Abstract
The aim of this work was to test the effectiveness of using enzymatically deantigenated equine bone block as a scaffold for guided bone regeneration (GBR) during a horizontal augmentation of the lower jaw. A partially edentulous atrophic mandible was augmented using an equine-derived block with an expanded polytetrafluoroethylene membrane. After 8.5 months, two bone core samples were collected at the augmentation site, and implants were placed. A definitive prosthesis delivered 6 months after implant placement provided excellent functional and aesthetic rehabilitation throughout the follow-up period. Histological and histomorphometrical analysis of the biopsies showed newly formed bone to be present and the residual biomaterial was still undergoing remodeling. Comparison of cone beam computed tomography scans taken before augmentation and 26 months later showed maintenance of ridge width and possible corticalization of the vestibular augmented ridge side. The equine-derived bone block placed in accordance with GBR principles provided a successful clinical, radiographic, and histological outcome.
Keywords: equine bone, guided bone regeneration, heterologous bone
Prosthetic-driven implantology implies the placement of implants as determined by the definitive prosthesis and not vice versa. This approach poses challenges when the patient has atrophic ridges. A lack of sufficient bone height and/or width, together with the need to place implants according to a predesigned rehabilitation plan, often makes bone regeneration unavoidable.
Various procedures can be used to augment bone successfully. In cases of severe atrophy, onlay augmentation using autogenous bone blocks has been shown to be predictable.1 Autogenous bone contains bone cells (osteoblasts, osteocytes, and stem cells) and growth factors that favor bone regeneration. However, harvesting autogenous blocks requires surgery and increases both morbidity and the risk of intra- and postsurgical complications.2 3 4
An alternative approach is to apply guided bone regeneration (GBR). The underlying principle of GBR is that bone cells have slower migration and proliferation rates than soft tissue cells, such as epithelial cells and fibroblasts.5 6 The GBR approach therefore calls for the application of a long-lasting membrane to prevent soft tissue cells from invading the regenerating site. Osteoprogenitors can then populate the site, differentiate, and form new bone tissue.7 8
GBR membrane materials vary. Collagen-based membranes are biocompatible and easily handled but, given their fast degradation rate, exhibit poor mechanical properties and stability.9 10 At present, expanded polytetrafluoroethylene (e-PTFE) barriers are used most commonly in GBR procedures. Such barriers show predictable outcomes, even though the fact that they are nonresorbable may increase the risk of wound dehiscence. Alternatively, titanium meshes may also provide predictable and reproducible results.11 12
Although the original GBR technique only envisions isolating the regenerating site from the surrounding soft tissues, a further development calls for placement of bone-graft material under the membrane to provide a scaffold for osteoprogenitor cells and blood vessels. In most cases, the graft material used is autologous bone collected from intraoral sites such as the chin,13 mandibular symphysis,14 15 16 and ramus,17 or from extraoral sites such as the iliac crest,2 calvaria, or tibia.18 19 Due to the increased risks for the patient and the limited availability of autogenous bone at intraoral sites, however, the use of alternative graft materials is highly advisable.
Both synthetic and natural biomaterials are available for this purpose.1 Among the latter, bone-graft materials processed from the bone of a mammal species to make it non-antigenic are regarded as the most promising, due to the similarities of bone architecture and collagen composition between human bone tissue and that of some other mammals. Among such xenografts, deproteinized bovine bone has been extensively studied, often in combination with GBR membranes.20 21 Thermally deproteinized bovine bone shows good osteoconductive properties but may also have a low resorption capacity.22 In particulate form, this material is used successfully with collagen membranes for horizontal crest increases.23 24 However, the same material in block form has a lower osteoconductive capacity when used in both vertical25 and lateral26 27 bone-augmentation procedures. Histological examination of such blocks has found them to be surrounded by connective tissue, with only a small quantity of newly formed bone at the base of the graft.26 28
An alternative xenograft is an enzymatically deantigenated form of equine bone. The enzymatic process preserves the type I bone collagen in its native, nondenatured state, which should allow for an improved bone-regeneration process, given collagen's well-known biological properties.29 30 31 32 33 34 35 36 Osteoclasts cultured over such equine, enzymatically deantigenated and collagen-preserving bone substitutes have significantly higher adhesion and activity than that found for osteoclasts grown over deproteinized bovine bone.37 38 The collagen component also gives the equine bone an elasticity that allows it more easily to be shaped to fit the defect and secured to the receiving bone surface by means of titanium osteosynthesis screws. Moreover, when sites augmented with equine bone alone are compared with others augmented with the same material added to autogenous bone, immunohistochemical tests shows no differences between the two regarding the expression of some biochemical markers of bone regeneration.39 Enzyme-deantigenic equine bone is used in clinical practice as a scaffold in bone regeneration of different bone defects40 41 42 and is applied in orthopedic regenerative surgery.43 It has been also recently used to treat cases in which implant thread exposure resulted from positioning44 and in cases of peri-implantitis resulting in significant bone loss.45
Partially demineralized blocks perform successfully in lateral ridge augmentation40 without any bone loss detectable on computed tomography (CT) scans with respect to the grafted volume. After 6 months, histological tests comparing bone cores collected from the grafted sites and nonregenerated adjacent sites show that newly formed bone in grafted sites is undistinguishable from bone undergoing remodeling in nonregenerated ones and that equine bone (∼30% of the core volume) is still undergoing remodeling.
The present article describes the treatment of a patient to increase the transverse thickness of the atrophic edentulous posterior mandibular ridge using a rigid enzymatically deantigenated equine bone block containing native type I collagen.
Materials and Methods
A 75-year-old partially edentulous patient presented, missing teeth #s #18, #19, and #20 according to the universal numbering system46 and asking for effective rehabilitation. The ridge featured Cawood and Howell class IV atrophy. A two-step procedure, calling for an onlay block ridge augmentation and consequent placement of two implants, was planned. The patient provided informed consent.
Cone beam CT (CBCT) scans were obtained, and the residual ridge was measured. A stereolithographic ridge model was prepared and used to shape a heterologous spongy bone block (Osteoxenon, OX52, Bioteck, Arcugnano, Italy), containing preserved bone collagen (Fig. 1a). After shaping, the block was sterilized by β-irradiation.
Fig. 1.
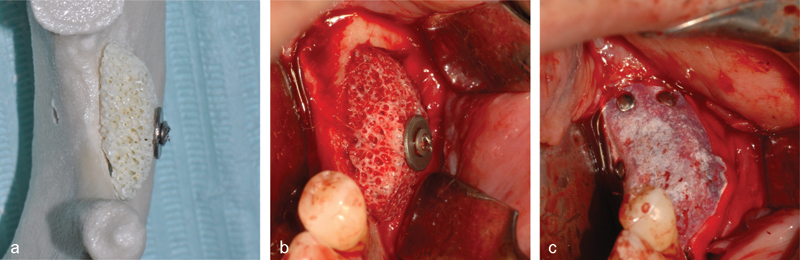
(a) The block adapted to the stereolithographic model of the jaw. (b) The block after placing and affixing it with an osteosynthesis screw. (c) The e-PTFE membrane placed according to GBR principles. e-PTFE, expanded polytetrafluoroethylene; GBR, guided bone regeneration.
Antibiotic prophylaxis (2 g amoxicillin/clavulanic acid) was prescribed to be taken 1 hour before surgery and then every 12 hours for 7 to 9 days, and the patient was subjected to mouth rinses with chlorhexidine 0.2%. Nimesulide (100 mg) was administered 1 hour before surgery and then twice a day for 5 days. Local anesthetic was administered by means of an infiltration with 1% articaine with adrenaline 1:100,000.
A full-thickness trapezoidal mucoperiosteal flap was detached to expose the bone ridge. After preparing the site with a bone scraper (Safe-twist, Meta, Reggio Emilia, Italy), the preshaped block was placed in position and affixed with an osteosynthesis screw (Fig. 1b). The block was then covered with a nonresorbable e-PTFE membrane (Gore-Tex Regenerative Membrane, Gore Medical, Flagstaff, AZ) (Fig. 1c) and secured with four pins (FRIOS Membrane Tacks, Dentsply, Rome, Italy).
At 8.5 months after grafting, the same antibiotic prophylaxis was begun, and another flap was raised. The membrane and pins were removed (Fig. 2a) and two bone samples were collected with a trephine, under irrigation, at implant placement sites corresponding to positions #19 and #20 (Fig. 2a, b). Two implants (Prime, Prodent Italia, Pero, Italy) were placed, and the osteosynthesis screw was removed (Fig. 2c).
Fig. 2.
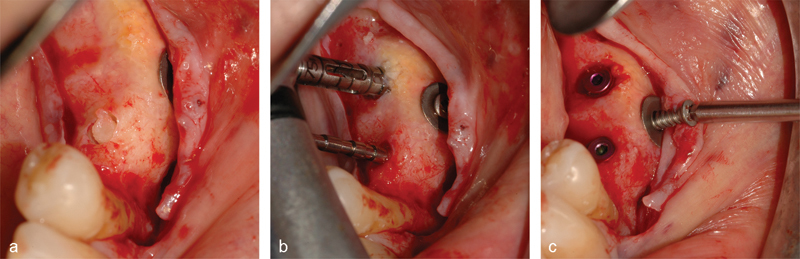
Bone biopsies collection and implant insertion. The bone block with the first bone core ready for extraction (a). A paralleling pin (b, lower) was inserted into the prepared implant tunnel to guide the drilling of the second one and the collection of the second bone core with a trephine blade (b, upper). Two implants were positioned and the osteosynthesis screw removed (c).
The implants were covered and allowed to heal for 3.5 months. They were then uncovered, and after additional 2 months, a provisional prosthesis was placed. One month after that, the definitive prosthesis was delivered (Fig. 3b). A radiograph was taken 5 months after the definitive prosthetic rehabilitation (Fig. 3c), and a follow-up CBCT scan was obtained 6 months after that, corresponding to 26 months after the grafting procedure (Fig. 4).
Fig. 3.
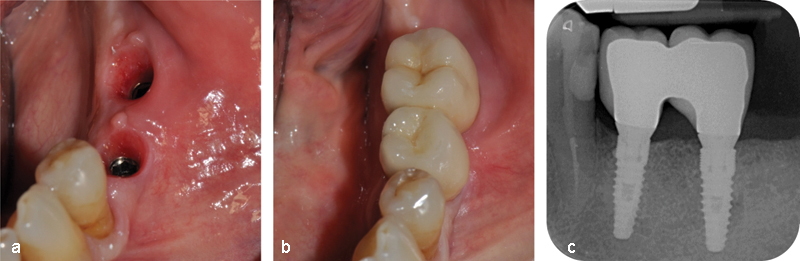
(a) Gum tissue after removal of the provisional prosthesis. (b) The definitive two-unit bridge after delivery. (c) Radiograph taken 5 months after delivery of the definitive prosthesis, showing the optimal peri-implant bone levels and the absence of any resorption cone around the implant heads.
Fig. 4.
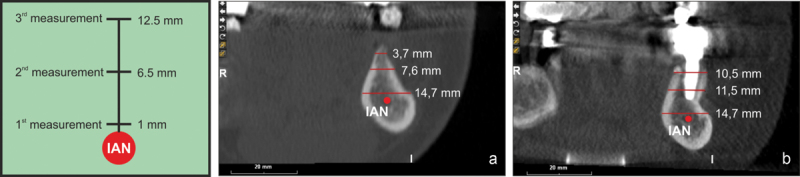
The CBCT scan obtained before augmentation (a) was compared with the scan taken 26 months later (b). The IAN was used as a reference point for the cortical bone thickness measurements, which were performed at a distance of 1, 6.5, and 12.5 mm from the IAN. (a) Before augmentation the ridge width ranged from 7.6 to 3.7 mm. (b) At 26 months after the augmentation surgery, the ridge width ranged from 11.5 to 10.5 mm. Regenerated volumes possibly display a cortical-like appearance on the vestibular side. CBCT, cone beam computed tomography; IAN, inferior alveolar nerve.
Histological Analysis
The bone cores were fixed in 4% formalin and decalcified for 21 days in a solution containing sodium formate 0.76 M and formic acid 1.6 M (Panreac Quimica, Barcelona, Spain). Subsequently, the sample was dehydrated in graded ethanol and embedded in paraffin. This procedure allowed for rapid infiltration of the tissue and the achievement of the right softness for cutting, with only minimal artifactual shrinking, thus providing a tissue morphology that was representative of the in vivo bone features. Five-µ-thick sections were obtained, mounted on slides, and stained with hematoxylin-eosin. Morphometrical measurements were performed on digital photomicrographs collected both at ×3.5 and ×10 magnification. Statistical analysis was performed using the GraphPad Prism 4.0 statistical program (GraphPad Software, San Diego, CA). All results are given as mean% ± standard deviation.
Results
The patient had no clinical symptoms during follow-up controls. At the time of bone core collection, the bone ridge volume appeared fully augmented, with no loss of volume as compared with site at the time of graft placement (Fig. 2a). All follow-up radiographs taken up to 2.2 years after graft placement showed optimal alveolar bone height and no cone resorption around the head of the implants (Fig. 3c).
The cortical bone thickness at the site of tooth #20 was measured by comparing the initial CBCT scan (taken before the augmentation surgery) to the one collected 26 months later. To ensure reproducibility of the measurements, the inferior alveolar nerve was located on the CBCT section, and the ridge thickness was measured at 1, 6.5, and 12.5 mm from the nerve (Fig. 4). The initial alveolar bone thickness ranged between 3.7 and 7.6 mm (Fig. 4a). The different degrees of translucency in the cross section may be caused by the different architecture and density of the external cortical layer (more white and opaque) and the inner spongy bone tissue (less white and more transparent). Twenty-six months after the graft, the alveolar bone thickness at the recipient site was properly retained (≈ 11 mm, Fig. 4b). Based on the different translucency of the tissues, the grafted bone seemed to have a physiological architecture, with recognizable cortical and trabecular compartments. This suggested that the spongy bone block used for the graft underwent a complete remodeling process.
Histological Analysis
As the bone cores were harvested completely in the augmented area (Fig. 2a, b), the histological findings of vital bone must be considered as newly formed bone. Newly formed vital bone—identified as acidophilic material, showing bone lacunae and osteocytes within—was found in all fields (Fig. 5) in both samples. Residual graft material was observed in both samples, with the particles being in strict contact with newly formed bone tissue, indicating the grafted material was biocompatible. Histomorphometrical analysis showed the following relative amounts: newly formed bone 36.76 ± 0.04%; residual biomaterial 38.20 ± 0.10%; and connective tissue/medullar spaces 25.04 ± 0.30%. The bone graft was, therefore, still undergoing remodeling and still acting as a space-maintaining material.
Fig. 5.
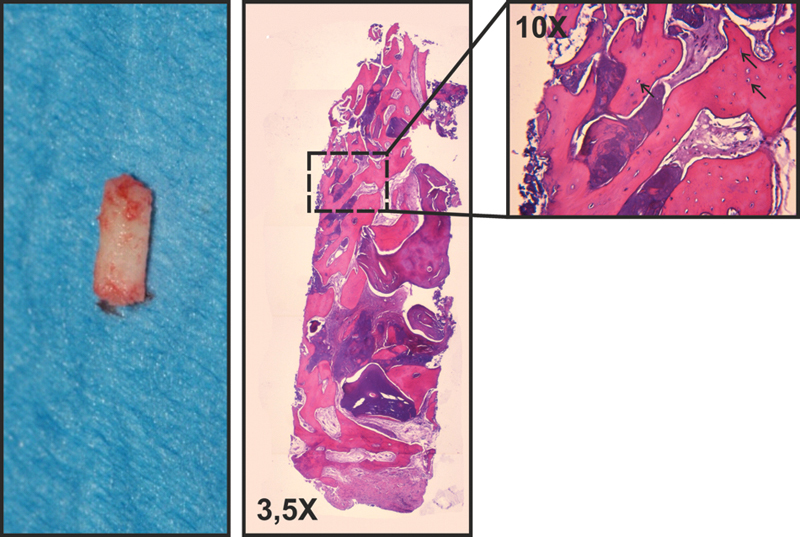
One of the two biopsies collected from the grafted site and the hematoxylin-eosin staining of the bioptic sample. The 10X magnification detail shows the absence of inflammatory reaction and the graft material undergoing remodeling; black arrows indicate the osteocytes into the newly formed bone.
Discussion
In the case described here, the equine bone block grafted according to GBR principles provided a highly satisfying clinical outcome, with the radiographic appearance confirming that peri-implant bone levels were maintained over time. Both the implants and prosthesis functioned perfectly throughout the entire observation period. The radiographic analysis at the final follow-up time indicated that both implants were osseointegrated and surrounded by a bone-like, structured tissue suggesting the bone block, which was still remodeling at implant placement, had possibly undergone complete replacement with newly formed bone. Comparison of the presurgical CBCT scan with the 26-month follow-up scan showed no horizontal resorption within the augmented area. Furthermore, the newly formed bone appeared to have undergone reorganization, developing a cortical-like, more radio-opaque structure on its vestibular side. The extent of bone remodeling observed at 26 months was consistent with the histological and histomorphometrical findings at 8 months, showing the bone graft was undergoing remodeling and substitution at an extent consistent with data previously collected by Di Stefano et al,40 using the same material (though partially demineralized).
Earlier studies of both bovine25 27 47 and equine28 48 bone grafts reported contrasting results with regard to the potential resorption of both materials. These differences may be due to the type of membrane used to cover the grafts. Nonresorbable membranes may protect the graft from resorption,49 50 whereas resorbable collagen membranes, as they deteriorate, may facilitate the resorption process.27
The method used to process the equine bone used in the present study preserves bone apatite and bone collagen, in an unaltered state. This may explain both the histological results observed 8.5 months after grafting and the long-term CBCT outcome, both of which were coherent with a previous case in which the same equine enzyme-deantigenic blocks were used to augment an atrophic maxilla.42 In the present case, reshaping the block on an accurate stereolithographic model of the jaw also may have contributed to the successful regenerative outcome; the best possible adaptation of the block to the receiving bone surface is, in fact, mandatory since any gap presents a barrier to blood vessels seeking to enter the grafted area.
As a final comment, it must be underlined that the principle guiding the surgical technique used in the present case was to carry out a standard GBR augmentation, isolating the regenerating area from the surrounding soft tissue. The surgery performed was not regarded as an onlay block augmentation in which an autogenous block was simply replaced with a heterologous substitute. A collagen-preserved heterologous block, though as easy to shape and manipulate as an autogenous one, does not provide any osteoprogenitor cells or growth factors and therefore must be regarded only as an inert, if biocompatible, scaffold.
Conclusion
This clinical case shows the successful reconstruction of an atrophic posterior partially edentulous ridge using a heterologous, collagen-preserved, equine bone block and following the principles of GBR. The successful bone regeneration allowed for the delivery of a definitive prosthesis that is, at present, still perfectly functional. Equine bone blocks, if applied according to coherent and logical GBR principles, seem to be able to provide excellent results. These encouraging results should be confirmed by prospective and randomly controlled clinical trials.
Acknowledgments
Bioteck partially supported this case report donating the xenograft block. Data belonged to the authors and by no means did the manufacturer interfere with the conduct of the case or the publication of its results.
References
- 1.Esposito M, Grusovin M G, Felice P, Karatzopoulos G, Worthington H V, Coulthard P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants - a Cochrane systematic review. Eur J Oral Implantology. 2009;2(3):167–184. [PubMed] [Google Scholar]
- 2.Nkenke E, Weisbach V, Winckler E. et al. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: a prospective study. Int J Oral Maxillofac Surg. 2004;33(2):157–163. doi: 10.1054/ijom.2003.0465. [DOI] [PubMed] [Google Scholar]
- 3.Buser D, Dula K, Hess D, Hirt H P, Belser U C. Localized ridge augmentation with autografts and barrier membranes. Periodontol 2000. 1999;19:151–163. doi: 10.1111/j.1600-0757.1999.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 4.Nkenke E, Schultze-Mosgau S, Radespiel-Tröger M, Kloss F, Neukam F W. Morbidity of harvesting of chin grafts: a prospective study. Clin Oral Implants Res. 2001;12(5):495–502. doi: 10.1034/j.1600-0501.2001.120510.x. [DOI] [PubMed] [Google Scholar]
- 5.Karring T, Nyman S, Lindhe J. Healing following implantation of periodontitis affected roots into bone tissue. J Clin Periodontol. 1980;7(2):96–105. doi: 10.1111/j.1600-051x.1980.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 6.Nyman S, Karring T, Lindhe J, Plantén S. Healing following implantation of periodontitis-affected roots into gingival connective tissue. J Clin Periodontol. 1980;7(5):394–401. doi: 10.1111/j.1600-051x.1980.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 7.Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81(5):672–676. doi: 10.1097/00006534-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Buser D, Brägger U, Lang N P, Nyman S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implants Res. 1990;1(1):22–32. doi: 10.1034/j.1600-0501.1990.010104.x. [DOI] [PubMed] [Google Scholar]
- 9.Rakhmatia Y D, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 2013;57(1):3–14. doi: 10.1016/j.jpor.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Gentile P, Chiono V, Tonda-Turo C, Ferreira A M, Ciardelli G. Polymeric membranes for guided bone regeneration. Biotechnol J. 2011;6(10):1187–1197. doi: 10.1002/biot.201100294. [DOI] [PubMed] [Google Scholar]
- 11.Roccuzzo M, Ramieri G, Spada M C, Bianchi S D, Berrone S. Vertical alveolar ridge augmentation by means of a titanium mesh and autogenous bone grafts. Clin Oral Implants Res. 2004;15(1):73–81. doi: 10.1111/j.1600-0501.2004.00998.x. [DOI] [PubMed] [Google Scholar]
- 12.Roccuzzo M, Ramieri G, Bunino M, Berrone S. Autogenous bone graft alone or associated with titanium mesh for vertical alveolar ridge augmentation: a controlled clinical trial. Clin Oral Implants Res. 2007;18(3):286–294. doi: 10.1111/j.1600-0501.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 13.Pikos M A. Chin grafts as donor sites for maxillary bone augmentation—Part II. Dent Implantol Update. 1996;7(1):1–4. [PubMed] [Google Scholar]
- 14.Garg A K, Morales M J, Navarro I, Duarte F. Autogenous mandibular bone grafts in the treatment of the resorbed maxillary anterior alveolar ridge: rationale and approach. Implant Dent. 1998;7(3):169–176. doi: 10.1097/00008505-199807030-00003. [DOI] [PubMed] [Google Scholar]
- 15.Sethi A, Kaus T. Ridge augmentation using mandibular block bone grafts: preliminary results of an ongoing prospective study. Int J Oral Maxillofac Implants. 2001;16(3):378–388. [PubMed] [Google Scholar]
- 16.Misch C M, Misch C E, Resnik R R, Ismail Y H. Reconstruction of maxillary alveolar defects with mandibular symphysis grafts for dental implants: a preliminary procedural report. Int J Oral Maxillofac Implants. 1992;7(3):360–366. [PubMed] [Google Scholar]
- 17.D'Addona A, Nowzari H. Intramembranous autogenous osseous transplants in aesthetic treatment of alveolar atrophy. Periodontol 2000. 2001;27:148–161. doi: 10.1034/j.1600-0757.2001.027001148.x. [DOI] [PubMed] [Google Scholar]
- 18.Verhoeven J W, Cune M S, Terlou M, Zoon M A, de Putter C. The combined use of endosteal implants and iliac crest onlay grafts in the severely atrophic mandible: a longitudinal study. Int J Oral Maxillofac Surg. 1997;26(5):351–357. doi: 10.1016/s0901-5027(97)80796-5. [DOI] [PubMed] [Google Scholar]
- 19.Garg A K. Lateral proximal tibia bone harvest for use in augmentation procedures. Interview. Dent Implantol Update. 2001;12(5):33–37. [PubMed] [Google Scholar]
- 20.Mellonig J T. Autogenous and allogeneic bone grafts in periodontal therapy. Crit Rev Oral Biol Med. 1992;3(4):333–352. doi: 10.1177/10454411920030040201. [DOI] [PubMed] [Google Scholar]
- 21.Zitzmann N U, Naef R, Schärer P. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration. Int J Oral Maxillofac Implants. 1997;12(6):844–852. [PubMed] [Google Scholar]
- 22.Zitzmann N U, Schärer P, Marinello C P, Schüpbach P, Berglundh T. Alveolar ridge augmentation with Bio-Oss: a histologic study in humans. Int J Periodontics Restorative Dent. 2001;21(3):288–295. [PubMed] [Google Scholar]
- 23.Hämmerle C H, Jung R E, Yaman D, Lang N P. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res. 2008;19(1):19–25. doi: 10.1111/j.1600-0501.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 24.Jensen S S, Broggini N, Hjørting-Hansen E, Schenk R, Buser D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res. 2006;17(3):237–243. doi: 10.1111/j.1600-0501.2005.01257.x. [DOI] [PubMed] [Google Scholar]
- 25.Rothamel D, Schwarz F, Herten M. et al. Vertical ridge augmentation using xenogenous bone blocks: a histomorphometric study in dogs. Int J Oral Maxillofac Implants. 2009;24(2):243–250. [PubMed] [Google Scholar]
- 26.Schwarz F, Rothamel D, Herten M, Ferrari D, Sager M, Becker J. Lateral ridge augmentation using particulated or block bone substitutes biocoated with rhGDF-5 and rhBMP-2: an immunohistochemical study in dogs. Clin Oral Implants Res. 2008;19(7):642–652. doi: 10.1111/j.1600-0501.2008.01537.x. [DOI] [PubMed] [Google Scholar]
- 27.Simion M, Rocchietta I, Kim D, Nevins M, Fiorellini J. Vertical ridge augmentation by means of deproteinized bovine bone block and recombinant human platelet-derived growth factor-BB: a histologic study in a dog model. Int J Periodontics Restorative Dent. 2006;26(5):415–423. [PubMed] [Google Scholar]
- 28.Fontana F, Rocchietta I, Dellavia C, Nevins M, Simion M. Biocompatibility and manageability of a new fixable bone graft for the treatment of localized bone defects: preliminary study in a dog model. Int J Periodontics Restorative Dent. 2008;28(6):601–607. [PubMed] [Google Scholar]
- 29.Baslé M F, Lesourd M, Grizon F, Pascaretti C, Chappard D. Type I collagen in xenogenic bone material regulates attachment and spreading of osteoblasts over the beta1 integrin subunit [in German] Orthopade. 1998;27(2):136–142. doi: 10.1007/s001320050211. [DOI] [PubMed] [Google Scholar]
- 30.Green J, Schotland S, Stauber D J, Kleeman C R, Clemens T L. Cell-matrix interaction in bone: type I collagen modulates signal transduction in osteoblast-like cells. Am J Physiol. 1995;268(5, Pt 1):C1090–C1103. doi: 10.1152/ajpcell.1995.268.5.C1090. [DOI] [PubMed] [Google Scholar]
- 31.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184(2):207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Hu Y Y, Zhao J N, Wu S J, Xiong Z, Lu R. Effect of type I collagen on the adhesion, proliferation, and osteoblastic gene expression of bone marrow-derived mesenchymal stem cells. Chin J Traumatol. 2004;7(6):358–362. [PubMed] [Google Scholar]
- 33.Güngörmüş M, Kaya O. Evaluation of the effect of heterologous type I collagen on healing of bone defects. J Oral Maxillofac Surg. 2002;60(5):541–545. doi: 10.1053/joms.2002.31852. [DOI] [PubMed] [Google Scholar]
- 34.Gungormus M. The effect on osteogenesis of type I collagen applied to experimental bone defects. Dent Traumatol. 2004;20(6):334–337. doi: 10.1111/j.1600-9657.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- 35.Regazzoni C, Winterhalter K H, Rohrer L. Type I collagen induces expression of bone morphogenetic protein receptor type II. Biochem Biophys Res Commun. 2001;283(2):316–322. doi: 10.1006/bbrc.2001.4813. [DOI] [PubMed] [Google Scholar]
- 36.Toroian D, Lim J E, Price P A. The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. J Biol Chem. 2007;282(31):22437–22447. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 37.Perrotti V, Nicholls B M, Piattelli A. Human osteoclast formation and activity on an equine spongy bone substitute. Clin Oral Implants Res. 2009;20(1):17–23. doi: 10.1111/j.1600-0501.2008.01608.x. [DOI] [PubMed] [Google Scholar]
- 38.Perrotti V, Nicholls B M, Horton M A, Piattelli A. Human osteoclast formation and activity on a xenogenous bone mineral. J Biomed Mater Res A. 2009;90(1):238–246. doi: 10.1002/jbm.a.32079. [DOI] [PubMed] [Google Scholar]
- 39.Artese L, Piattelli A, Di Stefano D A. et al. Sinus lift with autologous bone alone or in addition to equine bone: an immunohistochemical study in man. Implant Dent. 2011;20(5):383–388. doi: 10.1097/ID.0b013e3182310b3d. [DOI] [PubMed] [Google Scholar]
- 40.Di Stefano D A, Artese L, Iezzi G. et al. Alveolar ridge regeneration with equine spongy bone: a clinical, histological, and immunohistochemical case series. Clin Implant Dent Relat Res. 2009;11(2):90–100. doi: 10.1111/j.1708-8208.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 41.Di Stefano D A, Cazzaniga A, Andreasi Bassi M, Ludovichetti M, Ammirabile G, Celletti R. The use of cortical heterologous sheets for sinus lift bone grafting: a modification of Tulasne's technique with 7-year follow-up. Int J Immunopathol Pharmacol. 2013;26(2):549–556. doi: 10.1177/039463201302600231. [DOI] [PubMed] [Google Scholar]
- 42.Pistilli R, Signorini L, Pisacane A, Lizio G, Felice P. Case of severe bone atrophy of the posterior maxilla rehabilitated with blocks of equine origin bone: histological results. Implant Dent. 2013;22(1):8–15. doi: 10.1097/ID.0b013e3182777239. [DOI] [PubMed] [Google Scholar]
- 43.Santini S, Barbera P, Modena M, Schiavon R, Bonato M. Equine-derived bone substitutes in orthopedics and traumatology: authors' experience. Minerva Chir. 2011;66(1):63–72. [PubMed] [Google Scholar]
- 44.Di Stefano D A, Andreasi Bassi M, Cinci L, Pieri L, Ammirabile G. Treatment of a bone defect consequent to the removal of a periapical cyst with equine bone and equine membranes: clinical and histological outcome. Minerva Stomatol. 2012;61(11-12):477–490. [PubMed] [Google Scholar]
- 45.Materni A. Managing an extreme peri-implantitis. Minerva Stomatol. 2013;62(9):295–305. [PubMed] [Google Scholar]
- 46.Yurdukoru B. Standardization of the tooth numbering systems [in Turkish] Ankara Univ Hekim Fak Derg. 1989;16(3):527–531. [PubMed] [Google Scholar]
- 47.Araújo M G, Sonohara M, Hayacibara R, Cardaropoli G, Lindhe J. Lateral ridge augmentation by the use of grafts comprised of autologous bone or a biomaterial. An experiment in the dog. J Clin Periodontol. 2002;29(12):1122–1131. doi: 10.1034/j.1600-051x.2002.291213.x. [DOI] [PubMed] [Google Scholar]
- 48.Simion M, Nevins M, Rocchietta I. et al. Vertical ridge augmentation using an equine block infused with recombinant human platelet-derived growth factor-BB: a histologic study in a canine model. Int J Periodontics Restorative Dent. 2009;29(3):245–255. [PubMed] [Google Scholar]
- 49.Antoun H, Sitbon J M, Martinez H, Missika P. A prospective randomized study comparing two techniques of bone augmentation: onlay graft alone or associated with a membrane. Clin Oral Implants Res. 2001;12(6):632–639. doi: 10.1034/j.1600-0501.2001.120612.x. [DOI] [PubMed] [Google Scholar]
- 50.Buser D, Dula K, Belser U, Hirt H P, Berthold H. Localized ridge augmentation using guided bone regeneration. 1. Surgical procedure in the maxilla. Int J Periodontics Restorative Dent. 1993;13(1):29–45. [PubMed] [Google Scholar]


