Abstract
p38 MAPK has long been understood as an inducible kinase under conditions of cellular stress, but there is now increasing evidence to support its role in the regulation of neuronal function. Several phosphorylation targets have been identified, an appreciable number of which are ion channels, implicating the possible involvement of p38 MAPK in neuronal excitability. The KNa channel Slack is an important protein to be studied as it is highly and ubiquitously expressed in DRG neurons and is important in the maintenance of their firing accommodation. We sought to examine if the Slack channel could be a substrate of p38 MAPK activity. First, we found that the Slack C-terminus contains two putative p38 MAPK phosphorylation sites that are highly conserved across species. Second, we show via electrophysiology experiments that KNa currents and further, Slack currents, are subject to tonic modulation by p38 MAPK. Third, biochemical approaches revealed that Slack channel regulation by p38 MAPK occurs through direct phosphorylation at the two putative sites of interaction, and mutating both sites prevented surface expression of Slack channels. Based on these results, we conclude that p38 MAPK is an obligate regulator of Slack channel function via the trafficking of channels into the membrane. The present study identifies Slack KNa channels as p38 MAPK substrates.
Keywords: p38 MAPK, Slack, phosphorylation, regulation, DRG neurons, membrane expression
1. Introduction
The functions and signalling of p38 Mitogen-Activated Protein Kinase (MAPK) have been well studied under the broad categories of cellular stress, immune response and cell survival and differentiation. In parallel, recent work has put forth burgeoning evidence for the involvement of p38 MAPK in neuronal function, owing to a number of its identified neuronal targets being ion channels fundamental to central and peripheral nervous system functions. For example, the sodium channels Nav1.6 and Nav1.8 are important players in action potential generation and have been identified as substrates of p38 MAPK, wherein direct phosphorylation by the kinase decreased Nav1.6 and increased Nav1.8 current density (Wittmack et al. 2005, Hudmon et al. 2008). In neurons of the ciliary ganglion, surface expression of the Calcium-activated Potassium channel (KCa) is regulated by p38 MAPK via an F-actin dependent mechanism (Chae & Dryer 2005). The major contributor to the delayed outward current in neurons, Kv2.1, is phosphorylated by p38 MAPK as an apoptotic signal (Redman et al. 2007). Considering that these above-mentioned targets are also involved in neuronal excitability, it is not surprising that p38 MAPK has recently been linked to central and peripheral sensitization. It has now been established not only that activation of p38 MAPK occurs in the sensory neurons of the Dorsal Root Ganglion (DRG) during peripheral sensitization, but also that modulation of p38 MAPK activity appears to regulate sensitization induced pathological symptoms in neuropathic and inflammatory pain (Jin et al. 2003, Ji et al. 2002). More conclusively, administering p38 inhibitors intrathecally has been demonstrated to alleviate mechanical allodynia in various rodent models of neuropathic pain (Kumar et al. 2003, Schafers et al. 2003), suggesting the involvement of sensory neuron specific substrates in p38 MAPK’s mechanism of action. Collectively, the literature described above has put forth several such protein targets including ion channels such as Nav1.8, TRPV1 and TRPA, but there is as yet no existing link between p38 MAPK and KNa channels despite their well-known abundance of expression in DRG neurons.
The novel class of sodium-activated potassium (KNa) channels have gained much attention due to their role in maintaining the resting membrane potential and firing accommodation of the sensory neurons in the DRG. This ion channel family is comprised of two known members- Slack (Kcnt1, Slo2.2) and Slick (Kcnt2, Slo2.1). Specifically, the Slack channel is a slowly activating channel whose large conductance of potassium is thought to be primarily governed by the binding of cytoplasmic sodium (Kameyama et al. 1984). Of the two functionally characterized isoforms Slack-A and Slack-B, the latter has been identified as abundant in the nervous system, predominantly expressed in regions of the brain such as the cerebellum, brainstem and the olfactory bulb, and in the sensory DRG neurons (Bhattacharjee et al. 2002, Tamsett et al. 2009). In combination with localization studies showing Slack channels to be expressed in small, medium and large diameter DRG neurons, electrophysiological experiments investigating the role of Slack channels in these neurons have implicated them in the adaptation of neuronal firing rates (Tamsett et al. 2009). Indeed, siRNA mediated silencing of Slack channel expression or knockdown of the Slack gene in sensory neurons results in loss of firing accommodation and DRG neuronal hyperexcitability (Nuwer et al. 2010, Lu et al. 2015). These findings have established Slack as central to understanding sensory and nociceptive neuronal excitability.
Functional studies have revealed that Slack channels are subject to regulation by several intracellular proteins. Most recently, the little studied Anoctamin TMEM16C has been demonstrated to form a complex with Slack channels in nociceptor DRG neurons to enhance Slack channel function by facilitating both membrane expression and sodium-dependent channel opening (Huang et al. 2013). Using recombinant Xenopus oocytes, Barcia et al. have shown that activation of Protein Kinase C (PKC) up-regulates Slack current by 2–3 fold (Barcia et al. 2012). In primary cultures of DRG neurons, Slack channels are regulated by Protein Kinase A (PKA) such that activation of PKA leads to internalization of the channel and DRG hyperexcitability, essentially altering neuronal firing properties to resemble neurons in the sensitized state (Nuwer et al. 2010). The mRNA binding protein Fragile-X Mental Retardation Protein (FMRP) has been shown to act as a potent activator of Slack activity via a direct binding interaction in neurons of the auditory brainstem (Zhang et al. 2012). Thus, it appears that identifying regulatory protein interactions that the Slack channel participates in, either through a trafficking event leading to loss of channel numbers at the membrane or through a gating effect influencing channel properties at the membrane, is central to understanding neuronal excitability and function.
Considering that Slack channels are subject to dynamic and acute modulation by phosphorylation as evidenced by its PKA- and PKC-mediated regulation, we sought to examine whether p38 MAPK phosphorylation affects Slack channel functioning. In the present study, using a combination of electrophysiological, biochemical and molecular approaches on primary DRG neurons and heterologous expression systems, we demonstrate that the Slack channel is subject to tonic phosphorylation by p38 MAPK, the inhibition of which appears to reduce Slack channel numbers at the DRG membrane. These results are the first to directly demonstrate that Slack channels are p38 MAPK substrates and furthermore to suggest that obligate p38 phosphorylation is required for Slack channel membrane expression.
2. Methods
2.1 DRG Neuronal Culture
Timed pregnant Sprague-Dawley rats (Harlan, Indianapolis, IN) were used for all experiments. Animals were housed singly in a temperature and humidity controlled animal facility on a 12 h: 12 h light–dark schedule with food and water freely available. All procedures were approved by the University at Buffalo Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health guidelines for the use of laboratory animals in research. One the day of the dissection, rats were euthanized by CO2 asphyxiation and E15 embryos were extracted. DRG were dissected from the embryos and enzymatically digested with Trypsin (2.5mg/ml) at 37°C for 50 minutes, followed by mechanical dissociation and plating. DRG neurons were plated onto poly-D-lysine (Sigma; 100μg/ml) and laminin (Invitrogen; 3μg/ml) coated coverslips. Cells were maintained at 37°C in a 7% CO2 humidified incubator in serum-free medium, comprised of the trophic factors N2 (Gemini Bio products; 1%), L-Glutamine (Invitrogen; 200 μg/ml), and Nerve Growth Factor (NGF) (Harlan; 100 ng/ml; required for embryonic neuronal survival) in 50% DMEM and 50% F-12 (Nuwer et al. 2010). Of note, the dependency of embryonic DRG neurons on NGF selects for the small-diameter population that is thought to subserve nociception and thermoception (Ruit et al. 1992). For two days after the day of the dissection, cells were cultured in C2 media containing the anti-mitotic cytosine β-D-arabinofuranoside hydrochloride (Sigma; 3μM), followed by two days of recovery before use in experiments. All subsequent experiments were performed on days 5–8 of neuronal culture.
2.2. Cell Culture
Human Embryonic Kidney (HEK-293) cells that stably express Slack channels (Yang et al. 2006) were a kind gift from Dr. Fred Sigworth (Yale University, New Haven, CT). Cells were maintained at 37°C in a 5% CO2 humidified incubator in a low sodium DMEM medium containing 10% Fetal Bovine Serum (FBS) (Invitrogen; heat-inactivated) and 1% penicillin-streptomycin (Invitrogen). In the serum starvation experiment, Slack stable HEK cells maintained in the above described medium were rinsed with Phosphate Buffered Saline (PBS) twice and kept in serum-free medium for 18–20 hours before being used. Chinese Hamster Ovary (CHO) cells were cultured at 37°C in 5% CO2 in an IMDM medium supplemented with 10% FBS, 1% HT supplement (Life Technologies) and 1% penicillin-streptomycin. Both cell lines were plated in 35 mm dishes for all electrophysiology experiments, and in six-well culture plates for biochemical experiments.
2.3. Electrophysiology
All data was acquired using the Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) and Multiclamp-700B (Molecular Devices), digitized and filtered at 2 kHz. Data acquisition was monitored and controlled using pClamp 10.2 (Molecular Devices).
Whole-cell patch clamp recordings were performed on embryonic DRG neurons, Slack-stable HEK-293 cells, and CHO cells transiently transfected with WT or mutated Slack. Glass electrodes were pulled using a horizontal pipette puller (Sutter Instrument Company, Novato, CA) and fire polished to be of 5–12 M Ω resistance. Pipettes were filled with solution containing (in mM) 124 K-gluconate, 2 MgCl2, 13.2 NaCl, 1 EGTA, 10 HEPES, 4 Mg-ATP, and 0.3 Na-GTP at pH 7.2 (Wu & Pan 2007) for neuronal experiments, and with 110 KCl, 30 KGluconate, 10 HEPES, and 5 EGTA when testing cell lines. The bath solution for all cells contained (in mM) 140 NaCl, 5.4 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, and 10 Glucose. When examining sodium-dependent potassium currents in isolation, NaCl was completely replaced with the impermeable cation N-methyl-D-glucamine (NMG-Cl). Identical bath and pipette solutions were used in both the voltage clamp and current clamp modes. In voltage clamp mode, current was recorded at a holding potential of −70 mV, with 200 ms pulses between −120 and +120 mV. A current clamp protocol consisting of depolarizing steps in increments of 10 pA from −10 to 200 pA (20 ms duration) was used to examine action potential properties such as resting membrane potential, threshold potential and rheobase. Firing frequency was examined by measurement of repetitive discharge of each cell upon injecting 2.5X threshold stimulus for 1000 ms.
Patch perfusion experiments to examine single channel activity and sodium dependence of channels were conducted using Slack stable HEK cells under symmetric ion concentrations in the bath and the pipette solutions. Electrodes in these experiments had resistances in the range of 9–15 MΩ. Excised inside-out patches were perfused with solutions of increasing Na+ concentrations through the SmartSquirt small volume delivery system (Automate Scientific) using a 100 μm perfusion tip with a flow rate of 0.01 ml/min. The perfusates were prepared such that they contained an equimolar concentration of 40 mM [K+], while using NMG-Cl as a cationic replacement to maintain osmolarity at 0, 20, 50 and 90 mM of [Na+]. In experiments involving treatment with anisomycin (Life Technologies; 10 mg/ml) or p38 Inhibitor (Millipore; 10 μM), cells were incubated at 37°C for 90 minutes with the drug or vehicle (DMSO), followed by recording at 5–120 minutes after removal of the drug.
2.4. Site-Directed Mutagenesis
Site-directed mutagenesis at the putative p38 phosphorylation sites in the Slack C-terminus was performed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA.). rSlack-B cDNA cloned into the expression plasmid pTRACER (Invitrogen) was used as the template to introduce single or double mutations at Serines 736 and 742 in the Slack C-terminus. Serines were replaced with alanine to prevent phosphorylation or glutamate to mimic phosphorylation, using specific primer sets to introduce base mismatches at one or both sites. Pfu Turbo mediated PCR incorporation of the mutation(s) was followed by DpnI digestion to eliminate non-amplified cDNA. Remaining PCR products were transformed into XL-10 Gold Ultracompetent Cells (Agilent Technologies, Santa Clara, CA.) and mini-prepped (Qiagen Spin mini-prep kit). The final products were sequence verified to obtain the following six mutations: S736A, S742A, S736E, S742E, S736A & S742A and S736E & S742E. One day after plating at ~20% confluency, CHO cells were transiently transfected with 1 μg of either WT or mutated Slack pTracer DNA via the lipofectamine protocol (Invitrogen). Cells were used in experiments 48–72h after transfection.
2.5. Immunoprecipitation Experiments
Slack stable HEK cells or CHO cells in six-well plates were treated with 10 μM p38 Inhibitor/Vehicle for 90 minutes at 37°C and transiently transfected with WT or mutated Slack pTRACER DNA, respectively. Cells were then lysed with 100 μl/well of cold buffer containing RIPA and protease inhibitor cocktail (Sigma). 60 μl/well of Protein G-linked Sepharose bead slurry (GE Healthcare) was washed three times with cold lysis buffer and incubated on a rotator overnight at 4°C with 4 μg of pS-P phospho-specific antibody (Abcam) in PBS with 0.1% Tween and cell lysate (3 wells/sample). On the following day, samples were centrifuged and supernatants were stored separately. Pellets were washed three times with cold lysis buffer and bound protein was eluted via boiling three times at 95°C for 8 minutes each. Samples were centrifuged to separate proteins into the supernatant, which was then denatured with Sodium Dodecyl Sulfate (SDS) and loaded onto a Ready Gel (Bio-Rad) (4–15% Tris-HCl) as the immunoprecipitate. Supernatants collected earlier and whole cell lysate (total input) were also denatured with SDS and run as controls. After electrophoresis, the gel was then transferred onto a nitrocellulose membrane for 1 hour and incubated in 5% non-fat dry milk as a blocking agent for 1 hour. The monoclonal anti-Slack (NeuroMab) primary antibody was used to probe the blot for Slack channel, and signal was detected using horseradish peroxidase-conjugated mouse secondary antibody and chemiluminescent substrate kit (KPL).
2.6. Membrane Biotinylation Assays
For these experiments, CHO cells and Slack stable HEK cells in six-well plates were used at 48h after transient transfection and 90 minutes following treatment with p38 Inhibitor/Vehicle, respectively. 160 μl of 10mM Sulfo-NHS-SS-Biotin (Thermo Scientific) was added to each well and incubated at room temperature for 45 min. During this time, 100 μl of Pierce Streptavidin Magnetic Beads (Thermo Scientific) were washed with PBS + 0.1% Tween. After 45 min, the cells were rinsed with PBS and lysed with a buffer containing PBS, 150 mM NaCl, 1% Nonidet P-40, and 0.1% SDS, pH 8.0, and a protease inhibitor cocktail. Cells were then collected (3 wells/sample) in PBS and spun down such that the cell pellet was then re-suspended in the lysis buffer. Lysis was allowed to occur in a rotator for one hour at 4°C. Samples were then incubated with 50 μl of the streptavidin beads in the rotator overnight at 4°C. On the following day, the samples were washed twice in PBS before separating the supernatant to run as controls. Avidin beads with bound biotinylated protein were re-suspended in Laemmli sample buffer (Bio-Rad) containing 5% β-Mercaptaethanol and incubated at 37°C for 45 minutes. The resulting elute was loaded onto a Ready Gel and Western analysis was performed to detect membrane Slack as described earlier.
2.7. Data and Statistical Analysis
Clampfit (Molecular Devices) and Origin 8.0 (Origin Lab) software were used for all electrophysiology data analysis. Densitometry analyses of Western blots were done using Image J (NIH) software. Statistical analysis was done using GraphPad Prism 4 (GraphPad, San Diego, CA). Unpaired t-tests and one-way ANOVA analyses were used to determine statistical significance (p < 0.05).
2.8. Materials
Anisomycin was purchased from Life Technologies. The p38 MAPK Inhibitor (C20H13ClFN3O) was purchased from Millipore (de Laszlo et al. 1998).
3. Results
Since the cloning of the Slack channel in 1998, studies attempting to elucidate the structure of the channel have revealed that the KNa channels are set apart from the larger super-family of potassium channels by an extensive intracellular C-terminus (Joiner et al. 1998). The C terminus of Slack channels appears to be a regulatory hub, with two Regulator of Conductance of Potassium (RCK) domains comprising the predicted region for sodium binding (Jiang et al. 2001), as well as several conserved motifs for protein-protein interactions such as with PKA, PKC and FMRP (Nuwer et al. 2010, Barcia et al. 2012, Zhang et al. 2012). In our effort to identify if p38 MAPK can regulate Slack channels, we closely examined the C-terminus of the Slack channel for the putative p38 MAPK ‘proline-directed’ phosphorylation motif consisting of a Serine or Threonine residue followed by a Proline (Roux & Blenis 2004). The online resource Phosphosite (http://www.phosphosite.org) (Hornbeck et al. 2012) revealed two putative p38 MAPK phosphorylation sites located at Serines 736 and 742, both of which show high evolutionary conservation in our sequence alignment across species ranging from higher mammals like humans and rodents to fish (Fig. 1). Interestingly, both sites lie in the second of two known RCK domains in close proximity to residues 761–767 that comprise the putative Na+ binding region (Zhang et al. 2010). Based on the adjacency of the p38 MAPK sites to the region that’s critical for channel gating, we proposed the overarching hypothesis that a functionally significant interaction may occur between the Slack channel and p38 MAPK.
Fig. 1. Putative p38 MAPK phosphorylation sites are highly conserved in the Slack C-terminus across species.

Amino acid sequence alignment of the mammalian (rSlack- rat, hSlack- human, mSlack- mouse, cSlack- chicken), Xenopus (xSlack) and Zebrafish (zSlack) Slack channel. Serines at positions 736 and 742 (black, underlined) in the RCK2 domain of Slack are the putative p38 phosphorylation motifs, and are evolutionarily conserved across species. Also shown in red is the putative Na+ binding site in close proximity.
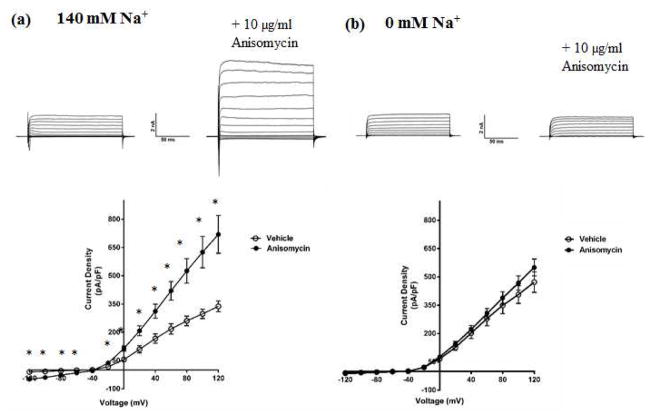
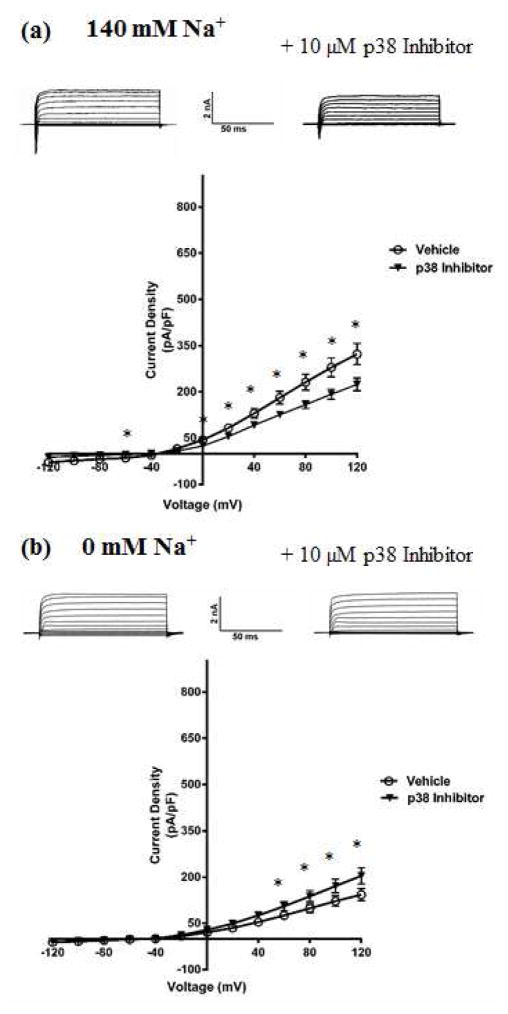
As the first step in testing this hypothesis, we examined the effect of pharmacological modulation of p38 MAPK on KNa and Slack currents. We used primary cultures of embryonic rat DRG neurons due to their endogenous expression of Slack channels (Grigaliunas et al. 2002, Nuwer et al. 2010). The protein synthesis inhibitor anisomycin has an indirect and non-specific mechanism of action, but is widely used as a p38 MAPK activator due to lack of more specific pharmacological activators. Incubating DRG neurons with 10 μg/ml of anisomycin for 90 minutes (Hudmon et al. 2008) produced a significant increase in total outward potassium current IK in whole-cell voltage clamp experiments (Fig. 2a). We also found anisomyin treatment to be associated with an increase in INa consistent with previously reported literature (Hudmon et al. 2008), with a statistically significant difference in current density between vehicle (−105.45 pA/pF; n=8; p<0.05) and anisomycin (−199.75 pA/pF; n=10) groups measured at +20 mV. Upon replacing sodium in the bath and pipette solution with the impermeant cation N-Methyl Glucamine (NMG) to isolate the sodium-dependent component (KNa) of IK, we found that anisomycin no longer increased the outward current, suggesting that p38 MAPK activation specifically increased the KNa current (Fig. 2b). To test if reduced p38 MAPK activity would produce the opposite effect, we used a selective inhibitor of p38 MAPK and found that incubating the cells with 10 μM of the drug for 90 minutes produced a decrease in total IK (Fig. 3a). This effect was not seen upon replacing Na+ with NMG, but in fact, we observed a small but statistically significant increase in the total outward current, implying the presence of other unidentified potassium current(s) that are likely subject to p38 MAPK modulation (Fig. 3b). Altogether, this set of results indicated that modulation of p38 MAPK activity affected the KNa component of the total IK in DRG neurons. Upon examining neuronal firing properties, we found that p38 MAPK inhibition caused the neurons to have a more depolarized resting membrane potential (RMP) (−44 ± 0.53 mV) as compared to vehicle treated neurons (−55± 0.81 mV) (n=10; p<0.05), but no differences were observed in the rheobase or firing frequency (data not shown). However, we observed that p38 MAPK inhibition also resulted in an opposite effect to anisomycin on the INa of DRG neurons, similar to what has been previously reported for p38 MAPK regulation of sodium channels (Hudmon et al. 2008). A significant decrease (Fig. 3a) on INa in p38 inhibitor treated neurons (−83.29 ± 8.21 pA/pF; n=10; p<0.001, measured at +20 mV) compared to vehicle (−167.0 ± 11.92 pA/pF; n=10) was observed. This in combination with the observed increase in the unidentified sodium-independent IK could underlie the surprising lack of changes in rheobase or excitability under conditions of depolarized RMP.
Fig. 2. Activation of p38 MAPK by anisomycin up-regulates the KNa component of IK in DRG neurons.
(a) Top- Representative traces from whole-cell recordings of DRG neurons treated with 10μg/ml anisomycin for 90 minutes at 37 °C, in bath solution containing sodium. Bottom- Current-Voltage relationship of IK measured from anisomycin-treated DRG neurons in bath solution containing sodium. (b) Top- Representative traces from whole-cell recordings of DRG neurons treated with 10μg/ml anisomycin for 90 minutes at 37 °C, in bath solution containing the impermeant cation NMG in place of sodium. Bottom, Current-Voltage relationship of the isolated KNa current measured from anisomycin-treated DRG neurons in bath solution containing NMG. Holding potential was −70 mV, and currents were elicited with voltage steps from −120 mV to +120 mV in 20 mV increments. Data are means ± SEM (n=8–12 per group). Statistical analysis was done using Student’s unpaired t-test (*p<0.05).
Fig. 3. DRG neurons have decreased KNa component of IK current upon inhibition of p38 MAPK.
(a) Top- Representative traces from whole-cell recordings of DRG neurons treated with 10 μM p38 Inhibitor for 90 minutes at 37 °C, in bath solution containing sodium. Bottom, Current-Voltage relationship of IK measured from p38 Inhibitor-treated DRG neurons in bath solution containing sodium. (b) Top- Representative traces from whole-cell recordings of DRG neurons treated with 10 μM p38 Inhibitor for 90 minutes at 37 °C, in bath solution containing the impermeant cation NMG in place of sodium. Bottom, Current-Voltage relationship of the isolated KNa current measured from Inhibitor-treated DRG neurons in bath solution containing NMG. Holding potential was −70 mV, and currents were elicited with voltage steps from −120 mV to +120 mV in 20 mV increments. Data are means ± SEM (n=8–12 per group). Statistical analysis was done using Student’s unpaired t-test (*p<0.05).
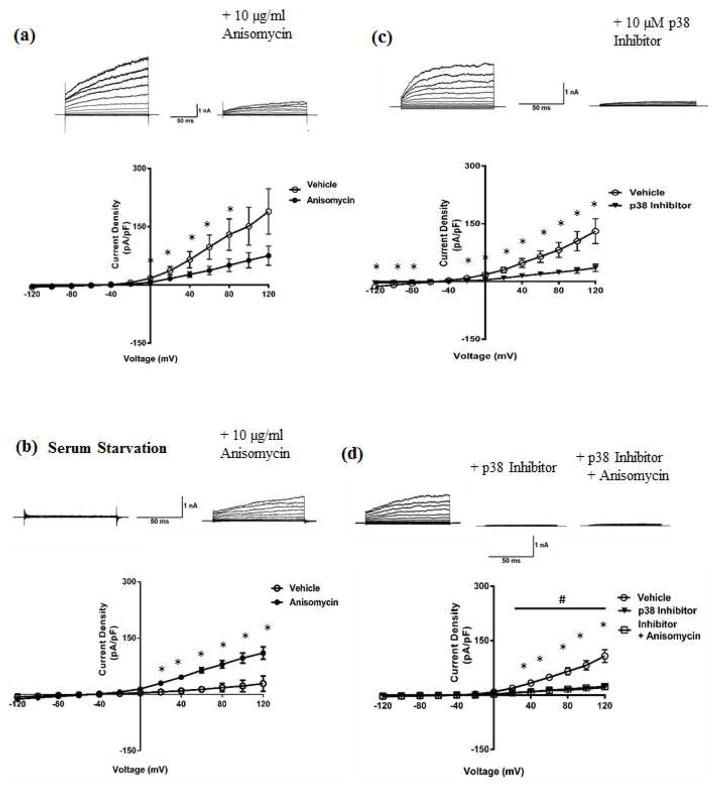
HEK-293 cells stably expressing the Slack protein are a useful model system to examine Slack KNa currents in isolation as the over-expression of Slack channels makes it the predominant contributor to the total outward potassium current. To probe for Slack channels’ contribution to the KNa current being modulated by p38 MAPK, we treated Slack stable HEK cells with anisomycin or p38 MAPK Inhibitor and measured the total outward current. In contrast to anisomycin’s up-regulation of KNa current in DRG neurons, Slack current decreased in response to 90 minutes in 10 μg/ml of the drug (Fig. 4a). The unexpected contrast of this result to the up-regulation of KNa induced by anisomycin in DRG neurons led us to suspect a cell type specific disparity in the action of anisomycin. Specifically, DRG neurons are post-mitotic while HEK cells are actively dividing mitotic cells. To ask if the mitotic state of the cell underlies the effect of anisomycin, we serum starved Slack stable HEK cells for 18 hours to induce cell cycle arrest and mimic the post-mitotic DRG neuronal state, following which the cells were treated with vehicle or anisomycin.
Fig. 4. p38 MAPK modulates Slack KNa current in Slack stable HEK cells.
Representative traces of whole-cell recordings (Top) and current voltage relationship of Slack current (Bottom) from (a) Slack Stable HEK cells treated with vehicle (0.01% DMSO) or anisomycin (10 μg/ml) for 90 minutes at 37°C, (b) Serum starved slack stable HEK cells treated with vehicle (0.01% DMSO) or anisomycin (10 μg/ml) for 90 minutes at 37°C, (c) Slack Stable HEK cells treated with vehicle (0.01% DMSO) or p38 Inhibitor (10 μM) for 90 minutes at 37°C. Data is means ± SEM via unpaired t-test (n=7–10 per group; *p<0.05 between vehicle and p38 Inhibitor/anisomycin groups). (d) Representative traces of whole-cell recordings (Top) and current voltage relationship of Slack current (Bottom) from Slack Stable HEK cells treated with vehicle or p38 Inhibitor alone or p38 Inhibitor following anisomycin treatment. Data is expressed as mean +/− SEM (n=7–10 for all groups). Statistics performed using One Way ANOVA followed by multiple comparisons using Tukey’s method (n=7–10 per group; *p<0.05 between vehicle and p38 Inhibitor/anisomycin groups, #p<0.05 between vehicle and Inhibitor + anisomycin groups.) Holding potential was −70 mV, and currents were elicited with voltage steps from −120 mV to +120 mV in 20 mV increments.
Serum starvation resulted in the reversal of the previously observed anisomycin effect on Slack current, now producing a significant increase in Slack current compared to vehicle (Fig. 4b) and suggesting the earlier observed disparity in results to indeed be dependent on the mitotic state. Furthermore, the more specific acting p38 MAPK Inhibitor produced a consistent and similar effect in that the drug significantly decreased Slack current compared to vehicle as seen for KNa currents in neurons (Fig. 4c). To determine if anisomycin was acting through p38 MAPK activation, we pre-treated Slack stable HEK cells with p38 Inhibitor before treating with anisomycin, and found that activation produced no further effect following that of the p38 Inhibitor (Fig. 4d).
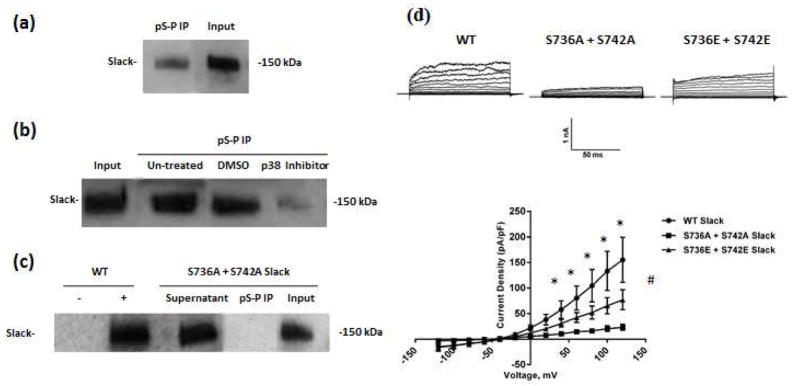
To ask the question if p38 MAPK induced modulation of Slack current was occurring through direct phosphorylation at the putative p38 MAPK phosphorylation sites in the Slack C-terminus, we used immunoprecipitation experiments followed by western blot analysis. To our advantage, the ‘S-P’ phosphorylation motif is uniquely found in the Slack sequence at positions 736 and 742, allowing the use of a phospho-specific pS-P antibody as a tool to detect phosphorylation specifically at the sites of interest. By performing a pull-down on Slack stable HEK cell lysates with the pS-P antibody followed by western blot analysis for Slack protein, we found that the sites were phosphorylated under basal conditions (Fig. 5a). When the experiment was repeated to include cells treated with the p38 Inhibitor, the amount of Slack protein in the pull-down significantly reduced, suggesting that the basal phosphorylation of Slack channels was mediated by p38 MAPK (Fig. 5b). We then asked if Serines 736 and 742 were necessary and sufficient for p38 MAPK phosphorylation of Slack channels. To address this question, we used the expression plasmid pTRACER carrying the Slack cDNA (Joiner et al. 1998) to express wild type (WT) Slack or mutant protein in which both serines were replaced with alanine (S736A + S742A) to prevent phosphorylation. We then used the immuno-precipitation and western blotting approach on CHO cells transiently transfected with either WT or the double mutant Slack pTRACER, and found that the double alanine mutation decreased the amount of Slack protein pulled down significantly (Fig. 5c). These experiments were performed twice to verify the specificity of the pS-P antibody. Additionally, the contribution of each individual site was tested by singly mutating serines 736 and 742. Slack protein pull-down in cells transfected with the single mutations was no different than in those transfected with WT Slack (data not shown), indicating the requirement for simultaneous phosphorylation of both sites. On this basis, subsequent experiments were performed using the double mutated Slack pTRACER constructs only. In combination, the electrophysiological and immunoprecipitation experimental results appear to suggest an obligate role for p38 MAPK in regulating the Slack channel, through a direct phosphorylation event involving both serines 736 and 742. It must be noted that in using the phospho-specific antibody in our pull-downs, we recognized the caveat that a phosphorylated population of channels was being selected for. Thus, we cannot rule out the contribution of a non-phosphorylated population of channels in our electrophysiology studies.
Fig. 5. Slack is basally regulated by p38 MAPK via phosphorylation at S736 and S742.
(a) Representative western blot of Slack protein from Slack stable HEK cell lysates. Pull-down using a phospho-specific Serine-Proline antibody was followed by western analysis using a Slack-specific monoclonal antibody. (b) Representative blot of Slack protein in Slack Stable HEK cells, as detected by western blotting using Slack antibody following pull-down by the pS-P antibody. Cells were untreated or treated with either Vehicle (0.01% DMSO) or p38 Inhibitor (10 μM) for 90 minutes at 37°C. (c) Representative blot of Slack protein in CHO cells transiently transfected with either wild type (WT) (‘-‘ and ‘+’ denoting IPs done in the absence and presence of pS-P antibody, respectively) or mutant Slack pTRACER expression plasmid in which Serines 736 and 742 were both replaced with Alanine. Cells were subjected to western analysis for Slack following pull-down using the pS-P antibody. (d) Top- Representative traces of whole-cell recordings from CHO cells transiently transfected with either WT (left), double alanine mutated (middle) or double glutamate mutated (right) Slack pTRACER. Bottom- Current voltage relationship of IK measured from WT, S736A+ S742A or S736E + S742E Slack pTRACER- transfected CHO cells. Holding potential was −70 mV, and and currents were elicited with voltage steps from −120 mV to +120 mV in 20 mV increments. Data is expressed as mean +/− SEM (n=10 for all groups). Statistics performed using One Way ANOVA followed by multiple comparisons using Tukey’s method (*p<0.05 between WT Slack and S736A + S742A groups, #p<0.05 between WT Slack and S736E + S742E groups).
We then further confirmed the critical requirement of phosphorylation by p38 MAPK at serines 736 and 742 via patch clamp experiments on CHO cells transiently transfected with WT Slack pTRACER or double mutant Slack pTRACER. We found that the double alanine mutation almost completely abolished WT Slack current measured at a holding potential of −70 mV (Fig. 5d). We then examined if mutating the serines to glutamate (S736E + S742E) to mimic phosphorylation would have a restorative effect on Slack current. While the glutamate mutation restored Slack current to a significant extent, there still remained a significant difference between the double glutamate mutation and WT Slack currents at more depolarized potentials. Electrostatic property changes stemming from the fact that the addition of a phosphate group has a negative charge of three while the glutamate residue has a negative charge of two may underlie this observation, indicating a basal functional requirement for phosphate groups at the two Serine residues.
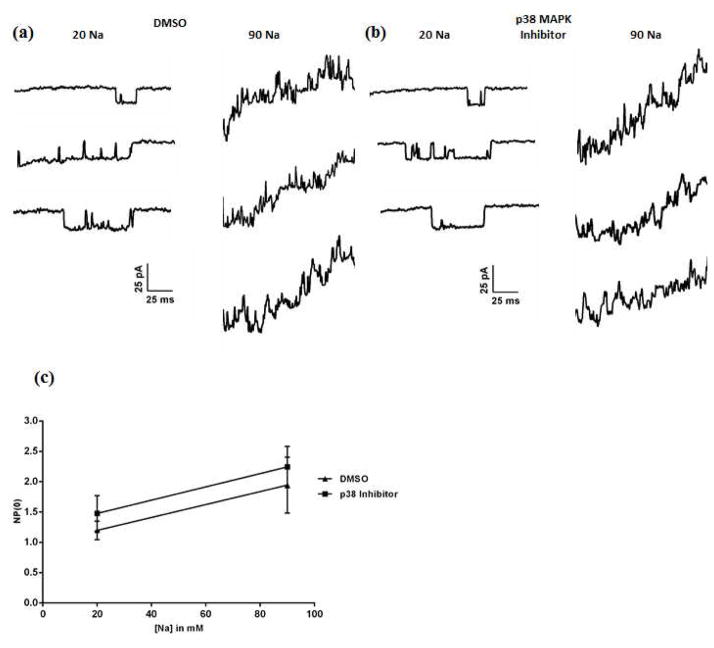
Next, we investigated the mechanism by which p38 MAPK phosphorylation mediates the modulation of Slack channel current. Based on the proximity of the phosphorylation sites to the putative sodium binding region, we first tested if modulation of p38 MAPK in turn modulates the gating of the Slack channel. Slack stable HEK cells were incubated in vehicle or p38 MAPK Inhibitor for 90 minutes, following which inside-out patches were excised and perfused with increasing concentrations of Na+. Patches excised from vehicle treated cells contained channels that exhibited unitary conductance, open probability (NP(0)) and sodium dependence similar to previous reports on Slack channel properties (Fig. 6a and 6c) (Tamsett et al. 2009, Gao et al. 2008). Surprisingly, inhibition of p38 MAPK produced no change in the channel properties, with the conductance and the sodium dependence resembling channels in the vehicle-treated cells and the open probability being slightly lower but not significantly different (Fig. 6b and 6c). These results led us to conclude that p38 MAPK phosphorylation does not contribute to the gating of the Slack channel.
Fig. 6. p38 MAPK does not affect gating of the Slack channel.
(a,b) Representative traces of channels recorded from an excised inside-out patch perfused with 20 mM and 90 mM Na+ in Slack stable HEK cells, after treatment with Vehicle (0.01% DMSO, A) or p38 Inhibitor (10 μM, B) for 90 minutes at 37°C. (c) Na+ dose response relationship of vehicle- and p38 Inhibitor-treated Slack HEK cells. NP (0) values were calculated from 33 sweeps over 2400 ms at each dose of Na+. Data are means ± SEM (n=4).
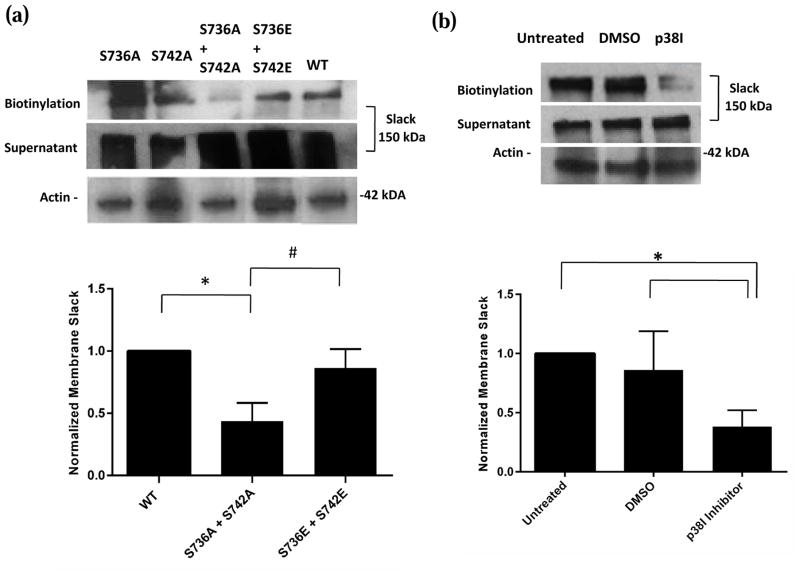
We then tested the second possibility in the mechanism by which p38 MAPK phosphorylation regulates Slack channels i.e. by modulating the number of Slack channels expressed at the cell membrane. Biotinylation assays were used to measure the surface expression of Slack channels in CHO cells transiently transfected with WT, double alanine or double glutamate mutant Slack channels. We were able to pull down significant amounts of biotinylated WT and S736E + S742E Slack, but the amount of S736A + S742A Slack at the membrane was much reduced (Fig. 7a). Furthermore, in agreement with our earlier electrophysiology data, singly mutating either site did not affect the amount of Slack protein pulled down. To confirm that the membrane Slack channel population was indeed being modulated by p38 MAPK, biotinylation assays on p38 MAPK Inhibitor or vehicle treated Slack stable HEK cells were performed. The amount of biotinylated Slack pulled down following incubation of the Slack stable HEK cells in 10 μM Inhibitor for 90 minutes was significantly diminished compared to cells in DMSO (Fig. 7b). Together, these results indicate that the prevention of p38 MAPK phosphorylation of Slack channels, either through mutation of the site or direct inhibition of the kinase, decreases Slack channel numbers at the membrane. This data correlates with the immunoprecipitation and electrophysiology results, suggesting that the Slack channel is phosphorylated by p38 MAPK in the resting cellular state. There appears to be an obligate requirement for p38 MAPK phosphorylation, the absence of which lowers the amount of Slack channel inserted into the membrane.
Fig. 7. p38 MAPK phosphorylation at Serines 736 and 742 is required for surface expression of Slack.
(a) Top- Representative images of western blots for membrane Slack following biotinylation assays on lysates of CHO cells transiently transfected with WT, double alanine mutant or double glutamate mutant Slack pTRACER. Actin was used as a loading control. Bottom- Densitometric analysis of represented western blots for Slack quantified as normalized to Actin. Data is expressed as mean +/− SEM (n=4 for all groups). Statistics performed using One Way ANOVA followed by multiple comparisons using Tukey’s method (*p<0.05 between WT and S736A+S742A groups, #p<0.05 between S736E+S742E and S736A+S742A groups). (b) Top- Representative images of western blots for membrane Slack following biotinylation assays on lysates of Slack stable HEK cells incubated in Vehicle (0.01% DMSO) or p38 MAPK Inhibitor (10 μM) for 90 minutes at 37 °C. Actin was used as a loading control. Bottom- Densitometric analysis of represented western blots for Slack quantified as normalized to Actin. Data is expressed as mean +/− SEM (n=3 for all groups). Statistics performed using One Way ANOVA followed by multiple comparisons using Tukey’s method (*p<0.05).
4. Discussion
The present study identifies p38 MAPK as an obligate regulator of the Slack channel and is the first direct demonstration of Slack channels as substrates of p38 MAPK. We identified two putative p38 MAPK phosphorylation sites in the intracellular Slack C-terminus to be conserved across species. Inhibition of p38 MAPK significantly decreased KNa and Slack current in DRG neurons and Slack stable HEK cells respectively. Immunoprecipitation followed by western blot analysis showed that the Slack channel is phosphorylated by p38 MAPK. Site-directed mutagenesis at the putative phosphorylation sites demonstrated that regulation of Slack current by p38 MAPK occurs through phosphorylation at S736 and S742 in the Slack channel C-terminus. Further examination of the functional significance of this phosphorylation event revealed that upon inhibition of p38 MAPK, surface expression of Slack channels is decreased, suggesting that p38 MAPK is involved in insertion of channels into the membrane. Overall, the results from the study indicate the critical requirement of p38 MAPK phosphorylation for tonic activity of the Slack channel at the cell membrane.
While our study brings to light an important novel regulatory mechanism of Slack, some key issues remain to be understood. First, the result that the p38 MAPK activator anisomycin produced contrasting and opposite effects on the KNa current in DRG neurons and Slack current in Slack stable HEK cells was unexpected, but based on the reversal in effect produced by serum starvation of the HEK cells, we surmise that the basis for the discrepancy lies in the difference between the two cell types in that DRG neurons are post-mitotic while the HEK cells are mitotic cells. Moreover, we observed in the serum starved state that the basal Slack current in the vehicle-treated cells was substantially reduced, consistent with our finding that Slack channel function is under tight regulation by p38 MAPK. Despite this result, the opposite effect in the mitotic HEK state is still unclear. It is well known that p38 MAPK is highly active during active mitosis performing its anti-proliferative, cell cycle check functions (Calabrese et al. 2010, Takenaka et al. 1998), so this may have been a confounding factor in the detection of anisomycin activation of p38 MAPK in the HEK cells. Secondly, there is the challenging fact that anisomycin’s mode of action- protein synthesis inhibition, is indirect and non-specific, activating not only p38 MAPK but also other MAPKs including c-Jun N-terminal protein Kinase (JNK) (Hazzalin et al. 1998). Crosstalk has been shown to exist between JNK and p38 MAPK specifically in mitotic cells; for example, in proliferating mouse cardiomyocytes, active JNK inhibits p38 MAPK and Extracellular signal-related Kinase (ERK) (Peng et al. 2009). There is recent evidence that demonstrates continued activity of JNK via a positive feedback loop in HEK-293 cells (Fey et al. 2012), so it is conceivable that anisomycin activation of JNK could lead to its sustained activity through the positive feedback loop, which could then mediate inhibition of basally active p38 MAPK and result in observed decreased Slack current in the Slack stable HEK cell line. The lack of evidence of such crosstalk in post-mitotic cells could explain the contrasting discrepancy between the neuronal and mitotic HEK-293 cell anisomycin results. Nonetheless, despite our being limited by the absence of more selective pharmacological activators of p38 MAPK, our conclusions are well substantiated by the consistent effects produced by the very specific acting p38 MAPK Inhibitor. A second key question that remains to be answered in future work is how p38 MAPK phosphorylation regulates Slack channel numbers at the cell membrane at the mechanistic level. The Slack C-terminus contains putative binding sites for scaffolding proteins such as 14-3-3, but whether these proteins interact with Slack and are involved in its trafficking mechanism is a matter of future interest.
Our findings imply the endogenous presence of a constitutively active p38 MAPK, which is a conflicting concept amidst the classical understanding of p38 MAPK as an inducible, stress-activated kinase. There is, however, some evidence in the literature to suggest such a role for p38 MAPK. In HEK 293 cells, Samuvel et al. demonstrated p38 MAPK inhibition via RNA interference to down-regulate Serotonin Transporter (SERT) activity, hence identifying SERT as a substrate of basally active p38 MAPK (Samuvel et al. 2005). Thus, we are not the first group to propose basal p38 MAPK activity in this cell line. However, our HEK-293 cell line stably expresses Slack channels, which allows for the following to be proposed. Since Slack channels are over-expressed in these cells, it is likely that not all of the synthesized protein is expressed at the membrane and that there exists a sizable cytoplasmic pool of channels whose insertion into the membrane is signal dependent. We speculate that increased p38 MAPK activation may act as the above-mentioned signal and that this may distinguish the effect of basally active vs. up-regulated p38 MAPK (such as when treated with anisomycin) in the HEK cells.
DRG neurons are an ideal choice for studies focused on Slack channels owing to their endogenous expression of the channel, but there is also sufficient evidence for constitutive neuronal activity of p38 MAPK. High basal expression of total p38 MAPK has been found specifically in small diameter DRG neurons, known to primarily comprise the nociceptors (Ji et al. 2002). This same study also showed that the active form of p38 MAPK, phospho-p38 (p-p38), is present constitutively in non-neuronal cells of the dorsal horn in the spinal cord, with cytokines and inflammatory conditions inducing only a modest increase in p-p38 levels. Also in DRG neurons, the isoform p38 MAPKa has been established as active and essential to neurite outgrowth and growth cone formation following sciatic nerve injury (Kato et al. 2013, Temporin et al. 2008). The DRG neurons used in our experiments are primarily small diameter nociceptive TrkA+ neurons that are responsive to NGF, so it is likely that p38 MAPK is in the active state in these cells. There is, in fact, considerable evidence for NGF activation of p38 MAPK- intrathecal administration of NGF leads to increased levels of p-p38 in DRG neurons (Ji et al. 2002) and treatment of PC-12 cells with NGF results in sustained p38 MAPK activation (Morooka & Nishida 1998). Thus, the very presence of NGF in the neuronal culture alone may be a contributing factor to the basal activation of p38 MAPK in these neurons.
While the association between p38 MAPK and Slack channels is a novel finding, there is, in fact, a precedent for its role in regulating neuronal IK currents via modulation of channel expression at the membrane. Similar to our findings but in rat cortical neurons, it has been shown that p38 MAPK phosphorylation is required for membrane insertion of de novo Kv2.1 channels (Redman et al. 2007). As in the case of Kv2.1, it appears that Slack channel phosphorylation by p38 MAPK is not required for normal function of the already inserted channel, as mutating S736 and S742 did not affect our ability to record Slack current at the whole cell level nor did inhibiting the kinase affect the conductance and sodium dependence of Slack at the single channel level in our Slack stable cell line. When extrapolated to the sensory neurons of the DRG, it appears that there is a subset of channels phosphorylated by p38 MAPK that is inserted into the membrane, which when increased in population can alleviate neuronal excitability, or when decreased in population, can aid in conditions of Slack over-activity. These exciting possibilities remain to be tested by future studies.
Our finding in DRG neurons that p38 MAPK basally regulates Slack channel activity alludes to clinical importance in the context of Slack’s predicted role in neuropathic and inflammatory pain. Neuronal simulation studies first revealed the importance of KNa channels to the maintenance of firing accommodation of DRG neurons (Brown et al. 2008b). In agreement, Nuwer et al. demonstrated that siRNA knockdown of the Slack channel subunit in DRG neurons produced a loss of firing accommodation and DRG hyperexcitability (Nuwer et al. 2010). In vivo evidence of the same has been provided by Lu et al., showing sensory neuron specific Slack knockout mice to have increased neuropathic pain behaviour, which was then alleviated using the Slack channel opener Loxapine (Lu et al. 2015). In agreement, increased membrane expression of Slack channels in DRG neurons via the ion channel TMEM16C leads to enhanced Slack current at the whole cell and single channel level and counters neuronal excitability and nociceptive behaviours (Huang et al. 2013). Thus, regulatory mechanisms that maintain or enhance Slack channel activity at the DRG membrane, one of which would be the p38 MAPK phosphorylation event reported in the present study, appear to be central to understanding pain signalling. Combined with the previously mentioned activity of p38 MAPK in nerve regeneration following neuropathy, it is conceivable to think of p38 MAPK phosphorylation of Slack channels as a neuro-protective mechanism to enhance K+ currents and counter the nociceptive state.
Also of strong therapeutic import is the recent association that has been reported between mutations in the human KCNT1 gene and epilepsy, with patients of three distinct epileptic conditions having mutations in the KCNT1 gene (Barcia et al. 2012, Heron et al. 2012, Martin et al. 2014). Specifically, Barcia et al. reported Slack to be a major disease target for Malignant Migrating Partial Seizures of Infancy (MMPSI), identifying two de novo gain-of-function mutations in the KCNT1 gene region encoding the C-terminus to cause constitutive activation of the channel and underlie the epileptic condition (Barcia et al. 2012). Our finding that p38 inhibition attenuates basal Slack channel activity provides a powerful clinical indication for the highly specific acting p38 Inhibitors in patients of MMPSI. Studies so far provide substantial in vitro evidence for such an application, but further work addressing the efficacy of the drug in vivo, such as in transgenic mice expressing the known MMPSI-related mutations, will be necessary in the near future.
Slack KNa channel is subject to regulation by intracellular proteins.
Two putative p38 MAPK phosphorylation sites reside in the Slack channel C terminus.
Pharmacological modulation of p38 MAPK alters tonic KNa and Slack current.
p38 MAPK regulates the Slack channel through direct phosphorylation at Serines 736 and S742.
p38 MAPK phosphorylation is required for surface expression of the Slack channel.
Acknowledgments
Funding
The research was supported by the National Institute of Health, Grant NS078184 (to A.B.)
The authors are grateful to Dr. Fred Sigworth (Yale University) for the Slack stable HEK-293 cell line.
Abbreviations
- MAPK
Mitogen-Activated Protein Kinase
- DRG
Dorsal Root Ganglion
- KNa
Sodium-activated Potassium Channel
- INa
Sodium Current
- TRPV1
Transient Receptor Potential Cation Channel V1
- TRPA
Transient Receptor Potential Cation Channel A
- PKC
Protein Kinase C
- PKA
Protein Kinase A
- RCK
Regulator of Conductance of K+
- siRNA
short interfering Ribo-Nucleic Acid
- FMRP
Fragile-X Mental Retardation Protein
- NGF
Nerve Growth Factor
- HEK
Human Embryonic Kidney
- CHO
Chinese Hamster Ovary
- DMEM
Dulbecco’s Modified Eagle Medium
- IMDM
Iscove’s Modified Dulbecco’s Medium
- FBS
Fetal Bovine Serum
- PBS
Phosphate Buffered Saline
- WT
Wild Type
- RMP
Resting Membrane Potential
- JNK
c-Jun N-terminal protein Kinase
- ERK
Extracellular signal related kinase
- SERT
Serotonin Transporter
- MMPSI
Malignant Migrating Partial Seizures of Infancy
Footnotes
Competing Interests
The authors declare no competing financial interests.
Author Contributions
A.B. designed all the experiments and edited the manuscript. S.G performed and analyzed the results of the experiments and wrote the manuscript. J.F. performed and analyzed some of the experiments. All authors approved of the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barcia G, Fleming MR, Deligniere A, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nature Genetics. 2012;44:1255–1259. doi: 10.1038/ng.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Gan L, Kaczmarek LK. Localization of the Slack potassium channel in the rat central nervous system. J Comp Neurol. 2002;454:241–254. doi: 10.1002/cne.10439. [DOI] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Spilianakis CG, Flavell RA, von Hehn CA, Bhattacharjee A, Kaczmarek LK. Amino-termini isoforms of the Slack K+ channel, regulated by alternative promoters, differentially modulate rhythmic firing and adaptation. J Physiol. 2008a;586:5161–5179. doi: 10.1113/jphysiol.2008.160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WM, Sasson A, Bellew DR, Hunsaker LA, Martin S, Leitao A, Deck LM, Vander Jagt DL, Oprea TI. Efficient calculation of molecular properties from simulation using kernel molecular dynamics. J Chem Inf Model. 2008b;48:1626–1637. doi: 10.1021/ci8001233. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Mancuso C, Lentile R, Stella AM, Butterfield DA. Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol Biol. 2010;610:285–308. doi: 10.1007/978-1-60327-029-8_17. [DOI] [PubMed] [Google Scholar]
- Chae KS, Dryer SE. The p38 mitogen-activated protein kinase pathway negatively regulates Ca2+-activated K+ channel trafficking in developing parasympathetic neurons. J Neurochem. 2005;94:367–379. doi: 10.1111/j.1471-4159.2005.03201.x. [DOI] [PubMed] [Google Scholar]
- de Laszlo SE, Visco D, Agarwal L, et al. Pyrroles and other heterocycles as inhibitors of p38 kinase. Bioorg Med Chem Lett. 1998;8:2689–2694. doi: 10.1016/s0960-894x(98)00495-8. [DOI] [PubMed] [Google Scholar]
- Fey D, Croucher DR, Kolch W, Kholodenko BN. Crosstalk and signaling switches in mitogen-activated protein kinase cascades. Front Physiol. 2012;3:355. doi: 10.3389/fphys.2012.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SB, Wu Y, Lu CX, Guo ZH, Li CH, Ding JP. Slack and Slick KNa channels are required for the depolarizing afterpotential of acutely isolated, medium diameter rat dorsal root ganglion neurons. Acta Pharmacol Sin. 2008;29:899–905. doi: 10.1111/j.1745-7254.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- Grigaliunas A, Bradley RM, MacCallum DK, Mistretta CM. Distinctive neurophysiological properties of embryonic trigeminal and geniculate neurons in culture. J Neurophysiol. 2002;88:2058–2074. doi: 10.1152/jn.2002.88.4.2058. [DOI] [PubMed] [Google Scholar]
- Hazzalin CA, Le Panse R, Cano E, Mahadevan LC. anisomycin selectively desensitizes signalling components involved in stress kinase activation and fos and jun induction. Mol Cell Biol. 1998;18:1844–1854. doi: 10.1128/mcb.18.4.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron SE, Smith KR, Bahlo M, et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 2012;44:1188–1190. doi: 10.1038/ng.2440. [DOI] [PubMed] [Google Scholar]
- Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2012;40:D261–270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Wang X, Ostertag EM, Nuwal T, Huang B, Jan YN, Basbaum AI, Jan LY. TMEM16C facilitates Na(+)-activated K+ currents in rat sensory neurons and regulates pain processing. Nat Neurosci. 2013;16:1284–1290. doi: 10.1038/nn.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, Dib-Hajj SD. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci. 2008;28:3190–3201. doi: 10.1523/JNEUROSCI.4403-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Pico A, Cadene M, Chait BT, MacKinnon R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 2001;29:593–601. doi: 10.1016/s0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Tang MD, Wang LW, Dworetzky SI, Boissard CG, Gan L, Gribkoff VK, Kaczmarek LK. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nature Neuroscience. 1998;1:462–469. doi: 10.1038/2176. [DOI] [PubMed] [Google Scholar]
- Kameyama M, Kakei M, Sato R, Shibasaki T, Matsuda H, Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984;309:354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Kato N, Matsumoto M, Kogawa M, Atkins GJ, Findlay DM, Fujikawa T, Oda H, Ogata M. Critical role of p38 MAPK for regeneration of the sciatic nerve following crush injury in vivo. Journal of Neuroinflammation. 2013;10:1. doi: 10.1186/1742-2094-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nature Reviews Drug Discovery. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- Lu R, Bausch AE, Kallenborn-Gerhardt W, et al. Slack channels expressed in sensory neurons control neuropathic pain in mice. J Neurosci. 2015;35:1125–1135. doi: 10.1523/JNEUROSCI.2423-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HC, Kim GE, Pagnamenta AT, et al. Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum Mol Genet. 2014;23:3200–3211. doi: 10.1093/hmg/ddu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morooka T, Nishida E. Requirement of p38 mitogen-activated protein kinase for neuronal differentiation in PC12 cells. J Biol Chem. 1998;273:24285–24288. doi: 10.1074/jbc.273.38.24285. [DOI] [PubMed] [Google Scholar]
- Nuwer MO, Picchione KE, Bhattacharjee A. PKA-induced internalization of slack KNa channels produces dorsal root ganglion neuron hyperexcitability. J Neurosci. 2010;30:14165–14172. doi: 10.1523/JNEUROSCI.3150-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Zhang T, Lu X, Feng Q. JNK1/c-fos inhibits cardiomyocyte TNF-alpha expression via a negative crosstalk with ERK and p38 MAPK in endotoxaemia. Cardiovasc Res. 2009;81:733–741. doi: 10.1093/cvr/cvn336. [DOI] [PubMed] [Google Scholar]
- Redman PT, He K, Hartnett KA, Jefferson BS, Hu L, Rosenberg PA, Levitan ES, Aizenman E. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc Natl Acad Sci U S A. 2007;104:3568–3573. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruit KG, Elliott JL, Osborne PA, Yan Q, Snider WD. Selective dependence of mammalian dorsal root ganglion neurons on nerve growth factor during embryonic development. Neuron. 1992;8:573–587. doi: 10.1016/0896-6273(92)90284-k. [DOI] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K, Moriguchi T, Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280:599–602. doi: 10.1126/science.280.5363.599. [DOI] [PubMed] [Google Scholar]
- Tamsett TJ, Picchione KE, Bhattacharjee A. NAD+ activates KNa channels in dorsal root ganglion neurons. J Neurosci. 2009;29:5127–5134. doi: 10.1523/JNEUROSCI.0859-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temporin K, Tanaka H, Kuroda Y, Okada K, Yachi K, Moritomo H, Murase T, Yoshikawa H. Interleukin-1 beta promotes sensory nerve regeneration after sciatic nerve injury. Neurosci Lett. 2008;440:130–133. doi: 10.1016/j.neulet.2008.05.081. [DOI] [PubMed] [Google Scholar]
- Wittmack EK, Rush AM, Hudmon A, Waxman SG, Dib-Hajj SD. Voltage-gated sodium channel Nav1.6 is modulated by p38 mitogen-activated protein kinase. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6621–6630. doi: 10.1523/JNEUROSCI.0541-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZZ, Pan HL. Role of TRPV1 and intracellular Ca2+ in excitation of cardiac sensory neurons by bradykinin. Am J Physiol Regul Integr Comp Physiol. 2007;293:R276–283. doi: 10.1152/ajpregu.00094.2007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brown MR, Hyland C, Chen Y, Kronengold J, Fleming MR, Kohn AB, Moroz LL, Kaczmarek LK. Regulation of neuronal excitability by interaction of fragile X mental retardation protein with slack potassium channels. J Neurosci. 2012;32:15318–15327. doi: 10.1523/JNEUROSCI.2162-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Rosenhouse-Dantsker A, Tang QY, Noskov S, Logothetis DE. The RCK2 domain uses a coordination site present in Kir channels to confer sodium sensitivity to Slo2.2 channels. J Neurosci. 2010;30:7554–7562. doi: 10.1523/JNEUROSCI.0525-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]