Abstract
The association between sex hormones and sex hormone binding globin (SHBG) with vertebral fractures in men is not well studied. In these analyses, we determined whether sex hormones and SHBG were associated with greater likelihood of vertebral fractures in a prospective cohort study of community dwelling older men. We included data from participants in MrOS who had been randomly selected for hormone measurement (N=1,463 including 1054 with follow-up data 4.6 years later.). Major outcomes included prevalent vertebral fracture (semi-quantitative grade ≥ 2, N=140, 9.6%); and new or worsening vertebral fracture (change in SQ grade ≥ 1, N=55, 5.2%). Odds ratios per SD decrease in sex hormones and per SD increase in SHBG were estimated with logistic regression adjusted for potentially confounding factors including age, bone mineral density, and other sex hormones. Higher SHBG was associated with a greater likelihood of prevalent vertebral fractures (OR: 1.38 per SD increase, 95% CI: 1.11, 1.72). Total estradiol analyzed as a continuous variable was not associated with prevalent vertebral fractures (OR per SD decrease: 0.86, 95% CI: 0.68 to 1.10). Men with total estradiol values ≤ 17 pg/ml had a borderline higher likelihood of prevalent fracture than men with higher values (OR: 1.46, 95% CI: 0.99, 2.16). There was no association between total testosterone and prevalent fracture. In longitudinal analyses, SHBG (OR: 1.42 per SD increase, 95% CI: 1.03, 1.95) was associated with new or worsening vertebral fracture, but there was no association with total estradiol or total testosterone. In conclusion, higher SHBG (but not testosterone or estradiol) is an independent risk factor for vertebral fractures in older men.
Keywords: osteoporosis, vertebral fracture, estradiol, testosterone, sex hormone binding globulin
Introduction
Estradiol, testosterone and their metabolites are known to influence skeletal health. Declines in estrogens due to menopause are linked to bone loss (1); there is significant bone loss in men with severe sex hormone deficiency (2). Testosterone and estradiol are predominantly bound to other proteins in the circulation, in particular sex hormone binding globulin (SHBG). Thus, SHBG levels influence the amount of the hormone that is freely able to enter cells and interaction with its receptor. Testosterone and estradiol levels decline with age, and SHBG levels increase, thus each of these factors are inter-related yet may have independent effects on health.
Current clinical practice guidelines state that men with androgen deficiency syndromes (primarily focusing on total testosterone), in certain circumstances, should be treated with testosterone, to improve bone mineral density (BMD), and thus presumably lower their risk of fractures.(3, 4) Low levels of testosterone (and estradiol) have been linked to non-vertebral fractures and osteoporosis, as well as frailty in older adults.(5–12) A recent report from the MrOS Swedish and Hong Kong cohorts of older men reported an association between higher levels of SHBG, but not testosterone or estradiol, and an increased risk of incident, clinically identified vertebral fractures (13); radiographic vertebral fractures were only ascertained in the Hong Kong men, but results were similar to those for clinical vertebral fractures. A few other smaller studies have also investigated this association in men and women, but these were limited to case-control studies(5, 14); small clinical populations (6); or cohort studies with relatively few fractures (11, 15). Whether sex hormones and SHBG are related to radiographic vertebral fractures in Caucasian men remains unclear.
Thus, we hypothesized that lower total estradiol, total testosterone, bioavailable estradiol, bioavailable testosterone and higher sex hormone binding globulin (SHBG) would be associated with greater likelihood of both prevalent and incident vertebral fractures in Caucasian older men. We tested this hypothesis using data from the United States cohort of the Osteoporotic Fractures in Men Study (MrOS), a cohort of older community dwelling men.
Materials and Methods
Participants
At the baseline visit in 2000–2002, 5,994 community dwelling men aged ≥ 65 years were recruited at six U.S. academic medical centers.(16, 17) To participate, men must have been free from bilateral hip replacements and able to walk without assistance. Men returned to the clinics for a second evaluation (Visit 2) an average of of 4.6±0.4SD years after baseline: 4,530 men returned to the clinical center for repeat x-rays and 699 provided questionnaire-based data only. All men provided written informed consent.
Measurement of sex hormones
A random sample of 1,602 men from the cohort was selected for assessment of sex hormone levels in fasting serum from the baseline visit, as previously described.(10) Total estradiol and total testosterone were analyzed centrally by gas chromatograph/mass spectrometry (GCMS) assay (Taylor Technology, Princeton NJ). Briefly, the analytes and their deuterated internal standards were extracted from 1.00 mL of human serum using BondElut Certify solid phase cartridges. Estradiol and testosterone were eluted from the solid phase cartridges with ethyl acetate. The analytes underwent three separate derivatization steps: (1) reaction with pentafluorobenzoyl chloride, (2) reaction with O (2,3,4,5,6 pentafluorobenzyl) hydroxylamine hydrochloride, and (3) reaction with N-Methyl-N-(trimethylsilyl)trifluoroacetamide. Then the derivatized analytes were separated by gas chromatography using a DB 17 fused silica capillary column and detected by tandem mass spectrometry using negative ion chemical ionization. The lower limit of detection for total estradiol was 0.625 pg/ml and for total testosterone, 2.5 ng/dl. Duplicate aliquots from each participant were assayed, and the two results were averaged. No sex hormone values fell below the assay sensitivity. The intra-assay CV for total estradiol was 6.4%; the inter-assay CV was 10.1%. The intra-assay CV for total testosterone was 2.5%; the inter-assay CV was 6.0%.
Sex hormone binding globulin (SHBG) was assayed by the Oregon Health & Sciences Center (OHSU) OCTRI labs using an Immulite Analyzer with chemiluminescent substrate (Diagnostic Products Corp., Los Angeles, CA) on the same samples previously thawed for the sex hormone measurements. The standard curve ranged from 0.2 – 180 nm/l. No values fell below the standard curve; only two participants with values above the standard curve were excluded from analyses. Only 21 men had insufficient serum to have SHBG assays. The intra-assay CV for SHBG was 4.6%; the inter-assay CV was 5.8%.
Bioavailable estradiol and testosterone were calculated using mass action equations described by Sodergard(18) with updated association constants for testosterone(19) and a fixed albumin level of 4.3 g/dl. The “bioavailable” amount of hormone refers to the amount that is either unbound (free) or loosely bound to albumin in the serum and excludes the amount of hormone that is tightly bound to SHBG.
Prevalent and incident radiographic vertebral fracture assessment
Vertebral fracture assessment on lateral and thoracic radiographs in MrOS has been described in detail elsewhere.(20) The general process for review of spine images in MrOS was as follows. First, all spine images were assessed for quality and underwent a “triage process” by trained technicians, designed to eliminate grossly normal images from semi-quantitative (SQ) scoring, thereby reducing the number of images that needed to be read by the physician reader (JS). In a quality assurance subset (random sample of 500 participants), sensitivity of the triage process was 97%; specificity was 46%. When triage was complete, all films from participants with a possible fracture or other abnormality (“triage positive”) were evaluated by a physician reader using the SQ method of Genant;(21) triage negative films were assumed to be fracture free and the SQ score was set to 0 for all levels. The triage process had few false negatives (that is, a high sensitivity: 96.8%) and the SQ scoring had excellent reproducibility as kappa scores ranged from 0.79–0.91 on a series of quality assurance readings. Of the 5,994 participants at baseline, 5,958 had radiographs of sufficient quality to assess vertebral fracture status. Of the 5,110 men who provided any data at Visit 2, 4,332 had radiographs of sufficient quality to assess vertebral fracture status at follow-up.
Clinical vertebral facture assessment
As described previously(22), participants self-reported (at four-month intervals), fractures for which medical records were subsequently obtained. Verification of clinical vertebral fractures and date of identification was completed by the central study radiologist; only clinically identified vertebral fractures identified in T4-T12 and L1-L4 were included in the analyses.
Other measures
Participants completed questionnaires that included information about age, race/ethnicity (white vs. non-white), smoking status (current/past/never), non-spine fractures since age 50, and history of falls in the past 12 months. Height and weight were measured to calculate body mass index (BMI) as weight (kg) / height2 (m2). Activity level was determined from the Physical Activity Scale for the Elderly (PASE)(23); a higher score indicated a higher activity level. Participants also reported a history of a physician diagnosis of the selected medical conditions (see Table 1 footnote). Bone mineral density (BMD) was measured using Hologic 4500 dual energy x-ray absorptiometry (DXA) machines (Hologic, Inc., Bedford, MA) as previously described.(24) At baseline, participants were asked to bring all prescription medications they had been taking for at least 1 month and medication use was coded using standard study procedures.(25) Physical performance was assessed,(22) including walking speed (m/s) at usual pace was determined over 6 meters
Table 1.
Characteristics of participants [mean ± SD or N (%)] by quartiles of sex hormone binding globulin (SHBG)
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
|---|---|---|---|---|---|
| < 35.7 nmol/liter | ≥35.7–<46.5 nmol/liter | ≥46.5–<59.3 nmol/liter | ≥59.3 nmol/liter | P value | |
| Age | 71.8 ± 5.0 | 73.2 ± 5.5 | 74.02 ± 6.0 | 75.7 ± 6.4 | <0.001 |
| Race/ethnicity | |||||
| Caucasian | 321 (88.4) | 345 (93.0) | 328 (89.4) | 326 (88.6) | 0.161 |
| African American | 15 (4.1) | 11 (3.0) | 16 (4.4) | 10 (2.7) | |
| Asian, Hispanic or Other | 27 (7.4) | 15 (4.0) | 23 (6.3) | 32 (8.7) | |
| BMI (kg/m2) | 28.9 ± 3.8 | 27.7 ± 4.0 | 26.7 ± 3.1 | 26.2 ± 3.6 | <0.001 |
| Height (cm) | 174.7 ± 6.3 | 174.9 ± 6.9 | 174.1 ± 7.2 | 173.9 ± 7.1 | 0.163 |
| Smoking | |||||
| Never | 116 (32.0) | 133 (35.9) | 146 (39.8) | 151 (41.0) | 0.002 |
| Past | 241 (66.4) | 224 (60.4) | 207 (56.4) | 194 (52.7) | |
| Current | 6 (1.7) | 14 (3.8) | 14 (3.8) | 23 (6.3) | |
| PASE score (activity level) | 148.7 ± 68.2 | 151.5 ± 70.8 | 143.6 ± 69.0 | 143.5 ± 67.5 | 0.299 |
| Fall history | 58 (16.0) | 73 (19.7) | 88 (24.0) | 89 (24.2) | 0.018 |
| Femoral neck BMD (g/cm2) | 0.818 ± 0.128 | 0.777 ± 0.123 | 0.785 ± 0.130 | 0.749 ± 0.122 | <0.001 |
| Lumbar spine BMD (g/cm2) | 1.080 ± 0.175 | 1.078 ± 0.194 | 1.069 ± 0.177 | 1.036 ± 0.177 | 0.003 |
| Walking speed (m/s) | 1.20 ± 0.23 | 1.20 ± 0.23 | 1.20 ± 0.22 | 1.18 ± 0.23 | 0.084 |
| Non-spine fracture after age 50 | 73 (20.2) | 87 (23.5) | 87 (23.7) | 96 (26.2) | 0.288 |
| Number of selected comorbidities* | |||||
| 0 | 166 (45.7) | 185 (49.9) | 189 (51.5) | 182 (49.5) | 0.608 |
| 1–2 | 173 (47.7) | 171 (46.1) | 161 (43.9) | 167 (45.4) | |
| 3+ | 24 (6.6) | 15( 4.0) | 17 (4.6) | 19 (5.2) | |
| Bioavailable Estradiol (pg/ml) | 15.1 ± 4.9 | 14.7 ± 4.8 | 14.2 ± 4.7 | 13.5 ± 4.7 | <0.001 |
| Estradiol (pg/ml) | 20.1 ± 6.6 | 21.7 ± 7.0 | 22.9 ± 7.5 | 25.2 ± 9.2 | <0.001 |
| Bioavailable Testosterone (ng/dl) | 179.0 ± 51.4 | 204.0 ± 57.9 | 215.0 ± 61.8 | 219.4 ± 78.0 | <0.001 |
| Testosterone (ng/dl) | 282.2 ± 83.4 | 367.9 ± 99.8 | 431.9 ± 118.7 | 535.5 ± 197.6 | <0.001 |
Medical conditions included self-report of a physician’s diagnosis of diabetes, stroke, low or high thyroid levels, heart attack, congestive heart failure, chronic obstructive pulmonary disease, Parkinson’s disease, and non-skin cancer.
BMD: Bone mineral density
Analysis sample
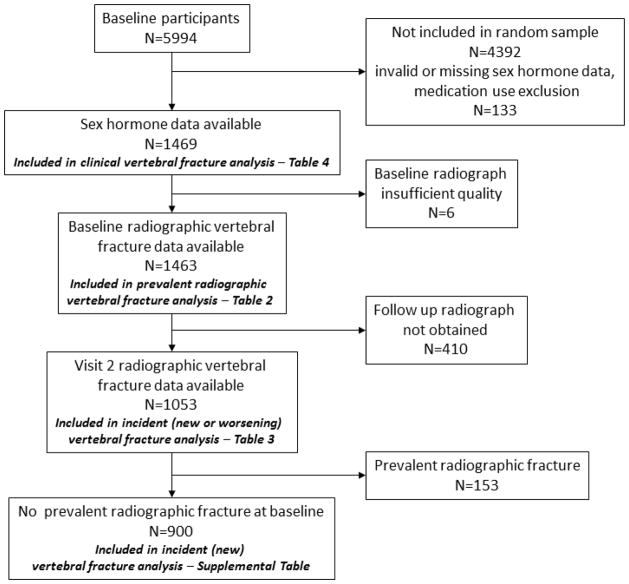
A random sample (N=1602) of the baseline participants (N=5994) was selected for measurement of sex hormones; 133 of these men were excluded due to missing or implausible values for sex hormones; medication use (reported taking androgens or antiandrogens, or missing medication use data); or surgical castration as treatment for prostate cancer. This left 1,469 men with complete sex hormone data. Follow-up for the clinical vertebral fracture outcome was available for all of these men. Of these 1,469 participants, 6 men had baseline radiographs of insufficient quality to assess vertebral fracture status, leaving 1,463 men for analyses of prevalent vertebral fractures. A total of 410 men did not have Visit 2 radiographs completed or of sufficient quality (158 died, 33 did not return for the visit, 219 returned for the visit but did not have radiographs taken), leaving 1,053 men for analyses of incident radiographic vertebral fractures (defined as new or worsening fracture). Of these, 153 had a prevalent fracture at baseline (SQ=1), leaving 900 men in analyses of new incident vertebral fractures. (Summarized in Figure 1).
Figure 1.
Participants selected for analysis.
Statistical analysis
Participant characteristics were compared by quartiles of SHBG, with ANOVA for continuous normally distributed variables, Kruskal-Wallis rank sum tests for continuous skewed variables and chi-square tests for categorical variables.
In the cross-sectional analyses, prevalent vertebral fractures were defined as moderate or worse (SQ≥2) compared to mild or no fracture (SQ≤1). In the prospective analyses, incident radiographic vertebral fractures were primarily defined as a new or worsening fracture at follow-up, defined as a change in SQ score of 1 or more without adjustment for baseline fracture status. In secondary analyses, we excluded participants with a prevalent radiographic vertebral fracture (SQ≥1 at baseline) and estimated the likelihood of developing a new radiographic vertebral fracture (SQ≥1) at follow-up.
Sex hormone levels were analyzed as quartiles and as continuous variables with the odds or hazard ratio reported per SD increase. We also analyzed total testosterone as dichotomous variables: ≤ 200 versus > 200 ng/dl and ≤ 300 versus >300 ng/dl We used two different cut-points as professional societies are equivocal on a single cut-point to use for defining testosterone deficiency.(3) We also analyzed total estradiol as a dichotomous variable: ≤ 17 vs. >17 pg/ml. Logistic regression models were used to estimate the likelihood of prevalent or incident radiographic vertebral fractures. Cox proportional hazards models were used to estimate the risk of incident clinical vertebral fractures.
Initially, each sex hormone was analyzed individually. Base models were adjusted for age and clinical center. Next, models were adjusted for age, clinical center, smoking status, non-white race, height, weight, history of falling, presence of at least one medical condition (see footnote Table 1), inability to complete the repeat chair stands, and physical activity level (PASE score). We then further adjusted the models for lumbar spine BMD (LS BMD). Finally, we ran additional fully adjusted multivariate models in which we simultaneously included total estradiol, total testosterone and SHBG as continuous variables. We did not consider models with bioavailable estradiol, bioavailable testosterone and SHBG simultaneously because the algorithms to calculate bioavailable hormones include SHBG, and inclusion of both SHBG and bioavailable hormones in the same model may constitute over-adjustment.
Results
Given the consistent association between SHBG and fracture risk, participant characteristics by quartiles of SHBG are shown in Table 1. Participants in higher quartiles of SHBG were likely to be older; have lower BMI, and BMD; and were more likely to report a history of smoking and falls than those in lower quartiles of SHBG. Several characteristics were not associated with SHBG quartiles, including race/ethnicity, height, activity level, medication use, physical performance (walking speed and chair stand ability), and comorbid medical conditions. Those with higher SHBG had lower bioavailable estradiol, higher total estradiol, higher bioavailable testosterone and higher total testosterone compared to those with lower SHBG. Characteristics of MrOS participants by bioavailable estradiol quartiles(26) and bioavailable testosterone quartiles(10) have been reported elsewhere.
Prevalent radiographic vertebral fractures
Of the 1,463 men included in the prevalent radiographic vertebral fracture analyses, 140 (9.6%) had an at least one vertebrae with a SQ grade of ≥ 2. There was an association between higher SHBG and greater likelihood of prevalent radiographic vertebral fracture when SHBG was analyzed as a continuous measure, although the analyses of SHBG as quartiles were not statistically significant (Table 2). These results were largely unchanged by adjustment for covariates including spine BMD. When further adjusted for total estradiol and total testosterone, the association of SHBG with prevalent vertebral fracture was somewhat stronger (OR: 1.38 per SD increase, 95% CI: 1.11, 1.72).
Table 2.
Likelihood (OR, 95% CI) of moderate or severe prevalent radiographic vertebral fracture (SQ grade ≥ 2) by sex hormone level
| Q1 | Q2 | Q3 | Q4 | Per SD increase | ||
|---|---|---|---|---|---|---|
| SHBG | < 35.7 nmol/liter | ≥35.7–<46.5 nmol/liter | ≥46.5–<59.3 nmol/liter | ≥59.3 nmol/liter | p trend | SD=19.78 |
| N with outcome | 31 (8.5) in 363 | 30 (8.1) in 370 | 35 (9.6) in 365 | 44 (12.1) in 365 | ||
| Age, clinical center adjusted | 0.74 (0.45, 1.22) | 0.67 (0.41, 1.10) | 0.78 (0.48, 1.25) | 1.0 (ref) | 0.174 | 1.19 (1.01, 1.40) |
| Multivariate Adjusted | 0.66 (0.39, 1.13) | 0.57 (0.34, 0.96) | 0.71 (0.44, 1.15) | 1.0 (ref) | 0.079 | 1.24 (1.04, 1.47) |
| Multivariate Adjusted + BMD | 0.70 (0.41, 1.20) | 0.61 (0.36, 1.04) | 0.75 (0.46, 1.23) | 1.0 (ref) | 0.128 | 1.20 (1.01, 1.43) |
| E2 | < 17.2 pg/ml | ≥17.2–<21.7 pg/ml | ≥21.7–<26.9 g/ml | ≥26.9 pg/ml | SD=7.86 | |
| N with outcome | 46 (12.8) in 359 | 30 (8.2) in 368 | 37 (10.1) in 368 | 27 (7.3) in 368 | ||
| Minimally Adjusted | 1.84 (1.11, 3.04) | 1.13 (0.65, 1.95) | 1.42 (0.84, 2.38) | 1.0 (ref) | 0.040 | 0.82 (0.68, 0.98) |
| Multivariate Adjusted | 1.78 (1.07, 2.96) | 1.10 (0.63, 1.93) | 1.39 (0.82, 2.36) | 1.0 (ref) | 0.058 | 0.82 (0.68, 0.99) |
| Multivariate Adjusted + BMD | 1.56 (0.93, 2.62) | 1.05 (0.60, 1.84) | 1.31 (0.76, 2.23) | 1.0 (ref) | 0.162 | 0.86 (0.71, 1.04) |
| T | < 298.0 ng/dl | ≥298.0–<388.0 ng/dl | ≥388.0–<488.0 ng/dl | ≥488.0 ng/dl | SD=161.31 | |
| N with outcome | 39 (10.7) in 366 | 22 (6.0) in 366 | 40 (11.0) in 365 | 39 (10.7) in 366 | ||
| Minimally Adjusted | 0.98 (0.61, 1.57) | 0.52 (0.30, 0.91) | 1.01 (0.63, 1.62) | 1.0 (ref) | 0.424 | 0.96 (0.80, 1.15) |
| Multivariate Adjusted | 0.95 (0.57, 1.58) | 0.53 (0.30, 0.92) | 0.97 (0.60, 1.58) | 1.0 (ref) | 0.394 | 0.96 (0.79, 1.17) |
| Multivariate Adjusted + BMD | 0.95 (0.57, 1.60) | 0.54 (0.31, 0.95) | 0.98 (0.60, 1.59) | 1.0 (ref) | 0.426 | 0.95 (0.78, 1.15) |
| BioE | < 11.3 pg/ml | ≥11.3–<14.0 pg/ml | ≥14.0–<17.0 pg/ml | ≥17.0 pg/ml | SD=4.80 | |
| N with outcome | 50 (13.7) in 365 | 29 (7.9) in 366 | 33 (9.0) in 366 | 28 (7.7) in 366 | ||
| Minimally Adjusted | 1.83 (1.12, 2.99) | 1.00 (0.58, 1.72) | 1.17 (0.69, 1.99) | 1.0 (ref) | 0.024 | 0.77 (0.64, 0.93) |
| Multivariate Adjusted | 1.80 (1.09, 2.97) | 0.96 (0.55, 1.68) | 1.13 (0.66, 1.94) | 1.0 (ref) | 0.031 | 0.77 (0.63, 0.93) |
| Multivariate Adjusted + BMD | 1.47 (0.88, 2.46) | 0.82 (0.47, 1.45) | 1.00 (0.58, 1.73) | 1.0 (ref) | 0.162 | 0.82 (0.67, 0.99) |
| BioT | < 165.4 ng/dl | ≥165.4–<201.5 ng/dl | ≥201.5–<241.9 ng/dl | ≥241.9 ng/dl | SD=64.89 | |
| N with outcome | 37 (10.1) in 367 | 35 (9.6) in 365 | 39 (10.6) in 367 | 29 (8.0) in 364 | ||
| Minimally Adjusted | 1.18 (0.70, 1.98) | 1.16 (0.69, 1.95) | 1.28 (0.77, 2.13) | 1.0 (ref) | 0.682 | 0.85 (0.71, 1.01) |
| Multivariate Adjusted | 1.20 (0.69, 2.10) | 1.18 (0.69, 2.01) | 1.29 (0.77, 2.17) | 1.0 (ref) | 0.633 | 0.82 (0.67, 1.00) |
| Multivariate Adjusted + BMD | 1.16 (0.66, 2.03) | 1.17 (0.68, 2.01) | 1.27 (0.75, 2.15) | 1.0 (ref) | 0.720 | 0.83 (0.68, 1.01) |
Minimally adjusted models include age and clinical center. Multivariate adjusted models include age, clinical center, smoking status, non-white race, height, weight, history of falling, presence of at least one medical condition.
In age and clinical center adjusted models, lower total estradiol and lower bioavailable estradiol were associated with an increased likelihood of prevalent radiographic vertebral fracture, although adjustment for lumbar spine BMD largely attenuated this association. The association between total estradiol and prevalent radiographic vertebral fracture was non-significant after further adjustment for total testosterone and SHBG (OR per SD increase 0.86, 95%CI: 0.68, 1.10). When total estradiol was analyzed as a dichotomous variable categorized as ≤ 17 vs >17 pg/ml, men with values ≤ 17 ng/dl were more likely to have a prevalent vertebral fracture than men with higher levels (OR: 1.68, 95% CI: 1.15, 2.45). This association remained after multivariate adjustment (OR: 1.62 for ≤ 17 vs >17 ng/dl, 95% CI 1.10, 2.39) but was attenuated and of borderline significance after adjusted for BMD (OR: 1.46 for ≤ 17 vs >17 ng/dl, 95% CI: 0.99, 2.16). Neither total testosterone nor bioavailable testosterone was associated with prevalent radiographic vertebral fractures in any model. There was no association between prevalent radiographic vertebral fractures and total testosterone analyzed as >200 ng/dl vs ≤ 200 ng/dl, or total testosterone was analyzed as >300 ng/dl vs. ≤ 300 ng/dl.
Incident radiographic vertebral fractures
Of the 1,053 men included in the longitudinal analysis, 55 (5.2%) men had new or worsening radiographic vertebral fracture (change in SQ grade≥1). Of these, 51 men had at least one new vertebral fracture (change in SQ grade from 0 at baseline to 1 or higher at follow-up) and 4 men had only a worsening vertebral fracture (SQ grade at least 1 at baseline, and increased by at least one point at follow-up). Higher SHBG was associated with an increased likelihood of new or worsening radiographic vertebral fracture, an association that was largely unchanged by adjustment for covariates including BMD (Table 3). When SHBG, total estradiol and total testosterone were included in the same fully adjusted model, each SD increase in SHBG was associated with an increased likelihood of new or worsening radiographic vertebral fracture (OR: 1.42, 95% CI: 1.03, 1.95), whereas total estradiol and total testosterone were not significantly related to new or worsening radiographic vertebral fracture. There was no association between total estradiol, bioavailable estradiol, total testosterone or bioavailable testosterone and new or worsening vertebral fracture regardless of the covariates included in the model. When analyses were restricted to men with no radiographic vertebral fracture at baseline (N=900, 36 [4.0%] who then developed a new fracture, Supplemental Table 1), the results were largely unchanged. In the analyses restricted to men with no radiographic vertebral fracture at baseline, higher SHBG was associated with an increased likelihood of fracture, although the association after adjustment for lumbar spine BMD was of borderline significance. In this subset of men without radiographic vertebral fracture at baseline, in models that simultaneously included SBHG, total estradiol and total testosterone and other covariates, the association between SHBG and new radiographic vertebral fracture was somewhat strengthened (OR: 1.72 per SD increase, 95% CI: 1.13, 2.62). There was no association between total estradiol, bioavailable estradiol, total testosterone or bioavailable testosterone and new radiographic vertebral fracture in the subset of men free of radiographic fracture at baseline. There was no association between incident radiographic vertebral fractures (in all participants, or on the subset of participants free from radiographic vertebral fracture at baseline) and total testosterone analyzed as >200 ng/dl vs ≤ 200 ng/dl, or total testosterone was analyzed as >300 ng/dl vs. ≤ 300 ng/dl, or estradiol analyzed as >17 vs ≤ 17 pg/ml.
Table 3.
Likelihood (OR, 95% CI) of new or worsening radiographic vertebral fracture (ΔSQ grade ≥ 1) by sex hormone level
| Q1 | Q2 | Q3 | Q4 | Per SD increase | ||
|---|---|---|---|---|---|---|
| SHBG | < 35.7 nmol/liter | ≥35.7–<46.5 nmol/liter | ≥46.5–<59.3 nmol/liter | ≥59.3 nmol/liter | p trend | SD=19.78 |
| N with outcome | 11 (3.9) in 279 | 5 (1.8) in 279 | 18 (7.0) in 259 | 21 (8.9) in 236 | ||
| Minimally Adjusted | 0.54 (0.25, 1.18) | 0.22 (0.08, 0.59) | 0.83 (0.43, 1.63) | 1.0 (ref) | 0.016 | 1.36 (1.07, 1.73) |
| Multivariate Adjusted | 0.54 (0.24, 1.22) | 0.21 (0.08, 0.58) | 0.85 (0.43, 1.68) | 1.0 (ref) | 0.019 | 1.35 (1.05, 1.74) |
| Multivariate Adjusted + BMD | 0.56 (0.24, 1.28) | 0.21 (0.08, 0.60) | 0.86 (0.43, 1.71) | 1.0 (ref) | 0.028 | 1.29 (1.00, 1.67) |
| E2 | < 17.2 pg/ml | ≥17.2–<21.7 pg/ml | ≥21.7–<26.9 pg/ml | ≥26.9 pg/ml | SD=7.83 | |
| N with outcome | 12 (4.8) in 249 | 12 (4.4) in 276 | 17 (6.4) in 266 | 14 (5.3) in 262 | ||
| Minimally Adjusted | 0.99 (0.44, 2.21) | 0.97 (0.43, 2.16) | 1.27 (0.61, 2.66) | 1.0 (ref) | 0.815 | 1.05 (0.81, 1.36) |
| Multivariate Adjusted | 1.00 (0.44, 2.24) | 0.98 (0.44, 2.21) | 1.30 (0.62, 2.75) | 1.0 (ref) | 0.831 | 1.04 (0.81, 1.34) |
| Multivariate Adjusted + BMD | 0.85 (0.37, 1.94) | 0.94 (0.41, 2.13) | 1.26 (0.59, 2.67) | 1.0 (ref) | 0.566 | 1.09 (0.85, 1.41) |
| T | < 298.0 ng/dl | ≥298.0–<388.0 ng/dl | ≥388.0–<488.0 ng/dl | ≥488.0 ng/dl | SD=160.9 | |
| N with outcome | 13 (5.1) in 255 | 14 (5.2) in 269 | 14 (5.4) in 261 | 14 (5.2) in 268 | ||
| Minimally Adjusted | 1.03 (0.47, 2.27) | 1.06 (0.49, 2.29) | 1.03 (0.48, 2.23) | 1.0 (ref) | 0.918 | 1.08 (0.83, 1.40) |
| Multivariate Adjusted | 1.05 (0.46, 2.40) | 1.05 (0.47, 2.32) | 1.03 (0.47, 2.25) | 1.0 (ref) | 0.906 | 1.07 (0.82, 1.41) |
| Multivariate Adjusted + BMD | 1.08 (0.47, 2.48) | 1.07 (0.48, 2.39) | 1.04 (0.47, 2.28) | 1.0 (ref) | 0.854 | 1.06 (0.80, 1.38) |
| BioE | < 11.3 pg/ml | ≥11.3–<14.0 pg/ml | ≥14.0–<17.0 pg/ml | ≥17.0 pg/ml | SD=4.79 | |
| N with outcome | 16 (6.4) in 249 | 9 (3.4) in 264 | 16 (6.0) in 266 | 14 (5.1) in 274 | ||
| Minimally Adjusted | 1.26 (0.59, 2.69) | 0.68 (0.29, 1.62) | 1.23 (0.58, 2.59) | 1.0 (ref) | 0.865 | 0.93 (0.71, 1.21) |
| Multivariate Adjusted | 1.26 (0.59, 2.72) | 0.67 (0.28, 1.62) | 1.30 (0.61, 2.78) | 1.0 (ref) | 0.900 | 0.93 (0.71, 1.22) |
| Multivariate Adjusted + BMD | 1.02 (0.46, 2.23) | 0.57 (0.23, 1.39) | 1.18 (0.55, 2.56) | 1.0 (ref) | 0.666 | 1.00 (0.76, 1.32) |
| BioT | < 165.4 ng/dl | ≥165.4–<201.5 ng/dl | ≥201.5–<241.9 ng/dl | ≥241.9 ng/dl | SD=64.81 | |
| N with outcome | 15 (6.4) in 234 | 14 (5.3) in 264 | 13 (4.8) in 270 | 13 (4.6) in 285 | ||
| Minimally Adjusted | 1.33 (0.60, 2.92) | 1.25 (0.57, 2.75) | 1.05 (0.47, 2.35) | 1.0 (ref) | 0.422 | 0.86 (0.65, 1.14) |
| Multivariate Adjusted | 1.31 (0.57, 3.02) | 1.27 (0.56, 2.85) | 1.07 (0.48, 2.39) | 1.0 (ref) | 0.459 | 0.86 (0.65, 1.15) |
| Multivariate Adjusted + BMD | 1.29 (0.55, 2.99) | 1.27 (0.56, 2.87) | 1.10 (0.49, 2.48) | 1.0 (ref) | 0.512 | 0.88 (0.66, 1.17) |
Minimally adjusted models include age and clinical center. Multivariate adjusted models include age, clinical center, smoking status, non-white race, height, weight, history of falling, presence of at least one medical condition.
Incident clinical vertebral fracture
Of the 1,469 men in the longitudinal analyses, only 3.1% (N=45) had a clinical vertebral fracture over 4.6 years of follow-up. Neither SHBG nor either of the sex hormones was significantly associated with clinical vertebral fractures in any model (Table 4).
Table 4.
Risk (HR, 95% CI) of clinical vertebral fracture by sex hormone level
| Q1 | Q2 | Q3 | Q4 | Per SD increase | ||
|---|---|---|---|---|---|---|
| SHBG | < 35.7 nmol/liter | ≥35.7–<46.5 nmol/liter | ≥46.5–<59.3 nmol/liter | ≥59.3 nmol/liter | p trend | |
| N with outcome | 8 (2.2) in 363 | 10 (2.7) in 371 | 10 (2.7) in 367 | 17 (4.6) in 368 | SD=19.78 | |
| Minimally Adjusted | 0.55 (0.23, 1.31) | 0.65 (0.29, 1.44) | 0.61 (0.28, 1.35) | 1.0 (ref) | 0.176 | 1.19 (0.92, 1.53) |
| Multivariate Adjusted | 0.53 (0.21, 1.31) | 0.65 (0.29, 1.45) | 0.60 (0.27, 1.32) | 1.0 (ref) | 0.167 | 1.19 (0.91, 1.55) |
| Multivariate Adjusted + BMD | 0.55 (0.22, 1.37) | 0.63 (0.28, 1.44) | 0.63 (0.29, 1.41) | 1.0 (ref) | 0.179 | 1.14 (0.88, 1.48) |
| E2 | < 17.2 pg/ml | ≥17.2–<21.7 pg/ml | ≥21.7–<26.9 pg/ml | ≥26.9 pg/ml | SD=7.86 | |
| N with outcome | 13 (3.6) in 361 | 8 (2.2) in 369 | 13 (3.5) in 368 | 11 (3.0) in 371 | ||
| Minimally Adjusted | 1.34 (0.60, 3.01) | 0.82 (0.33, 2.05) | 1.27 (0.57, 2.85) | 1.0 (ref) | 0.693 | 0.89 (0.66, 1.19) |
| Multivariate Adjusted | 1.40 (0.62, 3.14) | 0.86 (0.34, 2.16) | 1.36 (0.60, 3.05) | 1.0 (ref) | 0.631 | 0.88 (0.66, 1.17) |
| Multivariate Adjusted + BMD | 1.19 (0.53, 2.71) | 0.81 (0.32, 2.06) | 1.26 (0.56, 2.86) | 1.0 (ref) | 0.893 | 0.92 (0.69, 1.23) |
| T | < 298.0 ng/dl | ≥298.0–<388.0 ng/dl | ≥388.0–<488.0 ng/dl | ≥488.0 ng/dl | SD=161.31 | |
| N with outcome | 12 (3.3) in 366 | 11 (3.0) in 368 | 11 (3.0) in 365 | 11 (3.0) in 370 | ||
| Minimally Adjusted | 1.08 (0.47, 2.45) | 0.93 (0.40, 2.16) | 0.96 (0.42, 2.23) | 1.0 (ref) | 0.880 | 1.08 (0.82, 1.43) |
| Multivariate Adjusted | 1.11 (0.47, 2.64) | 0.94 (0.40, 2.23) | 0.99 (0.42, 2.31) | 1.0 (ref) | 0.844 | 1.08 (0.81, 1.44) |
| Multivariate Adjusted + BMD | 1.06 (0.44, 2.53) | 0.93 (0.39, 2.23) | 1.05 (0.45, 2.46) | 1.0 (ref) | 0.962 | 1.07 (0.81, 1.41) |
| BioE | < 11.3 pg/ml | ≥11.3–<14.0 pg/ml | ≥14.0–<17.0 pg/ml | ≥17.0 pg/ml | SD=4.80 | |
| N with outcome | 14 (3.8) in 367 | 15 (4.1) in 367 | 7 (1.9) in 367 | 9 (2.5) in 368 | ||
| Minimally Adjusted | 1.60 (0.69, 3.71) | 1.63 (0.71, 3.72) | 0.78 (0.29, 2.09) | 1.0 (ref) | 0.117 | 0.80 (0.59, 1.09) |
| Multivariate Adjusted | 1.69 (0.72, 3.97) | 1.69 (0.73, 3.94) | 0.84 (0.31, 2.26) | 1.0 (ref) | 0.102 | 0.79 (0.59, 1.07) |
| Multivariate Adjusted + BMD | 1.40 (0.59, 3.36) | 1.50 (0.63, 3.58) | 0.78 (0.28, 2.13) | 1.0 (ref) | 0.240 | 0.85 (0.62, 1.16) |
| BioT | < 165.4 ng/dl | ≥165.4–<201.5 ng/dl | ≥201.5–<241.9 ng/dl | ≥241.9 ng/dl | SD=64.89 | |
| N with outcome | 14 (3.8) in 367 | 10 (2.7) in 367 | 12 (3.3) in 367 | 9 (2.5) in 368 | ||
| Minimally Adjusted | 1.40 (0.60, 3.30) | 1.12 (0.45, 2.77) | 1.22 (0.51, 2.92) | 1.0 (ref) | 0.494 | 0.99 (0.73, 1.34) |
| Multivariate Adjusted | 1.46 (0.60, 3.57) | 1.20 (0.48, 3.02) | 1.26 (0.52, 3.05) | 1.0 (ref) | 0.453 | 0.98 (0.72, 1.34) |
| Multivariate Adjusted + BMD | 1.28 (0.52, 3.16) | 1.18 (0.47, 3.00) | 1.24 (0.51, 3.00) | 1.0 (ref) | 0.640 | 1.02 (0.75, 1.39) |
Discussion
In this cohort of community dwelling older men, higher SHBG was associated with a greater likelihood of both prevalent and incident radiographic vertebral fracture. This association was largely unchanged by adjustment for covariates including BMD and other sex hormones. However, the association between SHBG and incident clinical vertebral fractures was not statistically significant, although number and therefore power was much lower. We saw no association between total testosterone (or bioavailable testosterone) and prevalent radiographic vertebral fracture, incident radiographic vertebral fracture or incident clinical vertebral fracture. Lower total estradiol and lower bioavailable estradiol were associated with a greater likelihood of prevalent radiographic vertebral fracture, but there was no association between total estradiol or bioavailable estradiol and incident radiographic or clinical vertebral fracture.
Results from previous studies in men somewhat parallel our results: a previous report from the Swedish and Hong Kong cohorts of MrOS show an association between SHBG and incident clinically identified vertebral fractures (13); our results from the U.S. cohort demonstrate an association between SHBG and radiographic but not incident clinical vertebral fractures. In addition, we demonstrated the association of SHBG with radiographic fracture was independent of testosterone and estradiol. Identification of clinical vertebral fractures differed amongst the three countries in MrOS: in the US, clinically identified vertebral fractures were centrally reviewed by a study radiologist and compared to the study-acquired baseline radiograph. In Sweden, clinical vertebral fractures were identified in national registries, and in Hong Kong fractures were collected via hospital surveillance and confirmation of self-report of factures. Despite these differences, these studies taken together demonstrate an association between high SHBG and incident vertebral fracture, and little or no association between testosterone and estradiol and incident vertebral fractures. Other reports, including a case-control study(5) and a study of a small clinical population(6) both found higher SHBG in men with prevalent vertebral fractures than in men with no vertebral fractures. Neither study found a significant difference in estradiol or testosterone levels between men with fractures and men without vertebral fractures. For other bone outcomes, previous reports from this cohort(7) and the Tromso Study(8) consistently find that high SHBG is associated with increased risk of non-spine fracture in men; other studies (such as Framingham)(9) did not report the association between SHBG and non-spine or hip fractures in men. Congruent to our current findings, previous work in MrOS has also shown that high SHBG was associated with lower BMD and greater bone loss, but there was no independent association between bioavailable testosterone and BMD or BMD loss.(26)
The SHBG findings are puzzling. It is possible that SHBG has independent effects on risk of vertebral fractures by directly influencing skeletal tissues. Although it was long considered merely a transporter for sex hormones to target cell membranes, it is now considered likely that SHBG acts directly on a variety of cell types and can be found intracellularly.(27) Receptors for SHBG (RSHBG) have been found on cells of breast tissue, ovarian follicles, and placenta, for example, and its binding can affect both cell growth and biochemical activity. However, to our knowledge, there is no information regarding either RSHBG presence or SHBG production in cell types relevant to bone strength. Elucidating the biologic mechanisms of SHBG on bone health in vivo may be important for osteoporosis prevention. We cannot rule out, however, that higher levels SHBG may be a marker for poor health status, and that we were unable to completely control for such confounding influences. For example, men with higher SHBG levels were more likely to report history of falls and have lower lumbar spine BMD. We adjusted for these covariates, but other factors such as bone strength or complex measures of health status were not included in our multivariate models. Other reports that have found an association between SHBG and bone loss have postulated that SHBG is a biomarker for bone health (12), rather than a direct intermediate, and our data support this theory.
Total estradiol and bioavailable estradiol, was associated with prevalent radiographic vertebral fractures in multivariate models, although these associations were strongly attenuated after adjustment for bone mineral density. The association appeared strongest in the lowest quartile of total estradiol suggesting a threshold effect; however, even analyses comparing low (e.g. quartile 1, ≤ 17 pg/ml) versus higher (e.g. quartiles 2–4, >17 pg/ml) estradiol were attenuated to non-significance by adjustment for BMD. We did not observe an association between total or bioavailable estradiol and incident radiographic or incident clinical vertebral fractures. This suggests that at most, only very low total estradiol or bioavailable estradiol levels (which were not commonly found in MrOS) may increase the likelihood of a prevalent vertebral fracture, and it is unlikely that such an association would be independent of BMD. In contrast to our current results, previous reports in MrOS have demonstrated that bioavailable estradiol was consistently and strongly related to BMD and bone loss.(26) The reason for the discrepancy – that bioavailable estradiol is related to bone loss but not incident vertebral fractures – is not clear. We had relatively few incident radiographic vertebral fractures; however, the effect estimates for the association between bioavailable estradiol and incident radiographic vertebral fractures were close to null after adjusting for BMD suggesting that we did not miss a strong effect. It may be that any effect of bioavailable estradiol acts through BMD, and adjusting for BMD explains any effect of bioavailable estradiol on likelihood of vertebral fracture. Estradiol may be important for other fracture outcomes.
There was no association between total or bioavailable testosterone and vertebral fractures, a finding that does not provide direct evidence to support clinical practice guidelines that suggest men with androgen deficiency syndromes be supplemented with testosterone to improve (in part) their BMD and presumably subsequent fracture risk. Other studies of vertebral fractures in men parallel our findings of no association between testosterone and vertebral fractures(5, 6), and results for hip and non-vertebral fractures in men generally suggest that low testosterone is only a risk factor for non-spine fractures when levels of estradiol are also low and/or levels of SHBG are also high.(7–9) Given the lack of direct evidence from observational studies, randomized trials are needed to establish whether testosterone supplementation will decrease fracture risk.
Several alternative explanations are possible for our finding that only SHBG, but not the other sex hormones, was strongly related to prevalent and incident radiographic vertebral fractures. First, the lack of a significant association with estradiol and testosterone may be due to limited power. Relatively few men had vertebral fractures by any definition (<10%), and it is possible that the relatively limited sample size limited our ability to detect small, statistically significant effect sizes. Second, it is possible that the association between SHBG and vertebral fractures is due to residual confounding that did not influence the associations between the other sex hormones and vertebral fractures. If this were the case, then we would posit that higher SHBG is associated with greater frailty status, and the association between SHBG and fracture is due to this higher degree of frailty rather than SHBG levels per se. However, this is unlikely as we adjusted for several potential confounders related to physical function and activity, and these did not substantially alter the association between SHBG and radiographic vertebral fractures. Also, in previous analyses in this cohort, SHBG was not strongly related to frailty status.(10) Third, SHBG binds to testosterone and estradiol in serum, effectively reducing sex hormone concentrations and thus their ability to bind receptors. However, we did not see an association between bioavailable testosterone or bioavailable estradiol and vertebral fractures. We did not directly measure free or bioavailable sex hormones, but instead relied on a formula that depends on measurement of the sex hormones and SHBG. We acknowledge that there is considerable controversy about the accuracy of bioavailable calculations.(28–30) However, given the association between SHBG and fractures in our study, and the inability to directly measure bioavailable and free hormone levels in this study, we present the bioavailable data from calculations to provide a full picture of our results while acknowledging that such data have potential limitations. It is possible that our results would have differed had we used a direct assay to measure free hormone status. We found that SHBG, bioavailable estradiol and bioavailable testosterone levels are inter-related: those the highest SHBG levels had lower bioavailable estradiol yet higher bioavailable testosterone. Perhaps this seemingly incongruent fact is due to the very strong positive association between SHBG and total testosterone levels.
There are several factors that may explain the potential discrepancy between the positive association between SHBG and incident radiographic vertebral fractures and the lack of significant association between SHBG and incident clinical vertebral fractures. First, the number of clinical vertebral fractures in the dataset was small, limiting our power to detect an association. Second, there are differences in methods of ascertainment: radiographic vertebral fractures require collection of radiographs in our clinical centers, while clinical vertebral fracture must come to clinical attention, either due to symptoms or as incidental findings.
Our study has many strengths. It is a well characterized cohort with gold standard assessment of radiographic vertebral fractures and sex hormone levels. However a few limitations must be noted. First, the study results may not be generalizable to other populations including younger individuals, women, or those who are institutionalized. Second, we did not obtain follow-up radiographs for a small proportion of men, as some died before collection and others did not complete the radiographs at the clinic visit. This may have somewhat limited our ability to detect an effect. Third, we present models that simultaneously include total estradiol, total testosterone, and sex hormone binding globulin. These models are somewhat difficult to interpret, as estradiol and testosterone levels rise with greater SHBG levels and thus may somewhat overestimate the association between SHBG and vertebral fracture. However, these models did not suggest that the association between SHBG and vertebral fracture was explained by the level of total estradiol or total testosterone.
In summary, SHBG, but not estradiol or testosterone, was associated with prevalent and incident radiographic, but not clinically identified incident vertebral fractures. Further research should aim to identify the potential mechanism of action of SHBG on vertebral fracture risk.
Supplementary Material
Highlights.
Higher SHBG (but not testosterone or estradiol) is an independent risk factor for vertebral fractures in older men.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The sponsor had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Authors’ roles: Study design: PC, KE, JA, SC, ES. Study conduct: PC, KE, JA, SC, JS, ES. Data collection: PC, KE, JA, SC, ES. Data analysis: PC and SH. Data interpretation: All authors. Drafting manuscript: PC. Revising manuscript content: All authors. Approving final version of manuscript: All authors. SH takes responsibility for the integrity of the data analysis.
No authors report conflict of interests directly related to this work. JS has received personal fees from Merck, Inc. PC has received grant support from Merck, Eli Lilly and GlaskoSmithKline as well as personal fees from Eli Lilly. EO reports consulting for Eli Lilly. KE, JS, SH, DB, JA, SC, EL, GL, CN, AB, DK, AH, SJ, EBC report no other conflicts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cauley JA. Estrogen and bone health in men and women. Steroids. 2015;99(Pt A):11–5. doi: 10.1016/j.steroids.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Stepan JJ, Lachman M, Zverina J, Pacovsky V, Baylink DJ. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. The Journal of clinical endocrinology and metabolism. 1989;69(3):523–7. doi: 10.1210/jcem-69-3-523. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2010;95(6):2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 4.Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2012;97(6):1802–22. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 5.Tuck SP, Scane AC, Fraser WD, Diver MJ, Eastell R, Francis RM. Sex steroids and bone turnover markers in men with symptomatic vertebral fractures. Bone. 2008;43(6):999–1005. doi: 10.1016/j.bone.2008.08.123. [DOI] [PubMed] [Google Scholar]
- 6.El Maghraoui A, Ouzzif Z, Mounach A, Ben-Ghabrit A, Achemlal L, Bezza A, et al. The relationship between sex steroids, bone turnover and vertebral fracture prevalence in asymptomatic men. Bone. 2011;49(4):853–7. doi: 10.1016/j.bone.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 7.LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. The Journal of clinical endocrinology and metabolism. 2009;94(9):3337–46. doi: 10.1210/jc.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjornerem A, Ahmed LA, Joakimsen RM, Berntsen GK, Fonnebo V, Jorgensen L, et al. A prospective study of sex steroids, sex hormone-binding globulin, and non-vertebral fractures in women and men: the Tromso Study. Eur J Endocrinol. 2007;157(1):119–25. doi: 10.1530/EJE-07-0032. [DOI] [PubMed] [Google Scholar]
- 9.Amin S, Zhang Y, Felson DT, Sawin CT, Hannan MT, Wilson PW, et al. Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. The American journal of medicine. 2006;119(5):426–33. doi: 10.1016/j.amjmed.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 10.Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett-Connor E, et al. Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. The Journal of clinical endocrinology and metabolism. 2009;94(10):3806–15. doi: 10.1210/jc.2009-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, Rockwood AL, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med. 2008;168(1):47–54. doi: 10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 12.Hsu B, Cumming RG, Seibel MJ, Naganathan V, Blyth FM, Bleicher K, et al. Reproductive Hormones and Longitudinal Change in Bone Mineral Density and Incident Fracture Risk in Older Men: The Concord Health and Aging in Men Project. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015 doi: 10.1002/jbmr.2493. [DOI] [PubMed] [Google Scholar]
- 13.Vandenput L, Mellstrom D, Kindmark A, Johansson H, Lorentzon M, Leung J, et al. High Serum SHBG Predicts Incident Vertebral Fractures in Elderly Men. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015 doi: 10.1002/jbmr.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goderie-Plomp HW, van der Klift M, de Ronde W, Hofman A, de Jong FH, Pols HA. Endogenous sex hormones, sex hormone-binding globulin, and the risk of incident vertebral fractures in elderly men and women: the Rotterdam Study. The Journal of clinical endocrinology and metabolism. 2004;89(7):3261–9. doi: 10.1210/jc.2002-022041. [DOI] [PubMed] [Google Scholar]
- 15.Barrett-Connor E, Mueller JE, von Muhlen DG, Laughlin GA, Schneider DL, Sartoris DJ. Low levels of estradiol are associated with vertebral fractures in older men, but not women: the Rancho Bernardo Study. The Journal of clinical endocrinology and metabolism. 2000;85(1):219–23. doi: 10.1210/jcem.85.1.6327. [DOI] [PubMed] [Google Scholar]
- 16.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. The Journal of clinical endocrinology and metabolism. 1999;84(10):3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 20.Cawthon PM, Haslam J, Fullman R, Peters KW, Black D, Ensrud KE, et al. Methods and reliability of radiographic vertebral fracture detection in older men: the osteoporotic fractures in men study. Bone. 2014;67:152–5. doi: 10.1016/j.bone.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1993;8(9):1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 22.Cawthon PM, Fullman RL, Marshall L, Mackey DC, Fink HA, Cauley JA, et al. Physical performance and risk of hip fractures in older men. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23(7):1037–44. doi: 10.1359/JBMR.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J ClinEpidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 24.Cawthon PM, Ewing SK, McCulloch CE, Ensrud KE, Cauley JA, Cummings SR, et al. Loss of hip BMD in older men: the osteoporotic fractures in men (MrOS) study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(10):1728–35. doi: 10.1359/JBMR.090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 26.Cauley JA, Ewing SK, Taylor BC, Fink HA, Ensrud KE, Bauer DC, et al. Sex steroid hormones in older men: longitudinal associations with 4.5-year change in hip bone mineral density--the osteoporotic fractures in men study. The Journal of clinical endocrinology and metabolism. 2010;95(9):4314–23. doi: 10.1210/jc.2009-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol. 2010;316(1):79–85. doi: 10.1016/j.mce.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Egleston BL, Chandler DW, Dorgan JF. Validity of estimating non-sex hormone-binding globulin bound testosterone and oestradiol from total hormone measurements in boys and girls. Annals of clinical biochemistry. 2010;47(Pt 3):233–41. doi: 10.1258/acb.2010.009112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giton F, Fiet J, Guechot J, Ibrahim F, Bronsard F, Chopin D, et al. Serum bioavailable testosterone: assayed or calculated? Clinical chemistry. 2006;52(3):474–81. doi: 10.1373/clinchem.2005.052126. [DOI] [PubMed] [Google Scholar]
- 30.Laurent MR, Vanderschueren D. Reproductive endocrinology: functional effects of sex hormone-binding globulin variants. Nature reviews Endocrinology. 2014;10(9):516–7. doi: 10.1038/nrendo.2014.120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.