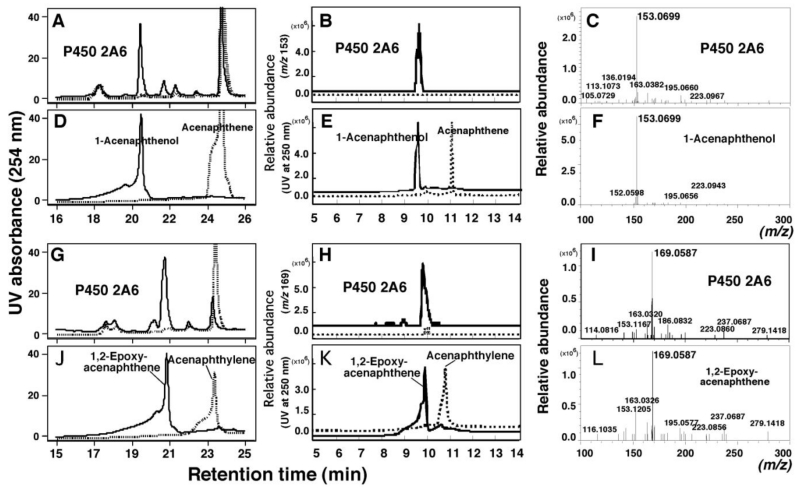
Bicistronic P450 2A6 membranes were incubated with acenaphthene and acenaphthylene and the products were examined using LC-UV and LC-MS, comparing the chromatograms with those of standard 1-acenaphthenol and 1,2-epoxyacenaphthene (Figure 5). LC chromatograms (Figure 5A) and mass spectra with m/z 153.0699 (Figure 5B and 5C), resulting from acenaphthene oxidation by P450 2A6, indicated the formation of 1-acenaphthenol as a major metabolite by comparison with those of standard acenaphthene and 1-acenaphthenol (Figure 5D, 5E, and 5F). Similarly, acenaphthylene oxidation by P450 2A6 showed the formation of 1,2-epoxyacenaphthene as a major product on analysis by LC (Figure 5G), with its mass spectrum with m/z 169.0587 (expected 169.0653 for C12H9O) (Figure 5H and 5I) and by comparison with those of standard acenaphthylene and 1,2-epoxyaxenaphthene (Figure 5J, 5K, and 5L). The co-incident elution and other properties clearly establish the product as 1,2-epoxyacenaphthene.
Figure 5.
HPLC and LC-MS analyses of oxidation of acenaphthene (A-F) and acenaphthylene (G-L) at 50µM substrate concentration by a bicistronic P450 2A6 system. HPLC analysis (by detecting at 254 nm) was carried out to determine the products of acenaphthene (A) and acenaphthylene (G) catalyzed by P450 2A6 in the presence (solid line) and absence (dotted line) of an NADPH-generating system. The standard acenaphthene and 1-acenaphthenol (D and E) and acenaphthylene and 1,2-epoxyacenaphthene (J and K) were analyzed using HPLC (D and J) and LC-MS (E and K). The same incubates were also analyzed with LC-MS at m/z 153.0783 (B) and 169.0649 (H) for detecting 1-acenaphthenol and 1,2-epoxyacenaphthene, respectively. MS analyses of a product of acenaphthene (C) and a 1-acenaphthenol standard (F) and of a product of acenaphthylene (I) and a 1,2-epoxyacenaphthene standard (L) were also determined.
Using authentic 1-acenaphthenol and 1,2-epoxyacenaphthene as standards, we detemined the formation of these products with six bicistronic P450 enzymes by both UV absorbance and MS (only the HPLC-UV results are presented) (Table 1). Formation of 1-acenaphthenol from acenaphthene was found to be rapid with P450 2A6, followed by P450 2A13, 1B1, and 1A2. Formation of 1,2-epoxyacenaphthene from acenaphthylene was much faster with P450 2A6, 1B1, 3A4, and 1A2 than with P450 2A13 and 2C9 (Table 1).
Table 1. Formation of 1-Acenaphthenol and 1.2-Epoxyacenaphthene from Oxidation of Acenaphthene and Acenaphthylene, respectively, by Human P450 Enzymes.
|
P450 |
oxidation of acenaphthene | oxidation of acenaphthylene |
|---|---|---|
| formation of 1-acenaphthenol (nmol/min/nmol P450) |
formation of 1,2-epoxyacenaphthene (nmol/min/nmol P450) |
|
| P450 2A13 | 4.5 ± 0.6 | 0.18 ± 0.03 |
| P450 2A6 | 6.7 ± 0.8 | 4.4 ± 0.3 |
| P450 1B1 | 3.6 ± 0.4 | 5.3 ± 0.6 |
| P450 1A2 | 1.5 ± 0.2 | 2.4 ± 0.3 |
| P450 2C9 | 0.81 ± 0.33 | 0.16 ± 0.03 |
| P450 3A4 | 0.39 ± 0.07 | 3.8 ± 0.4 |
Formation of 1-acenaphthenol from acenaphthene and formation of 1,2-epoxyacenaphthene from acenaphthylene by human P450 enzymes were determined with HPLC using a wavelength of 254 nm. Data are means ± SD (range) of duplicate determinations.
Further Oxidation of 1-Acenaphthenol and 1,2-Epoxyacenaphthene by P450 2A13 and 2A6
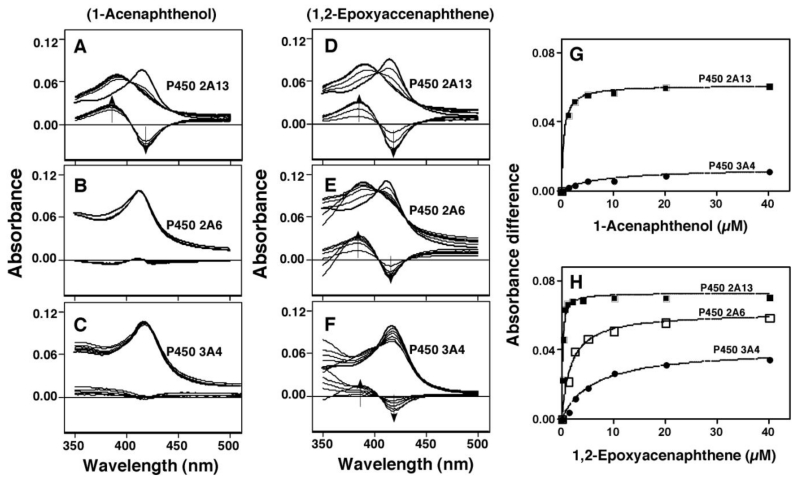
1,2-Epoxyacenaphthene was found to induce Type I binding spectra with P450 2A6, 2A13, and 3A4 with Ks values of 1.6. 0.16, and 6.2 µM, respectively (Figure 6). 1-Acenaphthenol also induced Type I binding spectra with P450 2A13 but it was very weak or undetectable with P450 2A6 and 3A4. P450 1B1, 1A2, and 2C9 did not show any spectral changes with these two chemicals. The spectral interactions of acenaphthene and acenaphthylene with P450 2A6 and 2A13 have previously been reported by our laboratories,17 and kinetic parameters for spectral interaction of acenaphthene, acenaphthylene, 1-acenaphthenol and 1,2-epoxyacenaphthene with P450 2A13 and 2A6 were compared with respect to Ks, ΔAmax, and ΔAmax /Ks values (Table 2).
Figure 6.
Spectral interaction of 1-acenaphthenol (A, B, and C) and 1,2-epoxyacenaphthene (D, E, and F) with P450 2A13 (A and D), 2A6 (B and E), and 3A4 (C and F). The P450 concentration used was 1 µM in 100 mM potassium phosphate buffer containing 20% gl;ycerol (v/v), and chemical concentrations used were 1.25-40 µM except that concentrations of 1,2-epoxyacenaphthene for P450 2A13 were 0.125. 0.25, 0.5, 1.0, 2.0, and 4.0 µM. Analysis of the interaction of 1-acenaphthenol (G) and 1,2-epoxyacenaphthene with P450s 2A13, 2A6, and 3A4 was determined with Graphpad Prism (Graphpad).
Table 2. Spectral Interaction of Acenaphthene, Acenaphthylene, 1-Acenaphthenol, and 1,2-Epoxyacenaphthene with P450s 2A6, 2A13, and 3A4a.
| P450 | ace- naphthene |
ace- naphthylene |
1-acenaphthenol | 1,2-epoxy- acenaphthene |
|
|---|---|---|---|---|---|
| P450 2A6 | Ks (μM) | 1.2±0.2 | 4.4±0.5 | ND | 1.78 ± 0.25 |
| ΔAmax | 0.066±0.002 | 0.076±0.002 | 0.062 ± 0.002 | ||
| ΔAmax /Ks | 0.055 | 0.017 | 0.035 | ||
| P450 2A13 | Ks (μM) | 0.48±0.10 | 0.61±0.21 | 0.48±0.03 | 0.16±0.03 |
| ΔAmax | 0.074±0.005 | 0.050±0.004 | 0.061±0.001 | 0.073 ± 0.002 | |
| ΔAmax / Ks | 0.15 | 0.081 | 0.13 | 0.46 | |
| P450 3A4 | Ks (μM) | 19±3.5 | 13±2.7 | 7.8 ± 2.2 | 6.20 ± 0.87 |
| ΔAmax | 0.040±0.003 | 0.030±0.002 | 0.013 ± 0.001 | 0.041 ± 0.002 | |
| ΔAmax / Ks | 0.002 | 0.002 | 0.002 | 0.007 |
The results for Ks and ΔAmax values indicate SE, obtained from fitting of hyperbolic plots in GraphPad Prism, and values for ΔAmax / Ks include further analysis of the SE of the quotients. ND, not detected even at concentration of 16 μM.
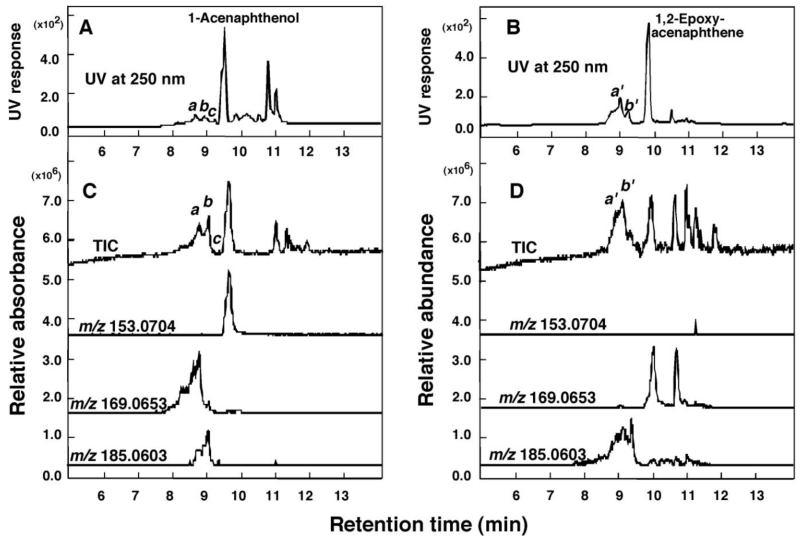
Because 1-acenaphthenol and 1,2-epoxyacenaphthene induced spectral interactions with P450 2A6 and 2A13, having different Ks values, we measured the oxidation of these chemicals by these P450 enzymes (Figure 7). 1-Acenaphthenol was oxidized by P450 2A13, but not by P450 2A6, to form different products with m/z 169.0704 and 185.0603, indicating further oxidation to form 2-electron-oxidized or mono-oxygenated products of 1-acenaphthenol by P450 2A13 (Figure 7A and 7C). 1,2-Epoxyacenaphthene was further oxidized by P450 2A6 (and by P450 2A13 to a small extent, results not shown) to form mono-oxygenated products with m/z 185.0603 (Figure 7B and 7D).
Figure 7.
Oxidation of 1-acenaphthenol (A) and 1,2-epoxyacenaphthene (B) at 50µM substrate concentration by a reconstituted monooxygenase system containing P450 2A13 or P450 2A6, respectively. Detection of oxidation products of 1-acenaphthenol and 1,2-epoxyacenaphthene was made using UV absorbance at 250 nm, TIC, and reconstructed chromatograms of m/z 153.0704, 168.0653, and 185.0903 (all in positive ion mode). Peaks obtained with UV and TIC were indicated a-d for 1-acenaphthenol products and a’ and b’ for 1,2-epoxyacenaphthene products and the mass spectra of these peaks are shown in Supporting Information Figure S6.
Docking Simulation of Interaction of Acenaphthene, Acenaphthylene, 1,2-Epoxyacenaphthene, and 1-Acenaphthenol with P450s 2A6 and 2A13
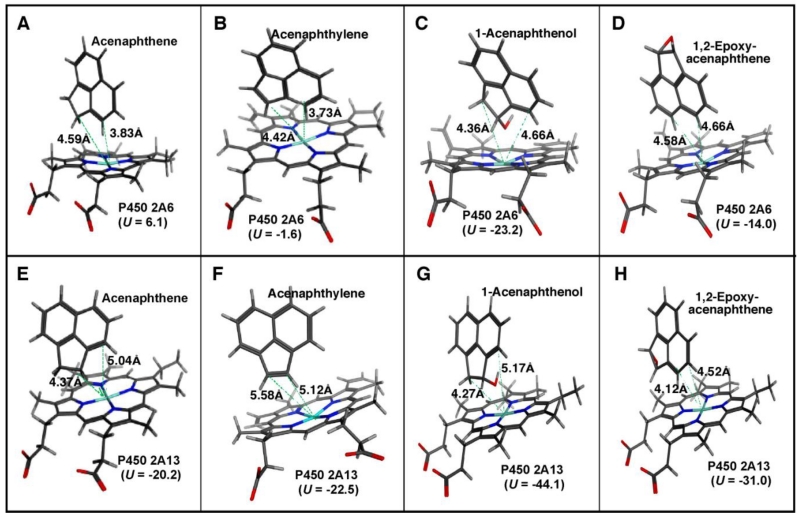
We selected P450s 2A6 and 2A13 to determine how acenaphthene, acenaphthylene, 1-acenaphthenol, and 1,2-epoxyacenaphthene interact with active sites of these P450 enzymes on molecular docking analysis (Figure 8). Interaction between acenaphthene and P450s 2A6 or 2A13 was relatively similar; in both cases C-2 and C-3 of acenaphthene oriented into the active site, but the U value was smaller in P450 2A13 than P450 2A6 (Figure 8A and 8E). Similarly, C-2 and C-3 positions of acenaphthylene faced into the active site of P450 2A6 heme, while C-1 and C-2 positions faced the P450 2A13 active site. The distances between C-2 and C-3 of acenaphthylene and the P450 2A6 heme iron were 4.42 and 3.73 Å, respectively, which were lower than those with P450 2A13 (Figure 8B and 8F). However, the U value was lower in P450 2A13 (U = −20.1) than P450 2A6 (U = −1.6).
Figure 8.
Docking simulation of interaction of acenaphthene (A and E), acenaphthylene (B and F), 1-acenaphthenol (C and G), and 1,2-epoxyacenaphthene (D and H) with P450 2A6 (A-D) and P450 2A13 (E-H).
1-Acenaphthenol, which was oxidized by and induced spectral change with P450 2A13 but not P450 2A6, interacted with the active sites of both P450 2A6 and 2A13 with similar but different orientations (Figure 8C and 8G). Interaction of 1,2-epoxyacenaphthene between P450 2A6 and 2A13 was very different; the C-5 and C-6 atoms of 1,2-epoxyacenaphthene were near the active site of P450 2A6 while C-3 and C-4 faced into the active site of P450 2A13.
Genotoxicity Asssays of Acenaphthene, Acenaphthylene, 1-Acenaphthenol, and 1,2-Epoxyacenaphthene in S. typhimurium
Acenaphthene, acenaphthylene, 1-acenaphthenol, and 1,2-epoxyacenaphthene were examined for their genotoxic activities in S. typhimurium strain NM2009,19 using concentrations of 0, 2.5, 5, 10, 20, 40, 80, and 160 µM. We found that none of these four chemicals showed any genotoxic activity, but they showed slight (10-20%) cytotoxic activities at concentrations of 80 and 160 µM, in this tester strain. We also examined metabolic activation of these chemicals with P450 2A6 and 2A13 in this tester strain and still found negative results with these compounds, although positive-control systems with P450 1B1 and benzo[a]pyrene-7,8-diol or other procarcinogens induced umu gene expression after metabolic activation (results not shown).
DISCUSSION
Among the various PAHs analyzed thus far, acenaphthene and acenaphthylene have been shown to be major contaminants in the environment, including coal tar, tobacco smoke, and ground water, and have been reported to be present in human milk.1-8 However, little is known about how these PAHs are transformed to biologically inert or active metabolites by xenobiotic-metabolizing enzymes, which is an important part of understanding their potential toxicity and carcinogenicity in humans.
We had previously examined the oxidative metabolism of acenaphthene and acenaphthylene by determining substrate disappearance with P450 2A13 and 2A6 and found that neither acenaphthene nor acenaphthylene was extracted quantitatively with organic solvents such as CH2Cl2 and ethyl acetate.17 In this study, we added CH3OH to the reaction mixtures and (after centrifugation and filtration) the fractions were subjected directly to HPLC analysis. Although several contaminants (as well as PAHs and their products) were found to be present in the aqueous methanolic solutions in reconstituted and bicistronic P450 enzyme systems, this method is applicable for use in determining product formation from acenaphthene and acenaphthylene with regard to reproducibility, speed, and simplicity.
The present results showed, for the first time, that acenaphthene and acenaphthylene are oxidized by human P450 enzymes to several mono- and di-oxygenated products on analysis with HPLC and LC-MS. On HPLC analysis, we detected three products—a, b, and c—from acenaphthene on incubation with P450 2A6 in a reconstituted system (Figures 2 and Supportive Information Figure S1). Product b, a major product, was identified as 1-acenaphthenol by LC-MS analysis (Figure 5). An m/z 153.0699 (positive ion mode) for 1-acenaphthenol was detected, which was 18 a.m.u. less than the exact molecular mass (171.0810), indicating the loss of water from the molecule (presuably dehydration to acenaphtylene in the source). Using 1-acenaphthenol as a standard, turnover numbers were calculated with six human P450 enzymes and we found that P450 2A13, 2A6, and 1B1 were efficient catalysts for this reaction. LC-MS analysis of acenaphthene products also showed the formation of other oxidized products (m/z 169.0653 and 185.0603), although the structures were not identified. Incubation of 1-acenaphthenol with P450 2A13, but not P450 2A6, produced further oxidation products. Acenaphthene was not oxidized to acenaphthylene by P450 enzymes in the present assay conditions.
HPLC analysis of acenaphthylene oxidation by P450 enzymes showed the formation of two products, a and b, with UV detection and two more products, c and d, with fluorescence detection. The major peak b was identified as 1,2-epoxyacenaphthene (Figure 5; also m/z 167.0500 in the negative ion mode on LC-MS analysis, data not presented); this 1,2-epoxide product was further oxidized by P450 2A6 (Figure 7). Other products (a, c, and d on HPLC) were not characterized in this study, but analysis with LC-MS indicated the formation of other mono- and di-oxygenated products with m/z 169.0653 and 185.0603, respectively.
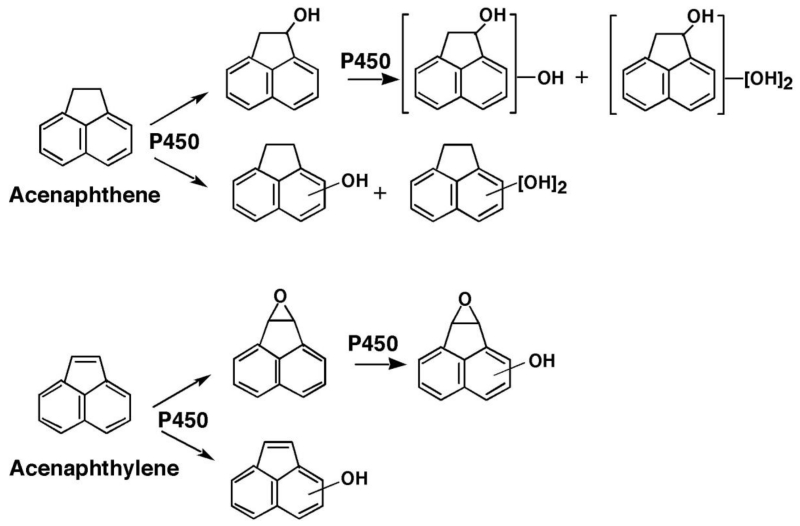
Pathways for acenaphthene metabolism by human P450 enzymes are proposed (Figure 9). Acenaphthene is first oxidized by P450s to form 1-acenaphthenol and other mono-oxygenated products by P450s 2A6, 2A13, 1B1, and 1A2 at higher rates than P450s 2C9, and 3A4. 1-Acenaphthenol and possibly other mono-oxygenated products appear to be further oxidized by P450 enzymes. The major product of acenaphthylene formed by P450s was identified as 1,2-epoxyacenaphthene, and the formation of this product was catalyzed by P450s 2A6, 1B1, 3A4, and 1A2 but not as well by P450 2A13 and 2C9. 1,2-Epoxyacenaphthene was further oxidized by P450 2A6, but not P450 2A13, to different oxidation products. Oxidation of 1,2-epoxyacenaphthene was faster with P450 2A6 than P450 2A13, while oxidation of 1-acenaphthenol was catalyzed by P450 2A13. 1-Acenaphthenol induced Type I binding spectra with P450 2A13, but not P450 2A6, supporting the role of oxidation of P450 2A13 in the reaction. Although 1,2-epoxyacenaphthene induced Type I binding spectra with P450 2A13 with a Ks value of 0.16 µM (~ 10-fold lower than that (1.8 µM) obtained with P450 2A6), the latter enzyme was more active than P450 2A13 in oxidizing 1,2-epoxyacenaphthene (indicating that spectral changes do not always relate to the rate of oxidation of 1,2-epoxyacenaphthene by P450 2A6 and 2A13).
Figure 9.
Proposed pathways for the oxidation of acenaphthene and acenaphthylene by human P450 enzymes.
Enzymes from the fungus C. elegans16 and the bacteria Beijerinckia sp.14 and B. megaterium15 oxidize acenaphthene to 1-acenaphthenol and then to several dioxygenated and other metabolites including cis- and trans-1,2-dihydroxy-acenaphthene, 1-acenaphthenone, and 1,5-dihydroxyacenaphthenone. P450 BM-3 (P450 102A1) and its mutants have been shown to oxidize acenaphthene and its oxidation products.14 Other products identified through metabolism of acenaphthene by fungal enzymes include 6-hydroxyacenaphtheneone and 1,2-acenaphthenedione.16 Formation of 1,2-epoxyacenaphthene by fungal and bacterial enzymes has not been reported. Thus, similar, but different, enzyme systems that oxidize acenaphthene appear to exist in human, bacterial, and fungal systems.
Molecular docking simulations of interaction of acenaphthylene suggest different orientations in interactions with P450 2A6 and 2A13; with acenaphthylene the atoms C-2 and C-3 were near the active site of P450 2A6, while with P450 2A13 the active site faced the C-1 and C-2 atoms of acenaphtylene. Acenaphthylene induced Type I binding spectra with P450 2A13 with a Ks value of 0.61 µM, much lower than with P450 2A6 (4.4 µM). Different orientations of acenaphthene and its metabolites, (1-acenaphthenol and 1,2-epoxyacenaphthene) with the active sites of P450 2A6 and 2A13 were also observed in this study. P450 2A6 and 2A13 have 94% sequence identity25,26 and have been reported to show overlapping but somewhat different substrate specificities.27-37 As discussed above, the intensities of spectral interaction of acenaphthene, acenaphthylene, 1-acenaphthenol, and 1,2-epoxyacenaphthene with P450 2A6 and 2A13 did not always correlate with oxidations by the P450 enzymes. It has been reported that structually diverse chemicals—including phenacetin, coumarin, nicotine, various indole derivatives, pilocarpine, tryptamine, benzylmorpholine derivatives, 8-methoxypsoralen, organoselenilum compounds, various flavonoids, and many PAHs—induce spectral changes with P450s 2A6 and 2A13 with different affinities, Many of these chemicals are substrates but others are tpotent inhibitors as well as substrates for P450 2A6 and 2A13.17,19, 20, 24, 31-22, 37-41
The biological significance of the major metabolites of acenaphthene and acenaphthylene—1-acenaphthenol and 1,2-epoxyacenaphthene, respectively—is not known at present, although our present studies showed that these metabolites (as well as the parent compounds) were not genotoxic in the S. typhimuroum NM 2009 tester strain. 1-Acenaphthenol and 1,2-epoxyacenaphthene seem to be chemically stable and not reactive with DNA. It is not known at present whether or not 1,2-epoxyacenaphthene is transformed to more reactive metabolites, such as diol-epoxides. Further studies are required to examine any potential toxic and carcinogenic activities of these PAHs and their metabolites in mammalian systems.
In conclusion, these studies show, for apparently the first time, that acenaphthene and acenaphthylene are oxidized by different forms of human P450 enzymes to various oxygenated products. We identified 1-acenaphthenol and 1,2-epoxyacenaphthene as initial oxidation products of acenaphthene and acenaphthylene, respectively, by P450s and found that these products are further oxidized by P450s to form several unidentified oxidized products, upon analysis with LC-MS. The importance of P450 2A6 in the oxidations of acenaphthene and acenaphthylene is suggested in this study; other forms of P450s—including 2A13, 1B1, 1A2, 2C9, and 3A4—play significant but sometimes minor roles that depend on the reaction steps in the metabolic pathway. The present results may be of use for further studies of metabolism of acenaphthene and acenaphthylene and the biological and toxicological significance in humans.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported in part by Grants from the Ministry of Education, Science, and Culture of Japan, the Ministry of Health and Welfare of Japan (T.S., S.T., N.M., H.Y., M.K.), and NIH grant R37 CA090426 (F.P.G.).
ABBREVIATIONS
- APCI
atmospheric pressure chemical ionization
- b5
cytochrome b5
- PAHs
polycyclic aromatic hydrocarbons
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- TIC
total ion current
Footnotes
ASSOCIATED CONTENT
Spectral interaction of acenaphthene and acenaphthylene with P450 2A6, 2A13, and 3A4; effects of deletion of b5 or NADPH-P450 reductase on P450 2A6-dependent oxidation of acenaphthene in reconstituted monooxygenase system; effects of anti-P450 2A1 IgG on acenapththylene oxidation by P450 2A6 in bicistronic E. coli membranes; mass spectra of enzyme-generated reaction products. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- (1).Fieser LF, Fieser M. Advanced Organic Chemistry. Reinhold Publishing Co.; New York: 1961. [Google Scholar]
- (2).Neurath GB. Recent advances in knowledge of the chemical composition of tobacco smoke. In: Schmeltz I, editor. The Chemistry of Tobacco and Tobacco Smoke. Plenum Publishing; New York: 1972. pp. 77–97. [Google Scholar]
- (3).Mattox CF, Humenick MJ. Organic groundwater contaminants from UCG. In Situ. 1980;4:129–151. [Google Scholar]
- (4).Kaur S, Senthilkumar K, Verma VK, Kumar B, Kumar S, Katnoria JK, Sharma CS. Preliminary analysis of polycyclic aromatic hydrocarbons in air particles (PM10) in Amritsar, India: sources, apportionment, and possible risk implications to humans. Arch. Environ. Contam. Toxicol. 2013;65:382–395. doi: 10.1007/s00244-013-9912-6. [DOI] [PubMed] [Google Scholar]
- (5).Nguyen T, Hlangothi D, Martinez RA, Jacob D, Anthony K, Nance H, Saleh MA. Charcoal burning as a source of polyaromatic hydrocarbons in waterpipe smoking. J. Environ. Sci. Health B. 2013;48:1097–1102. doi: 10.1080/03601234.2013.824300. [DOI] [PubMed] [Google Scholar]
- (6).Martorell I, Nieto A, Nadal M, Perello G, Marce RM, Domingo JL. Human exposure to polycyclic aromatic hydrocarbons (PAHs) using data from a duplicate diet study in Catalonia, Spain. Food Chem. Toxicol. 2012;50:4103–4108. doi: 10.1016/j.fct.2012.08.011. [DOI] [PubMed] [Google Scholar]
- (7).Grova N, Feidt C, Creineau C, Laurent C, Lafargue PE, Hachimi A, Rychen G. Detection of polycyclic aromatic hydrocarbon levels in milk collected near potential contamination sources. J. Agric. Food Chem. 2002;50:4640–4642. doi: 10.1021/jf0201071. [DOI] [PubMed] [Google Scholar]
- (8).Del Bubba M, Zanieri L, Galvan P, Donzelli GP, Checchini L, Lepri L. Determination of polycyclic aromatic hydrocarbons (PAHs) and total fats in human milk. Ann. Chim. 2005;95:629–641. doi: 10.1002/adic.200590074. [DOI] [PubMed] [Google Scholar]
- (9).Gatehouse D. Mutagenicity of 1,2 ring-fused acenaphthenes against S. typhimurium TA1537 and TA1538: structure-activity relationship. Mutat. Res. 1980;78:121–35. doi: 10.1016/0165-1218(80)90091-9. [DOI] [PubMed] [Google Scholar]
- (10).Babich H, Sardana MK, Borenfreund E. Acute cytotoxicities of polynuclear aromatic hydrocarbons determined in vitro with the human liver tumor cell line, HepG2. Cell. Biol. Toxicol. 1988;4:295–309. doi: 10.1007/BF00058738. [DOI] [PubMed] [Google Scholar]
- (11).Yan J, Wang L, Fu PP, Yu H. Photomutagenicity of 16 polycyclic aromatic hydrocarbons from the US EPA priority pollutant list. Mutat. Res. 2004;557:99–108. doi: 10.1016/j.mrgentox.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kaden DA, Hites RA, Thilly WG. Mutagenicity of soot and associated polycyclic aromatic hydrocarbons to Salmonella typhimurium. Cancer Res. 1979;39:4152–4159. [PubMed] [Google Scholar]
- (13).International Agency for Research on Cancer . IARC Evaluation of Carcinogenic Risk of Chemicals to Man. Vol. 3. International Agency for Research on Cancer; Lyon, France: 1973. [Google Scholar]
- (14).Schocken MJ, Gibson DT. Bacterial oxidation of the polycyclic aromatic hydrocarbons acenaphthene and acenaphthylene. Appl. Environ. Microbiol. 1984;48:10–16. doi: 10.1128/aem.48.1.10-16.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Li QS, Ogawa J, Schmid RD, Shimizu S. Engineering cytochrome P450 BM-3 for oxidation of polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 2001;67:5735–5739. doi: 10.1128/AEM.67.12.5735-5739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pothuluri JV, Freeman JP, Evans FE, Cerniglia CE. Fungal metabolism of acenaphthene by Cunninghamella elegans. Appl. Environ. Microbiol. 1992;58:3654–3659. doi: 10.1128/aem.58.11.3654-3659.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Shimada T, Kim D, Murayama N, Tanaka K, Takenaka S, Nagy LD, Folkmann LM, Foroozesh MK, Komori M, Yamazaki H, Guengerich FP. Binding of diverse environmental chemicals with human cytochromes P450 2A13, 2A6, and 1B1 and enzyme inhibition. Chem. Res. Toxicol. 2013;26:517–528. doi: 10.1021/tx300492j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Imuta M, Ziffer H. Convenient method for preparation of reactive oxiranes by direct epoxidation. J. Org. Chem. 1979;44:1351–1352. [Google Scholar]
- (19).Shimada T, Murayama N, Yamazaki H, Tanaka K, Takenaka S, Komori M, Kim D, Guengerich FP. Metabolic activation of polycyclic aromatic hydrocarbons and aryl and heterocyclic amines by human cytochromes P450 2A13 and 2A6. Chem. Res. Toxicol. 2013;26:529–537. doi: 10.1021/tx3004906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Shimada T, Murayama N, Tanaka K, Takenaka S, Guengerich FP, Yamazaki H, Komori M. Spectral modification and catalytic inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2A6, and 2A13 by four chemopreventive organoselenium compounds. Chem. Res. Toxicol. 2011;24:1327–1337. doi: 10.1021/tx200218u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Shimada T, Tanaka K, Takenaka S, Murayama N, Martin M,V, Foroozesh MK, Yamazaki H, Guengerich FP, Komori M. Structure-function relationships of inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2C9, and 3A4 by 33 flavonoid derivatives. Chem. Res. Toxicol. 2010;23:1921–35. doi: 10.1021/tx100286d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yun CH, Shimada T, Guengerich FP. Purification and characterization of human liver microsomal cytochrome P-450 2A6. Mol Pharmacol. 1991;40:679–685. [PubMed] [Google Scholar]
- (23).Yamazaki H, Gillam EM, Dong MS, Johnson WW, Guengerich FP, Shimada T. Reconstitution of recombinant cytochrome P450 2C10(2C9) and comparison with cytochrome P450 3A4 and other forms: effects of cytochrome P450-P450 and cytochrome P450-b5 interactions. Arch Biochem Biophys. 1997;342:329–337. doi: 10.1006/abbi.1997.0125. [DOI] [PubMed] [Google Scholar]
- (24).Yamazaki H, Nakamura M, Komatsu T, Ohyama K, Hatanaka N, Asahi S, Shimada N, Guengerich FP, Shimada T, Nakajima M, Yokoi T. Roles of NADPH-P450 reductase and apo- and holo-cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli. Protein Expr. Purif. 2002;24:329–37. doi: 10.1006/prep.2001.1578. [DOI] [PubMed] [Google Scholar]
- (25).Sansen S, Hsu MH, Stout CD, Johnson EF. Structural insight into the altered substrate specificity of human cytochrome P450 2A6 mutants. Arch. Biochem. Biophys. 2007;464:197–206. doi: 10.1016/j.abb.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Smith BD, Sanders JL, Porubsky PR, Lushington GH, Stout CD, Scott EE. Structure of the human lung cytochrome P450 2A13. J. Biol. Chem. 2007;282:17306–17313. doi: 10.1074/jbc.M702361200. [DOI] [PubMed] [Google Scholar]
- (27).Chiang HC, Wang CY, Lee HL, Tsou TC. Metabolic effects of CYP2A6 and CYP2A13 on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK-induced gene mutation-a mammalian cell-based mutagenesis approach. Toxicol. Appl. Pharmacol. 2011;253:145–152. doi: 10.1016/j.taap.2011.03.022. [DOI] [PubMed] [Google Scholar]
- (28).Fukami T, Katoh M, Yamazaki H, Yokoi T, Nakajima M. Human cytochrome P450 2A13 efficiently metabolizes chemicals in air pollutants: naphthalene, styrene, and toluene. Chem. Res. Toxicol. 2008;21:720–725. doi: 10.1021/tx700325f. [DOI] [PubMed] [Google Scholar]
- (29).Wong HL, Murphy SE, Hecht SS. Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N’-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem. Res. Toxicol. 2005;18:61–69. doi: 10.1021/tx0497696. [DOI] [PubMed] [Google Scholar]
- (30).Wong HL, Zhang X, Zhang QY, Gu J, Ding X, Hecht SS, Murphy SE. Metabolic activation of the tobacco carcinogen 4-(methylnitrosamino)-(3-pyridyl)-1-butanone by cytochrome P450 2A13 in human fetal nasal microsomes. Chem. Res. Toxicol. 2005;18:913–918. doi: 10.1021/tx0500777. [DOI] [PubMed] [Google Scholar]
- (31).DeVore NM, Smith BD, Wang JL, Lushington GH, Scott EE. Key residues controlling binding of diverse ligands to human cytochrome P450 2A enzymes. Drug Metab. Dispos. 2009;37:1319–1327. doi: 10.1124/dmd.109.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).DeVore NM, Scott EE. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone binding and access channel in human cytochrome P450 2A6 and 2A13 enzymes. J. Biol. Chem. 2012;287:26576–26585. doi: 10.1074/jbc.M112.372813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).DeVore NM, Smith BD, Urban MJ, Scott EE. Key residues controlling phenacetin metabolism by human cytochrome P450 2A enzymes. Drug Metab. Dispos. 2008;36:582–5890. doi: 10.1124/dmd.108.023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Su T, Bao Z, Zhang Q-Y, Smith TJ, Hong J-Y, Ding X. Human cytochrome P450 CYP2A13: Predominant exression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000;60:5074–5079. [PubMed] [Google Scholar]
- (35).Chiang H-C, Wang C-K, Tsou T-C. Differential distribution of CYP2A6 and CYP2A13 in the human respiratory tract. Respiration. 2012;84:319–326. doi: 10.1159/000339591. [DOI] [PubMed] [Google Scholar]
- (36).Zhu LR, Thomas PE, Lu G, Reuhl KR, Yang GY, Wang LD, Wang SL, Yang CS, He XY, Hong JY. CYP2A13 in human respiratory tissues and lung cancers: an immunohistochemical study with a new peptide-specific antibody. Drug Metab. Dispos. 2006;34:1672–1676. doi: 10.1124/dmd.106.011049. [DOI] [PubMed] [Google Scholar]
- (37).Yano JK, Denton TT, Cerny MA, Zhang X, Johnson EF, Cashman JR. Synthetic inhibitors of cytochrome P-450 2A6: inhibitory activity, difference spectra, mechanism of inhibition, and protein cocrystallization. J. Med. Chem. 2006;49:6987–7001. doi: 10.1021/jm060519r. [DOI] [PubMed] [Google Scholar]
- (38).Stephens ES, Walsh AA, Scott EE. Evaluation of inhibition selectivity for human cytochrome P450 2A enzymes. Drug Metab. Dispos. 2012;40:1797–802. doi: 10.1124/dmd.112.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Blake LC, Roy A, Neul D, Schoenen FJ, Aube J, Scott EE. Benzylmorpholine analogs as selective inhibitors of lung cytochrome P450 2A13 for the chemoprevention of lung cancer in tobacco users. Pharm. Res. 2013;30:2290–302. doi: 10.1007/s11095-013-1054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Wu ZL, Podust LM, Guengerich FP. Expansion of substrate specificity of cytochrome P450 2A6 by random and site-directed mutagenesis. J. Biol. Chem. 2005;280:41090–41100. doi: 10.1074/jbc.M508182200. [DOI] [PubMed] [Google Scholar]
- (41).Kim D, Wu Z-L, Guengerich FP. Analysis of coumarin 7-hydroxylation activity of cytochrome P450 2A6 using random mutagenesis. J. Biol. Chem. 2005;280:40319–40327. doi: 10.1074/jbc.M508171200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.