Abstract
The purpose of this article is to describe a technique for secondary reconstruction of traumatic orbital wall defects using titanium implants that act as three-dimensional (3D) puzzle pieces. We present three cases of large defect reconstruction using implants produced by Xilloc Medical B.V. (Maastricht, the Netherlands) with a 3D printer manufactured by LayerWise (3D Systems; Heverlee, Belgium), and designed using the biomedical engineering software programs ProPlan and 3-Matic (Materialise, Heverlee, Belgium). The smaller size of the implants allowed sequential implantation for the reconstruction of extensive two-wall defects via a limited transconjunctival incision. The precise fit of the implants with regard to the surrounding ledges and each other was confirmed by intraoperative 3D imaging (Mobile C-arm Systems B.V. Pulsera, Philips Medical Systems, Eindhoven, the Netherlands). The patients showed near-complete restoration of orbital volume and ocular motility. However, challenges remain, including traumatic fat atrophy and fibrosis.
Keywords: orbital fractures, surgery, computer-assisted, printing, three-dimensional
Orbital wall fractures can result in increased orbital volume, tissue herniation into the maxillary sinus, fat atrophy, loss of ligament support, and scar contracture leading to enophthalmos and diplopia.1 Reconstruction of large defects remains challenging since anatomical landmarks are lost, particularly the posteromedial orbital bulge and orbital apex.2 In approximately 8.5% of patients, increased orbital volume remains after treatment with traditional reconstruction techniques.3 This may be due to treatment by inexperienced surgeons, especially when damage involves the posterior orbit, which is particularly difficult to reconstruct.
To design implants for orbital reconstruction, rapid prototype models can be derived from Digital Imaging and Communications in Medicine (DICOM) data obtained from the patient's computed tomography (CT) scan. The model is used to create the implant by mirroring data from the unaffected orbit, and reconstruction is performed with prebent plates. Although this technique is not commonly used, advantages include a true-to-original anatomical repair,4 restoration of orbital volume,5 and superior ophthalmological rehabilitation when evaluating for binocular single vision and ocular motility.6 From a surgical point of view, insertion is simplified by the precise fit, and no operating time is wasted in shaping the implant.
The orbital floor can be accessed using a transconjunctival approach, which allows exploration of both medial and lateral walls and leaves no scar on the skin. However, extensive defects inevitably call for a larger reconstruction plate, which often requires a transcaruncular or a lateral canthotomy extension of the transconjunctival approach. Transcutaneous approaches, including the subciliary approach (which can be extended laterally), the subtarsal approach, and the infraorbital approach, all leave a cutaneous scar. To prevent cutaneous scarring in late primary and secondary reconstruction and to achieve perfect shape and volume, the three-dimensional (3D) puzzle-solving technique was developed.
Technique
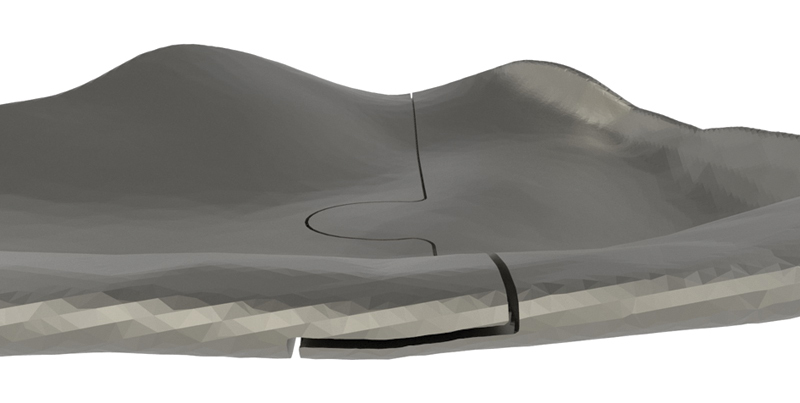
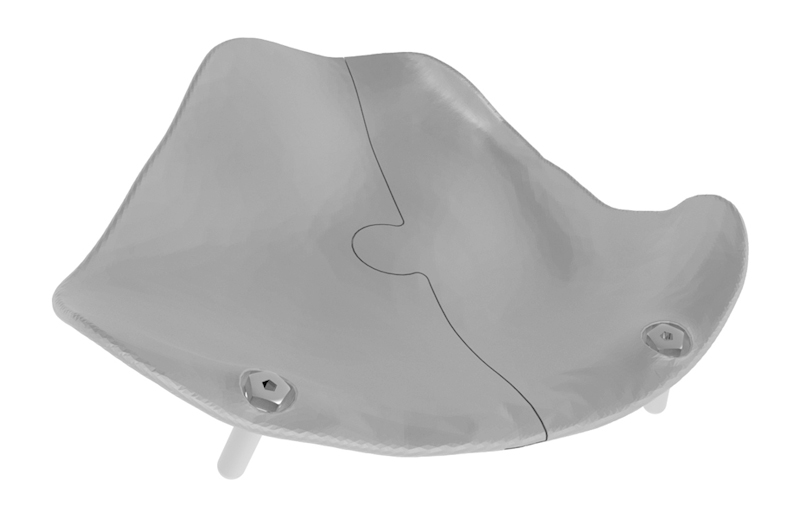
The puzzle-like interlocking of the implant pieces allows unambiguous anteroposterior positioning of one piece relative to the other. Overlapping edges of the connection provide an interlock in the coronal plane, avoiding downward rotation of the connection when manually adapted (Fig. 1).
Fig. 1.

Case 3: The two-piece puzzle design with overlap at the rim. Positions of implant screw holes took into account the underlying structures (orbital mesh, infraorbital nerve, lacrimal system). Information regarding the ideal screw length was provided.
Our first design did not include the overlap. In the second design, the overlap was added, and screw heads were partially sunk into the implant. In the third design, vertical flanges were added anteriorly to determine depth position; they were removed once the implants were in position. The ability of the implant shape to provide stable support can be determined by overlapping the margins of the reconstructed and the defective orbit. The screw positions must take into account the infraorbital nerve, lacrimal apparatus, bone quantity, and insertion angle.
The stereolithography (STL) files of the implant design were sent to the additive manufacturing company LayerWise (3D Systems; Heverlee, Belgium), which created the 3D printed implant from titanium (Ti6A14V ELI, grade 23). For the first and second designs, a porous structure consisting of diamond unit cells was added to enable bony ingrowth. However, this porosity hampered the production and accuracy of the interlock system, where bone contact is limited. Because of the low chance of bone ingrowth and higher infection risk in the region of the interlocking, the puzzle rim was made nonporous in the third design. Flanges projecting over the orbital rim with screws inserted can cause pain and irritation.7 Flanges were added to the third design to ensure perfect sagittal positioning but were removed once the 0.6-mm-thick implants were fixed with a microscrew within the orbit.
For each case, the surgeon uploaded patient data for the implant design requirements using MedX online collaboration software (Xilloc Medical B.V.; Maastricht, the Netherlands). All three implants were placed using a transconjunctival approach without the need for a medial transcaruncular or lateral canthotomy incision. We learned the following lessons: a three-piece design is not necessary; the puzzle design allows for accurate interlocking and good fit; implant overlap with the surrounding bone and overlap between pieces ensure a proper fit without the need for intraoperative navigation; a fixation screw inside the orbital rim stabilizes the first implant while the second piece is fitted; two screw holes per piece are optimal because the first microscrew inserted may not hold in thin bone; removable anterior lips (removed after positioning when appropriate) dictate the proper depth; a landmark (e.g., maxillozygomatic suture or lacrimal crest) can be used to identify the proper transverse position; and porosity enables bone ingrowth but can also result in soft tissue entrapment (3D printing cannot currently create smooth upper surfaces and porous under surfaces).
Discussion
3D printing technology offers a distinct advantage in complex reconstruction cases. These techniques enable a precise fit of the implant, decreasing surgical time because intraoperative modeling of the implant is unnecessary.
The anatomy of the orbit makes a true-to-original reconstruction challenging. Secondary reconstructions can be even more challenging as intraoperative anatomical landmarks may be lost. The mirroring technique enables digital reconstruction by projection of the unaffected side onto the affected side. Patient-specific implants can then be created by using a digital workflow or by manually bending the implants. Metzger et al described a procedure for producing anatomically adapted mesh, which is bent using a STL template of the digitally reconstructed orbit.8 Navigation-aided procedures guarantee precise intraoperative placement of the manually preformed mesh. The usefulness of this strategy in complex orbital trauma was confirmed in a larger patient series (n = 15) by Bell and Markiewicz.9 In our opinion, adaptation of the titanium plate to a solid model introduces small inaccuracies caused by the DICOM to STL conversion10 and bigger errors introduced by manual bending. To avoid the latter, a complete digital workflow is preferred.
In 2012, Salmi et al described a workflow for 3D modeling and 3D printing of a titanium orbital implant.11 After digital reconstruction of the affected orbit without mirroring of the contralateral side, a STL solid model was fabricated using fine polyamide, and a verification model in stainless steel was created by selective laser sintering. The 3D modeling of the orbital implant required the use of two software programs: the shape was determined using the first software program and surface modeling with the second. The implant was created with titanium Ti64 ELI mesh (0.4-mm net thickness, 3-mm hole) to correct a defect of the orbital floor with extension to the medial wall. Using an infraorbital approach, the implant was positioned without the need for an external guidance device because of the precise anatomical fit and fixed with two titanium screws. The technique described in this article uses a single software program for preoperative planning and modeling.
Gander et al12 and Rana et al13 described modifications of the workflow proposed by Salmi et al.11 A 3D reconstruction of the affected orbit used the mirrored unaffected orbit as template. After the digital design, three landmarks were positioned on the implant to facilitate control of the implant position by intraoperative navigation, making an external guidance device or STL solid model with a verification model unnecessary. We believe that intraoperative navigation will also become unnecessary because the patient-specific design should enable a precise fit.
In 2014, Stoor et al presented a case series of 12 orbital reconstructions (including combined maxillary and orbital defects) with Ti6AI4V ELI laser-sintered implants.14 The implants were designed using a single software program, with the size and thickness dependent on the defect (0.5–0.8 mm). Most of the implants were placed through a subciliary incision and fixed with self-drilling 2 mm screws; positioning did not require intraoperative navigation. The authors report that 16% of procedures resulted in misfit, probably due to errors in data processing of the thin orbital walls. Incorrect placement of the implant occurred in 24% of the cases, as assessed by overlapping the preoperative planning and postoperative CT images. The saving of surgical time was confirmed.
Use of two-piece puzzle 3D printed implants simplifies the surgical approach. The smaller implants allow for sequential implantation via a normal transconjunctival incision. The transconjunctival approach appears to have an overall low complication rate (0.3%).15 However, adding a lateral canthotomy is associated with the highest rate of lower eyelid malposition and is best avoided.15 Salgarelli et al advocate the subciliary approach when wider exposure is needed; however, this approach increases the probability of visible scarring, ectropion, and eyelid malpositioning compared with the transconjunctival approach.15 Although the transcaruncular approach leaves no visible scar, potential complications include injury to the globe, eyelid, lacrimal apparatus, inferior oblique muscle, medial canthal tendon, trochlea of superior oblique, and ethmoidal vessels.16
Printed patient-specific implants have increased stiffness, preventing deformation during placement. The more rigid structure of the titanium implant avoids intraoperative bending and loss of shape, which can occur when using the more flexible traditional prebent mesh plates. In our small series, no intraoperative corrections were necessary for correct adaptation, whereas Gander et al reported an intraoperative correction rate of 16.6%.12
Gander et al reported that digital planning required 30 to 36 minutes, and the manufacturing process took 4 to 6 days.12 However, the main disadvantage of our approach is the planning time, which required approximately 2 weeks for a custom design, followed by 2 to 3 weeks of production time. In addition, the implant must be sterilized the day before surgery. This increased time can be explained by the learning curve required for the planning and printing of the interlocking puzzle mechanism. After optimization, this procedure will still require 2 days for data upload and implant design, 1.5 days for implant production, and 1 day for shipment according to Xilloc Medical B.V. (January 2015), limiting but not excluding the use of this technique in primary reconstruction. For the moment being, we suggest reserving this technique for complex secondary reconstruction.
Case Reports
Case 1
A 12-year-old boy presented with a left orbital floor defect extending into the medial wall due to a traffic accident that occurred 1 month earlier. After rendering of the high-resolution CT DICOM data, mirroring was performed using Mimics Innovation Suite (Materialise, Heverlee, Belgium), which showed extension of the dislocation into the medial wall (Fig. 2). Two interlocking porous pieces were designed for transconjunctival insertion (Fig. 3). The surgery was performed 3 months after the accident. Screw position was determined after taking into account infraorbital nerve position and bone thickness. Microscrews (1.2-mm diameter; Surgi-Tec, Sint-Denijs-Westrem, Belgium) were used for plate fixation. The pieces did not overlap vertically, resulting in unfavorable rotational movement around the sagittal axis upon insertion (Fig. 4). Two months postoperatively, there was normal ocular motility, no enophthalmos, and no V2 disturbance.
Fig. 2.
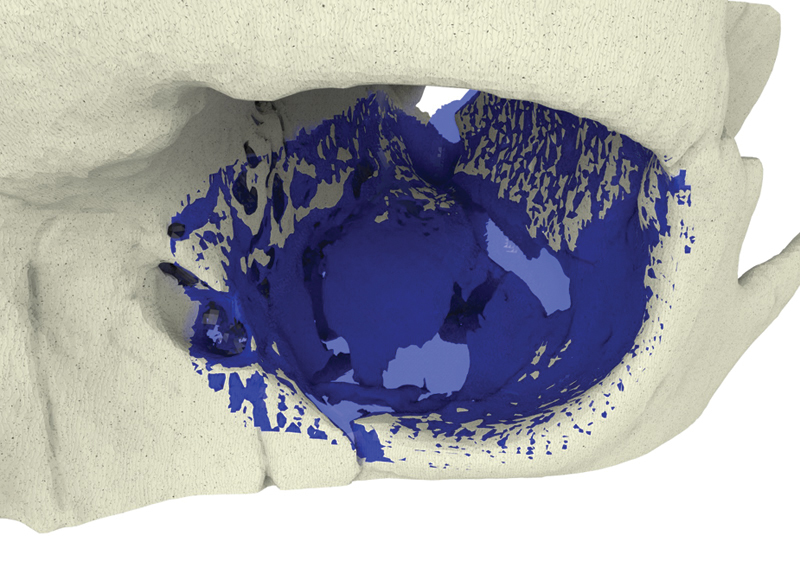
Case 1: Mirrored contralateral orbit (blue) overlying the left side orbital floor defect, extending medially in a hinge-door fashion.
Fig. 3.

Case 1: Two-piece puzzle design (double tongue-in-groove).
Fig. 4.
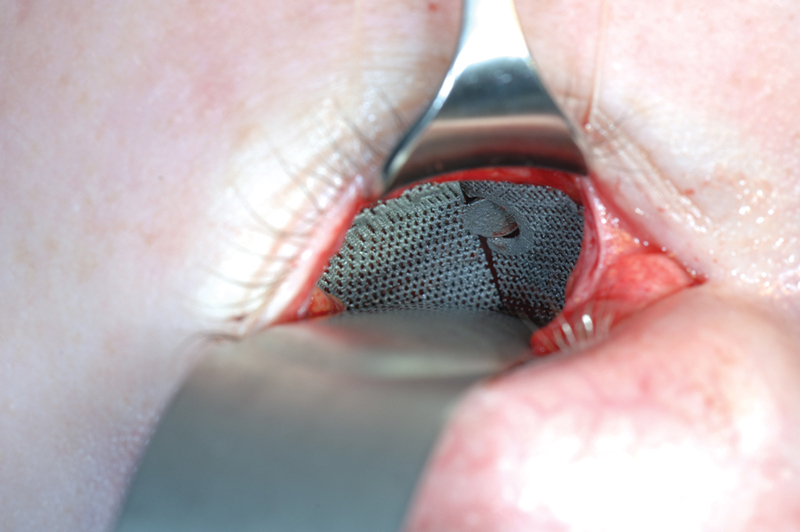
Case 1: Lack of overlap in this first two-piece puzzle implant resulted in insufficient vertical position control between the two plates.
Case 2
A 46-year-old man was referred for evaluation of debilitating diplopia with enophthalmos, lagophthalmos, and downward displacement of the globe caused by trauma that had occurred 7 months previously. The mirror image technique was used to facilitate implant design. Because of the large caudal dislocation in the posterior area, support there was not possible. However, lateral extension of the borders provided support and enabled a proper fit. A single tongue-in-groove structure guaranteed the reciprocal anteroposterior positions of the pieces, and the overlap provided vertical fit and stability (Fig. 5). After the screw position was determined, the flanges were made thicker to sink the screw heads (Fig. 6). The implant was inserted using a transconjunctival approach without extensions. Postoperative CT images showed good anatomical reconstruction and restoration of orbital volume (Fig. 7). Minimal diplopia in extreme upward gaze, slight enophthalmos, and lagophthalmos persisted because of fibrosis and fat atrophy (Fig. 8).
Fig. 5.
Case 2: Overlap between the implants.
Fig. 6.
Case 2: Single tongue-in-groove design and reinforcement of the anterior border to allow countersinking of the microscrew heads.
Fig. 7.
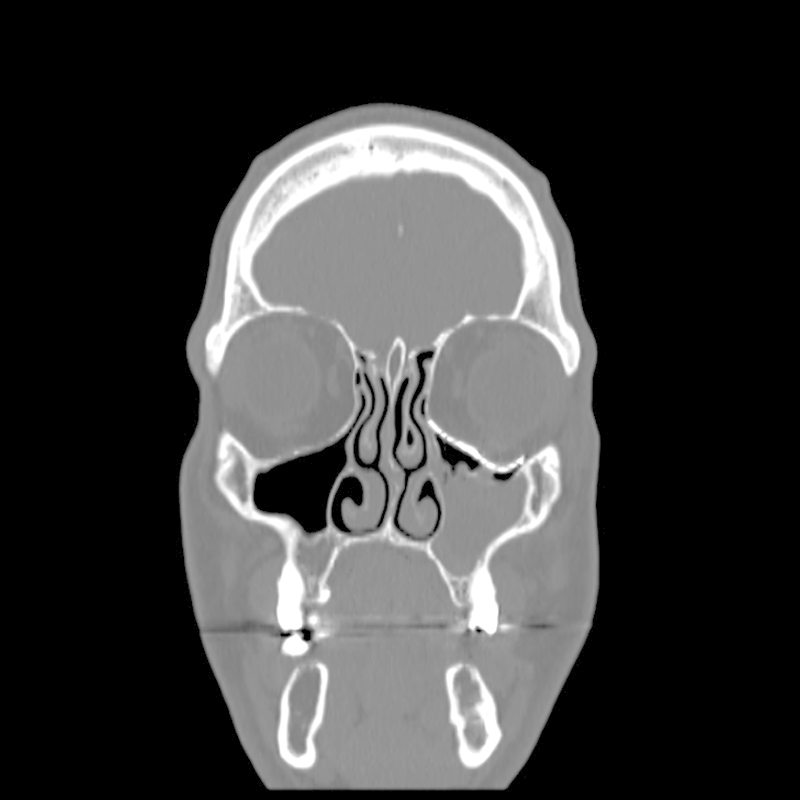
Case 2: Postoperative coronal CT scan, demonstrating near-perfect fit of the implant and symmetrical intraorbital content.
Fig. 8.
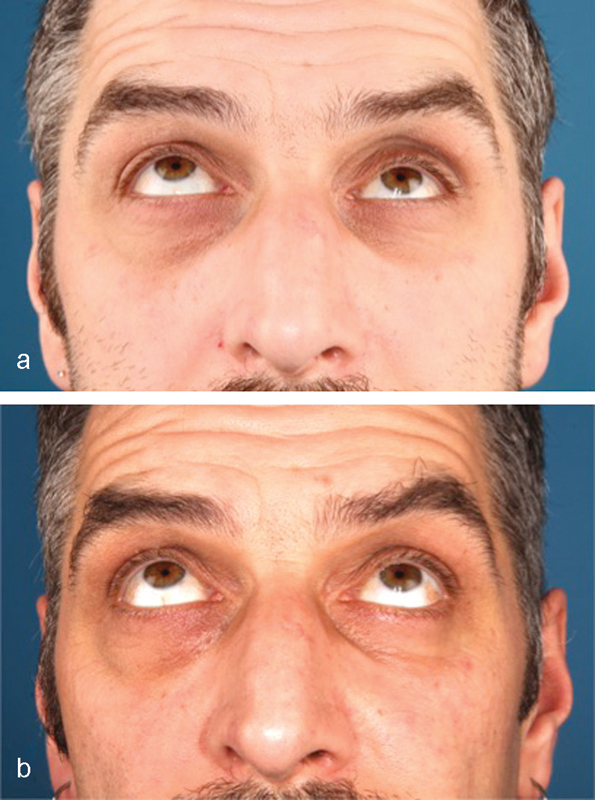
Case 2: Patient looking upward preoperatively (a) and 4 months postoperatively (b).
Case 3
A 28-year-old man was referred with disabling diplopia and severe globe malposition after two repairs of an impure orbital floor fracture using a stock titanium orbital mesh plate (Fig. 9). He had been hit by a pulley in a work accident. The presence of the previously inserted titanium mesh slightly hindered implant design because of the scatter effect on CT scan. Mirroring revealed a height difference of 7 mm between the unaffected side and the mesh on the affected side (Figs. 10 11 12). The 3D model had to take into account the lower position of the orbital rim, and the screw position was based on the amount of remaining bone and positions of the mesh, infraorbital canal, and lacrimal system. The porous implants were printed with a double tongue-in-groove design (Figs. 1 and 10). The puzzle-piece design allowed precise fit between the first and second implants (Fig. 12). Anterior flanges were not necessary as the shape of the reconstructed infraorbital rim provided anteroposterior guidance. Slight diplopia in extreme upward gaze and lagophthalmos remained because of previous fibrosis (Fig. 9).
Fig. 9.

Case 3: Patient looking upward preoperatively (a) and 1 month postoperatively (b).
Fig. 10.
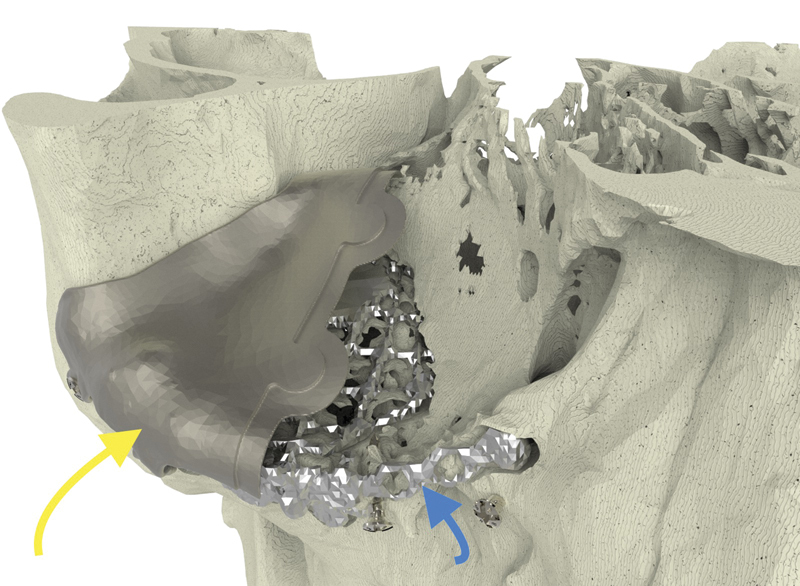
Case 3: The long yellow arrow indicates the lateral implant. The small blue arrow indicates the previously inserted titanium mesh.
Fig. 11.
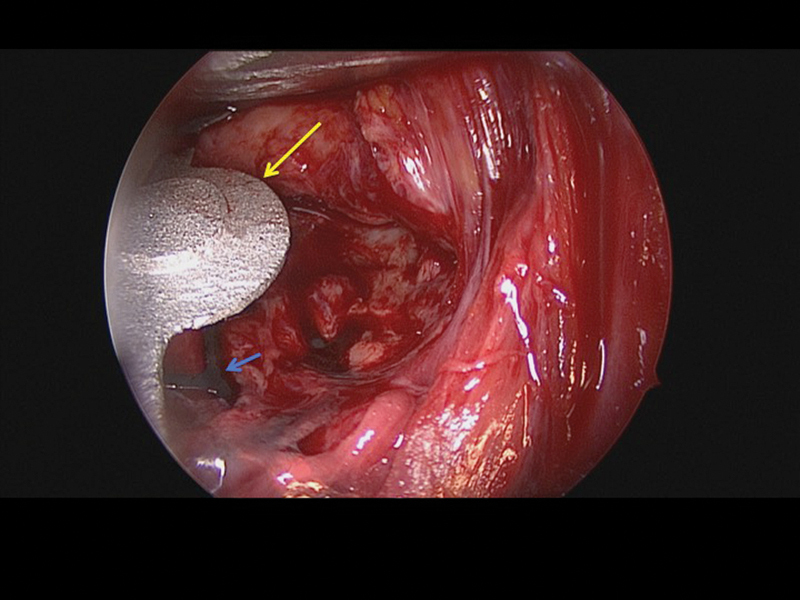
Case 3: Intraoperative endoscopic view of the lateral plate in situ (long yellow arrow) and the previously adapted titanium mesh (small blue arrow).
Fig. 12.
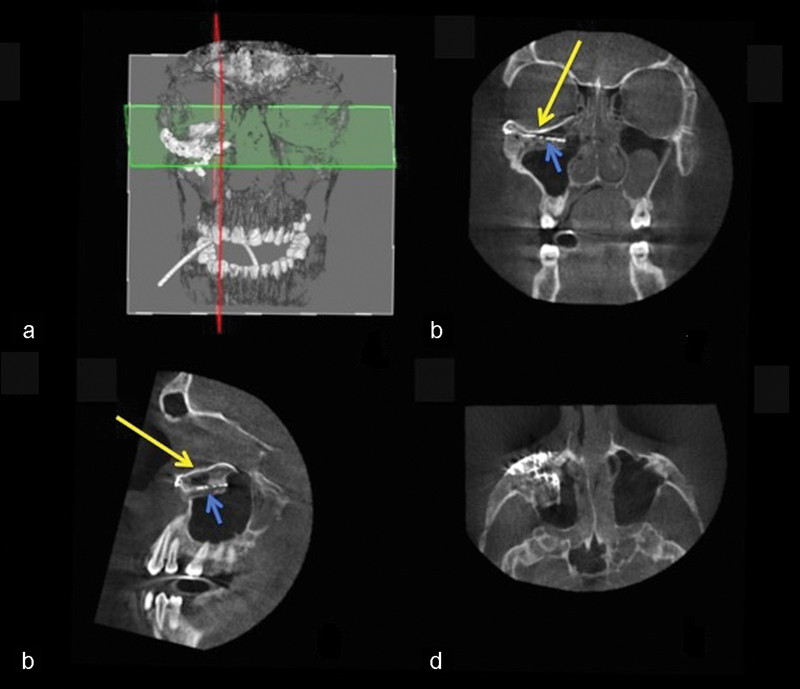
Case 3: Intraoperative 3D imaging using the Pulsera fluoroscopy system. (a) 3D reconstruction and section plane indication. (b) Good fit of the two puzzle pieces (long yellow arrow). Malpositioned mesh (short blue arrow). (c) Good fit of the medial puzzle-piece (long yellow arrow). The titanium mesh was not prebent and was 7 mm too low at the posterior sigmoid bulge (short blue arrow). (d) Axial view of the titanium mesh. The two-piece puzzle implants are visible only where they overlap the infraorbital rim.
Conclusion
The two-piece puzzle 3D printed implant appears to be useful for reconstruction of extensive orbital wall damage. This approach makes use of a limited surgical access, and the precise fit greatly decreases operation time. Because of planning cost and time, cases should be selected based on reconstruction difficulty and extent of the defect.
References
- 1.Clauser L, Galiè M, Pagliaro F, Tieghi R. Posttraumatic enophthalmos: etiology, principles of reconstruction, and correction. J Craniofac Surg. 2008;19(2):351–359. doi: 10.1097/SCS.0b013e3180534361. [DOI] [PubMed] [Google Scholar]
- 2.Hammer B, Kunz C, Schramm A, deRoche R, Prein J. Repair of complex orbital fractures: technical problems, state-of-the-art solutions and future perspectives. Ann Acad Med Singapore. 1999;28(5):687–691. [PubMed] [Google Scholar]
- 3.Hammer B, Prein J. Correction of post-traumatic orbital deformities: operative techniques and review of 26 patients. J Craniomaxillofac Surg. 1995;23(2):81–90. doi: 10.1016/s1010-5182(05)80453-6. [DOI] [PubMed] [Google Scholar]
- 4.Schön R, Metzger M C, Zizelmann C, Weyer N, Schmelzeisen R. Individually preformed titanium mesh implants for a true-to-original repair of orbital fractures. Int J Oral Maxillofac Surg. 2006;35(11):990–995. doi: 10.1016/j.ijom.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Tang W, Guo L, Long J. et al. Individual design and rapid prototyping in reconstruction of orbital wall defects. J Oral Maxillofac Surg. 2010;68(3):562–570. doi: 10.1016/j.joms.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 6.Kozakiewicz M, Elgalal M, Piotr L, Broniarczyk-Loba A, Stefanczyk L. Treatment with individual orbital wall implants in humans - 1-Year ophthalmologic evaluation. J Craniomaxillofac Surg. 2011;39(1):30–36. doi: 10.1016/j.jcms.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Mustafa S F, Evans P L, Bocca A, Patton D W, Sugar A W, Baxter P W. Customized titanium reconstruction of post-traumatic orbital wall defects: a review of 22 cases. Int J Oral Maxillofac Surg. 2011;40(12):1357–1362. doi: 10.1016/j.ijom.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Metzger M C, Schön R, Schulze D, Carvalho C, Gutwald R, Schmelzeisen R. Individual preformed titanium meshes for orbital fractures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(4):442–447. doi: 10.1016/j.tripleo.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Bell R B, Markiewicz M R. Computer-assisted planning, stereolithographic modeling, and intraoperative navigation for complex orbital reconstruction: a descriptive study in a preliminary cohort. J Oral Maxillofac Surg. 2009;67(12):2559–2570. doi: 10.1016/j.joms.2009.07.098. [DOI] [PubMed] [Google Scholar]
- 10.Huotilainen E, Jaanimets R, Valášek J. et al. Inaccuracies in additive manufactured medical skull models caused by the DICOM to STL conversion process. J Craniomaxillofac Surg. 2014;42(5):e259–e265. doi: 10.1016/j.jcms.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Salmi M, Tuomi J, Paloheimo K S. et al. Patient-specific reconstruction with 3D modeling and DMLS additive manufacturing. Rapid Prototyping J. 2012;18:209–214. [Google Scholar]
- 12.Gander T, Essig H, Metzler P. et al. Patient specific implants (PSI) in reconstruction of orbital floor and wall fractures. J Craniomaxillofac Surg. 2015;43(1):126–130. doi: 10.1016/j.jcms.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Rana M, Gellrich M M, Gellrich N C. Customised reconstruction of the orbital wall and engineering of selective laser melting (SLM) core implants. Br J Oral Maxillofac Surg. 2015;53(2):208–209. doi: 10.1016/j.bjoms.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Stoor P, Suomalainen A, Lindqvist C. et al. Rapid prototyped patient specific guiding implants for reconstruction of orbital wall defects. J Craniomaxillofac Surg. 2014;42(8):1644–1649. doi: 10.1016/j.jcms.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Salgarelli A C, Bellini P, Landini B, Multinu A, Consolo U. A comparative study of different approaches in the treatment of orbital trauma: an experience based on 274 cases. Oral Maxillofac Surg. 2010;14(1):23–27. doi: 10.1007/s10006-009-0176-2. [DOI] [PubMed] [Google Scholar]
- 16.Edgin W A, Morgan-Marshall A, Fitzsimmons T D. Transcaruncular approach to medial orbital wall fractures. J Oral Maxillofac Surg. 2007;65(11):2345–2349. doi: 10.1016/j.joms.2006.06.270. [DOI] [PubMed] [Google Scholar]


