Abstract
It is well established that HIV-1-infected mononuclear phagocytes release platelet activating factor (PAF) and elevated levels of PAF have been detected in blood and in the cerebrospinal fluid (CSF) of acquired immunodeficiency syndrome (AIDS) patients with HIV-associated neurocognitive disorders (HAND). It is our hypothesis that the elevated levels of PAF alter long-term potentiation (LTP) in the hippocampus, leading to neurocognitive dysfunction. To test this hypothesis, we studied the effects of PAF on LTP in the CA1 region of rat hippocampal slices. Our results showed incubation of hippocampal slices with PAF attenuated LTP. The PAF-mediated attenuation was blocked by ginkgolide B, a PAF receptor antagonist, suggesting PAF attenuation of LTP via PAF receptors. Application of lyso-PAF, an inactive PAF analog, had no apparent effect on LTP. Further investigation revealed an involvement of tyrosine kinase in PAF attenuation of LTP, which was demonstrated by lavendustin A (a specific protein tyrosine kinase inhibitor) blockage of PAF attenuation of LTP. As LTP is widely considered as the cellular and synaptic basis for learning and memory, the attenuation of LTP by PAF may contribute at least in part to the HAND pathogenesis.
Keywords: Platelet activating factor, Hippocampal slice, LTP, HIV, HIV-associated dementia
1. Introduction
Brain infection of human immunodeficiency virus type 1 (HIV-1) often results in a spectrum of neurological complications that range from asymptomatic cognitive impairments to severe dementia, which are termed collectively as HIV-1-associated neurocognitive disorders (HAND) [2, 16]. Although the advent of combined antiretroviral therapy (cART) has resulted in a dramatic decline in all forms of neurological complications of HIV-1 infection, it is becoming clear that cART cannot fully control HAND and the prevalence of HAND continues to rise due to sustained HIV-1-infection of mononuclear phagocytes (MP, brain macrophages and microglia), the emergence of resistant viral phenotypes, poor drug penetration of blood-brain barrier, drug toxicities and increase in patient lifespan [1, 30]. Nevertheless, the mechanisms underlying HAND pathogenesis, especially in the era of cART, are not fully understood. Many studies suggest an indirect mechanism, in addition to a direct HIV-1 neurotoxicity, by which the HIV-1-infected and immune activated MPs release soluble viral and cellular factors leading to development of HAND[23, 46]. Amongst the soluble cellular factors is platelet-activating factor (PAF), a candidate HIV-1-induced neurotoxin [15, 23].
PAF (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is a potent phospholipid inflammatory mediator, which seems to play a role in HIV-1-associated neurotoxicity and HAND pathogenesis [6, 15, 29, 38]. It has been shown that elevated levels of PAF were detected in blood of naïve HIV-infected patients [44], in the cerebrospinal fluid (CSF) of acquired immunodeficiency syndrome (AIDS) patients with HAND [15], in brain tissues of mice with an immunodeficiency syndrome (murine AIDS)[31] and in the culture supernatants of HIV-1-infected MPs [15, 32, 35]. The elevated levels of PAF in the CSF and brain tissues are potentially toxic to neurons and can cause neuronal and synaptic dysfunction or injury, resulting in an impairment of memory as seen in patients with HAND. However, how elevated PAF induces memory impairment remains an interesting area of investigation. To this end, we studied effects of PAF on long-term potentiation (LTP), an activity-dependent increase in synaptic efficacy that has been proposed as the cellular and molecular basis for learning and memory, in rat hippocampal brain slices. Our results revealed that PAF attenuated LTP via protein tyrosine kinase signaling.
2. Materials and Methods
2.1 Preparation of hippocampal slices
Male Sprague-Dawley rats (two- to four-week old), purchased from Charles River Laboratories (Wilmington, MA) were used for preparation of hippocampal slices. All animal use procedures were strictly reviewed by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center (IACUC No. 00-062-07). On the experimental day, an animal was anesthetized with isoflurane and decapitated. Brain was quickly removed from the cranial cavity and placed into an ice-cold (4°C), pre-oxygenated artificial cerebrospinal fluid (ACSF). Hippocampi were dissected out and transverse hippocampal slices (400μm in thickness) were cut using a tissue chopper. Slices were kept in a humidified/oxygenated holding chamber at room temperature for at least 1 h before experiment. In the recording chamber, a single hippocampal slice was submerged and continuously perfused (2.0ml/min) with ACSF contained (in mM): NaCl (124.0), KCl (3.0), CaCl2 (2.0), MgCl2 (2.0), NaH2PO3 (1.0), NaHCO3 (26.0) and glucose (10.0), equilibrated with 95% O2 and 5% CO2, pH 7.40–7.50. The temperature of the perfusate was maintained at 30 ± 1°C. Drugs were applied onto slices via incubation (1 h) or otherwise as indicated.
2.2 Electrophysiology
Field excitatory postsynaptic potentials (EPSPs), elicited by a constant current stimulation (0.05 Hz, 50–400 μA) of Schaffer collateral-commissural fibers using an insulated bipolar tungsten electrode, were recorded in the CA1 dendrite field (stratum radium) with an Axopatch-1D amplifier (Molecular Devices, Sunnyvale, CA). Intensity and duration of stimulation were adjusted to generate approximately 30–40% of a maximal response. Glass recording electrodes had a tip diameter of 2.5–5.0μ and a resistance of 1–5MΩ when filled with ACSF. A 15 min control recording was conducted in each experiment once the adjustment of stimulation parameters was achieved. Each recording trial was an average of 3 consecutive sweeps. High frequency stimulation (HFS, 100Hz, 500ms) was delivered twice in a 20 s interval at the same intensity as that employed in test pulses. Electrical signals were filtered at 1 kHz and digitized at 2.5 kHz using a Digidata 1320 interface (Molecular Devices). Data were stored on a desktop PC and analyzed off-line using pCLAMP 10 software (Molecular Devices). The initial slope of the EPSPs was analyzed and expressed as percentage of basal level (the average of initial slope from the first 15 min was treated as 100%). In bar graphs, the magnitudes of LTP were quantified at the time point of 35 min after HFS. All data were expressed as the mean + SEM (unless otherwise indicated) and graphed using OriginLab®8.5 (OriginLab Corp, Northampton, MA). Statistical analyses were made by two-tailed t-tests. Differences were considered significant if p < 0.05.
3. Results
3.1 PAF attenuation of hippocampal LTP
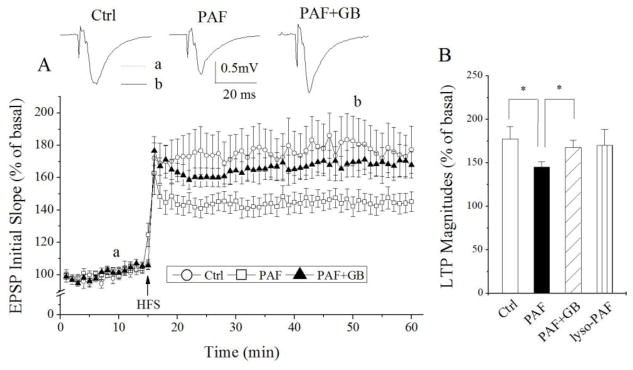
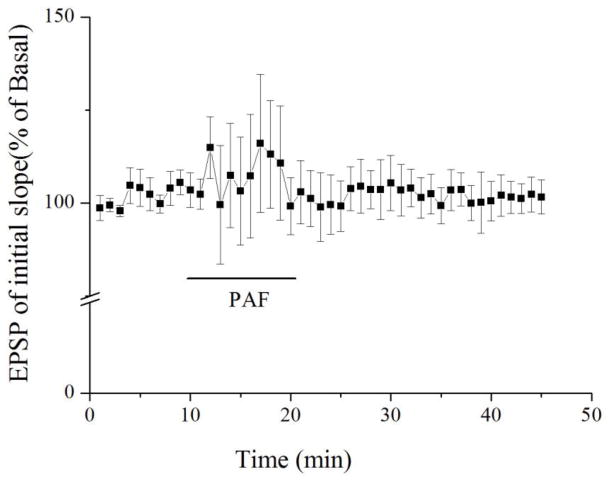
PAF has been proposed as an HIV-1-induced neurotoxin produced by infected and immune activated macrophages and microglia and elevated levels of PAF were detected in the CSF of HIV-1-infected patients with neurocognitive dysfunction [15, 44]. To determine whether PAF is involved in the mediation of HIV-1-associated neurocognitive dysfunction, we studied the effects of PAF on LTP in rat hippocampus, a brain region widely believed to be involved in learning and memory. Incubation of hippocampal slices with PAF (20μM, 1 h) attenuated LTP recorded in the CA1 region of hippocampal slices. The average LTP magnitude was 145.2 ± 6.1% of basal level (n=14) when measured 35 min after HFS. In contrast, the magnitude of LTP in non-treated (control) slices was 177.3 ± 14.6% of basal level (n=10). The difference was statistically significant (t(22)=0.034; p<0.05), suggesting PAF attenuation of LTP in the hippocampus (Fig. 1). The PAF-induced attenuation of LTP was blocked by a PAF receptor antagonist ginkgolide B (5μM, n=12), indicating PAF attenuation of LTP via PAF receptors expressed in the hippocampus [25] (Fig. 1). To examine if PAF attenuation of LTP was a result of suppression of basal synaptic transmission, PAF (20μM) was applied onto hippocampal brain slices via bath perfusion. As shown in Figure 2, bath application of PAF for 10 min had no significant effect on basal synaptic transmission (n=5). Moreover, incubation of lyso-PAF C-16, an inactive PAF, had no apparent effect on LTP, suggesting a specific effect of PAF on LTP (Fig. 1B, n=6). These results demonstrated PAF reduction of LTP via PAF receptors in the CA1 region of hippocampal slices.
Figure 1.
PAF attenuation of LTP induced by HFS in CA1 region of rat hippocampal slices. Panel A exhibits the time course and average magnitude of LTP in the Schaffer-collateral to CA1 synapses recorded from control slices (○) and PAF-treated slices (□) and slices treated with PAF and ginkgolide B (GB), a specific PAF receptor antagonist (▲). The graph plots the initial slope of the evoked EPSPs recorded from the CA1 dendrite field (stratum radium) in response to constant current stimuli. High frequency stimulation (HFS, 100 Hz, 500ms × 2) was delivered at the time indicated by an arrow. Each data point in the graph is an average of 3 consecutive sweeps. Note that incubation of hippocampal slices with PAF significantly attenuated LTP, and such PAF-induced attenuation was blocked by PAF receptor antagonist ginkgolide B. Above the time course are representative individual field EPSPs taken from different time points marked a (dot line) and b (solid line), respectively, under different experimental conditions as indicated. The bar graph in the panel B shows the average LTP magnitudes measured at 45 min after HFS in slices of control, treated with PAF, treated with PAF plus ginkgolide B or treated with lyso-PAF, an inactive PAF analog. * denotes p<0.05.
Figure 2.
Bath application of PAF had no significant effect on synaptic transmission. Each data point in the graph is an average of 3 consecutive sweeps as indicated in Figure 1. Note that bath application of PAF (20μM), as indicated by a horizontal bar, had no significant effect on basal synaptic transmission (n=5).
3.2 Involvement of tyrosine kinase signaling in PAF attenuation of LTP
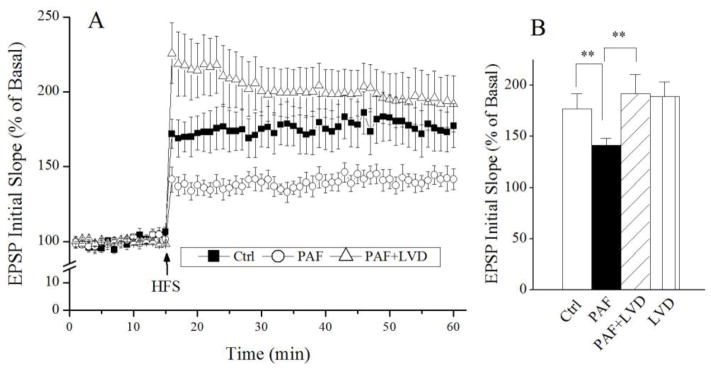
It has been shown that PAF induces protein tyrosine phosphorylation in hippocampus [9]. To investigate whether PAF attenuation of LTP was mediated via tyrosine kinase signaling, we examined effects of a specific tyrosine kinase inhibitor, lavendustin A, on PAF-induced attenuation of LTP in the hippocampal slices. Lavendustin A (20μM) had no apparent effect on basal synaptic transmission when applied to the hippocampal slices alone, it significantly blocked PAF-induced attenuation of LTP when it was co-incubated with PAF, indicating the PAF attenuation of LTP via tyrosine kinase signaling.
4. Discussion
In the present study, we examined effects of PAF on synaptic transmission and plasticity in the CA1 region of rat hippocampal slices. Treatment of hippocampal slices with PAF for 1 h via incubation attenuated the induction of LTP. Such an inhibitory effect was not a result of PAF inhibition of basal synaptic transmission since bath perfusion of hippocampal slices with PAF for 10 min did not produce a significant change on EPSP initial slope. The PAF-mediated attenuation of LTP was blocked by ginkgolide B, a PAF receptor antagonist, indicating PAF attenuation of LTP via PAF receptors expressed in the hippocampal neurons [27]. Our results also showed that PAF attenuated LTP via tyrosine kinase signaling pathway as the PAF-mediated attenuation on LTP was blocked by a tyrosine kinase inhibitor lavendustin A [33].
It has been shown that PAF modulates synaptic plasticity and memory formation [17, 28, 45], in addition to its platelet-activating properties and involvement in the mediation of neuro-inflammatory responses [29, 38]. On one hand, PAF induces LTP in mouse hippocampal slices [45] and in rat somatosensory cortex in vitro [17], enhances memory in rat performing an inhibitory avoidance task [20] as well as in water maze task [34]. Blockade of PAF receptors by specific antagonists attenuated LTP in the hippocampal slices [3, 22] and induced amnesia in mice in elevated plus-maze tests [41]. In parallel, knockout PAF receptor in mice attenuated LTP in the hippocampal slices [11], despite a study demonstrating that LTP in the hippocampal CA1 region was normal in mice lacking the PAF receptors [24]. On the other hand, PAF induces neuronal injury or death in immune and inflammatory conditions [5, 6, 15, 43] and inhibits LTP in rat hippocampal slices at higher doses [13]. Studies using animal models revealed that mice with elevated levels of PAF in the frontal cortex and hippocampus developed an impaired spatial learning and memory [31] and that systemic administration of a PAF antagonist attenuated HIV-1-associated neuropathology [36]. In this study, we observed an inhibitory effect of PAF on LTP in rat hippocampus. As LTP is a widely-accepted cellular and synaptic mechanism for learning and memory and the hippocampus is one of the brain regions involved in learning and memory, the attenuation of LTP in the hippocampus by PAF may reflect a role for PAF in the disruption of learning and memory processes. Taken together, these results indicate that PAF may play a role in HIV-1-associated neurocognitive dysfunction.
The mechanisms underlying the aforementioned discrepancy are unclear. It may be the consequence of concentration difference. At physiological (low) concentrations, PAF enhances synaptic transmission and induces LTP, while at pathological (high) concentrations it inhibits LTP. Therefore, the elevated levels of PAF in the CSF and brain tissues are potentially toxic to neurons and can cause neuronal and synaptic dysfunction as well as injury leading to neurocognitive dysfunction as seen in HIV-1-infected individuals with HAND [15, 31, 32, 35]. This notion is supported by the results obtained in a multicenter clinical trial study demonstrating that a PAF antagonist improved neuropsychological test scores, especially in verbal memory, in HIV-1-infected individuals [39].
Many cellular events are critically regulated by tyrosine kinases and tyrosine phosphorylation signaling is important for both synaptic transmission and synaptic plasticity [7, 42]. High levels of protein tyrosine kinases are expressed in the hippocampus [18, 27] and PAF was found to stimulate tyrosine phosphorylation in this brain region [9, 40]. Studies have shown that tyrosine phosphorylation either enhances the induction of LTP [19, 26] or inhibits the induction of LTP [12]. Our results demonstrated that the PAF-induced inhibition of LTP can be blocked by a specific tyrosine kinase inhibitor, lavendustin A, suggesting PAF-induced inhibition of LTP was mediated via tyrosine kinase signaling pathways. This finding was in an agreement with the results illustrating tyrosine phosphorylation-dependent inhibition of hippocampal LTP in young adult rats [12].
The biological significance for PAF inhibition of LTP in the hippocampus remains to be determined. As a higher concentration of PAF produces neuronal injury [8, 37] and elevated levels of PAF have been detected in the CSF and brain tissues from AIDS patients with HAND [15, 21] and other neurological disorders [4, 10], the inhibition of LTP may reflect, at least in part, a role PAF may play in HAND pathogenesis because LTP is an activity-dependent enhancement of synaptic strength which may underlie learning and memory. This assumption is supported by experimental results demonstrating that PAF antagonists exhibited therapeutic effects for HIV-1-associated neurocognitive impairment in both HIV-1-infected individuals [39] and SCID mice with HIV encephalitis [14, 36].
In summary, we demonstrated that PAF, when applied via incubation, attenuated the LTP in the CA1 region of rat hippocampal slices. The PAF-induced attenuation of LTP was blocked by ginkgolide B, a PAF receptor antagonist, suggesting that PAF-mediated attenuation of LTP was achieved via acting on PAF receptors. The PAF-induced attenuation of LTP was also blocked by a tyrosine kinase inhibitor, lavendustin A, indicating an involvement of tyrosine kinase signaling in PAF-associated attenuation of LTP. As elevated levels of PAF were detected in HIV-infected patients with HAND and LTP has been proposed as the cellular basis for learning and memory, the attenuation of LTP by PAF in the hippocampus may represent, at least in part, a potential mechanism for HAND pathogenesis.
Figure 3.
Involvement of protein tyrosine kinase in PAF-induced attenuation of LTP. Panel A shows the time course and average magnitude of LTP recorded from control slices (■), slices treated with PAF (○) and slices treated with PAF plus lavendustin A (LVD), a specific protein tyrosine kinase (△). As shown in the panel A, PAF decreased the magnitude of LTP and the PAF-mediated decrease of LTP was blocked by lavendustin A, a specific protein tyrosine kinase inhibitor. Panel B is a summary bar graph showing the average LTP magnitudes measured at 45 min after HFS. Note that lavendustin A blockade of PAF-mediated decrease of LTP. Application of lavendustin A along had no significant effect on LTP. ** denotes p<0.01.
Highlights.
PAF attenuated LTP in rat hippocampal slices.
PAF attenuation of LTP was mediated via PAF receptors.
Tyrosine kinase signaling was involved in PAF-mediated reduction of LTP.
Acknowledgments
The authors thank Mr. Reed C. Felderman for reading the manuscript. This work was supported by NIH grant R01 NS063878.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alfahad TB, Nath A. Update on HIV-associated neurocognitive disorders. Curr Neurol Neurosci Rep. 2013;13:387. doi: 10.1007/s11910-013-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai A, Lynch G. Antagonists of the Platelet-activating Factor Receptor Block Long-term Potentiation in Hippocampal Slices. Eur J Neurosci. 1992;4:411–419. doi: 10.1111/j.1460-9568.1992.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 4.Arditi M, Manogue KR, Caplan M, Yogev R. Cerebrospinal fluid cachectin/tumor necrosis factor-alpha and platelet-activating factor concentrations and severity of bacterial meningitis in children. J Infect Dis. 1990;162:139–147. doi: 10.1093/infdis/162.1.139. [DOI] [PubMed] [Google Scholar]
- 5.Bazan NG. The neuromessenger platelet-activating factor in plasticity and neurodegeneration. Prog Brain Res. 1998;118:281–291. doi: 10.1016/s0079-6123(08)63215-x. [DOI] [PubMed] [Google Scholar]
- 6.Bellizzi MJ, Lu SM, Masliah E, Gelbard HA. Synaptic activity becomes excitotoxic in neurons exposed to elevated levels of platelet-activating factor. J Clin Invest. 2005;115:3185–3192. doi: 10.1172/JCI25444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boxall AR, Lancaster B. Tyrosine kinases and synaptic transmission. Eur J Neurosci. 1998;10:2–7. doi: 10.1046/j.1460-9568.1998.00009.x. [DOI] [PubMed] [Google Scholar]
- 8.Brewer C, Bonin F, Bullock P, Nault MC, Morin J, Imbeault S, Shen TY, Franks DJ, Bennett SA. Platelet activating factor-induced apoptosis is inhibited by ectopic expression of the platelet activating factor G-protein coupled receptor. J Neurochem. 2002;82:1502–1511. doi: 10.1046/j.1471-4159.2002.01094.x. [DOI] [PubMed] [Google Scholar]
- 9.Calcerrada MC, Catalan RE, Martinez AM. PAF-stimulated protein tyrosine phosphorylation in hippocampus: involvement of NO synthase. Neurochem Res. 2002;27:313–318. doi: 10.1023/a:1014911329489. [DOI] [PubMed] [Google Scholar]
- 10.Callea L, Arese M, Orlandini A, Bargnani C, Priori A, Bussolino F. Platelet activating factor is elevated in cerebral spinal fluid and plasma of patients with relapsing-remitting multiple sclerosis. J Neuroimmunol. 1999;94:212–221. doi: 10.1016/s0165-5728(98)00246-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Magee JC, Marcheselli V, Hardy M, Bazan NG. Attenuated LTP in hippocampal dentate gyrus neurons of mice deficient in the PAF receptor. J Neurophysiol. 2001;85:384–390. doi: 10.1152/jn.2001.85.1.384. [DOI] [PubMed] [Google Scholar]
- 12.Coussens CM, Williams JM, Ireland DR, Abraham WC. Tyrosine phosphorylation-dependent inhibition of hippocampal synaptic plasticity. Neuropharmacology. 2000;39:2267–2277. doi: 10.1016/s0028-3908(00)00087-3. [DOI] [PubMed] [Google Scholar]
- 13.Dong J, Lu DX, Yan L, Zhang SM. Effect of platelet-activating factor on long-term potentiation in rat hippocampal slices. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2005;21:133–136. [PubMed] [Google Scholar]
- 14.Eggert D, Dash PK, Serradji N, Dong CZ, Clayette P, Heymans F, Dou H, Gorantla S, Gelbard HA, Poluektova L, Gendelman HE. Development of a platelet-activating factor antagonist for HIV-1 associated neurocognitive disorders. J Neuroimmunol. 2009;213:47–59. doi: 10.1016/j.jneuroim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelbard HA, Nottet HS, Swindells S, Jett M, Dzenko KA, Genis P, White R, Wang L, Choi YB, Zhang D, et al. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol. 1994;68:4628–4635. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heusler P, Boehmer G. Platelet-activating factor contributes to the induction of long-term potentiation in the rat somatosensory cortex in vitro. Brain Res. 2007;1135:85–91. doi: 10.1016/j.brainres.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Hirano AA, Greengard P, Huganir RL. Protein tyrosine kinase activity and its endogenous substrates in rat brain: a subcellular and regional survey. J Neurochem. 1988;50:1447–1455. doi: 10.1111/j.1471-4159.1988.tb03029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang CC, Hsu KS. Protein tyrosine kinase is required for the induction of long-term potentiation in the rat hippocampus. J Physiol. 1999;520(Pt 3):783–796. doi: 10.1111/j.1469-7793.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izquierdo I, Fin C, Schmitz PK, Da Silva RC, Jerusalinsky D, Quillfeldt JA, Ferreira MB, Medina JH, Bazan NG. Memory enhancement by intrahippocampal, intraamygdala, or intraentorhinal infusion of platelet-activating factor measured in an inhibitory avoidance task. Proc Natl Acad Sci U S A. 1995;92:5047–5051. doi: 10.1073/pnas.92.11.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jett M, Ionin B, Bernton E, Swindells WS, GHE Platelet activating factor: Identification of an HIV-induced neurotoxin in cerebrospinal fluids of patients with HIV-associated dementia/encephalitis. The First National Conference on Human Retroviruses and Related Infections; Sheraton Washington Hotel. 1993. [Google Scholar]
- 22.Kato K, Zorumski CF. Platelet-activating factor as a potential retrograde messenger. J Lipid Mediat Cell Signal. 1996;14:341–348. doi: 10.1016/0929-7855(96)00543-3. [DOI] [PubMed] [Google Scholar]
- 23.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K, Ishii S, Kume K, Takahashi T, Shimizu T, Manabe T. Platelet-activating factor receptor is not required for long-term potentiation in the hippocampal CA1 region. Eur J Neurosci. 1999;11:1313–1316. doi: 10.1046/j.1460-9568.1999.00538.x. [DOI] [PubMed] [Google Scholar]
- 25.Maclennan KM, Smith PF, Darlington CL. Platelet-activating factor in the CNS. Prog Neurobiol. 1996;50:585–596. doi: 10.1016/s0301-0082(96)00047-0. [DOI] [PubMed] [Google Scholar]
- 26.Maguire C, Casey M, Kelly A, Mullany PM, Lynch MA. Activation of tyrosine receptor kinase plays a role in expression of long-term potentiation in the rat dentate gyrus. Hippocampus. 1999;9:519–526. doi: 10.1002/(SICI)1098-1063(1999)9:5<519::AID-HIPO5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 27.Mori M, Aihara M, Kume K, Hamanoue M, Kohsaka S, Shimizu T. Predominant expression of platelet-activating factor receptor in the rat brain microglia. J Neurosci. 1996;16:3590–3600. doi: 10.1523/JNEUROSCI.16-11-03590.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriguchi S, Shioda N, Yamamoto Y, Fukunaga K. Platelet-activating factor-induced synaptic facilitation is associated with increased calcium/calmodulin-dependent protein kinase II, protein kinase C and extracellular signal-regulated kinase activities in the rat hippocampal CA1 region. Neuroscience. 2010;166:1158–1166. doi: 10.1016/j.neuroscience.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Musto AE, Samii M. Platelet-activating factor receptor antagonism targets neuroinflammation in experimental epilepsy. Epilepsia. 2011;52:551–561. doi: 10.1111/j.1528-1167.2010.02920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nath A. Eradication of human immunodeficiency virus from brain reservoirs. J Neurovirol. 2015;21:227–234. doi: 10.1007/s13365-014-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishida K, Markey SP, Kustova Y, Morse HC, 3rd, Skolnick P, Basile AS, Sei Y. Increased brain levels of platelet-activating factor in a murine acquired immune deficiency syndrome are NMDA receptor-mediated. J Neurochem. 1996;66:433–435. doi: 10.1046/j.1471-4159.1996.66010433.x. [DOI] [PubMed] [Google Scholar]
- 32.Nottet H, Jett M, Flanagan C, Zhai QH, Persidsky Y, Rizzino A, Bernton E, Genis P, Baldwin T, Schwartz J, LaBenz C, Gendelman H. A regulatory role for astrocytes in HIV-1 encephalitis: an overexpression of eicosanoids, platelet-activating factor, and tumor necrosis factor-a by activated HIV-1infected monocytes is attenuated by primary human astrocytes. J Immunol. 1995;154:3567–3581. [PubMed] [Google Scholar]
- 33.Onoda T, Iinuma H, Sasaki Y, Hamada M, Isshiki K, Naganawa H, Takeuchi T, Tatsuta K, Umezawa K. Isolation of a novel tyrosine kinase inhibitor, lavendustin A, from Streptomyces griseolavendus. J Nat Prod. 1989;52:1252–1257. doi: 10.1021/np50066a009. [DOI] [PubMed] [Google Scholar]
- 34.Packard MG, Teather LA, Bazan NG. Effects of intrastriatal injections of platelet-activating factor and the PAF antagonist BN 52021 on memory. Neurobiol Learn Mem. 1996;66:176–182. doi: 10.1006/nlme.1996.0058. [DOI] [PubMed] [Google Scholar]
- 35.Perry SW, Hamilton JA, Tjoelker LW, Dbaibo G, Dzenko KA, Epstein LG, Hannun Y, Whittaker JS, Dewhurst S, Gelbard HA. Platelet-activating factor receptor activation. An initiator step in HIV-1 neuropathogenesis. J Biol Chem. 1998;273:17660–17664. doi: 10.1074/jbc.273.28.17660. [DOI] [PubMed] [Google Scholar]
- 36.Persidsky Y, Limoges J, Rasmussen J, Zheng J, Gearing A, Gendelman HE. Reduction in glial immunity and neuropathology by a PAF antagonist and an MMP and TNFalpha inhibitor in SCID mice with HIV-1 encephalitis. J Neuroimmunol. 2001;114:57–68. doi: 10.1016/s0165-5728(00)00454-9. [DOI] [PubMed] [Google Scholar]
- 37.Ryan SD, Harris CS, Mo F, Lee H, Hou ST, Bazan NG, Haddad PS, Arnason JT, Bennett SA. Platelet activating factor-induced neuronal apoptosis is initiated independently of its G-protein coupled PAF receptor and is inhibited by the benzoate orsellinic acid. J Neurochem. 2007;103:88–97. doi: 10.1111/j.1471-4159.2007.04740.x. [DOI] [PubMed] [Google Scholar]
- 38.Sattayaprasert P, Choi HB, Chongthammakun S, McLarnon JG. Platelet-activating factor enhancement of calcium influx and interleukin-6 expression, but not production, in human microglia. J Neuroinflammation. 2005;2:11. doi: 10.1186/1742-2094-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schifitto G, Sacktor N, Marder K, McDermott MP, McArthur JC, Kieburtz K, Small S, Epstein LG. Randomized trial of the platelet-activating factor antagonist lexipafant in HIV-associated cognitive impairment. Neurological AIDS Research Consortium. Neurology. 1999;53:391–396. doi: 10.1212/wnl.53.2.391. [DOI] [PubMed] [Google Scholar]
- 40.Shukla S. Platelet activating factor receptor and signal transduction mechanisms. FASEB J. 1992;6:2296–2301. doi: 10.1096/fasebj.6.6.1312046. [DOI] [PubMed] [Google Scholar]
- 41.Singh N, Sharma A, Singh M. Effects of BN-50730 (PAF receptor antagonist) and physostigmine (AChE inhibitor) on learning and memory in mice. Methods Find Exp Clin Pharmacol. 1997;19:585–588. [PubMed] [Google Scholar]
- 42.Suzuki T. Protein kinases involved in the expression of long-term potentiation. Int J Biochem. 1994;26:735–744. doi: 10.1016/0020-711x(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 43.Teather LA, Afonso VM, Wurtman RJ. Inhibition of platelet-activating factor receptors in hippocampal plasma membranes attenuates the inflammatory nociceptive response in rats. Brain Res. 2006;1097:230–233. doi: 10.1016/j.brainres.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 44.Tsoupras AB, Chini M, Mangafas N, Tsogas N, Stamatakis G, Tsantila N, Fragopoulou E, Antonopoulou S, Gargalianos P, Demopoulos CA, Lazanas MC. Platelet-activating factor and its basic metabolic enzymes in blood of naive HIV-infected patients. Angiology. 2012;63:343–352. doi: 10.1177/0003319711420608. [DOI] [PubMed] [Google Scholar]
- 45.Wieraszko A, Li G, Kornecki E, Hogan MV, Ehrlich YH. Long-term potentiation in the hippocampus induced by platelet-activating factor. Neuron. 1993;10:553–557. doi: 10.1016/0896-6273(93)90342-o. [DOI] [PubMed] [Google Scholar]
- 46.Xiong H, Zeng YC, Lewis T, Zheng J, Persidsky Y, Gendelman HE. HIV-1 infected mononuclear phagocyte secretory products affect neuronal physiology leading to cellular demise: relevance for HIV-1-associated dementia. J Neurovirol. 2000;6(Suppl 1):S14–23. [PubMed] [Google Scholar]