Abstract
Anorectal surgery is well tolerated. Rates of minor complications are relatively high, but major postoperative complications are uncommon. Prompt identification of postoperative complications is necessary to avoid significant patient morbidity. The most common acute complications include bleeding, infection, and urinary retention. Pelvic sepsis, while may result in dramatic morbidity and even mortality, is relatively rare. The most feared long-term complications include fecal incontinence, anal stenosis, and chronic pelvic pain.
Keywords: anorectal surgery, complications, hemorrhage, urinary retention, pelvic sepsis
Anorectal pathology is prevalent throughout the world, with most anorectal complaints being transient and without the need for formal medical evaluation. For those that do require surgical intervention for their anorectal pathology, the surgery can usually be done safely in the outpatient setting with minimal morbidity. However, no intervention is without risk, and complications frequently arise after anorectal surgery, with rates upward of 50% in some studies.1 The purpose of this chapter is to review the short- and long-term complications that can arise after anorectal surgery, including the diagnostic approach, interventions, and prevention strategies for these complications.
Short-Term/Acute Complications
Postoperative Hemorrhage
Minor bleeding after anorectal surgery is common. Since we expect patients to continue with normal bowel function, the already disturbed anorectal mucosal becomes further irritated with activity and bowel movements. Since some bloody discharge is normal, the patient should be appropriately counseled on what to expect so as to avoid unnecessary anxiety and phone calls. It may also be helpful to remove/wash out clots from the rectum while still in the operating room to minimize confusion after surgery. However, major bleeding can also occur, albeit rarely, and may require further intervention. While the presentation of major bleeding is not uniform, patients often report frequent passing of small to moderate amounts of clot and bright red blood starting after the first bowel movement.
Hemorrhoid surgery involves the vascular cushions of the anus, so not surprisingly, hemorrhoidectomy is associated with higher rates of bleeding when compared with other anorectal procedures.1 2 3 Bleeding after other anorectal procedures such as procedures for anal fistula or fissure is very low (0.4–1.2%).4 5
Rates of clinically significant bleeding after hemorrhoid surgery vary based on type of the procedure. For conventional hemorrhoidectomy (Milligan–Morgan and Ferguson) and bipolar energy device hemorrhoidectomy (Ligasure), rates of clinically significant hemorrhage has been reported in the range of 0.3 to 6%, with an average of around 2%.1 2 3 6 7 There does not seem to be a significant difference in rates of bleeding between conventional hemorrhoidectomy and bipolar energy device assisted procedures.
The timing of bleeding after hemorrhoidectomy varies, and can be generally divided into immediate and delayed.5 Immediate bleeding occurs within 24 to 48 hours of a procedure and is likely related to loss of control of the vascular pedicle. Delayed bleeding is defined as bleeding reported up to 2 weeks postprocedure, and is more often related to infection or local trauma.4 5 Delayed bleeding may be influenced by post-operative pain medications. Hemorrhoidectomy is associated with significant postoperative pain, and multimodality management is routinely employed to help alleviate discomfort. NSAIDS are an integral part of this pain management and can increase the incidence of bleeding.8
Thankfully, most bleeding will resolve spontaneously. For bleeding that does not resolve, the treatment depends on the location of the bleeding and the degree of blood loss. If the bleeding is more external in nature, holding pressure with gauze, cauterization, or suture ligation at the bedside are all acceptable interventions with high rates of success. Injection of local anesthetic with epinephrine can also be performed in clinic or on the ward; however, this can be uncomfortable and there are no data available on the success of this approach. For bleeding that is located within or above the anal canal, bedside intervention is more difficult, and the first approach is typically to tamponade the bleed with a finger or a piece of Vaseline gauze. Often, the patient's own sphincter tone acts as a tamponade on bleeding vessels within the anal canal, which can explain the episodic nature of postoperative bleeding. In more severe cases, tamponade with a Foley balloon catheter can be employed, possibly in conjunction with Vaseline gauze and Surgicel.4 5 Tamponade can be quite uncomfortable for the patient, and is often used as a temporizing measure while a more definitive plan is being activated.
On an average, 15 to 33% of patients with bleeding after hemorrhoidectomy will require a return to the operating room for control of the hemorrhage.3 9 Interestingly, most will not have an identifiable source of bleeding by the time they are examined in the operating room. However, these bleeding episodes can be significant and a return to the operating room for the second look may be justified.
Bleeding after stapled hemorrhoidectomy (procedure for prolapse and hemorrhoids, PPH) is slightly more common than for excisional hemorrhoidectomy, with rates as high as 9.6%.1 2 3 At the same time, rates of reintervention for bleeding are lower for PPH compared with conventional hemorrhoidectomy.3 Bleeding after Doppler-guided hemorrhoidal de-arterialization has been reported to be low (4.3%); however, this needs to be balanced with the chance of long-term recurrence.10
Special consideration should be given to patients with an increased risk for bleeding after anorectal surgery. Patients on hemodialysis have reported rates of postoperative bleeding as high as 11.1% after conventional hemorrhoidectomy.11 For patients on systemic anticoagulation, there are limited published data on postoperative bleeding. A study by Nelson et al focused on rubber band ligation for patients on antithrombotic prophylaxis, and reported minimal risk of bleeding for patients on aspirin, nonsteroidal anti-inflammatory drugs (NSAIDS) or coumadin.12 However, this group found that clopidogrel carried a higher risk of bleeding (8.6%), even if it was held prior to the banding and restarted in a delayed fashion. Data on newer antithrombotic medications and hemorrhoid procedures are not available.
There is significant controversy regarding whether or not antithrombotic therapy should be stopped prior to the surgical treatment of hemorrhoids. Most would consider aspirin and NSAIDS generally are safe to continue through the perioperative period. Coumadin at low doses seems to be safe as well, although several surgeons would recommend stopping it prior to surgery and restarting when chance of bleeding after the procedure decreases. The risk of bleeding while on other antiplatelet therapy such as clopidogrel is significant, and these medications should be stopped, or the procedure should be delayed until it can be stopped if at all possible.
Infection
Infectious complications after anorectal surgery are thankfully uncommon, but can be significant when they do occur. Since painful drainage (including more fibrinous material) and swelling is expected after most anorectal procedures, the diagnosis of an infection may be difficult and is often delayed. Fever, worsening pain after initial improvement in symptoms, and the development of delayed urinary retention are three very important warning signs. Patients exhibiting these three symptoms should be examined promptly to avoid worsening complications. Imaging, including computerized tomography or magnetic resonance imaging may be considered especially if a deeper abscess or pelvic sepsis is suspected. When an infection following anorectal surgery has been diagnosed, the surgeon should have low threshold for a quick return to the operating room for control of infection and debridement of any devitalized tissue.
Transient bacteremia after hemorrhoidectomy is common, and has been reported in up to 8% of cases,13 but clinically significant infections are extremely uncommon. Given the location, it is expected that all wounds will get colonized by bacteria shortly after surgery. The exact rate of infectious complications after anorectal surgery is difficult to interpret. The development of severe pelvic sepsis (Fournier's gangrene) following anorectal surgery has only been described as individual case reports, with 24 published cases between 1978 and 2004.14 15 There are a few reports of development of liver abscess and septic emboli after anorectal surgery.14 Rates of abscess formation after hemorrhoidectomy have been reported between 0.5 and 4%1 3 4 16 17 with most studies reporting a rate of 1%. Patients who are immunosuppressed seem to be at higher risk.18 19 There does not seem to be a significant difference between open and closed hemorrhoidectomy or between traditional hemorrhoidectomy and PPH.1 15 Several studies also report wound complications (drainage, nonhealing, etc.), but it is possible that these were caused/resulted from infection that was not clinically evident.
There does not seem to be an association between performing a perianal block with local anesthetic and postoperative infection.14 Rates of infection after procedures for anal fistula seem to be similarly low, but even harder to interpret since localized infection is an indication for surgery and these data are often combined with recurrence/reoperation.11 12 There has been no convincing evidence to suggest that either preoperative or postoperative antibiotics decrease rates of infectious complications for anorectal surgery.20 Exceptions to this are discussed in article “Perioperative Management of the Ambulatory Anorectal Surgery Patient” on pp. 7–13.
Urinary Retention
Urinary retention is the most common complication after anorectal surgery, with rates varying between 3 and 50%21 22 23 24 25 26 with most studies reporting a rate around 15%.22 23 24 25 Postsurgical urinary retention is multifactorial with contributions from irritation/blockade of pelvic nerves and pain evoked reflexes.24 27 The multifactorial nature of urinary retention makes it a difficult problem to deal with after anorectal surgery. Pain is a major issue and pain treatment strategies can exacerbate the problem. For example, local anesthetic can significantly improve postoperative pain and nerve irritation; however, it can also lead to decreased sensation of the urge to micturate leading to bladder distention.27
Several risk factors have been identified over the years that increase the likelihood of retention. Some of these factors are not modifiable, including age, male sex, and type of surgery.23 24 25 26 27 Other factors are modifiable and lead to changes in practices associated with anorectal surgery. In general, epidural and spinal anesthesia have been associated with higher rates of urinary retention25 26 27 compared with monitored anesthesia care. Opioids, often needed after anorectal surgery, can also contribute to the problem.
Excess intravenous fluid has also been shown to significantly increase the risk of urinary retention, and strategies for intravenous fluid restriction are typically employed.22 25 A more detailed discussion of fluid restriction can be found in the chapter on ambulatory anorectal surgery. Of note, several medications have been employed in attempts to reduce urinary retention, but the data are mixed and for the most part disappointing. Although well-designed, the previous studies on this topic suffered from small numbers and the use of older medications. Urecholine and prazosin have both been shown to be very effective in treating established urinary retention, but not in preventing it in anorectal surgery.22 24
Typical symptoms of urinary retention include pain, pressure, discomfort, an unproductive urge to urinate, and frequent, small volume micturition with persistent feeling of incomplete evacuation. In patients with good pelvic nerve blockade and decreased sensation, abdominal pressure may be the only early symptoms. Urinary retention can lead to urinary tract infections. If not addressed in the timely manner, retention can result in over distention of the bladder that can further exacerbate the problem and in some cases lead to acute renal injury from postrenal obstruction.27 Clinical symptoms as well as noninvasive bladder scanners are very effective in diagnosing this problem.
Thankfully, most issues with urinary retention are self-limited, and will resolve without major intervention. Since inflammation and swelling seem to contribute to the problem, patients with mild retention are often counseled to sit in a bath of very warm water, filled above the waist, to see if this can alleviate swelling and facilitate urination. When this is unsuccessful, patients may require bladder catheterization. This may involve intermittent straight catheterization or a temporary indwelling catheter, which can typically be removed after a few days without further testing. α1 antagonists such as tamsulosin can be helpful, and attempts to minimize opioid intake is also worthwhile.27 While these measures alone will aid most patients, a referral to a urologist for further studies is indicated if prolonged urinary retention occurs.
Other Acute Complications
Thrombosed hemorrhoids and fissures following anorectal surgery have been described by several papers1 2 3 4 5 18 19 and are likely related to local tissue trauma, injection of local anesthetic with epinephrine, and constipation. Incidences of these complications in all the studies reviewed are very low (although possibly underreported), so it is hard to make meaningful conclusions about true risks and preventive strategies. In general, sitz baths and avoidance of constipation can be helpful in avoiding fissures and thrombosed hemorrhoids, as well as in the treatment of these conditions when they develop.
Severe constipation is common after anorectal surgery, with rates between 15 and 30%.1 2 3 Hemorrhoidectomy has the highest rates reported. A fear of bowel movements and the associated pain can lead to functional constipation. Opioid consumption also plays a major role. While fecal impaction can develop, this is less common, and disimpaction is rarely required, with most cases treated on an outpatient basis. A solution to postoperative constipation is the combination of a strict bowel regimen with a multimodality pain regimen that limits opioid consumption. It is important to note that several patients have pre-existing constipation as a cause of their underlying anorectal disease, and vigilance is needed to avoid exacerbating this problem after surgery.16
Anal fistulas have also been reported after anorectal procedures. In cases where fistula-in-ano was not a primary problem, it is either the result of infection, trauma to the anal canal, or abnormal healing. The treatment of these fistulas varies significantly based on the degree of sphincter muscle involvement. Rectovaginal and ano-vaginal fistulas have also been reported with higher prevalence in PPH procedures. This can occur when the rectovaginal septum incurs damage, so it is more common in surgeries that involve the anterior anal canal and rectum, including hemorrhoidectomies and full-thickness trans-anal excisions.3 For this reason, careful dissection and postprocedure examination of the rectovaginal septum is warranted to avoid fistula.
Long-Term Complications
Complications after anorectal surgery are not always immediate, and can instead take months or years to fully develop. In general, these complications are more severe and more difficult to treat than those that occur in the acute postoperative period. We will discuss the most common and most feared long-term complications below.
Anal Stricture/Stenosis
If excluding coloanal anastomoses, anal stricture and stenosis are most commonly seen after hemorrhoidectomy, but can occur after any surgery within the anal canal. Stenosis can complicate a stapled or radical amputative hemorrhoidectomy in 1 to 7.5% of cases.10 27 28 29 In these patients, the normal pliable anoderm is replaced by cicatrized tissue due to excessive removal of the anoderm and distal rectal mucosa. The patient may also suffer from injury to the underlying anal sphincter muscle, leading to severe and progressive stenosis. Patients with anal stenosis often report straining to have a bowel movement, smaller caliber stools, and pain with defecation. Anal stenosis may also lead to fecal impaction and overflow incontinence.
A review of the patient's previous operative reports along with a detailed anorectal exam can confirm the diagnosis. Office evaluation with a digital exam, anoscopy, and proctoscopy are usually adequate, but patients with severe stenosis or pain may require an exam under anesthesia. An examination under anesthesia may also be helpful in determining how much of the stricture is from anatomic distortion versus a functional problem leading to muscle hypertonicity. With functional stenosis, the anus will relax under anesthesia while anatomic stricturing will not change. If the etiology of the stenosis is unclear, biopsies of the area are appropriate to exclude neoplastic or inflammatory etiologies.
Anal stenosis may be classified by the severity of the stricture as well as the level of involvement of the anal canal (Table 1).30 The management of anal stenosis is determined by the degree of symptoms rather than the degree of stenosis, and an asymptomatic patient does not necessarily require intervention, given that a malignant cause of the stenosis has been excluded.
Table 1. Classification of anal strictures.
| Severity of stricture | |
| Mild | Tight anal canal can be examined by a well-lubricated index finger or a medium Hill–Ferguson retractor |
| Moderate | Forceful dilation is required to insert the index finger or a medium Hill–Ferguson retractor |
| Severe | Neither the little finger nor the small Hill–Ferguson retractor can be inserted without forceful dilation |
| Level of stricture | |
| Low | At least 0.5 cm below the dentate line |
| Middle | 0.5 cm proximal or distal to the dentate line |
| High | More than 0.5 cm proximal to the dentate line |
Mild strictures can often be treated with dietary modifications, stool softeners, or fiber supplements. The regular passage of stool provides the most “natural” stretching possible. Digital dilatation or the use of anal dilators can be part of the treatment plan if medical management is not sufficient. The initial dilation should be performed under anesthesia and patients should continue daily dilations using a digit or a plastic dilator at home. The author recommends the use of the plastic insert from a disposable anoscope with lidocaine jelly as an effective dilator for home use. If patients remain symptomatic with these measures, it is important to ensure that the symptoms are not due other causes, such as an anal fissure.
Patients with moderate or severe strictures who have failed conservative management require surgical intervention. To determine the proper surgical procedure, the differential involvement of the anoderm compared with the underlying anal sphincter complex must be determined. A patient with a healthy anoderm and underlying fibrotic internal sphincter may only need a unilateral or bilateral sphincterotomy.31 32 Treatment of a fibrotic anal sphincter with sphincterotomy alone is most successful if the stricture is mild and low in the anal canal. Results of sphincterotomy alone for anal stenosis are limited but good results have been reported in up to 67% of patients.30
Patients with stenosis of the anoderm require the introduction of healthy tissue into the anal canal, replacing lost or diseased nonpliable anoderm with elastic and compliant neoanoderm.32 33 Several advancement flaps are described below. Patients with scarring of the anal sphincter muscle as well as stenosis of the anoderm should undergo a flap procedure combined with an internal sphincterotomy. Simple release of a stricture may provide temporary relief of symptoms but generally should be avoided because of the high rate of recurrent stricture.
There are multiple types of flaps for anal stenosis, which are generally classified as advancement, rotational, or adjacent tissue transfer flaps.32 The choice of flap is influenced by the location of the stricture. Mid and upper anal canal stenoses are optimally treated with mucosal advancement flaps. For stenoses below the dentate line, a dermal advancement flap anoplasty is recommended. The V–Y advancement flap is used for strictures at the dentate line. Longer stenoses are best treated with diamond or house flap. Very large defects may require multiple house flaps or an S-plasty.31 32
Mucosal Advancement Flap
Mucosal advancement flaps are best suited for treating midlevel and upper anal canal stenosis. The scar tissue is excised and a unilateral or bilateral internal sphincterotomy is performed if the underlying anal sphincter complex is also scarred and stenotic. A flap of healthy proximal rectal and anal mucosa with the underlying muscle is then undermined for 2 to 5 cm and advanced over the defect. Advancing the flap too far and suturing it to the anal verge may result in ectropion, leading to difficulty with incontinence and mucus discharge. Studies demonstrate success rates of up to 90% with the main complications being abscess, fecal leakage, and restenosis.34
Anoplasty
Several options for flap configuration exist, of which the most common flaps will be described below (Figs. 1 and 2). In general, the operative techniques include adequate mobilization to avoid tension and the maintenance of a healthy blood supply to the flap. The pedicled flap should typically retain a wide base, with care taken not to cone in when dissecting deep to the anoderm.
Fig. 1.
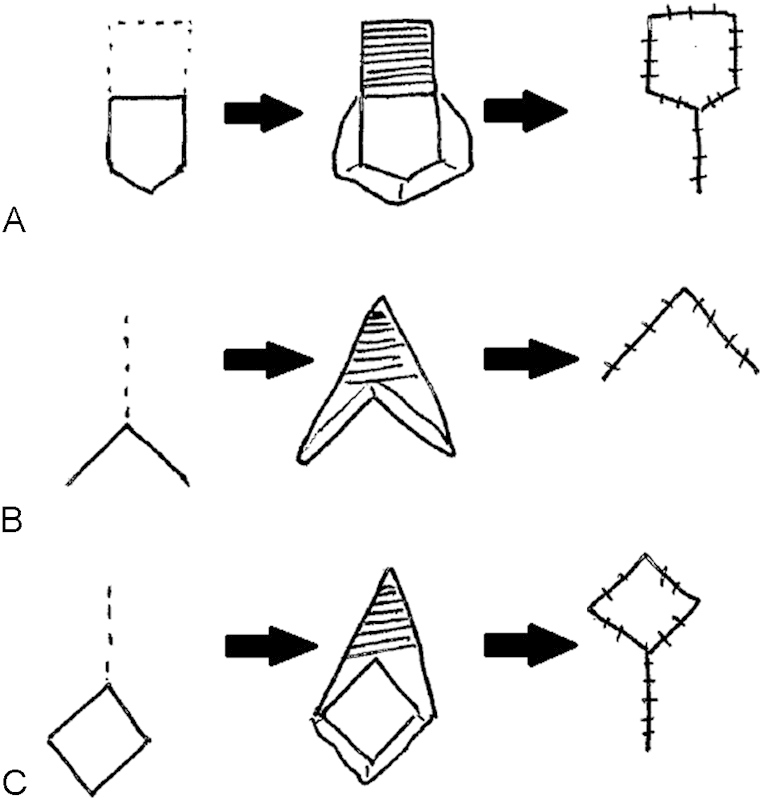
Anoplasty (A: house flap, B: Y–V flap, C: diamond flap).
Fig. 2.
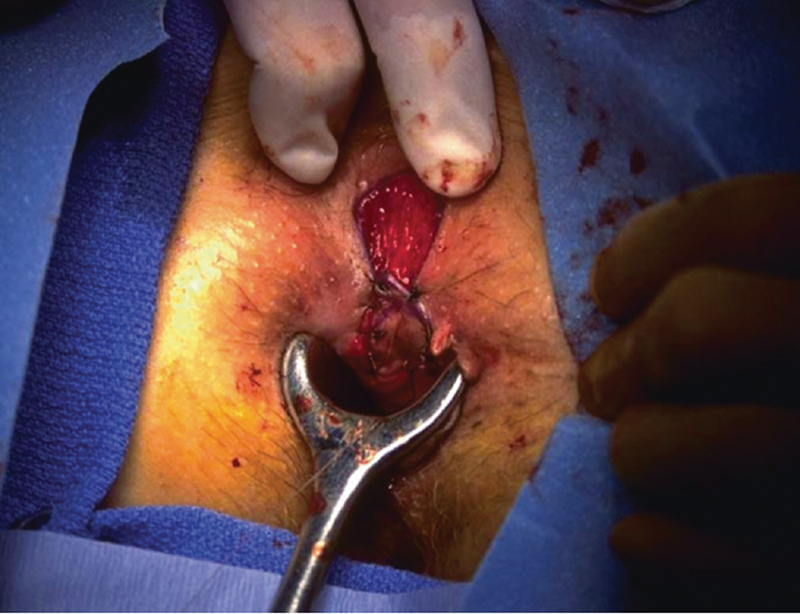
Anoplasty. (Image courtesy of W. Brian Sweeney, MD.)
The Y–V advancement flap is performed by making a Y-shaped incision with the vertical limb of the Y extending into the area of stenosis and the two arms of the Y extending widely out onto the perianal skin to form the V. The V flap is mobilized, generally down to the underlying fascia to maintain its subdermal vascular plexus. This is then sutured into the vertical limb of the Y incision. If the proximal portion of the flap is too narrow, the patient may have insignificant widening of the stenosis.
V–Y advancement flap is performed by making the V-shaped incision with the wide base oriented toward the dentate line. Once again, the flap is mobilized ensuring that the subdermal vascular plexus is preserved. The donor site is closed primarily, creating the vertical limb of the Y. The flap is then secured proximally to replace the area of excised scar.
To create a diamond flap, the scar tissue is incised and a diamond-shaped flap is created on the perianal skin at the distal end of the incision. Again, the flap is advanced over the area of incised scar and the donor site is closed primarily. Similar to the diamond-shaped flap, the U-shaped flap is created on the perianal skin after the anal stenosis scar tissue is incised and the flap is advanced over the area of incised scar. The donor site is left open to heal.35
The house flap anoplasty is used for anal stenosis when a V–Y advancement flap may not provide adequate tissue coverage. It has the advantage of a larger, flat proximal segment without a corner that can be susceptible to ischemia. A longitudinal incision is made from the dentate line to the end of the stenosis. The flap is created in the shape of an inverted house with the base oriented proximally and is advanced into the anal canal and secured in place. It is of paramount importance that the flap be created with an adequate length and width to cover the defect. The donor site can be left open or closed primarily. Bilateral house flaps can be used for severe stenosis or ectropion.36 37 38
Rotational S Flap
The rotational S-plasty flap is well suited for coverage of large areas but it does not work as well to open strictures as an advancement flap. Rotational S-plasty flaps are full-thickness flaps with the length of the base equivalent to its length. After the scar at the anal canal is excised, the flaps are rotated such that the apex is sutured to the opposite side of the anal canal and the side of the flap is sutured to the lateral wall.
Farid et al compared 63 consecutive patients with anal stenosis by utilizing the house flap, rhomboid flap, and V–Y anoplasty. A total of 90% of patients with a house flap had clinical improvement at 1 year compared with 60% of patients with a rhomboid flap and 30% of patients with Y–V anoplasty. The incidence of complications was lowest in the house flap cohort and included ischemia of the flap in one patient, delayed healing in one patient, and sepsis in one patient. Patient satisfaction was significantly higher among patients with a house flap compared with a rhomboid flap or Y–V anoplasty.37
Fecal Incontinence
Fecal incontinence following anorectal surgery can result from several issues. In cases such as fistulotomy, sphincter muscle may have been intentionally divided with an underestimation of the functional consequence. At other times, damage to the anal sphincter or associated nerves occurs unintentionally. This can be due to direct mechanical or thermal trauma, or due to subsequent infection. Meticulous surgical technique is paramount in avoiding unintentional damage to the anal sphincter. It is also essential that the risk of fecal incontinence be included in the informed consent prior to surgery. Incontinence related to specific anorectal procedures, as well as the approach to diagnosis and treatment of incontinence, is included below.
To prevent fecal incontinence after fistula surgery, the integrity of the anal sphincters prior to surgery must be kept in mind, as many patients undergo multiple surgeries to treat their anal fistula. It is extremely important to document an objective assessment of the patient's preoperative fecal incontinence, as this may aid in your surgical decision making, and also allows a more accurate assessment of postoperative disturbances in continence.
In select cases, it may also be beneficial to obtain preoperative imaging of the sphincter complex prior to fistula surgery. In a prospective study of 120 patients undergoing preoperative endorectal ultrasound, 37 (30.8%) patients had an internal anal sphincter defect and 17 (15.9%) had an external anal sphincter defect at baseline. Of the 83 patients with no preoperative internal anal sphincter defect, 47 (56.5%) had an internal defect after surgery. Of 103 patients with no previous external defect, 20 (19.4%) were found to have a postoperative external defect.39
Some degree of new-onset fecal incontinence has been reported in 8% of patients following a fistulotomy for a simple fistula, 24% of patients following a fistulotomy for a complex fistula, 25% of patients after a fistulectomy and sphincter repair, and 52% of patients after fistulectomy and advancement flap.39 40 41 Fistula characteristics, the number of abscesses incised, the number of fistulotomies performed, and the number of sphincter-sparing procedures are associated with the presence of fecal incontinence during follow-up. As expected, patients treated for a subcutaneous fistula tract have a lower risk of fecal incontinence than those with more complex fistulas.40 41
Although endorectal advancement flap is considered a sphincter preserving technique, the sphincter complex can be injured by stretch during the operation, the proximal internal sphincter could be disrupted by the raising of the flap, and an ectropion caused by advancing the flap beyond the internal fistula opening at the dentate line can cause moisture and fecal leakage. In one study, fecal incontinence after endorectal advancement flap for cryptoglandular fistulas was 13.2% and for Crohn fistulas it was 12% at an average follow-up of 28.9 months.42
Incontinence after hemorrhoidectomy is associated with a high incidence of partial or full-thickness internal anal sphincter injury and occasionally external sphincter defects.43 44 45 Incontinence has also been seen with intact sphincters, as the hemorrhoidal cushions are known to provide 15% of the patient's resting anal tone, and removal can unmask issues with incontinence that were being aided by these cushions. Excision of hemorrhoids with secondary healing may also cause decreased sensitivity and reduced capacity for rectoanal discrimination.46
Fecal incontinence can also occur after PPH, and is usually related to a low-placed staple line or by injury to the internal sphincter due to the large diameter of the circular stapler. In a prospective, randomized trial of 134 patients, de novo fecal incontinence at 1 year was reported in 2.5% of patients undergoing a stapled hemorrhoidopexy compared with 7.5% of patients who underwent a Milligan–Morgan hemorrhoidectomy.47 In another study of 257 patients undergoing stapled hemorrhoidopexy with a mean duration of follow-up of 6.3 ± 1.2 years, 11 patients (4.9%) reported newly developed fecal incontinence.48
Fecal incontinence is seen in 1.5 to 8% of patients after lateral internal sphincterotomy.49 50 In a study of 31 women who underwent lateral internal sphincterotomy (LIS) for a chronic anal fissure, continence scores were significantly correlated with the extent of division of the internal anal sphincter muscle. Division of less than 25% of the internal anal sphincter (IAS) was correlated with a minimal risk of incontinence.51
The evaluation of patients with fecal incontinence should start with a thorough history and physical examination. Further studies including anorectal manometry, endorectal ultrasound, and pudendal nerve testing can assist in determining the cause of fecal incontinence. Anorectal manometry is a useful objective measure of the power of the internal sphincter and the external sphincter during voluntary contraction. Endoanal ultrasound is useful for the identification and detection of defects in the anal sphincter muscles. Magnetic resonance imaging of the anal sphincter complex is an alternative to ultrasound with equivalent accuracy, but it is significantly more expensive than ultrasound, and should be reserved for select cases.
Medical management is the best treatment for the majority of patients with fecal incontinence after anorectal surgery. Bulking of the stool with fiber or antidiarrheals can make the stool easier to control and decrease the frequency of incontinence episodes. Biofeedback and sacral neuromodulation have also been shown to decrease fecal incontinence severity and improve quality of life.52 53 However, this has not been well studied in patients with iatrogenic injury to the anal sphincter complex. For patients with sphincter disruption, sphincteroplasty may also be helpful,54 although this surgery is not typically associated with long-term durability.
Chronic Pain
Chronic anal pain after anorectal surgery can be disabling for the patient and difficult to treat. To some degree, acute anal pain is common following anorectal surgery, particularly after hemorrhoidectomy, but this generally resolves completely within 3 to 4 weeks. Prior to surgery, patients should be counseled about the anticipated duration and intensity of postoperative pain.
The causes of chronic pain after anorectal surgery are numerous. This pain can be related to residual underlying pathology, new or ongoing fissures and/or thrombosed hemorrhoid, or subtle anal infections. If postoperative pain persists beyond what is expected, the patient should undergo a detailed evaluation focused on the above-mentioned causes, with special attention paid to the possibility of an occult infection or a nonhealing wound. If a thorough exam cannot be completed in clinic, an exam under anesthesia may be helpful to determine the source of pain.
Chronic pain syndromes after stapled hemorrhoidopexy are uncommon but well described. Patients undergoing stapled hemorrhoidopexy generally have less immediate postoperative pain and less chronic pain than open hemorrhoidectomy.28 55 In a prospective randomized trial at a mean follow-up of 16 months, 14/50 patients undergoing an open hemorrhoidectomy and 9/50 patients undergoing stapled hemorrhoidopexy complained of occasional long-term pain.55 When chronic pain does occur after PPH, it may be due to smooth muscle incorporation into the staple line. It has also been attributed to persistent hemorrhoidal disease, sphincter spasm, anal fissure, anorectal sepsis, or retained staples. Overall rates of chronic pain after PPH range from 1.6 to 31%.56
Treatment of chronic pain following anorectal surgery should be targeted to the underlying source. Warm sitz baths and nonsteroidals can relieve mild pain. Antispasmodics such as diazepam or cyclobenzaprine may be added if levator spasm is noted. Anismus may be treated with botulinum toxin injection.57 Sacral neuromodulation has also been described for chronic pelvic pain after anorectal surgery.58 If retained staples after PPH are identified, an exam under anesthesia with staple removal is appropriate. Thankfully, many patients with pain will slowly improve over time. Overall, chronic pain after anorectal surgery can be quite difficult to manage, which reinforces the importance of proper knowledge of the anatomy and use of meticulous surgical technique.
Conclusion
While anorectal surgery is generally well tolerated, short- and long-term complications frequently occur. To battle this, the surgeon should perform a thorough preoperative work-up of the patient's baseline disability, along with a detailed discussion of complications during the informed consent process. When complications do occur, prompt identification and elimination of the offending pathology can limit the long-term impact on the patient's quality of life.
References
- 1.Senagore A J, Singer M, Abcarian H. et al. A prospective, randomized, controlled multicenter trial comparing stapled hemorrhoidopexy and Ferguson hemorrhoidectomy: perioperative and one-year results. Dis Colon Rectum. 2004;47(11):1824–1836. doi: 10.1007/s10350-004-0694-9. [DOI] [PubMed] [Google Scholar]
- 2.MacRae H M, McLeod R S. Comparison of hemorrhoidal treatment modalities. A meta-analysis. Dis Colon Rectum. 1995;38(7):687–694. doi: 10.1007/BF02048023. [DOI] [PubMed] [Google Scholar]
- 3.Nisar P J, Acheson A G, Neal K R, Scholefield J H. Stapled hemorrhoidopexy compared with conventional hemorrhoidectomy: systematic review of randomized, controlled trials. Dis Colon Rectum. 2004;47(11):1837–1845. doi: 10.1007/s10350-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 4.Hyman N, O'Brien S, Osler T. Outcomes after fistulotomy: results of a prospective, multicenter regional study. Dis Colon Rectum. 2009;52(12):2022–2027. doi: 10.1007/DCR.0b013e3181b72378. [DOI] [PubMed] [Google Scholar]
- 5.Hall J F, Bordeianou L, Hyman N. et al. Outcomes after operations for anal fistula: results of a prospective, multicenter, regional study. Dis Colon Rectum. 2014;57(11):1304–1308. doi: 10.1097/DCR.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 6.Cintron J, Abcarian H. New York, NY: Springer; 2007. Benign anorectal: hemorrhoids; pp. 156–177. [Google Scholar]
- 7.Rosen L, Sipe P, Stasik J J, Riether R D, Trimpi H D. Outcome of delayed hemorrhage following surgical hemorrhoidectomy. Dis Colon Rectum. 1993;36(8):743–746. doi: 10.1007/BF02048364. [DOI] [PubMed] [Google Scholar]
- 8.Place R J, Coloma M, White P F, Huber P J, Van Vlymen J, Simmang C L. Ketorolac improves recovery after outpatient anorectal surgery. Dis Colon Rectum. 2000;43(6):804–808. doi: 10.1007/BF02238018. [DOI] [PubMed] [Google Scholar]
- 9.Chen H H, Wang J Y, Changchien C R, Yeh C Y, Tsai W S, Tang R. Effective management of posthemorrhoidectomy secondary hemorrhage using rectal irrigation. Dis Colon Rectum. 2002;45(2):234–238. doi: 10.1007/s10350-004-6154-8. [DOI] [PubMed] [Google Scholar]
- 10.Giordano P, Overton J, Madeddu F, Zaman S, Gravante G. Transanal hemorrhoidal dearterialization: a systematic review. Dis Colon Rectum. 2009;52(9):1665–1671. doi: 10.1007/DCR.0b013e3181af50f4. [DOI] [PubMed] [Google Scholar]
- 11.Sheikh F, Khubchandani I T, Rosen L, Sheets J A, Stasik J J. Is anorectal surgery on chronic dialysis patients risky? Dis Colon Rectum. 1992;35(1):56–58 k. doi: 10.1007/BF02053339. [DOI] [PubMed] [Google Scholar]
- 12.Nelson R S Ewing B M Ternent C Shashidharan M Blatchford G J Thorson A G Risk of late bleeding following hemorrhoidal banding in patients on antithrombotic prophylaxis Am J Surg 20081966994–999., discussion 999 [DOI] [PubMed] [Google Scholar]
- 13.Bonardi R A, Rosin J D, Stonesifer G L Jr, Bauer F W. Bacteremias associated with routine hemorrhoidectomies. Dis Colon Rectum. 1976;19(3):233–236. doi: 10.1007/BF02590908. [DOI] [PubMed] [Google Scholar]
- 14.Guy R J, Seow-Choen F. Septic complications after treatment of haemorrhoids. Br J Surg. 2003;90(2):147–156. doi: 10.1002/bjs.4008. [DOI] [PubMed] [Google Scholar]
- 15.McCloud J M, Jameson J S, Scott A N. Life-threatening sepsis following treatment for haemorrhoids: a systematic review. Colorectal Dis. 2006;8(9):748–755. doi: 10.1111/j.1463-1318.2006.01028.x. [DOI] [PubMed] [Google Scholar]
- 16.Bouchard D, Abramowitz L, Castinel A. et al. One-year outcome of haemorrhoidectomy: a prospective multicentre French study. Colorectal Dis. 2013;15(6):719–726. doi: 10.1111/codi.12090. [DOI] [PubMed] [Google Scholar]
- 17.Sielezneff I, Salle E, Lécuyer J, Brunet C, Sarles J C, Sastre B. Early postoperative morbidity after hemorrhoidectomy using the Milligan–Morgan technic. A retrospective studies of 1,134 cases. J Chir (Paris) 1997;134(5–6):243–247. [PubMed] [Google Scholar]
- 18.Grewal H, Guillem J G, Quan S H, Enker W E, Cohen A M. Anorectal disease in neutropenic leukemic patients. Operative vs. nonoperative management. Dis Colon Rectum. 1994;37(11):1095–1099. doi: 10.1007/BF02049810. [DOI] [PubMed] [Google Scholar]
- 19.Morandi E, Merlini D, Salvaggio A, Foschi D, Trabucchi E. Prospective study of healing time after hemorrhoidectomy: influence of HIV infection, acquired immunodeficiency syndrome, and anal wound infection. Dis Colon Rectum. 1999;42(9):1140–1144. doi: 10.1007/BF02238565. [DOI] [PubMed] [Google Scholar]
- 20.Khan K I, Akmal M, Waqas A, Mahmood S. Role of prophylactic antibiotics in Milligan–Morgan hemorrhoidectomy – a randomized control trial. Int J Surg. 2014;12(8):868–871. doi: 10.1016/j.ijsu.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Cataldo P A, Senagore A J. Does alpha sympathetic blockade prevent urinary retention following anorectal surgery? Dis Colon Rectum. 1991;34(12):1113–1116. doi: 10.1007/BF02050073. [DOI] [PubMed] [Google Scholar]
- 22.Petros J G, Bradley T M. Factors influencing postoperative urinary retention in patients undergoing surgery for benign anorectal disease. Am J Surg. 1990;159(4):374–376. doi: 10.1016/s0002-9610(05)81274-7. [DOI] [PubMed] [Google Scholar]
- 23.Bowers F J, Hartmann R, Khanduja K S, Hardy T G Jr, Aguilar P S, Stewart W R. Urecholine prophylaxis for urinary retention in anorectal surgery. Dis Colon Rectum. 1987;30(1):41–42. doi: 10.1007/BF02556921. [DOI] [PubMed] [Google Scholar]
- 24.Zaheer S, Reilly W T, Pemberton J H, Ilstrup D. Urinary retention after operations for benign anorectal diseases. Dis Colon Rectum. 1998;41(6):696–704. doi: 10.1007/BF02236255. [DOI] [PubMed] [Google Scholar]
- 25.Toyonaga T, Matsushima M, Sogawa N. et al. Postoperative urinary retention after surgery for benign anorectal disease: potential risk factors and strategy for prevention. Int J Colorectal Dis. 2006;21(7):676–682. doi: 10.1007/s00384-005-0077-2. [DOI] [PubMed] [Google Scholar]
- 26.Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110(5):1139–1157. doi: 10.1097/ALN.0b013e31819f7aea. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland L M Burchard A K Matsuda K et al. A systematic review of stapled hemorrhoidectomy Arch Surg 2002137121395–1406., discussion 1407 [DOI] [PubMed] [Google Scholar]
- 28.Shao W J, Li G C, Zhang Z H, Yang B L, Sun G D, Chen Y Q. Systematic review and meta-analysis of randomized controlled trials comparing stapled haemorrhoidopexy with conventional haemorrhoidectomy. Br J Surg. 2008;95(2):147–160. doi: 10.1002/bjs.6078. [DOI] [PubMed] [Google Scholar]
- 29.Tjandra J J, Chan M K. Systematic review on the procedure for prolapse and hemorrhoids (stapled hemorrhoidopexy) Dis Colon Rectum. 2007;50(6):878–892. doi: 10.1007/s10350-006-0852-3. [DOI] [PubMed] [Google Scholar]
- 30.Milsom J W, Mazier W P. Classification and management of postsurgical anal stenosis. Surg Gynecol Obstet. 1986;163(1):60–64. [PubMed] [Google Scholar]
- 31.Katdare M V, Ricciardi R. Anal stenosis. Surg Clin North Am. 2010;90(1):137–145. doi: 10.1016/j.suc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Liberman H, Thorson A G. How I do it. Anal stenosis. Am J Surg. 2000;179(4):325–329. doi: 10.1016/s0002-9610(00)00344-5. [DOI] [PubMed] [Google Scholar]
- 33.Brisinda G, Vanella S, Cadeddu F. et al. Surgical treatment of anal stenosis. World J Gastroenterol. 2009;15(16):1921–1928. doi: 10.3748/wjg.15.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakhmanine M, Rosen L, Khubchandani I, Stasik J, Riether R D. Lateral mucosal advancement anoplasty for anal stricture. Br J Surg. 2002;89(11):1423–1424. doi: 10.1046/j.1365-2168.2002.02230.x. [DOI] [PubMed] [Google Scholar]
- 35.Pearl R K, Hooks V H III, Abcarian H, Orsay C P, Nelson R L. Island flap anoplasty for the treatment of anal stricture and mucosal ectropion. Dis Colon Rectum. 1990;33(7):581–583. doi: 10.1007/BF02052210. [DOI] [PubMed] [Google Scholar]
- 36.Christensen M A, Pitsch R M Jr, Cali R L, Blatchford G J, Thorson A G. “House” advancement pedicle flap for anal stenosis. Dis Colon Rectum. 1992;35(2):201–203. doi: 10.1007/BF02050680. [DOI] [PubMed] [Google Scholar]
- 37.Farid M, Youssef M, El Nakeeb A, Fikry A, El Awady S, Morshed M. Comparative study of the house advancement flap, rhomboid flap, and y-v anoplasty in treatment of anal stenosis: a prospective randomized study. Dis Colon Rectum. 2010;53(5):790–797. doi: 10.1007/DCR.0b013e3181d3205a. [DOI] [PubMed] [Google Scholar]
- 38.Sentovich S M, Falk P M, Christensen M A, Thorson A G, Blatchford G J, Pitsch R M. Operative results of house advancement anoplasty. Br J Surg. 1996;83(9):1242–1244. [PubMed] [Google Scholar]
- 39.Roig J V, Jordán J, García-Armengol J, Esclapez P, Solana A. Changes in anorectal morphologic and functional parameters after fistula-in-ano surgery. Dis Colon Rectum. 2009;52(8):1462–1469. doi: 10.1007/DCR.0b013e3181a80e24. [DOI] [PubMed] [Google Scholar]
- 40.Visscher A P, Schuur D, Roos R, Van der Mijnsbrugge G J, Meijerink W J, Felt-Bersma R J. Long-term follow-up after surgery for simple and complex cryptoglandular fistulas: fecal incontinence and impact on quality of life. Dis Colon Rectum. 2015;58(5):533–539. doi: 10.1097/DCR.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 41.Jordán J, Roig J V, García-Armengol J, García-Granero E, Solana A, Lledó S. Risk factors for recurrence and incontinence after anal fistula surgery. Colorectal Dis. 2010;12(3):254–260. doi: 10.1111/j.1463-1318.2009.01806.x. [DOI] [PubMed] [Google Scholar]
- 42.Soltani A, Kaiser A M. Endorectal advancement flap for cryptoglandular or Crohn's fistula-in-ano. Dis Colon Rectum. 2010;53(4):486–495. doi: 10.1007/DCR.0b013e3181ce8b01. [DOI] [PubMed] [Google Scholar]
- 43.Lindsey I, Jones O M, Smilgin-Humphreys M M, Cunningham C, Mortensen N J. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum. 2004;47(10):1643–1649. doi: 10.1007/s10350-004-0651-7. [DOI] [PubMed] [Google Scholar]
- 44.Johannsson HÖ, Påhlman L, Graf W. Functional and structural abnormalities after milligan hemorrhoidectomy: a comparison with healthy subjects. Dis Colon Rectum. 2013;56(7):903–908. doi: 10.1097/DCR.0b013e31828deb6d. [DOI] [PubMed] [Google Scholar]
- 45.Felt-Bersma R J, van Baren R, Koorevaar M, Strijers R L, Cuesta M A. Unsuspected sphincter defects shown by anal endosonography after anorectal surgery. A prospective study. Dis Colon Rectum. 1995;38(3):249–253. doi: 10.1007/BF02055596. [DOI] [PubMed] [Google Scholar]
- 46.Bharucha A E, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130(5):1510–1518. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 47.Gravié J F, Lehur P A, Huten N. et al. Stapled hemorrhoidopexy versus Milligan–Morgan hemorrhoidectomy: a prospective, randomized, multicenter trial with 2-year postoperative follow up. Ann Surg. 2005;242(1):29–35. doi: 10.1097/01.sla.0000169570.64579.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ommer A, Hinrichs J, Möllenberg H, Marla B, Walz M K. Long-term results after stapled hemorrhoidopexy: a prospective study with a 6-year follow-up. Dis Colon Rectum. 2011;54(5):601–608. doi: 10.1007/DCR.0b013e3182098df2. [DOI] [PubMed] [Google Scholar]
- 49.Oh C, Divino C M, Steinhagen R M. Anal fissure. 20-year experience. Dis Colon Rectum. 1995;38(4):378–382. doi: 10.1007/BF02054225. [DOI] [PubMed] [Google Scholar]
- 50.García-Aguilar J, Belmonte Montes C, Perez J J, Jensen L, Madoff R D, Wong W D. Incontinence after lateral internal sphincterotomy: anatomic and functional evaluation. Dis Colon Rectum. 1998;41(4):423–427. doi: 10.1007/BF02235754. [DOI] [PubMed] [Google Scholar]
- 51.Murad-Regadas S M, Fernandes G O, Regadas F S. et al. How much of the internal sphincter may be divided during lateral sphincterotomy for chronic anal fissure in women? Morphologic and functional evaluation after sphincterotomy. Dis Colon Rectum. 2013;56(5):645–651. doi: 10.1097/DCR.0b013e31827a7416. [DOI] [PubMed] [Google Scholar]
- 52.Altomare D F, Ratto C, Ganio E, Lolli P, Masin A, Villani R D. Long-term outcome of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2009;52(1):11–17. doi: 10.1007/DCR.0b013e3181974444. [DOI] [PubMed] [Google Scholar]
- 53.Wexner S D, Coller J A, Devroede G. et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251(3):441–449. doi: 10.1097/SLA.0b013e3181cf8ed0. [DOI] [PubMed] [Google Scholar]
- 54.Glasgow S C, Lowry A C. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55(4):482–490. doi: 10.1097/DCR.0b013e3182468c22. [DOI] [PubMed] [Google Scholar]
- 55.Ganio E, Altomare D F, Gabrielli F, Milito G, Canuti S. Prospective randomized multicentre trial comparing stapled with open haemorrhoidectomy. Br J Surg. 2001;88(5):669–674. doi: 10.1046/j.0007-1323.2001.01772.x. [DOI] [PubMed] [Google Scholar]
- 56.Pescatori M, Gagliardi G. Postoperative complications after procedure for prolapsed hemorrhoids (PPH) and stapled transanal rectal resection (STARR) procedures. Tech Coloproctol. 2008;12(1):7–19. doi: 10.1007/s10151-008-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ron Y, Avni Y, Lukovetski A. et al. Botulinum toxin type-A in therapy of patients with anismus. Dis Colon Rectum. 2001;44(12):1821–1826. doi: 10.1007/BF02234461. [DOI] [PubMed] [Google Scholar]
- 58.Martellucci J, Naldini G, Del Popolo G, Carriero A. Sacral nerve modulation in the treatment of chronic pain after pelvic surgery. Colorectal Dis. 2012;14(4):502–507. doi: 10.1111/j.1463-1318.2011.02659.x. [DOI] [PubMed] [Google Scholar]


