Abstract
Background
In malaria-endemic countries in West Africa, sickle cell disease (SCD) contributes to childhood mortality. Historically, Liberia had regions wherein hemoglobin S and beta-thalassemia trait were mutually exclusive. Data on hemoglobinopathies in the Monrovia, the capital, are outdated and do not reflect urban migration. Updating the epidemiology of SCD is necessary to plan a public health and clinical agenda. Neither newborn screening (NBS) nor screening tools were available in-country. This pilot study aimed to determine the feasibility of NBS using a South-South partnership and define the incidence of sickle cell trait (SCT) and SCD in Monrovia.
Procedure
This descriptive epidemiologic feasibility study collected dried blood spots from 2785 consecutive newborns delivered at a hospital in Monrovia. Samples were analyzed by isoelectric focusing at a regional reference laboratory. Infants with SCD were referred for preventive care.
Results
SCT occurred in 10.31% of infants screened. SCD occurred in 33 infants screened [1.19% (95% CI: 0.79%–1.59%)](FS: 28/33, FSB: 2/33, FSA: 2/33, FSX 1/33). There were no infants with FSC phenotype observed. Non-sickling hemoglobin phenotypes ‘FC’ and ‘F’ were each present in 3 infants screened. Seventy-six percent of infants with SCD were brought to care, demonstrating the feasibility of our approach.
Conclusions
The incidence of SCD and other hemoglobinopathies remain high in Liberia. Additional studies are needed to clarify sickle genotypes and identify the contribution of silent beta-thalassemia alleles. By developing regional partnerships, countries similar to Liberia can acquire current data to inform NBS as an important public health initiative toward improving child health.
Keywords: Sickle Cell Disease, Hemoglobinopathies, Pediatric hematology/oncology, Epidemiology
INTRODUCTION
In 2006, the World Health Organization described sickle cell anemia (SCA) as a public health priority for sub-Saharan Africa, as SCA contributes to 9–16% of deaths in West African children under 5 years of age [1, 2]. Regional investigations suggest that 50–90% of undiagnosed children with SCA die by age 5 years [2]. In the carrier state, hemoglobin S and other hemoglobin genotypes (e.g. hemoglobin C, beta-thalassemia, hereditary persistence of fetal hemoglobin) confer a survival advantage against Plasmodium falciparum malaria. For this reason, these hemoglobinopathies are highly prevalent in malaria endemic regions. In Western nations, inexpensive preventive interventions such as newborn screening, penicillin prophylaxis, and pneumococcal vaccination have tremendously reduced mortality associated with all phenotypes of sickle cell disease (SCD) [3, 4]. In regions where SCD is most prevalent, persons with the disease often go undiagnosed and receive no preventive care. Broad implementation of newborn screening for sickling disorders in sub-Saharan Africa could save the lives of over 9.8 million newborns by 2050 [5].
Pioneering investigations of hemoglobinopathies in West Africa conducted prior to 1980 revealed a hemoglobin S carrier prevalence in Liberia ranging from 7–28%, varying amongst studies of adults, children or both [6,7], and by region [8]. These investigations highlighted Liberia as unique in West Africa with beta-thalassemia trait present in up to 9% in some regions, localized to ethnic groups and regions where the allelic frequency of hemoglobin S was low [7, 9]. Specifically, hemoglobin S and hemoglobin C were more common in the west of the nation, whereas beta-thalassemia trait was more common in the east [9]. In the north and central portions of Liberia, all hemoglobinopathies were seen. Though ethnic groups historically clustered regionally in Liberia, these groupings did not align with county borders. A lengthy war began in 1989, leading Liberians to flee warfronts on borders with Sierra Leone and Ivory Coast for safety in the capital, Monrovia. The resulting extensive internal displacement accelerated urban migration and may have altered the distribution of hemoglobinopathies, particularly by uniting previously distinct ethnic groups harboring different hemoglobinopathies. The effect of war on public health and health care infrastructure has been devastating, leaving a system with limited human resources and limited access to testing for many disorders, including hemoglobinopathies.
The two objectives of this investigation were to: determine the feasibility of conducting a newborn screening program in Monrovia, where a feasible program would have the diagnosis of SCD communicated to a majority of families before the subject is three months old; and to define the incidence of hemoglobinopathies and abnormal hemoglobins in post-war, post-migration Monrovia. By screening a newborn cohort, we designed an incidence study, eliminating bias toward survivors of childhood and infancy that may have confounded earlier prevalence studies. Without accurate data on incidence of SCD, describing a public health agenda and targeted interventions for SCD in Liberia is challenging. While several sub-Saharan African countries have developed localized newborn screening programs [10–12], Liberia had no screening program or established clinical care protocols for SCD at a time when the health care and public health systems were being rebuilt. In this manuscript, we present our approach to developing a successful pilot and the resulting epidemiologic data.
METHODS
Location
Liberia is a nation in West Africa that was founded by indigenous Africans and repatriated U.S. and Caribbean blacks in 1847. Approximately 95% of the population is comprised of indigenous Liberians, many of whom associate with one of 16 ethnic groups. The remaining 5% is comprised of descendants of repatriated peoples and non-Liberian foreigners. Liberia’s development was abruptly halted during a civil war waged from 1989 to 2003. Over 250,000 people were killed and over one million were displaced, migrating out of the country or internally to reach Monrovia [13]. Liberia’s current population is an estimated 4 million; following the war, the population in Monrovia swelled from 166,507 in 1972 to 1,010,970 in 2008 [14, 15].
Study Design
This epidemiological study defined the incidence of SCD in a subset of the Liberian population. All consecutive newborns delivered at the Liberian-Japanese Friendship Maternity Hospital of the John F. Kennedy Medical Center (JFK-MC) during a 13-month period were assessed for eligibility. This national referral hospital is public and centrally located in the city. Well-appearing infants born in the hospital to mothers residing in Greater Monrovia were included in the study. Newborns who were clinically unstable after birth and died during the hospitalization were excluded. Newborns who recovered and survived to discharge were eligible to be enrolled in the study. Demographic data including maternal ancestral county, home address, and telephone contact information for the family were reported on the screening card at enrollment by the mother. To enable comparison with older studies [6], mothers were also asked to report with which ethnic group each parent associated. The rate of consanguinity is expected to be low in this population, but such information was not collected.
Ethics
Local physicians and nurses participated in human subjects research training for Liberia prior to the initiation of the study. Pre-screening education and counseling was provided to pregnant women in group format as well as in private sessions. Verbal informed consent was documented prior to testing each newborn. The research protocol was conducted in accordance with ethical research practices and was reviewed by Institutional Review Boards at University of Liberia – PIRE and Boston Children’s Hospital.
Sample Collection and Processing
Whole blood samples were collected within 48 hours of birth by heel prick using the BD Microtainer Contact-Activated Lancet (Becton, Dickinson and Company, New Jersey, USA). Blood from a single heel prick was impregnated on standardized screening cards with filter paper (Association of Public Health Laboratories, Maryland, USA). Prior publications have demonstrated that low humidity and low temperature storage reduces sample degradation. Samples stored at room temperature for 6 weeks or at −20°C for up to 13 months maintain fidelity [16, 17]. Samples in this study were allowed to air dry completely prior to being stored in a sealed container at 4°C, as this temperature was most reliably available. Samples were batched every 4 weeks and shipped at room temperature to a regional reference laboratory, the Noguchi Memorial Institute for Medical Research/NMIMR at the University of Ghana in Legon, Ghana, that we contracted for this study. Prior to shipping, an image of each card was stored in a secure database. Each sample was analyzed by isoelectric focusing (IEF; Perkin Elmer, Finland) to identify hemoglobins A, S, C, and F and their relative proportions by visual inspection. Confirmatory testing by IEF was performed for all samples with a result suggestive of a variant hemoglobin.
Results Communication
IEF results were tabulated with 4 identifiers (mother’s name, newborn’s gender, newborn’s date of birth, and study card number) and communicated by secure file transfer from the regional reference laboratory to the investigators. Contact and demographic information were crosschecked with the database of screening card images. All families with a result suggestive of any hemoglobinopathy were contacted by phone and referred to the medical center for post-test counseling and care. When indeterminate results were returned or samples were inadequate for testing, subjects were invited to return for repeat testing.
Treatment
All newborns with a result suggestive of SCD were referred to an outpatient pediatrics clinic at JFK-MC that serves as a medical home for children with chronic health care needs. Newborns were seen by a local physician and local nurses who are trained to provide care for these patients. The clinical care provided to SCD patients was determined by the treating physicians and was not dictated by the study. Preventive care and medications were provided free-of-charge for all patients under age 5 years. In accordance with the clinic standard, infants diagnosed with SCD were treated with penicillin prophylaxis and received standard childhood vaccinations. Hydroxyurea and pneumococcal polysaccharide vaccination were not routinely available in Monrovia during the study period.
Statistical Methods
The primary endpoints of the study were: the presence or absence of a) hemoglobin S trait; b) hemoglobin C trait; c) SCD; d) whether a SCD screening result could be returned within three months. The incidence of hemoglobin S trait, hemoglobin C trait, and SCD were calculated from the total screened population. Historical data for incidence were not available; therefore, historical prevalence data are presented herein for a descriptive comparison to the incidence data from this study. The sample size calculation was based on providing sufficient precision for placement of a 95% confidence interval (CI) on the incidence of sickle trait or SCD in Monrovia. This pilot-scale study was not designed or powered to detect statistically significant differences in the demographics of the study population versus the population of Liberia.
Using the maternal ancestral county, the proportion of study population by ancestral county was calculated and descriptively compared to the 2008 National Census [18]. The portion of subjects with an S allele (i.e. subjects with hemoglobin S trait/ FAS) by county was assessed using maternal ancestral county. As fathers in this study were not routinely available for family studies, the S allele was assigned to the mother’s ancestral county. The proportion of newborns by maternal ethnic group was calculated and also compared to the census. The study was not designed or powered to detect statistically significant differences in sickle cell trait incidence between ethnic groups or counties.
To identify a potential role for beta-thalassemia alleles, we evaluated all possible ways to restore Hardy-Weinberg Equilibrium (HWE) by reassigning homozygous genotypes (i.e. FA, FC, FS) to a corresponding heterozygous genotype with a silent allele such as beta-0-thalassemia or HPFH. We calculated the Hardy-Weinberg permutation p-value for each combination. For the combinations satisfying HWE (p>0.05), we calculated the allele frequency of the silent allele, and determined the range of allele frequencies necessary to restore HWE.
RESULTS
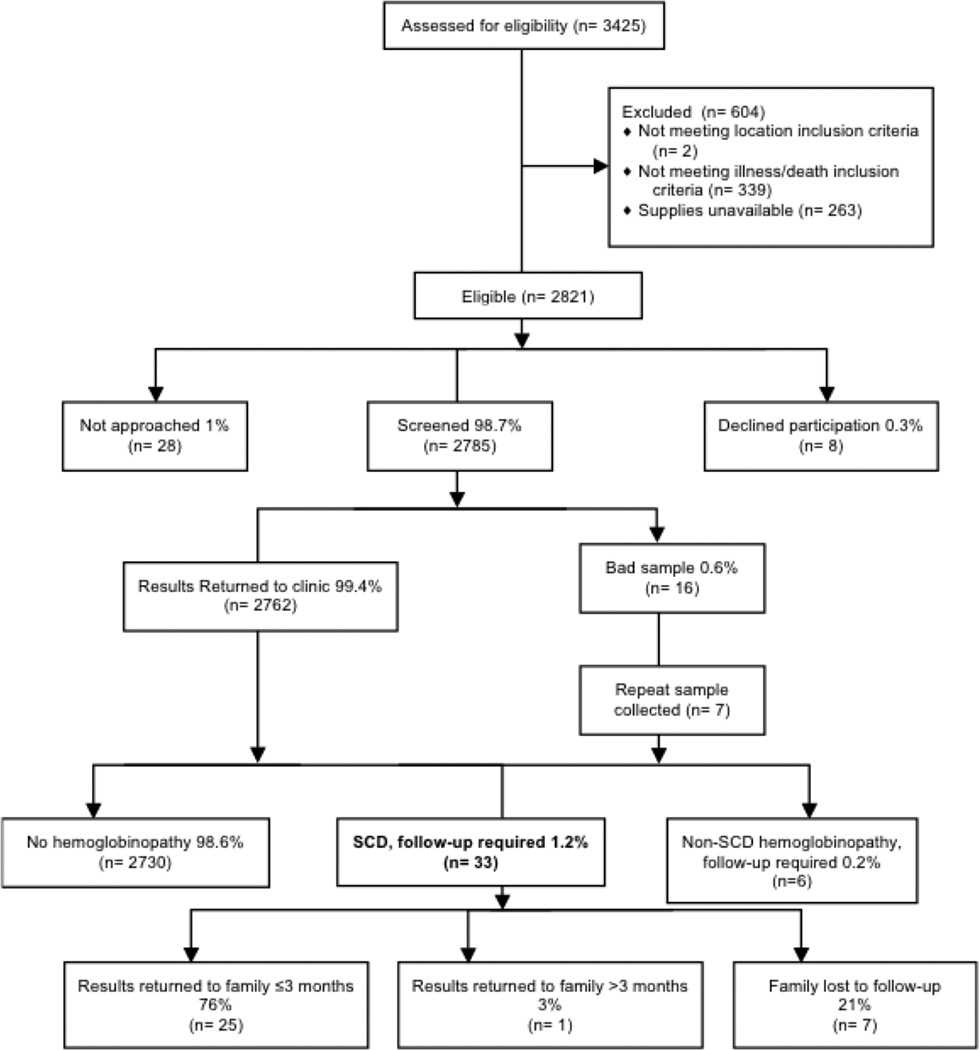
To determine the feasibility of newborn screening, all live births in the hospital during the study period were tracked and assessed for eligibility (Figure 1). Newborns born during a 3-week administrative hold due to a screening supply shortage were ineligible. During the study period, 2785 (98.7% of births) were eligible, consented, and screened for variant hemoglobins, and 99.4% of those had results returned to the clinic. Less than 1% of families declined participation. Sixteen samples were inadequate due to insufficient blood collection. Seven of those subjects provided a second sample leaving nine inadequate samples. Samples were shipped in batches and were received by the reference lab in an average 25 days (s.d. 14 days) and processed within average 4 days of receipt (s.d. 2 days). Results were processed at the reference lab and returned to the medical center as described above within average 12 days (s.d. 7 days) of being analyzed. Study staff successfully contacted all families of a child with SCD. Among subjects with SCD, 76% received results within three months during an initial clinic visit. One family did not return for results until the fourth month of life. Twenty-one percent of families did not return to clinic within six months and were considered lost to follow-up.
Figure 1. CONSORT diagram of newborns in Liberia screened for SCD.
Demographics of the Study Population
To describe the representativeness of our study population, ethnic groups as reported by mothers were tabulated and descriptively compared to the 2008 National Census (Supplement I). Paternal ethnic groups were excluded, though 30% of mothers reported the same ethnic group as the father (data not shown). The relative proportions of the largest ethnic groups in the study appeared to be similar to the national census.
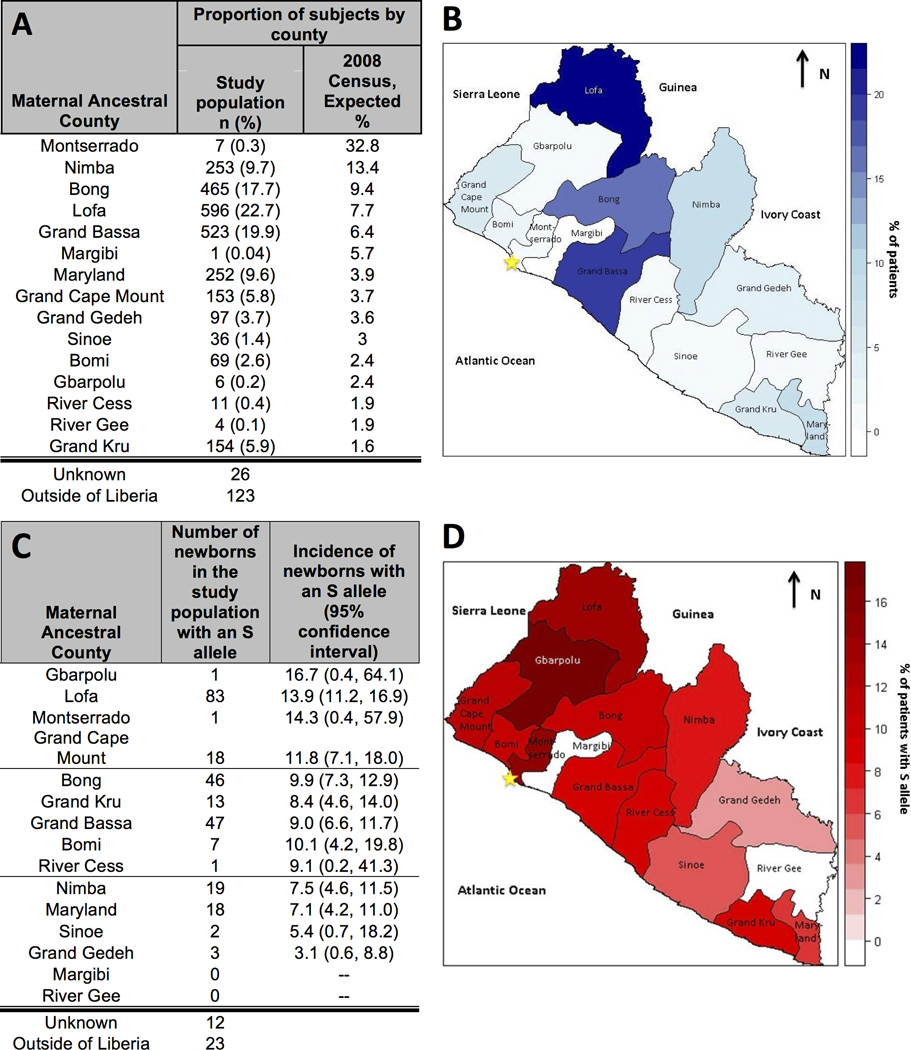
To estimate the effect of urban migration, maternal ancestral county was recorded. All counties were represented in the study population (Figure 2A, 2B). Four of the five largest counties in the national census are also the most frequently reported in the survey. Only seven subjects named Montserrado, the most populous county in Liberia, as their ancestral county, despite the fact that JFK-MC is located in Montserrado. Census data did not include non-Liberians, thus no comparator was available for the ‘outside of Liberia’ group.
Figure 2. Study Participants and Distribution of S Alleles by Ancestral County relative to the 2008 National Census.
The distribution of the maternal ancestral county in the study population versus the proportion that would have been expected had the study been representative of the 2008 national census (panel A). The blue geomap presents the distribution of the study population by county, from dark blue (counties with the highest proportion of the study population) to white (counties with the lowest proportion of the study population) (panel B). The observed number and proportions of newborns on this study with an S allele, excluding SCD patients (i.e., those with 2 alleles; panel C). The red geomap presents the distribution of the proportion of subjects with S alleles by county, from dark red (counties with the highest proportion of patients with an S allele) to white (counties with the lowest proportion of patients with an S allele) (panel D). A star marks the location of Monrovia (panels B, D).
Incidence of sickle cell trait and sickle cell disease in Monrovia
Based on IEF results, the incidence of sickle cell trait in Monrovia was 10.3% (Table I). Hemoglobin C trait was observed in 0.11% of screened newborns. IEF results consistent with SCD were observed in 1.2% (95% confidence interval: 0.8–1.6) of newborns. The most common phenotype of SCD was ‘FS’. The phenotype suggestive of compound heterozygous sickle/beta-+-thalassemia (“FSA”) was rarely observed, and no newborns with hemoglobin SC phenotype (“FSC”) were observed. One newborn that had a large portion of hemoglobin S and an indeterminate variant hemoglobin (FS“X”), was included in the SCD group. These observed data do not satisfy HWE (p<0.0001). However, in the beta-thalassemia allele model, if one additional silent beta-thalassemia or HPFH allele was present in our population with an allelic frequency of 2.4–8.2%, HWE is can be restored.
Table I.
The distribution of newborn screen results in the study population
| Phenotype* | Study population (n) |
Study population incidence (%) (95% CI) |
Historical Prevalence** (%) |
|---|---|---|---|
| Sickle Cell Trait (+/− alpha-thalassemia) | 287 | 10.3 (9.2–11.4) | 12.8 |
| FAS / AS | 274 | 9.9 | |
| FASB | 10 | 0.4 | |
| FASX | 1 | 0.04 | |
| FBAS | 1 | 0.04 | |
| ASF | 1 | 0.04 | |
| Hemoglobin C trait | 28 | 1.0 (0.6–1.4) | 0.6 |
| FAC / ACF | 28 | 1.0 | |
| Sickle Cell Disease | 33 | 1.2 (0.8–1.6) | 3.0 |
| FS§ | 28 | 1.0 | |
| FSA | 2 | 0.07 | |
| FSB | 2 | 0.07 | |
| FSX | 1 | 0.04 | |
| Non-SCD Hemoglobinopathy§ | 6 | 0.2 (0–0.4) | 0.6 |
| FC§ | 3 | 0.1 | |
| F§ | 3 | 0.1 | 0.6 |
| Normal Adult beta-globins | 2418 | 87.1 (85.9–88.4) | 82.3 |
| FA / AF / A | 2348 | 84.6 | |
| FAB | 70 | 2.5 | |
| Indeterminate variant hemoglobin | 4 | 0.1 (0–0.3) | 0.7 |
| FXA | 1 | 0.04 | |
| FAX | 3 | 0.1 | |
| Inadequate Samples without Retests | 9 | ||
| Total | 2785 | 100 |
Indeterminate variant hemoglobins are denoted by ‘X’.
The historical values are taken from Simbeye, 1979 [8]. Hemoglobin Barts was not reported in that study.
These results may represent homozygous (e.g. hemoglobin C disease, beta thalassemia major, HPFH) or heterozygous disorders with a silent allele.
Other hemoglobinopathies in Monrovia
Five additional samples with an indeterminate variant were observed, including one that likely represents an alpha-chain variant (‘FASX’) and was thus classified as sickle cell trait. (Table I) Other non-sickling hemoglobinopathies identified in the study population included ‘F’ and ‘FC’. Hb Bart’s, suggesting alpha-thalassemia trait, was present in 4% of subjects screened.
Distribution of S Alleles
To determine whether the distribution of S alleles in Monrovia by ancestry reflected the geographic distribution of alleles described in an earlier publication [6], S alleles in the study were mapped according to maternal ancestral county (Figure 2). The counties with the highest portion of subjects with an S allele were in the north and west of Liberia, including Lofa and Grand Cape Mount. Counties with the lowest portion of subjects with an S allele were in the south and east of the country. We observed very few subjects reporting four counties as their ancestral home: Montserrado, Margibi, River Gee, and Gbarpolu. This distribution of S alleles mirrors prior publications of national studies despite this study being done in one major city, consistent with urban migration.
DISCUSSION
By conducting a pilot newborn screening program at a single center in Monrovia and reporting results to 76% of subjects with SCD prior to three months of age, we established that newborn screening in Monrovia is feasible. This study exemplified a successful South-South partnership between Ghana and Liberia, which built capacity for providing care to patients with SCD in Liberia and expanded the reach of NMIMR [19]. An estimated 242,200 infants are born with SCD in sub-Saharan Africa annually [5], and the majority of those do not have access to newborn screening. Many countries lack laboratory facilities for newborn screening. We demonstrated how a regional partnership could be employed to expand access to newborn screening and facilitate acquisition of more current epidemiologic data. Future studies will explore approaches to increase the fraction of infants being brought back for care after newborn screening.
During the 30 years between the last prevalence study and our investigation, undoubtedly exacerbated by the conflict, the population of Monrovia increased 6-fold. Our demographic data reflect extensive migration that led to such dramatic population increase. For example, although all subjects reside in Montserrado county and thus would be included in Montserrado for census purposes, (Figure 2A) Montserrado was underrepresented as an ancestral county. Intermarriage over several generations has made identity by ethnic group genetically artificial, though many people associate culturally with specific ethnic groups through language.
Whereas malaria is holoendemic in Liberia, one might expect a uniform distribution of hemoglobin S alleles around the country. Thus, the historic finding of a geographical distribution of beta-thalassemia alleles and S alleles [7, 9], and the apparent persistence of this distribution by maternal ancestral county in our study population, is intriguing. In the Mediterranean, similar findings have been described and attributed to epistatic interactions which prevent the co-existence of hemoglobin S, beta thalassemia, and alpha thalassemias at high frequencies [20]. Similar interactions may be at play in Liberia, although our sampling method has some important limitations (Figure 2C) [9]. In our study, S alleles in subjects with sickle cell trait were assigned to mothers in the study, as fathers were not reliably available. Although the percentage of subjects with an S allele in Montserrado and Gbarpolu appear high and the percentage of subject from Margibi and River Gee appear low, the sample sizes from those counties was too small to provide accurate precision of an effect size. Family studies or a nationwide screening program are necessary to quantify this effect.
Surprisingly, our newborn screening observed an incidence of Hb S trait and SCD that appears to be lower than the prevalence reported in Monrovian school-aged children in 1979 (Table I) [8]. Given the reported high mortality of undiagnosed SCD, we expected the prevalence of Hb S trait and other asymptomatic hemoglobinopathies to be enriched in the prior reference population that included older children. This would be consistent with hypothesized survival advantage associated with being a carrier of these hemoglobinopathies. However, if the incidence underlying the 1979 prevalence study in school-aged children was the same as the incidence observed in our study of newborns (10.3%), then the higher prevalence in school-aged children (12.8%) would suggest an unexpected survival advantage for children with SCD in Monrovia. While incidence cannot be statistically compared to prevalence, a more reasonable inference of this unexpected outcome is that the incidence of SCD in Monrovia in 1979 was higher than it is in 2013.
Our data did not exhibit HWE, suggesting the presence of an additional unidentified beta globin allele, which provides a possible explanation for a change in incidence over time. Silent beta-thalassemia or HPFH alleles in Monrovia could explain this difference: if one additional silent allele was present in our population with an allelic frequency of between 2.4 and 8.2%, HWE could be restored. This is supported by historical data describing beta-thalassemia alleles in Liberia with a prevalence of 1–8% in various regions [9]. The non-sickling newborn screen hemoglobin phenotype of ‘F’ represents diagnoses of either beta-thalassemia major, homozygous hereditary persistence of fetal hemoglobin (HPFH), or heterozygous beta-zero thalassemia with HPFH, confirming that these silent alleles exist within the study population [21]. As children screened through this study age and as hemoglobin diagnostics become available locally, a confirmatory hemoglobin phenotype or globin genotyping should be obtained.
In Liberia, as in many other low-income nations in sub-Saharan Africa, SCD exacts a significant and underappreciated burden on the health care system. This pilot newborn screening program in one hospital over 13 months identified 36 newborns (SCD and other hemoglobinopathies) in need of preventative care, close health supervision, and advocacy. If these data are extrapolated nationwide using the 2013 crude birth rate and estimated population of 4.3 million, approximately 1700 Liberian infants are born annually with SCD and are not receiving treatment or preventive care [22]. A nationwide study is necessary to confirm the incidence of disease however, using available local and regional resources, we have generated up-to-date incidence data to inform public health interventions aimed at rebuilding Liberia’s health care system and reducing childhood mortality. If the WHO predictions for the role of SCD in childhood mortality are accurate, expanding capacity for newborn screening and preventative care will be important steps toward continued reduction of under 5 year mortality in Liberia and other sub-Saharan African nations.
Supplementary Material
Acknowledgements
This work was produced with support from the Thrasher Research Fund Early Career Award, NIH T32 HL007574, the Association of Public Health Laboratories, and the Sickle Cell Foundation of Ghana. Special recognition is due to the Department of Pediatrics at JFK-MC, Jill Falcone, Rays Jiang, PhD, and Ellis Neufeld, MD, PhD.
Footnotes
Contributors
VNT, RM, WJ, KOF, WBL, MMH conceived and designed the protocol. DG generated geomaps. WBL, DG, and CM performed the statistical analysis. All authors contributed to the writing of the manuscript.
Conflicts of interest
We declare that we have no conflicts of interest.
REFERENCES
- 1.World Health Organization. Sickle cell anaemia: Report by the Secretariat. Geneva. Fifty-ninth World Health Assembly WHA59.20.2006. [Google Scholar]
- 2.Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2011;41:S398–S405. doi: 10.1016/j.amepre.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King L, Knight-Madden J, Reid M. Newborn screening for sickle cell disease in Jamaica: a review - past, present and future. [Internet] West Indian Med J. 2014;63:147–150. doi: 10.7727/wimj.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10:e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingstone FB. The distribution of the sickle cell gene in Liberia. Am J Hum Genet. 1958;10:33–41. [PMC free article] [PubMed] [Google Scholar]
- 7.Willcox MC, Yekepa L, Airport RI. Thalassaemia in northern Liberia. A Mount Nimba area. J Med Genet. 1975;12:55–63. doi: 10.1136/jmg.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simbeye A. The Distribution of Haemoglobin S and Other Haemoglobin Variants in a Sample of Liberian Pediatric Subjects. East Afr Med J. 1979;56:223–225. [PubMed] [Google Scholar]
- 9.Willcox MC, Beckman L. Haemoglobin variants, beta-thalassaemia and G-6-PD types in Liberia. Hum Hered. 1981;31:339–347. doi: 10.1159/000153235. [DOI] [PubMed] [Google Scholar]
- 10.Rahimy MC, Gangbo A, Ahouignan G, Alihonou E. Newborn screening for sickle cell disease in the Republic of Benin. J Clin Path. 2009;62:46–48. doi: 10.1136/jcp.2008.059113. [DOI] [PubMed] [Google Scholar]
- 11.Tshilolo L, Kafando E, Sawadogo M, Cotton F, Vertongen F, Ferster A, Gulbis B. Neonatal screening and clinical care programmes for sickle cell disorders in sub-Saharan Africa: lessons from pilot studies. Public Health. 2008;122:933–941. doi: 10.1016/j.puhe.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Kafando E, Nacoulma E, Ouattara Y, Ayéroué J, Cotton F, Sawadogo M, Gulbis B. Neonatal haemoglobinopathy screening in Burkina Faso. J Clin Path. 2009;62:39–41. doi: 10.1136/jcp.2008.058966. [DOI] [PubMed] [Google Scholar]
- 13.Norwegian Refugee Council/Global IDP Project. Profile of Internal Displacement : Liberia 2005. [Accessed March 12, 2014];Internal Displacement Monitoring Centre. http://www.internal-displacement.org/sub-saharan-africa/liberia/2005/profile-of-internal-displacement-liberia-august-2005. Published August 2005.
- 14.Liberia Institute of Statistics and Geo-Information Services. Republic of Liberia 2008 Population and Housing Census: Final Results. 2009 [Google Scholar]
- 15.Campbell PR. Country Demographic Profiles: Liberia. Washington, DC: U.S. Department of Commerce; 1982. [Google Scholar]
- 16.Roa PD, Turner EA, Aguinaga M del P. Hemoglobin variant detection from dried blood specimens by high performance liquid chromatography. Ann Clin Lab Sci. 1993;23:433–438. [PubMed] [Google Scholar]
- 17.Murray C, Hall SK, Griffiths P. An evaluation of the Sebia Capillarys Neonat Haemoglobin FAST™ system for routine newborn screening for sickle cell disease. Int J Lab Hematol. 2011;33:533–539. doi: 10.1111/j.1751-553X.2011.01315.x. [DOI] [PubMed] [Google Scholar]
- 18.Liberia Institute of Statistics and Geo-Information Services (LISGIS) Government of the Republic of Liberia 2008 National Population and Housing Census 2008. Monrovia: LISGIS; 2011. [Google Scholar]
- 19.Tubman VN, Archer NM. Building partnerships to target sickle cell anemia in Africa. Am J Hematol. 2013;88:983. doi: 10.1002/ajh.23602. [DOI] [PubMed] [Google Scholar]
- 20.Penman BS, Gupta S, Buckee CO. The emergence and maintenance of sickle cell hotspots in the Mediterranean. Infect Genet Evol. 2012;12:1543–1550. doi: 10.1016/j.meegid.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heeney MM, Delgrosso K, Robinson R, Johnson CA, Daeschner CW, Campbell TA, Surrey S, Ware RE. Interpretation of fetal hemoglobin only on newborn screening for hemoglobinopathy. J Pediatr Hematol Oncol. 2002;24:499–502. doi: 10.1097/00043426-200208000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Liberia Institute of Statistics and Geo-Information Services (LISGIS) Liberia Demographic and Health Survey 2013. Monrovia: LISGIS; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.