Abstract
Rectovaginal fistulae are abnormal epithelialized connections between the rectum and vagina. Fistulae from the anorectal region to the posterior vagina are truly best characterized as anovaginal or very low rectovaginal fistulae. True rectovaginal fistulae are less common and result from inflammatory bowel disease, trauma, or iatrogenic injury. A very few patients are asymptomatic, but the symptoms of rectovaginal fistula are incredibly distressing and unacceptable. Diagnostic approach, timing, and choice of surgical intervention, including sphincteroplasty, gracilis flaps, Martius flaps, and special circumstances are discussed.
Keywords: anovaginal fistula, rectovaginal fistula, advancement flap, Martius flap, fistula plug
Rectovaginal fistulae are a challenge of both diagnosis and treatment for surgeons and a misery to bear for patients. Successful management requires the understanding and accounting of many patient variables including tissue quality, etiology, size, and location of the fistula.
Etiology
Causes of rectovaginal fistulae are listed in Table 1. The most common cause of rectovaginal fistulae is obstetrical trauma; prolonged and obstructed labor creates an impaction of the presented part of the child against the soft tissues of the pelvis and a widespread ischemic injury that results in tissue necrosis and weakness that leads to fistula formation. Third- and fourth-degree lacerations, along with episiotomies due to difficult labor, are also a well-known cause of fistula formation. While these lacerations typically undergo primary repair after childbirth, this can break down due to infection or poor wound healing.
Table 1. Common causes of rectovaginal fistulae.
| Obstetrical Injury |
| Crohn disease |
| Trauma, including previous surgeries |
| Infection/cryptoglandular abscess |
| Neoplasm |
| Radiation injury |
These issues being noted, a remarkably small number of patients suffer from fourth-degree lacerations and far fewer from anovaginal fistulae. In one historical report, out of 24,000 patients, 1.7% of patients suffered fourth-degree lacerations, and 0.5% of patients subsequently suffered rectovaginal fistulae.1 In a review of the literature, Homsi et al found that rectovaginal fistulae were reported in the range of 0.1% of patients who underwent episiotomy during delivery. Notably, it was found that rectovaginal fistulae develop in 0.05% of patients who undergo a median episiotomy but in 1% of those who suffer third- and fourth-degree lacerations.2 As expected, these fistulae are more common in developing countries due to less resources to aid with the process of childbirth.3
The next most common cause of rectovaginal fistulae is Crohn disease. The incidence has historically been ∼ 10% with Radcliffe et al reporting an incidence of 9.8% in women with Crohn and Schwartz et al noting an incidence of 9%.4 5 Additional causes include pelvic irradiation (particularly irradiation for cervical or endometrial cancer), malignant processes, and postsurgical complications (stapler misfire from low anterior resection (LAR), hysterectomy, rectocele repair, and proctocolectomy with ileoanal pouch anastomosis). Malignancies may cause rectovaginal fistulae. These fistulae are usually seen in the setting of rectal, uterine, cervical, or vaginal malignancies that have significant local extension or have been treated with radiation therapy. Following radiation therapy, the patient may develop proctitis followed by ulceration of the anterior rectal wall. Rectal ulcers then progress to fistula formation around 6 months to 2 years posttherapy. The incidence of rectovaginal fistula increases with high-dose radiation and previous hysterectomy.6 7
Presentation and Evaluation
Patients with rectovaginal fistulae can have a varying degree of symptoms based on the location, size, and etiology of the rectovaginal fistula and on the patient's tolerance of the condition. Symptoms include the obvious passage of flatus or stool through the vagina, with more subtle presentations being slight discharge, a feculent odor, or recurrent vaginal mucosal inflammation. In these latter situations, stool may only be noted per vagina when the bowel movements are liquid in nature. The presence of fecal incontinence is also of paramount importance, and obtaining a history regarding sphincter function will direct the clinician to further appropriate investigation and help guide the operative approach.
Examination is critical to confirm the presence, size, and location of the fistula, as well as the integrity of the anal sphincter. In addition, direct exam allows the surgeon to evaluate for fistulae to other organs, and the presence of absence of inflammation that could suggest Crohn disease, radiation injury, or incompletely controlled anovaginal sepsis.
A low fistula/anovaginal fistula is identified on digital examination with a dimple on palpation that can be confirmed easily on anoscopic and speculum examination. Stool is often seen in the vagina, which may be the site of active infection. Notably, the dark red rectal mucosa contrasts with the lighter vaginal mucosa, and a small fistula may appear only as a depression or a pit as a defect in the mucosa. Digital examination should be performed with one finger in the rectum and the other in the vagina, thus allowing an assessment of tissue induration, as well as the width and bulk of the anterior perineal body. Sphincter injury and perineal thinning upon examination should prompt documenting the extent of the prior injury. Examination under anesthesia with biopsies may be necessary in patients with prior irradiation or suspicion for malignancy. Overall, confirmatory diagnostic studies are only necessary when the rectovaginal fistula eludes identification on physical examination or if the extent of underlying disease is unknown.
Multiple office maneuvers have been advocated for the identification of more difficult rectovaginal fistulae.8 The patient can be placed in lithotomy position with a Trendelenburg positioning, placing a proctoscope, and filling the vagina with warm water; the proctoscope then insufflates the rectum, allowing air to traverse through a possible fistulous tract into the vagina to produce bubbling. Alternatively, a tampon can be placed in the vagina, and a methylene blue retention enema can be administered. The tampon is then removed after 1 hour. Blue on the tampon indicates the presence of a rectovaginal fistula.8
A fistula to other parts of the bowel should also be excluded; vesicovaginal, rectovesical, rectoperineal, and other fistulae can accompany rectovaginal fistulae. Contrast media fistulography, vaginography, barium enemas, and other endoscopic procedures may be of value. Fig. 1 demonstrates a classic type of enterovaginal fistula: diverticular inflammation has created a fistula to the vaginal cuff of a prior hysterectomy.
Fig. 1.
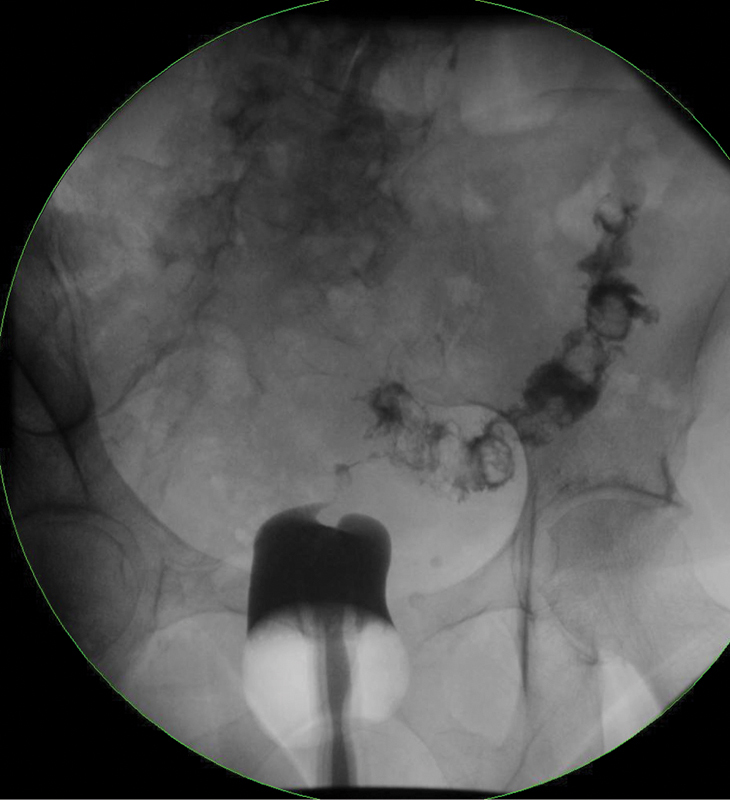
Enterovaginal fistula. Contrast administered per vagina directly communicates to segment of sigmoid colon, inflamed by diverticulitis.
Endoanal ultrasound, with or without the addition of hydrogen peroxide, is also useful in the evaluation of rectovaginal fistulae. It can identify the location and characteristics of the fistula tract, while simultaneously assessing the width of the perineal body, and the integrity of the internal and external anal sphincters.9 An alternative to ultrasound is magnetic resonance imaging, which is less operator-dependent with similar diagnostic accuracy.10
Classification
Classification of rectovaginal fistulae is based on location, size, and etiology. Admittedly, these methods are arbitrary and not well grounded in the literature, but such rubrics are helpful when comparing operative approaches. Traditionally, a “low” fistula is located at or just slightly above the dentate line with the vaginal opening just inside the vaginal fourchette. “High” fistulae are noted as vaginal openings behind or near the cervix, and “middle” when the fistula is noted between the “high” and the “low” areas. The higher the fistula the more difficult it can be to diagnose, and these “high” fistulae are often resultant from a surgical procedure such as hysterectomy or LAR with a stapled anastomosis. Sizes of the fistulae are classified as “small” if < 0.5 cm, “medium” if 0.5 to 2.5 cm, and “large” if > 2.5 cm.11 Fistulae are considered “complex” if they are large, high, or caused by inflammatory bowel disease or other pelvic processes (diverticulitis), including irradiation. Recurrent fistulae are also considered complex due to their association with tissue scarring and decreased blood supply.
Treatment
A reasonable algorithm for the treatment of rectovaginal fistula was presented by Dr. Hull, and is shown in Fig. 2.
Fig. 2.
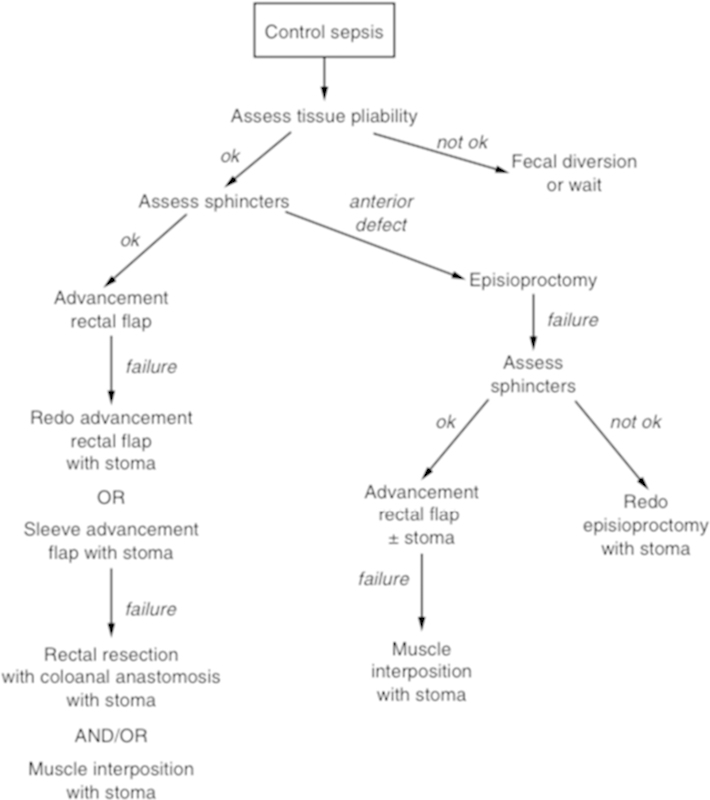
Algorithm for the management of rectovaginal fistula. (From: Hull T. Rectovaginal Fistula. In: Fazio VF, Church JM, Delaney CP, eds. Current Therapy in Colon and Rectal Surgery. 2nd ed. Philadelphia, PA: Mosby, Inc.; 2005:39.)
Watchful Waiting
Appropriate timing of surgical repair is crucial to successful healing. For small or minimally symptomatic fistulae, it is appropriate to start with a period of watchful waiting. Some women with fistulae related to obstetric trauma will experience spontaneous closure within the early postpartum period, and a waiting period of 6 to 9 months is warranted if symptoms remain manageable.12 Mattingly noted that 50% of small rectovaginal fistulae secondary to obstetric trauma heal spontaneously, and he recommended watchful waiting for at least 6 months.13 In our experience we have seen similar progress, but interval follow-up throughout the healing process is warranted.
Antibiotic and Seton Use
Prior to any attempt at fistula repair, the surgeon must also ensure that infection and local inflammation have resolved. Antibiotic therapy with appropriate drainage of any related abscess is often the mainstay of therapy. A draining seton may also be necessary to eliminate infection. In our practice, loose setons are used selectively for unresolved sepsis and to control ongoing symptoms of pain and drainage. We have found setons more useful in complex, high rectovaginal fistulae compared with lower, simpler fistulae. If a seton is required, it is our practice to leave this in for 8 or more weeks, with removal at the time of fistula repair.
Selection of Operative Technique
Many factors must be considered when choosing an operative approach to rectovaginal fistulae. The number and type of previous repairs, patient's risk factors, additional concomitant fistulae, and sphincter integrity are all concerns. Of these factors, the cause of the rectovaginal fistula and the status of the external anal sphincter are the most important.
Sphincteroplasty
A sphincteroplasty is advocated for rectovaginal fistula when the patient has a defect in the anterior sphincter complex. In this technique, a circumlinear incision is made on the anterior perineal body, and dissection is taken in the plane between the rectum and vagina through the fistula to the level of the levator ani. It is our practice to perform routine levatorplasty at this point. The ends of the damaged external sphincter are mobilized, and brought together in an overlapping fashion using several absorbable monofilament sutures. Success using this technique has been well documented in the literature.
Tsang et al14 conducted a study to determine the effect of a sphincter defect on the outcome of rectovaginal fistula repair. A total of 52 women underwent 62 repairs of simple obstetrical rectovaginal fistulae. They noted results that were better after sphincteroplasties versus endorectal advancement flaps (ERAFs) in patients with sphincter defects, identified by endorectal ultrasound (88 vs. 33%) and by manometry (86 vs. 33%). Poor results correlated with prior operations in patients undergoing endoanal advancement flaps (45 vs. 25%) but not sphincteroplasties (80 vs. 75%). It was their belief that rectovaginal fistulae should undergo preoperative evaluation for occult sphincter defects by ultrasound, manometry, or both.
Advancement Flap
When the anal sphincter complex is intact, and tissues are readily viable, a common and durable approach is the ERAF. In this technique, a wide-based flap of rectal mucosa, along with a small amount of underlying sphincter muscle, is mobilized and advanced over the fistula's internal opening ( Fig. 3a, b). Adequate perfusion and lack of tension are key to flap success.
Fig. 3.
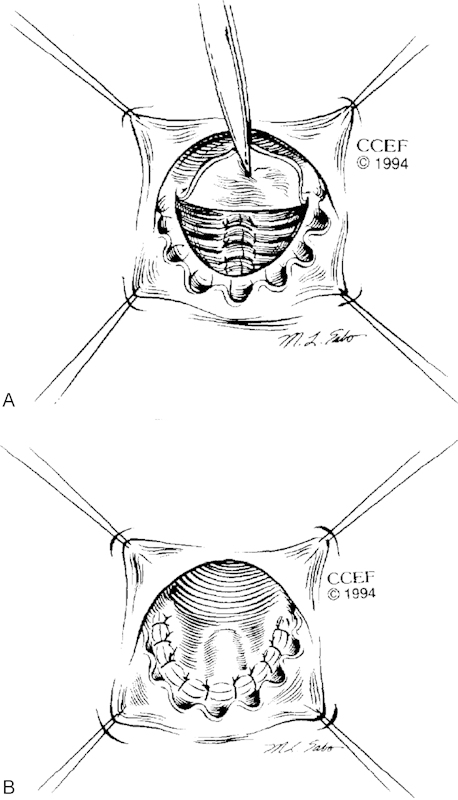
a, b Endorectal advancement flap. (From: Hull TL, Fazio VW. Surgical approaches to low anovaginal fistula in Crohn's disease. Am J Surg 1997;173:95–98.)
Lowry et al15 described results for 81 ERAFs in women with simple rectovaginal fistulae. Their overall success rate was 83%, with success correlating to the number of previous repairs. Patients who had no previous repair had an 88% success rate; one prior repair had an 85% success rate; and two prior repairs had a 55% success rate. This study suggests that a prior failure should not prevent the surgeon from trying the approach again; however, with two prior failed attempts one should be reluctant to perform a mucosal advancement.
An alternative approach to flap closure is the dermal advancement flap anoplasty. This technique involves the creation of a pedicled flap of adjacent anoderm, which is mobilized and brought into the anal canal to cover the fistula opening. This was initially conceived for the treatment of anal stenosis, but has since been described for the treatment of both rectovaginal and cryptoglandular anal fistulae.16 17 18 Several flap configurations have been described, including V-Y flaps, diamond-shaped flaps, rhomboid flaps, and inverted house-shaped flaps.19
In our experience, both anodermal and ERAFs may be used for this purpose; however, historically the vast predominance of the literature describes ERAF to treat these fistulae, and so the dermal advancement flap has much less supportive literature. However, we have changed our practice to using an anodermal advancement flap in lieu of an ERAF when the skin flap can be raised without tension. After over 200 cases with the anodermal advancement flap for fistula-in-ano, pouch-vaginal fistulae, and rectovaginal fistulae, the anoderm allows better affixation, an excellent and durable blood supply, as well as possibly less retraction than a mucosal flap. Publication of our case series is pending.
Vaginal Repair
One of the traditional tenets of fistula repair is the elimination of inflow into the fistula tract. For rectovaginal fistulae, this means closure on the rectal side of the fistula, which is the side of higher intraluminal pressure. However, gynecologists have long reported on outcomes of transvaginal repairs with relatively impressive reported outcomes. Rahman et al reported a 100% healing rate among 39 patients treated with a transvaginal approach.12 Bauer et al repaired 13 patients transvaginally with diverting stomas for low or middle septal fistulae. A total of 12 out of 13 patients succeeded during an average follow-up period of 50 months.20 In this study, fecal diversion may have eliminated the pressure gradient alluded to above.
Transvaginal repairs have traditionally been described in two main techniques. If the fistula is low and small, the fistula can be “inverted,” in which a circular incision is made around the vaginal introitus, and a mucosal flap is mobilized. Purse-string sutures are placed to invert the fistula into the rectum, and then the vaginal mucosa is reapproximated with the flap that is mobilized.12 Another method of transvaginal closure is the creation of a mucosal flap with the dissection extended laterally to the ischial tuberosities and then cephalad, with the vaginal defect previously closed with interrupted suture.20
Fibrin Glue
Fibrin glue injection has been extensively studied for cryptoglandular anal fistulae, with reported success rates as low as 10%21 and as high as 64%.22 In a small series of five patients with rectovaginal fistulae, Abel et al treated four patients with fibrin glue, and reported a 100% healing rate.23 Another series from Loungnarath et al showed one successful outcome out of three patients treated with fibrin glue.24 Currently, the role for fibrin glue in the definitive treatment of rectovaginal fistulae appears to be very limited.
Fistula Plug
The fistula plug has also been extensively studied for use in cryptoglandular anal fistulae, with much less supportive evidence for use in rectovaginal fistulae. The technical steps to fistula plug placement are relatively simple, and involve debridement of the tract followed by suture fixation of the plug within the fistula tract.
This technique was described by Dr. Ellis in 2008 with primary healing in six out of seven (86%) patients.25 In 2009 Gonsalves et al26 reported on the use of the fistula plug for rectovaginal fistulae and ileoanal pouch-vaginal fistulae. A total of 60% (three out of five) of rectovaginal fistulae experienced healing, along with 57% (four out of seven) of the pouch-vaginal fistulae. Of note, several repeat plug procedures were needed, with a first-time procedural success rate of 35% and overall success rate of 58%.
In general, outcomes with fistula plug for cryptoglandular fistulae were less impressive as studies emerged with longer periods of follow-up. This makes the authors skeptical that the fistula plug will have an enduring role on the repair of rectovaginal fistulae. However, undeniable benefits of the approach include its simplicity, patient tolerance, and the lack of disturbance to fecal continence. In our practice, the fistula plug is reserved for patients where the tissue is largely healed from prior repairs, and the plug can create pliability for fibrosis to allow closure. Such a use allows the plug to spare precious tissue for future attempts, and it is well tolerated by the patient with minimal associated risk.
Special Situations
Crohn Disease
Fistulae as a result of Crohn disease are particularly difficult to manage. As with Crohn fistulae-in-ano, asymptomatic or limited symptoms from fistulae do not require operative care, whereas severely symptomatic patients may require a proctocolectomy. Local repair may be warranted in the absence of active proctitis, but high rates of recurrence are reported. Often, patients with Crohn related fistulae will require tissue interposition and temporary fecal diversion, which will be discussed shortly. Persistently active disease refractory to medical therapy or with destruction of the anal sphincter with persistent disease and subsequent inflammation warrants proctectomy.
In our practice, Crohn patients with minimal anorectal disease, excellent tissue pliability, and minimal stricturing disease are offered an endorectal or anodermal advancement flap. In two Crohn patients with rectovaginal fistulae, we have performed a fistula plug closure, which was successful. Some centers prefer a transvaginal approach to prevent manipulation on the anorectal aspect; however, we feel that the pressure gradient is not hospitable for this type of approach.
Active Crohn disease elsewhere in the bowel should be treated medically and seems to correlate with rectovaginal fistula recurrence when flaring after treatment.
Radiation-Induced Fistulae
Pelvic radiation is a part of the treatment of several malignancies, including squamous cell carcinoma of the anus and cervix. Radiation can cause significant damage to the tissue adjacent to these cancers, causing fibrosis and endarteritis results in vascular compromise. Tissue loss and difficult dissection plains often preclude local repair of rectovaginal fistulae. Definitive repair of these fistulae often requires the interposition of healthy, nonirradiated, well-vascularized tissue to the rectovaginal septum. A host of tissue transfer options are available, with the most common approaches listed below.
Tissue Interposition
When a rectovaginal fistula is recurrent or refractory to sphincteroplasty and/or advancement flap, or when the surrounding tissue is heavily damaged or scarred, the best approach to definitive fistula management is the interposition of healthy, well-perfused tissue. This is particularly important for patients with a very thin rectovaginal septum, and for patients with a history of radiation injury.
When a tissue interposition is planned, it is often accompanied by temporary fecal diversion. While previous studies have not shown a definitive benefit to diversion, they were retrospective in nature, and it is likely that the lack of benefit can be explained by selection bias, with the diverted patients have more complex fistulae or more severe baseline disease.27 It is our practice to routinely protect these complex repairs with a temporary loop ileostomy.
Gracilis Flap
The gracilis muscle is often used as an interposition flap. The procedure can be performed by the fistula surgeon or in conjunction with a plastic surgeon with similar results.28 29 The surgery is typically performed in the lithotomy position, with the gracilis muscle being harvested from a 8 to 10 cm incision on the medial thigh. A perineal incision is then made, dissecting well above the fistula itself with care taken not to violate the rectal or vaginal mucosa. The pedicled muscle flap is tunneled to the perineal incision and secured into place with absorbable sutures (Fig. 4).
Fig. 4.
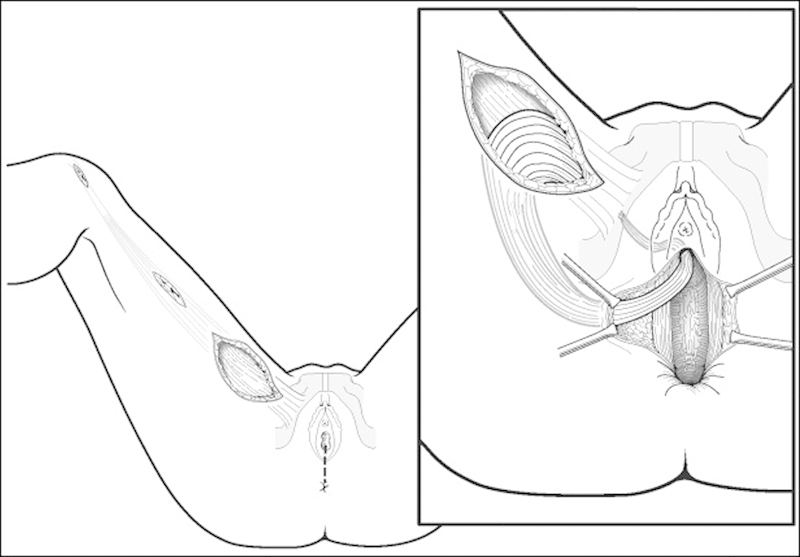
Gracilis muscle interposition flap. (From: Keighley MRB, Williams NS, Church JM Pahlman L, Sholefield JH, Scott NA. Surgery of the Colon, Rectum, and Anus. 3rd ed. Philadelphia, PA: Elsevier Limited; 2008:509.)
Reported primary healing rates for gracilis interposition grafts generally range from 75 to 92%.30 31 It should be noted that most case series on this topic involve complex fistulae with multiple previous attempts at repair. A large number of these fistulae were secondary to Crohn disease and radiation injury, with a small number being secondary to obstetrical injury. In addition, most case series employed routine fecal diversion.
Wexner et al noted healing rates to be lower for patients with Crohn disease (33%) when compared with other etiologies such as pouch-vaginal fistulae and radiation-induced fistulae (75%),28 whereas other series report healing rates in Crohn disease to be equivalent to other etiologies.30
Martius Flap
Another well-described tissue transfer and local flap repair is the use of the Martius or bulbocavernosus flap. (Fig. 5) Using a longitudinal incision over the labia majora, skin flaps are raised laterally and medially, with dissection continuing to the periosteum of the pubis and to the pubic symphysis. Once the entire fat pad with the bulbocavernosus muscle is mobilized, the anterior aspect is cut and used as a vascularized pedicled flap (perineal branch of the pudendal artery) and tunneled subvaginally. The flap is then sutured to the posterior vaginal wall to interpose it over the closed rectal aspect of the fistula.
Fig. 5.
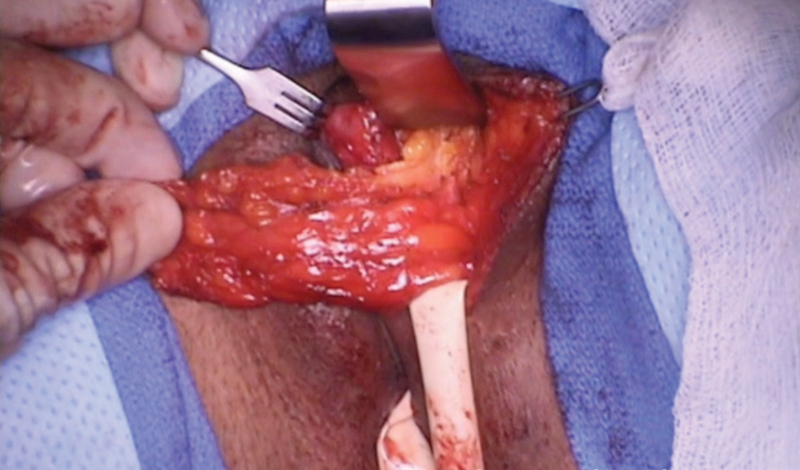
Martius flap with the bulbocavernosus flap from the left labia exposed.
Aartsen and Sindram32 initially reported 100% success in 14 patients with fistulae secondary to radiation damage, but after a 10-year follow-up, 8 of the 14 patients required diversion for continued damage from radiation. Pitel et al33 reported a 65% overall healing rate for Martius flap, with a 50% rate in patients with Crohn disease.
Conclusion
In general, rectovaginal fistulae are difficult to manage, and the level of evidence for surgical approaches to this disease remains poor, consisting primarily of case series.34 It is important that the surgeon and the patient alike be prepared for possible treatment failures and the need for further interventions. When a sphincter defect exists, the best approach is sphincteroplasty, while an advancement flap is appropriate for patient with intact sphincters. Complex recurrent fistulae, especially in patients with Crohn disease or radiation injury, often require fecal diversion and tissue interposition to achieve long-term healing.
References
- 1.Goldaber K G, Wendel P J, McIntire D D, Wendel G D Jr. Postpartum perineal morbidity after fourth-degree perineal repair. Am J Obstet Gynecol. 1993;168(2):489–493. doi: 10.1016/0002-9378(93)90478-2. [DOI] [PubMed] [Google Scholar]
- 2.Homsi R, Daikoku N H, Littlejohn J, Wheeless C R Jr. Episiotomy: risks of dehiscence and rectovaginal fistula. Obstet Gynecol Surv. 1994;49(12):803–808. [PubMed] [Google Scholar]
- 3.Wall L L, Karshima J A, Kirschner C, Arrowsmith S D. The obstetric vesicovaginal fistula: characteristics of 899 patients from Jos, Nigeria. Am J Obstet Gynecol. 2004;190(4):1011–1019. doi: 10.1016/j.ajog.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Radcliffe A G, Ritchie J K, Hawley P R, Lennard-Jones J E, Northover J M. Anovaginal and rectovaginal fistulas in Crohn's disease. Dis Colon Rectum. 1988;31(2):94–99. doi: 10.1007/BF02562636. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz D, Loftus E, Tremaine W. et al. The natural history of fistulizing Crohn's disease: a population based study. Gastroenterology. 2000;118(4):A337. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 6.Perez C A, Breaux S, Bedwinek J M. et al. Radiation therapy alone in the treatment of carcinoma of the uterine cervix. II. Analysis of complications. Cancer. 1984;54(2):235–246. doi: 10.1002/1097-0142(19840715)54:2<235::aid-cncr2820540210>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Boronow R C. Repair of the radiation-induced vaginal fistula utilizing the Martius technique. World J Surg. 1986;10(2):237–248. doi: 10.1007/BF01658140. [DOI] [PubMed] [Google Scholar]
- 8.Beck D E, Roberts P L, Saclarides T J, Senagore A J, Stamos M J, Wexner S eds. New York, NY: Springer; 2011. The ASCRS Textbook of Colon and Rectal Surgery. 2nd ed; pp. 245–260. [Google Scholar]
- 9.Sudoł-Szopińska I, Jakubowski W, Szczepkowski M. Contrast-enhanced endosonography for the diagnosis of anal and anovaginal fistulas. J Clin Ultrasound. 2002;30(3):145–150. doi: 10.1002/jcu.10042. [DOI] [PubMed] [Google Scholar]
- 10.Dwarkasing S, Hussain S M, Hop W C, Krestin G P. Anovaginal fistulas: evaluation with endoanal MR imaging. Radiology. 2004;231(1):123–128. doi: 10.1148/radiol.2311021190. [DOI] [PubMed] [Google Scholar]
- 11.Rothenberger D A, Goldberg S M. The management of rectovaginal fistulae. Surg Clin North Am. 1983;63(1):61–79. doi: 10.1016/s0039-6109(16)42930-0. [DOI] [PubMed] [Google Scholar]
- 12.Rahman M S, Al-Suleiman S A, El-Yahia A R, Rahman J. Surgical treatment of rectovaginal fistula of obstetric origin: a review of 15 years' experience in a teaching hospital. J Obstet Gynaecol. 2003;23(6):607–610. doi: 10.1080/01443610310001604349. [DOI] [PubMed] [Google Scholar]
- 13.Mattingly R ed. Philadelphia, PA: JB Lippincott; 1992. Anal incontinence and rectovaginal fistulas. In: Telinde's Operative Gynecology. 5th ed; pp. 618–626. [Google Scholar]
- 14.Tsang C B, Madoff R D, Wong W D. et al. Anal sphincter integrity and function influences outcome in rectovaginal fistula repair. Dis Colon Rectum. 1998;41(9):1141–1146. doi: 10.1007/BF02239436. [DOI] [PubMed] [Google Scholar]
- 15.Lowry A C, Thorson A G, Rothenberger D A, Goldberg S M. Repair of simple rectovaginal fistulas. Influence of previous repairs. Dis Colon Rectum. 1988;31(9):676–678. doi: 10.1007/BF02552581. [DOI] [PubMed] [Google Scholar]
- 16.Del Pino A, Nelson R L, Pearl R K, Abcarian H. Island flap anoplasty for treatment of transsphincteric fistula-in-ano. Dis Colon Rectum. 1996;39(2):224–226. doi: 10.1007/BF02068080. [DOI] [PubMed] [Google Scholar]
- 17.Alver O, Ersoy Y E, Aydemir I. et al. Use of “house” advancement flap in anorectal diseases. World J Surg. 2008;32(10):2281–2286. doi: 10.1007/s00268-008-9699-1. [DOI] [PubMed] [Google Scholar]
- 18.Hesterberg R, Schmidt W U, Müller F, Röher H D. Treatment of anovaginal fistulas with an anocutaneous flap in patients with Crohn's disease. Int J Colorectal Dis. 1993;8(1):51–54. doi: 10.1007/BF00341278. [DOI] [PubMed] [Google Scholar]
- 19.Farid M, Youssef M, El Nakeeb A, Fikry A, El Awady S, Morshed M. Comparative study of the house advancement flap, rhomboid flap, and y-v anoplasty in treatment of anal stenosis: a prospective randomized study. Dis Colon Rectum. 2010;53(5):790–797. doi: 10.1007/DCR.0b013e3181d3205a. [DOI] [PubMed] [Google Scholar]
- 20.Bauer J J, Sher M E, Jaffin H, Present D, Gelerent I. Transvaginal approach for repair of rectovaginal fistulae complicating Crohn's disease. Ann Surg. 1991;213(2):151–158. doi: 10.1097/00000658-199102000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damin D C, Rosito M A, Contu P C, Tarta C. Fibrin glue in the management of complex anal fistula. Arq Gastroenterol. 2009;46(4):300–303. doi: 10.1590/s0004-28032009000400010. [DOI] [PubMed] [Google Scholar]
- 22.Cintron J R Park J J Orsay C P et al. Repair of fistulas-in-ano using fibrin adhesive: long-term follow-up Dis Colon Rectum 2000437944–949., discussion 949–950 [DOI] [PubMed] [Google Scholar]
- 23.Abel M E, Chiu Y S, Russell T R, Volpe P A. Autologous fibrin glue in the treatment of rectovaginal and complex fistulas. Dis Colon Rectum. 1993;36(5):447–449. doi: 10.1007/BF02050009. [DOI] [PubMed] [Google Scholar]
- 24.Loungnarath R, Dietz D W, Mutch M G, Birnbaum E H, Kodner I J, Fleshman J W. Fibrin glue treatment of complex anal fistulas has low success rate. Dis Colon Rectum. 2004;47(4):432–436. doi: 10.1007/s10350-003-0076-8. [DOI] [PubMed] [Google Scholar]
- 25.Ellis C N. Outcomes after repair of rectovaginal fistulas using bioprosthetics. Dis Colon Rectum. 2008;51(7):1084–1088. doi: 10.1007/s10350-008-9339-8. [DOI] [PubMed] [Google Scholar]
- 26.Gonsalves S, Sagar P, Lengyel J, Morrison C, Dunham R. Assessment of the efficacy of the rectovaginal button fistula plug for the treatment of ileal pouch-vaginal and rectovaginal fistulas. Dis Colon Rectum. 2009;52(11):1877–1881. doi: 10.1007/DCR.0b013e3181b55560. [DOI] [PubMed] [Google Scholar]
- 27.Gaertner W B, Madoff R D, Spencer M P, Mellgren A, Goldberg S M, Lowry A C. Results of combined medical and surgical treatment of recto-vaginal fistula in Crohn's disease. Colorectal Dis. 2011;13(6):678–683. doi: 10.1111/j.1463-1318.2010.02234.x. [DOI] [PubMed] [Google Scholar]
- 28.Wexner S D, Ruiz D E, Genua J, Nogueras J J, Weiss E G, Zmora O. Gracilis muscle interposition for the treatment of rectourethral, rectovaginal, and pouch-vaginal fistulas: results in 53 patients. Ann Surg. 2008;248(1):39–43. doi: 10.1097/SLA.0b013e31817d077d. [DOI] [PubMed] [Google Scholar]
- 29.Ulrich D, Roos J, Jakse G, Pallua N. Gracilis muscle interposition for the treatment of recto-urethral and rectovaginal fistulas: a retrospective analysis of 35 cases. J Plast Reconstr Aesthet Surg. 2009;62(3):352–356. doi: 10.1016/j.bjps.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 30.Lefèvre J H, Bretagnol F, Maggiori L, Alves A, Ferron M, Panis Y. Operative results and quality of life after gracilis muscle transposition for recurrent rectovaginal fistula. Dis Colon Rectum. 2009;52(7):1290–1295. doi: 10.1007/DCR.0b013e3181a74700. [DOI] [PubMed] [Google Scholar]
- 31.Fürst A, Schmidbauer C, Swol-Ben J, Iesalnieks I, Schwandner O, Agha A. Gracilis transposition for repair of recurrent anovaginal and rectovaginal fistulas in Crohn's disease. Int J Colorectal Dis. 2008;23(4):349–353. doi: 10.1007/s00384-007-0413-9. [DOI] [PubMed] [Google Scholar]
- 32.Aartsen E J, Sindram I S. Repair of the radiation induced rectovaginal fistulas without or with interposition of the bulbocavernosus muscle (Martius procedure) Eur J Surg Oncol. 1988;14(2):171–177. [PubMed] [Google Scholar]
- 33.Pitel S, Lefevre J H, Parc Y, Chafai N, Shields C, Tiret E. Martius advancement flap for low rectovaginal fistula: short- and long-term results. Colorectal Dis. 2011;13(6):e112–e115. doi: 10.1111/j.1463-1318.2011.02544.x. [DOI] [PubMed] [Google Scholar]
- 34.Göttgens K W, Smeets R R, Stassen L P, Beets G, Breukink S O. The disappointing quality of published studies on operative techniques for rectovaginal fistulas: a blueprint for a prospective multi-institutional study. Dis Colon Rectum. 2014;57(7):888–898. doi: 10.1097/DCR.0000000000000147. [DOI] [PubMed] [Google Scholar]


