Abstract
Complex anal fistulas require careful evaluation. Prior to any attempts at definitive repair, the anatomy must be well defined and the sepsis resolved. Several muscle-sparing approaches to anal fistula are appropriate, and are often catered to the patient based on their presentation and previous repairs. Emerging technologies show promise for fistula repair, but lack long-term data.
Keywords: transsphincteric fistula, advancement flap, anal fistula
The management of perianal fistulas has been documented in the literature for thousands of years, dating back to the time of Hippocrates in 400 bc.1 A simple or low fistula-in-ano is best treated with a primary fistulotomy, with excellent healing rates and functional outcomes. However, the approach to complex anal fistulas is more difficult, with higher rates of failure and functional disability.
Instead of summarizing the anatomy and pathophysiology of fistula-in-ano, which is well known to our audience, this article focuses on the diagnostic approach to the complex anal fistula, evidence-based treatment strategies, response to treatment failures, and approach to special situations including Crohn disease.
Definition of a “Complex” Fistula
Many definitions exist for a “complex” anal fistula. In 1961, Dr. Parks divided fistulas into intersphincteric, transsphincteric, suprasphincteric, and extrasphincteric.2 The Standards Committee for the American Society of Colon and Rectal Surgeons (ASCRS) published practice parameters for the management of perianal abscess and fistula-in-ano in 2011,3 in which they define “simple” fistulas as those that are intersphincteric or low transsphincteric involving less than 30% of the external sphincter. “Complex” fistulas included those with more muscle involvement, or anterior fistulas in female patients, as well as recurrent fistulas, and those associated with preexisting fecal incontinence, inflammatory bowel disease, or radiation.3
Office Exam
Patients with a complex anal fistula should have a full history and physical examination focusing on prior surgical history including prior anorectal operations. In particular, understanding of baseline bowel function and continence, prior obstetric and anorectal surgical history, and any type of radiation to the deep pelvis is of critical importance. A detailed anorectal exam should be performed to delineate the trajectory of the primary and possible secondary fistula tracts, and identify any evidence of ongoing sepsis (as evidenced by fluctuance, purulence, or skin changes). This should include anoscopy to assess the internal fistula opening, as well as proctoscopy to assess for proctitis and other proximal diseases. Bimanual examination may assist in this assessment. If there is a palpable cord of a chronic fistula tract, the amount of muscle involved can be inferred. It is of critical importance to thoroughly describe the location of all perceived openings (anterior/posterior, right or left lateral), as this aids operative planning and positioning for definitive surgery.4
Preoperative Imaging
For situations where the fistula origin or trajectory is unclear, additional imaging should be considered. This includes previous failed repairs, inflammatory bowel disease, and situation in which multiple or unusual fistula tracts are suspected, such as tuberculosis.
Endoanal ultrasound has been shown to be an effective tool in defining complex fistula tracts, and correlation with intraoperative examination is 90 to 94%.5 The injection of hydrogen peroxide is a useful adjunct, with potential for better delineated secondary tracts and abscess cavities.6 The main disadvantage of ultrasound is patient discomfort, but it is generally well tolerated.
Pelvic magnetic resonance imaging (MRI) has also been widely utilized for the imaging of complex fistulas, with the benefit of being less operator-dependent than ultrasound. Though certainly more expensive, this modality correlates with correct anatomy in up to 90% of cases, and studies have shown it can impact therapeutic decision making up to 75% of the time.7 8 Interpretation may take some additional expertise, but there are several techniques that improve the yield of pelvic MRI. The field should be tilted forward to coincide with the axis of the anal canal. Fat-saturated (T2-weighted) images along with single tau inversion recovery(STIR) sequences are of particular value as they help delineate fistula inflammation from other ongoing processes. If differentiation is needed between scarring and ongoing inflammation, gadolinium-enhanced T1-weighted images are most helpful.9 These aspects and technical features make MRI the authors' preference for fistula mapping in appropriately selected cases.
Fistulography and computed tomography (CT) scans are regarded as obsolete due to the increased radiation exposure and suboptimal delineation of the fistula tract anatomy compared with other modalities.10 While CT imaging can be helpful in the evaluation of acute infectious processes such as supralevator abscess, it is not as useful when evaluating complex fistula tracts.
Treatment of Complex Fistulas
The initial management strategy prior to any definitive treatment is to have local control of perianal sepsis, particularly if an abscess exists. This may include draining an abscess cavity or placing a draining seton into the fistula to allow the area to “cool off.” Definitive repair in the setting of active infection often leads to lower healing rates.11 This is not a new concept, and it has been advocated for some time.12 Some authors believe, however, that fistula can be appropriately managed definitively at the time of abscess drainage in experienced hands.13 14 It is our preference to treat complex fistulas with drainage of abscess and resolution of sepsis prior to definitive repair.
Definitive management requires addressing the underlying fistula tract itself. Options include setons (temporary draining, cutting), fistulotomy or fistulectomy (primary or staged, with or without sphincteroplasty), endorectal advancement flap, anocutaneous advancement flap, fistula plug, fibrin glue, electrocauterization of tract/laser (fistula laser closure [FiLaC]), and ligation of intersphincteric fistula tract (LIFT). Each of these will be discussed individually in the text that follows.
Draining Setons
Draining setons are an effective way to control perianal sepsis.15 They can serve to prevent blockage of the fistula tract and allow it to mature, and may be particularly useful if a complex repair is planned in the future.16 17 It can also be used as a means to shorten the fistula tract to decrease the size of the wound over time, particularly if the external opening is located some distance away from the anal verge.
Often, a loose draining seton is placed through a complex fistula during the first evaluation as a means to stent the fistula tract open, and prevent closure of the external opening, which may lead to recurrent abscess. Many different materials can be used as a draining seton. One of the most common approaches is the use of a Silastic vessel loop tied loosely to itself with silk suture.
It should be noted that while the draining seton is often seen as a bridge to a more definitive therapy, it is also an acceptable long-term therapy for complex anal fistulas. For patients with multiple failed repairs or multiple synchronous fistulas, or for those who simply do not wish to undergo any further intervention, a well-constructed draining seton can be kept in the fistula for years, hopefully limiting fistula-associated symptoms and preventing recurrent infection.
Cutting Setons
Cutting setons have been used for many years to manage complex fistula-in-ano. In this procedure, the seton is secured tightly within the fistula tract, with intentional pressure placed on the tract itself. The seton can then be serially tightened in the office over time. The theory behind this procedure is similar to that of regelation, where a frozen object melts under pressure, but then freezes again when the pressure is released. This is a common high-school science project, where a wire or paper clip is placed on an ice cube, and the cube retains its form as the wire advances through.18 For a cutting seton, the slow division of muscle as the seton becomes more superficial allows buildup of a fibrotic tract, with less muscle separation than if a primary fistulotomy was performed.
It is a simple procedure to perform, but takes between 12 and 16 weeks to heal.19 Also, the regelation is better in theory than in actuality, and fecal incontinence rates range from 12 to 26%,20 21 22 with incontinence to flatus being the most common, followed by incontinence to liquid stool. A review article suggested the average rate of incontinence to be 9.7% for flatus and 5% for solid stool.21 While some enthusiasts remain,23 the cutting seton has been abandoned in most centers for use in complex fistulas.
Fistulotomy
Primary fistulotomy has been the mainstay of treatment for the simple anal fistula, and remains extremely useful for intersphincteric and low transsphincteric fistulas. Higher fistulas are potentially a much different problem, though good evidence is lacking. A small series including only high, complex, and recurrent fistulas demonstrated a 4% recurrence risk and continence disturbances in 36%, the majority of which was to flatus or minor fecal staining.24 Another series by the same authors reported results of 50 patients, and reported a recurrence rate of 7%, with minor control problems in 40%.25
Primary fistulotomy with end-to-end primary sphincteroplasty is another treatment option. In 2013, Ratto et al reported on this technique for 72 patients with complex anal fistula of cryptoglandular origin. After a mean follow-up of 29.4 months (range: 6–26), there was a primary healing rate of 95.8%, with no significant difference between the preoperative and postoperative Wexner Fecal Incontinence Scores.26 Another study looked specifically at anal manometry before and after the procedure in 35 patients, and found that anal continence and manometric values actually improved in patients who had prior incontinence. Patients with no prior incontinence did not worsen. Recurrence rate in this study was 5.7%.27 Another study reported similar findings for 70 patients with 81 months of follow-up, with improvement in fecal continence after surgery and a recurrence rate of 8.6%.28 These studies suggest that sphincter repair in the setting of primary fistulotomy is a viable option for complex fistulas. However, it should be noted that this is a very specialized technique, and it is practiced in relatively few centers internationally.
Endorectal Advancement Flap
The endorectal advancement flap involves mobilizing a partial-thickness flap comprising rectal mucosa, submucosa, and some muscle fibers. To ensure adequate blood supply, the base should be wider than the tip with at least a 2:1 ratio. The fistula tract can then be either cored out or curetted to remove epithelial lining and any residual debris, followed by suture closure of the internal opening and placement of the advancement flap over the defect, with care taken to avoid tension. Partial-thickness flaps appear to be better than full-thickness flaps according to a review of a total of 1,654 patients.29 As with other modalities, reported success rates are from small series and vary widely. Primary healing rates most commonly range between 65 and 93%.30 31 32
It remains unclear whether or not a draining seton is required prior to the flap.33 34 35 As many studies are not randomized, this is a difficult question to answer, as bias may be present in series that use setons selectively, reserving this extra step for the more complex fistulas. It is the authors' belief that with any evidence of ongoing inflammation or sepsis, preoperative drainage with a seton is beneficial.
If the first attempt fails, it is reasonable to try a second advancement flap. Mitalas et al reported their experience in 26 patients with redo advancement flaps, with a healing rate of 69% on the second attempt.36 When combined with those successfully treated during the first attempt, healing rates were >90%. Furthermore, the same study suggested the effect of an endorectal advancement flap on continence seems to be minimal.
Injection of fibrin glue was initially advocated as a treatment to use in conjunction with advancement flap. However, this hybrid approach was associated with increased recurrence compared with flap alone in case series.37 38 It was thought that the glue prevented adequate drainage of the fistula tract during healing, explaining the high failure rates.
Advancement flaps may also be used in Crohn disease, albeit with more limited success. It should not be performed in the setting of active mucosal disease. Published results suggest a fistula recurrence risk of 46 to 57%, even in the setting of a diverting stoma in a significant portion of patients.39 40
Dermal Advancement Flap/Island Advancement Flap Anoplasty
Endorectal advancement flaps have been associated with symptomatic mucosal ectropions, and are also thought to be more technically challenging when there is dense scarring within the anal canal. For these reasons, an advancement flap moving in from the outside of the anal canal has been developed. This technique, known as the dermal advancement flap or island advancement flap anoplasty, was initially borrowed from studies focusing on the treatment of anal stenosis, but has proven to be a viable alternative for definitive anal fistula management as well.
Several different flap configurations have been described, including V-Y flaps, Y-V flaps, house flaps, S-flaps, and many more.41 The typical approach involves debridement of the mucosa overlying the internal opening, debridement of the fistula tract and closure of the internal opening, and mobilization of a pedicled flap from the anal margin with advancement into the anal canal, once again with care taken to avoid tension or tissue devascularization.
Reported success rates are mostly in the 70 to 80% range.42 43 44 Most case series are small, so results must be interpreted accordingly. The success rates are not as good if patients have failed prior repairs.43 Data are mixed once again as to the effect on continence rates.45 46 Overall, the dermal advancement flap appears to have functional outcomes and healing rates similar to the endorectal advancement flap, and is an appropriate first-line therapy for patients with complex anal fistulas.
Fibrin Sealants
Treatment of anal fistulas with fibrin glue injection gained significant popularity during the technique's infancy, as it seemed to be a simple and well-tolerated approach to a complex problem. The technique consists of filling the fistula tract with the glue, and is often accompanied by tract debridement and/or suture closure of the internal opening. The glue allows for a provisional matrix formation during healing. It provides a plug to prevent fecal contamination and a scaffold for native tissue ingrowth.47
Many small case series were subsequently reported, most of which showed a primary healing rate of 30 to 60%, with lower healing rates reported within the larger series with longer periods of follow-up.48 49 50 51 A prospective study looking at long-term healing rates found that only 14% of patients experienced enduring freedom from fistula at 16 months after glue injection.52
In general, the use of fibrin glue and other sealants has fallen out of favor for the treatment of complex fistulas, but the ASCRS practice parameters still list it as an acceptable approach to fistulas, giving it a grade of recommendation of 2C.3 Some practitioners may try fibrin glue as an initial modality in complex fistulas as it does not alter any anatomy. It has been the author's experience, however, that if this fails (as it usually does), the definitive treatment is subsequently more difficult.
Fistula Plug
The anal fistula plug (AFP) technique was first described in 2004, with the first case series published in 2006.53 The technique involves debridement of the fistula tract followed by placement of a bioprosthetic plug within the tract, securing it at the internal opening and cutting it flush with the skin at the external opening. It was initially described using a rolled-up piece of biologic material, but was later studied with the commercially available Surgisis AFP (Cook Medical, Canton, IN), which is a bioabsorbable scaffold made of porcine submucosa. The technique gained instant favor due to its simplicity, ease of performance, lack of disturbance to the surrounding tissue, and relatively high patient tolerance.
The initial reported healing rates for AFP were 85 to 87%.53 54 However, these rates did not survive more rigorous study, and subsequent case series were unable to reproduce these high rates of success. Most studies with an adequate period of follow-up reported healing rates below 50%, with some as low as 24%.3 55 56 57 58 There are also data that argue against repeat plug placement after initial plug failure, showing that such an approach is very unlikely to be successful.59 Success rates for complex fistula ranged from 35 to 87%.
Recently, a second commercially available AFP has been introduced. The Gore Bio-A fistula plug (Gore Medical, Flagstaff, AZ) is a synthetic bioabsorbable scaffold that acts in a manner similar to the Surgisis plug, but for a reportedly cheaper price, and with the addition of several “tentacles” that can be removed or adjusted in a fashion tailored to the size and number of fistula tracts.60 Initial primary healing with this plug was reported at 69%,61 but a subsequent multicenter case series reported a 12-month healing rate of 49%.60
Overall, the AFP appears to be an acceptable approach to complex anal fistulas, as it is generally well tolerated with minimal side effects. However, failure rates are high, and the patient must be counseled accordingly.
Ligation of Intersphincteric Fistula Tract
The technique that has perhaps gained the most traction in recent years is the LIFT procedure. First described in 1993 by Dr. Robin Phillips,62 it gained popularity after it was revised and reborn in 2007 by Dr. Arun Rojanasakul.63 The LIFT technique involves dissection into the intersphincteric groove, identification and encirclement of the transsphincteric fistula tract, ligation and division of the tract (plus or minus short tract excision), and debridement of the external opening. The intersphincteric incision is then closed loosely.
Several variations of the LIFT technique exist. Some authors recommend routine seton placement prior to LIFT,64 while others use setons selectively. The classic technique does not involve suturing within the anal canal, but other series have advocated for debridement and closure of the internal opening within the anal canal.65
There have now been over 30 publications discussing LIFT within the past 9 years, with healing rates that range from 40 to 95%.64 65 66 67 68 In general, these case series have low rates of minor complications, without reports of any major complications.67 Median follow-up in these studies ranged from 6 to 78 weeks, with a median healing time of 8 weeks (range: 2–24), with recurrences typically occurring 2 to 8 months postprocedure.66 67 68 Functional outcomes after LIFT have been excellent, with minimal reported disturbances in fecal continence. Wexner Fecal Incontinence Scores do not appear to be affected by LIFT (median: 0–1),69 and anorectal manometry also shows no significant change after LIFT.70
After LIFT failure, a repeat LIFT appears to be an appropriate approach, with a healing rate of 54% (7/13 patients) in one series.64 Of note, early reports suggested that LIFTs tend to fail within the intersphincteric space, thus downgrading the fistula from transsphincteric to intersphincteric. However, more recent reports suggest that this downgrading occurs only 10 to 16% of the time.64 65
LIFT is also an acceptable approach to patients with Crohn disease, with a primary healing rate of 67% at 1 year of follow-up.71 LIFT has been compared with endorectal advancement flap in two separate randomized controlled trials, both of which showed the two procedures to have equivalent healing rates16 72 LIFT has also been suggested for other fistulas including rectovaginal fistula, but no high-quality data exist on this topic.
Several additions to the LIFT procedure have been suggested, including the addition of a bioprosthetic mesh to the intersphincteric groove (BioLIFT73), performing simultaneous LIFT and endorectal advancement flap,74 and the addition of a fistula plug going from the intersphincteric groove to the external opening (LIFT-Plug75). While the results of these techniques are promising, the case series are small, and there is no definitive benefit over the more traditional LIFT approach.
Emerging Technologies for Fistula Treatment
One of the newest technologies on the market is the use of a laser to ablate the fistula tract. This FiLaC system uses a radial emitting laser probe to cause denaturation, scarring, and shrinkage of tissue along the fistula. This technique was presented as a video at the 2014 ASCRS Annual Meeting,76 and there has been a single published case series as well.77 In this series of 45 patients, the primary healing rate was 71% at a median follow-up of 30 months, with no reported disturbances in fecal continence.
Video-assisted anal fistula treatment (VAAFT) is a similar technique that destroys the fistula tract under direct visualization and allows closure of the internal opening. It may be good for complex fistulas as the fistula tract can be navigated under direct vision and it does not involve cutting tissue, thus preserving sphincter function. Studies are limited, with some initial reports describing a 13 to 16% recurrence rate.78 79 Certainly, the FiLaC and VAAFT approaches to complex fistulas are interesting, but more robust data are warranted prior to widespread adoption of these techniques.
Fistula after Ileal Pouch-Anal Anastomosis
Anal fistulas after ileal pouch-anal anastomosis are particularly complex, usually resulting from an anastomotic defect. Gaertner et al80 describe their experience with several repairs including gracilis muscle flap, fistula plug, advancement flap, glue, seton, Martius flap (bulbocavernosus), or fistulotomy. Healing rates were only 64% in this study, and required an average of 2.8 local procedures to eradicate the fistula. The healing rate with complex or multiple fistulas was 0%. Pouch-related fistulas have a high failure rate regardless of chosen intervention, and they continue to challenge surgeons who deal with these complex problem.
Summary
There are multiple techniques available for the repair of complex anal fistulas. The best technique is not known, and the available evidence suffers from a lack of high-quality data, with very few large randomized studies. The technique of choice will depend on appropriate delineation of the anatomy, surgeon preference, and familiarity with the different techniques. In general, failure is common, and one should be prepared to perform multiple procedures if required. The authors propose an algorithm for management of complex anal fistula, which was modified from a published version by Dudukgian and Abcarian1 (Fig. 1).
Fig. 1.
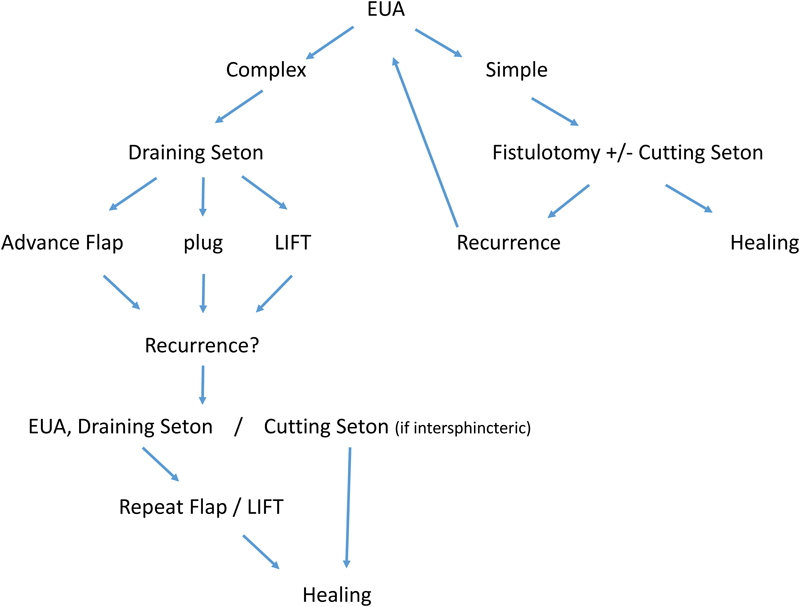
Algorithm for the management of anal fistula based on complexity. EUA: examination under anesthesia; LIFT: ligation of intersphincteric fistula tract.
References
- 1.Dudukgian H, Abcarian H. Why do we have so much trouble treating anal fistula? World J Gastroenterol. 2011;17(28):3292–3296. doi: 10.3748/wjg.v17.i28.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parks A G. Pathogenesis and treatment of fistuila-in-ano. BMJ. 1961;1(5224):463–469. doi: 10.1136/bmj.1.5224.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steele S R Kumar R Feingold D L Rafferty J L Buie W D; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of perianal abscess and fistula-in-ano Dis Colon Rectum 201154121465–1474. [DOI] [PubMed] [Google Scholar]
- 4.Ortega A E, Cologne K G. New York: Springer New York; 2014. Classification and treatment of anorectal infections; pp. 17–26. [Google Scholar]
- 5.Ratto C, Grillo E, Parello A, Costamagna G, Doglietto G B. Endoanal ultrasound-guided surgery for anal fistula. Endoscopy. 2005;37(8):722–728. doi: 10.1055/s-2005-870155. [DOI] [PubMed] [Google Scholar]
- 6.Nagendranath C, Saravanan M N, Sridhar C, Varughese M. Peroxide-enhanced endoanal ultrasound in preoperative assessment of complex fistula-in-ano. Tech Coloproctol. 2014;18(5):433–438. doi: 10.1007/s10151-013-1067-y. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan G N, Halligan S, Bartram C I, Williams A B, Tarroni D, Cohen C R. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology. 2004;233(3):674–681. doi: 10.1148/radiol.2333031724. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan G, Halligan S, Williams A. et al. Effect of MRI on clinical outcome of recurrent fistula-in-ano. Lancet. 2002;360(9346):1661–1662. doi: 10.1016/S0140-6736(02)11605-9. [DOI] [PubMed] [Google Scholar]
- 9.Gage K L, Deshmukh S, Macura K J, Kamel I R, Zaheer A. MRI of perianal fistulas: bridging the radiological-surgical divide. Abdom Imaging. 2013;38(5):1033–1042. doi: 10.1007/s00261-012-9965-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahni V A, Ahmad R, Burling D. Which method is best for imaging of perianal fistula? Abdom Imaging. 2008;33(1):26–30. doi: 10.1007/s00261-007-9309-y. [DOI] [PubMed] [Google Scholar]
- 11.Sileri P, Cadeddu F, D'Ugo S. et al. Surgery for fistula-in-ano in a specialist colorectal unit: a critical appraisal. BMC Gastroenterol. 2011;11:120. doi: 10.1186/1471-230X-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasilevsky C A, Gordon P H. The incidence of recurrent abscesses or fistula-in-ano following anorectal suppuration. Dis Colon Rectum. 1984;27(2):126–130. doi: 10.1007/BF02553995. [DOI] [PubMed] [Google Scholar]
- 13.McElwain J W, MacLean M D, Alexander R M, Hoexter B, Guthrie J F. Anorectal problems: experience with primary fistulectomy for anorectal abscess, a report of 1,000 cases. Dis Colon Rectum. 1975;18(8):646–649. doi: 10.1007/BF02604266. [DOI] [PubMed] [Google Scholar]
- 14.Ramanujam P S, Prasad M L, Abcarian H, Tan A B. Perianal abscesses and fistulas. A study of 1023 patients. Dis Colon Rectum. 1984;27(9):593–597. doi: 10.1007/BF02553848. [DOI] [PubMed] [Google Scholar]
- 15.Ramanujam P S, Prasad M L, Abcarian H. The role of seton in fistulotomy of the anus. Surg Gynecol Obstet. 1983;157(5):419–422. [PubMed] [Google Scholar]
- 16.Mushaya C, Bartlett L, Schulze B, Ho Y H. Ligation of intersphincteric fistula tract compared with advancement flap for complex anorectal fistulas requiring initial seton drainage. Am J Surg. 2012;204(3):283–289. doi: 10.1016/j.amjsurg.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Aboulian A, Kaji A H, Kumar R R. Early result of ligation of the intersphincteric fistula tract for fistula-in-ano. Dis Colon Rectum. 2011;54(3):289–292. doi: 10.1007/DCR.0b013e318203495d. [DOI] [PubMed] [Google Scholar]
- 18.Regelation Available at: http://en.wikipedia.org/wiki/Regelation. Accessed May 24, 2015
- 19.García-Aguilar J, Belmonte C, Wong D W, Goldberg S M, Madoff R D. Cutting seton versus two-stage seton fistulotomy in the surgical management of high anal fistula. Br J Surg. 1998;85(2):243–245. doi: 10.1046/j.1365-2168.1998.02877.x. [DOI] [PubMed] [Google Scholar]
- 20.Isbister W H, Al Sanea N. The cutting seton: an experience at King Faisal Specialist Hospital. Dis Colon Rectum. 2001;44(5):722–727. doi: 10.1007/BF02234574. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie R D, Sackier J M, Hodde J P. Incontinence rates after cutting seton treatment for anal fistula. Colorectal Dis. 2009;11(6):564–571. doi: 10.1111/j.1463-1318.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 22.Chuang-Wei C, Chang-Chieh W, Cheng-Wen H, Tsai-Yu L, Chun-Che F, Shu-Wen J. Cutting seton for complex anal fistulas. Surgeon. 2008;6(3):185–188. doi: 10.1016/s1479-666x(08)80117-5. [DOI] [PubMed] [Google Scholar]
- 23.Kamrava A, Collins J C. A decade of selective use of adjustable cutting seton combined with fistulotomy for anal fistula. Am Surg. 2011;77(10):1377–1380. [PubMed] [Google Scholar]
- 24.Atkin G K, Martins J, Tozer P, Ranchod P, Phillips R K. For many high anal fistulas, lay open is still a good option. Tech Coloproctol. 2011;15(2):143–150. doi: 10.1007/s10151-011-0676-6. [DOI] [PubMed] [Google Scholar]
- 25.Tozer P, Sala S, Cianci V. et al. Fistulotomy in the tertiary setting can achieve high rates of fistula cure with an acceptable risk of deterioration in continence. J Gastrointest Surg. 2013;17(11):1960–1965. doi: 10.1007/s11605-013-2198-1. [DOI] [PubMed] [Google Scholar]
- 26.Ratto C, Litta F, Parello A, Zaccone G, Donisi L, De Simone V. Fistulotomy with end-to-end primary sphincteroplasty for anal fistula: results from a prospective study. Dis Colon Rectum. 2013;56(2):226–233. doi: 10.1097/DCR.0b013e31827aab72. [DOI] [PubMed] [Google Scholar]
- 27.Perez F, Arroyo A, Serrano P, Candela F, Sanchez A, Calpena R. Fistulotomy with primary sphincter reconstruction in the management of complex fistula-in-ano: prospective study of clinical and manometric results. J Am Coll Surg. 2005;200(6):897–903. doi: 10.1016/j.jamcollsurg.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Arroyo A, Pérez-Legaz J, Moya P. et al. Fistulotomy and sphincter reconstruction in the treatment of complex fistula-in-ano: long-term clinical and manometric results. Ann Surg. 2012;255(5):935–939. doi: 10.1097/SLA.0b013e31824e9112. [DOI] [PubMed] [Google Scholar]
- 29.Soltani A, Kaiser A M. Endorectal advancement flap for cryptoglandular or Crohn's fistula-in-ano. Dis Colon Rectum. 2010;53(4):486–495. doi: 10.1007/DCR.0b013e3181ce8b01. [DOI] [PubMed] [Google Scholar]
- 30.van Onkelen R S, Gosselink M P, Schouten W R. Is it possible to improve the outcome of transanal advancement flap repair for high transsphincteric fistulas by additional ligation of the intersphincteric fistula tract? Dis Colon Rectum. 2012;55(2):163–166. doi: 10.1097/DCR.0b013e31823c0f74. [DOI] [PubMed] [Google Scholar]
- 31.Schouten W R Zimmerman D D Briel J W Transanal advancement flap repair of transsphincteric fistulas Dis Colon Rectum 199942111419–1422., discussion 1422–1423 [DOI] [PubMed] [Google Scholar]
- 32.Ortíz H, Marzo J. Endorectal flap advancement repair and fistulectomy for high trans-sphincteric and suprasphincteric fistulas. Br J Surg. 2000;87(12):1680–1683. doi: 10.1046/j.1365-2168.2000.01582.x. [DOI] [PubMed] [Google Scholar]
- 33.Sonoda T, Hull T, Piedmonte M R, Fazio V W. Outcomes of primary repair of anorectal and rectovaginal fistulas using the endorectal advancement flap. Dis Colon Rectum. 2002;45(12):1622–1628. doi: 10.1007/s10350-004-7249-y. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman D D, Delemarre J B, Gosselink M P, Hop W C, Briel J W, Schouten W R. Smoking affects the outcome of transanal mucosal advancement flap repair of trans-sphincteric fistulas. Br J Surg. 2003;90(3):351–354. doi: 10.1002/bjs.4044. [DOI] [PubMed] [Google Scholar]
- 35.van Koperen P J, Wind J, Bemelman W A, Bakx R, Reitsma J B, Slors J F. Long-term functional outcome and risk factors for recurrence after surgical treatment for low and high perianal fistulas of cryptoglandular origin. Dis Colon Rectum. 2008;51(10):1475–1481. doi: 10.1007/s10350-008-9354-9. [DOI] [PubMed] [Google Scholar]
- 36.Mitalas L E, Gosselink M P, Zimmerman D D, Schouten W R. Repeat transanal advancement flap repair: impact on the overall healing rate of high transsphincteric fistulas and on fecal continence. Dis Colon Rectum. 2007;50(10):1508–1511. doi: 10.1007/s10350-007-9015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Koperen P J, Wind J, Bemelman W A, Slors J F. Fibrin glue and transanal rectal advancement flap for high transsphincteric perianal fistulas; is there any advantage? Int J Colorectal Dis. 2008;23(7):697–701. doi: 10.1007/s00384-008-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis C N, Clark S. Fibrin glue as an adjunct to flap repair of anal fistulas: a randomized, controlled study. Dis Colon Rectum. 2006;49(11):1736–1740. doi: 10.1007/s10350-006-0718-8. [DOI] [PubMed] [Google Scholar]
- 39.Makowiec F, Jehle E C, Becker H D, Starlinger M. Clinical course after transanal advancement flap repair of perianal fistula in patients with Crohn's disease. Br J Surg. 1995;82(5):603–606. doi: 10.1002/bjs.1800820509. [DOI] [PubMed] [Google Scholar]
- 40.Mizrahi N, Wexner S D, Zmora O. et al. Endorectal advancement flap: are there predictors of failure? Dis Colon Rectum. 2002;45(12):1616–1621. doi: 10.1097/01.DCR.0000037654.01119.CD. [DOI] [PubMed] [Google Scholar]
- 41.Farid M, Youssef M, El Nakeeb A, Fikry A, El Awady S, Morshed M. Comparative study of the house advancement flap, rhomboid flap, and y-v anoplasty in treatment of anal stenosis: a prospective randomized study. Dis Colon Rectum. 2010;53(5):790–797. doi: 10.1007/DCR.0b013e3181d3205a. [DOI] [PubMed] [Google Scholar]
- 42.Del Pino A, Nelson R L, Pearl R K, Abcarian H. Island flap anoplasty for treatment of transsphincteric fistula-in-ano. Dis Colon Rectum. 1996;39(2):224–226. doi: 10.1007/BF02068080. [DOI] [PubMed] [Google Scholar]
- 43.Ellis C N, Clark S. Effect of tobacco smoking on advancement flap repair of complex anal fistulas. Dis Colon Rectum. 2007;50(4):459–463. doi: 10.1007/s10350-006-0829-2. [DOI] [PubMed] [Google Scholar]
- 44.Nelson R L, Cintron J, Abcarian H. Dermal island-flap anoplasty for transsphincteric fistula-in-ano: assessment of treatment failures. Dis Colon Rectum. 2000;43(5):681–684. doi: 10.1007/BF02235588. [DOI] [PubMed] [Google Scholar]
- 45.Sungurtekin U, Sungurtekin H, Kabay B. et al. Anocutaneous V-Y advancement flap for the treatment of complex perianal fistula. Dis Colon Rectum. 2004;47(12):2178–2183. doi: 10.1007/s10350-004-0744-3. [DOI] [PubMed] [Google Scholar]
- 46.Hossack T, Solomon M J, Young J M. Ano-cutaneous flap repair for complex and recurrent supra-sphincteric anal fistula. Colorectal Dis. 2005;7(2):187–192. doi: 10.1111/j.1463-1318.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 47.Singer M, Cintron J. New techniques in the treatment of common perianal diseases: stapled hemorrhoidopexy, botulinum toxin, and fibrin sealant. Surg Clin North Am. 2006;86(4):937–967. doi: 10.1016/j.suc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Loungnarath R, Dietz D W, Mutch M G, Birnbaum E H, Kodner I J, Fleshman J W. Fibrin glue treatment of complex anal fistulas has low success rate. Dis Colon Rectum. 2004;47(4):432–436. doi: 10.1007/s10350-003-0076-8. [DOI] [PubMed] [Google Scholar]
- 49.Singer M, Cintron J, Nelson R. et al. Treatment of fistulas-in-ano with fibrin sealant in combination with intra-adhesive antibiotics and/or surgical closure of the internal fistula opening. Dis Colon Rectum. 2005;48(4):799–808. doi: 10.1007/s10350-004-0898-z. [DOI] [PubMed] [Google Scholar]
- 50.Zmora O, Neufeld D, Ziv Y. et al. Prospective, multicenter evaluation of highly concentrated fibrin glue in the treatment of complex cryptogenic perianal fistulas. Dis Colon Rectum. 2005;48(12):2167–2172. doi: 10.1007/s10350-005-0199-1. [DOI] [PubMed] [Google Scholar]
- 51.Witte M E, Klaase J M, Gerritsen J J, Kummer E W. Fibrin glue treatment for simple and complex anal fistulas. Hepatogastroenterology. 2007;54(76):1071–1073. [PubMed] [Google Scholar]
- 52.Buchanan G N, Bartram C I, Phillips R K. et al. Efficacy of fibrin sealant in the management of complex anal fistula: a prospective trial. Dis Colon Rectum. 2003;46(9):1167–1174. doi: 10.1007/s10350-004-6708-9. [DOI] [PubMed] [Google Scholar]
- 53.Johnson E K, Gaw J U, Armstrong D N. Efficacy of anal fistula plug vs. fibrin glue in closure of anorectal fistulas. Dis Colon Rectum. 2006;49(3):371–376. doi: 10.1007/s10350-005-0288-1. [DOI] [PubMed] [Google Scholar]
- 54.Champagne B J, O'Connor L M, Ferguson M, Orangio G R, Schertzer M E, Armstrong D N. Efficacy of anal fistula plug in closure of cryptoglandular fistulas: long-term follow-up. Dis Colon Rectum. 2006;49(12):1817–1821. doi: 10.1007/s10350-006-0755-3. [DOI] [PubMed] [Google Scholar]
- 55.Lewis R, Lunniss P J, Hammond T M. Novel biological strategies in the management of anal fistula. Colorectal Dis. 2012;14(12):1445–1455. doi: 10.1111/j.1463-1318.2012.03192.x. [DOI] [PubMed] [Google Scholar]
- 56.Ortiz H, Marzo J, Ciga M A, Oteiza F, Armendáriz P, de Miguel M. Randomized clinical trial of anal fistula plug versus endorectal advancement flap for the treatment of high cryptoglandular fistula in ano. Br J Surg. 2009;96(6):608–612. doi: 10.1002/bjs.6613. [DOI] [PubMed] [Google Scholar]
- 57.Ellis C N, Rostas J W, Greiner F G. Long-term outcomes with the use of bioprosthetic plugs for the management of complex anal fistulas. Dis Colon Rectum. 2010;53(5):798–802. doi: 10.1007/DCR.0b013e3181d43b7d. [DOI] [PubMed] [Google Scholar]
- 58.Adamina M, Ross T, Guenin M O. et al. Anal fistula plug: a prospective evaluation of success, continence and quality of life in the treatment of complex fistulae. Colorectal Dis. 2014;16(7):547–554. doi: 10.1111/codi.12594. [DOI] [PubMed] [Google Scholar]
- 59.Ky A J, Sylla P, Steinhagen R, Steinhagen E, Khaitov S, Ly E K. Collagen fistula plug for the treatment of anal fistulas. Dis Colon Rectum. 2008;51(6):838–843. doi: 10.1007/s10350-007-9191-2. [DOI] [PubMed] [Google Scholar]
- 60.Stamos M J, Snyder M, Robb B W. et al. Prospective multicenter study of a synthetic bioabsorbable anal fistula plug to treat cryptoglandular transsphincteric anal fistulas. Dis Colon Rectum. 2015;58(3):344–351. doi: 10.1097/DCR.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 61.Heydari A, Attinà G M, Merolla E, Piccoli M, Fazlalizadeh R, Melotti G. Bioabsorbable synthetic plug in the treatment of anal fistulas. Dis Colon Rectum. 2013;56(6):774–779. doi: 10.1097/DCR.0b013e3182839824. [DOI] [PubMed] [Google Scholar]
- 62.Matos D, Lunniss P J, Phillips R K. Total sphincter conservation in high fistula in ano: results of a new approach. Br J Surg. 1993;80(6):802–804. doi: 10.1002/bjs.1800800651. [DOI] [PubMed] [Google Scholar]
- 63.Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K. Total anal sphincter saving technique for fistula-in-ano; the ligation of intersphincteric fistula tract. J Med Assoc Thai. 2007;90(3):581–586. [PubMed] [Google Scholar]
- 64.Wallin U G, Mellgren A F, Madoff R D, Goldberg S M. Does ligation of the intersphincteric fistula tract raise the bar in fistula surgery? Dis Colon Rectum. 2012;55(11):1173–1178. doi: 10.1097/DCR.0b013e318266edf3. [DOI] [PubMed] [Google Scholar]
- 65.Liu W Y, Aboulian A, Kaji A H, Kumar R R. Long-term results of ligation of intersphincteric fistula tract (LIFT) for fistula-in-ano. Dis Colon Rectum. 2013;56(3):343–347. doi: 10.1097/DCR.0b013e318278164c. [DOI] [PubMed] [Google Scholar]
- 66.Yassin N A, Hammond T M, Lunniss P J, Phillips R K. Ligation of the intersphincteric fistula tract in the management of anal fistula. A systematic review. Colorectal Dis. 2013;15(5):527–535. doi: 10.1111/codi.12224. [DOI] [PubMed] [Google Scholar]
- 67.Alasari S, Kim N K. Overview of anal fistula and systematic review of ligation of the intersphincteric fistula tract (LIFT) Tech Coloproctol. 2014;18(1):13–22. doi: 10.1007/s10151-013-1050-7. [DOI] [PubMed] [Google Scholar]
- 68.Madbouly K M, El Shazly W, Abbas K S, Hussein A M. Ligation of intersphincteric fistula tract versus mucosal advancement flap in patients with high transsphincteric fistula-in-ano: a prospective randomized trial. Dis Colon Rectum. 2014;57(10):1202–1208. doi: 10.1097/DCR.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 69.Madbouly K M, El Shazly W, Abbas K S, Hussein A M. Ligation of intersphincteric fistula tract versus mucosal advancement flap in patients with high transsphincteric fistula-in-ano: a prospective randomized trial. Dis Colon Rectum. 2014;57(10):1202–1208. doi: 10.1097/DCR.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 70.Tsunoda A, Sada H, Sugimoto T, Nagata H, Kano N. Anal function after ligation of the intersphincteric fistula tract. Dis Colon Rectum. 2013;56(7):898–902. doi: 10.1097/DCR.0b013e31828d2e29. [DOI] [PubMed] [Google Scholar]
- 71.Gingold D S, Murrell Z A, Fleshner P R. A prospective evaluation of the ligation of the intersphincteric tract procedure for complex anal fistula in patients with Crohn's disease. Ann Surg. 2014;260(6):1057–1061. doi: 10.1097/SLA.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 72.Tan K K, Alsuwaigh R, Tan A M. et al. To LIFT or to flap? Which surgery to perform following seton insertion for high anal fistula? Dis Colon Rectum. 2012;55(12):1273–1277. doi: 10.1097/DCR.0b013e31826dbff0. [DOI] [PubMed] [Google Scholar]
- 73.Ellis C N. Outcomes with the use of bioprosthetic grafts to reinforce the ligation of the intersphincteric fistula tract (BioLIFT procedure) for the management of complex anal fistulas. Dis Colon Rectum. 2010;53(10):1361–1364. doi: 10.1007/DCR.0b013e3181ec4470. [DOI] [PubMed] [Google Scholar]
- 74.van Onkelen R S, Gosselink M P, Schouten W R. Is it possible to improve the outcome of transanal advancement flap repair for high transsphincteric fistulas by additional ligation of the intersphincteric fistula tract? Dis Colon Rectum. 2012;55(2):163–166. doi: 10.1097/DCR.0b013e31823c0f74. [DOI] [PubMed] [Google Scholar]
- 75.Han J G, Yi B Q, Wang Z J. et al. Ligation of the intersphincteric fistula tract plus a bioprosthetic anal fistula plug (LIFT-Plug): a new technique for fistula-in-ano. Colorectal Dis. 2013;15(5):582–586. doi: 10.1111/codi.12062. [DOI] [PubMed] [Google Scholar]
- 76.FiLaC [video online] Available at: https://www.youtube.com/watch?v=8M-k0zEx1jo. Accessed May 24, 2015
- 77.Giamundo P, Esercizio L, Geraci M, Tibaldi L, Valente M. Fistula-tract Laser Closure (FiLaC™): long-term results and new operative strategies. Tech Coloproctol. 2015;19(8):449–453. doi: 10.1007/s10151-015-1282-9. [DOI] [PubMed] [Google Scholar]
- 78.Meinero P, Mori L. Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving procedure for treating complex anal fistulas. Tech Coloproctol. 2011;15(4):417–422. doi: 10.1007/s10151-011-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kochhar G Saha S Andley M et al. Video-assisted anal fistula treatment JSLS 2014183e2014.00127. doi:10.4293/JSLS.2014.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaertner W B, Witt J, Madoff R D, Mellgren A, Finne C O, Spencer M P. Ileal pouch fistulas after restorative proctocolectomy: management and outcomes. Tech Coloproctol. 2014;18(11):1061–1066. doi: 10.1007/s10151-014-1197-x. [DOI] [PubMed] [Google Scholar]


