Abstract
Orofacial clefting is a common birth defect with significant morbidity. A panoply of candidate genes have been discovered through synergy of animal models and human genetics. Among these, variants in Interferon Regulatory Factor 6 (IRF6) cause syndromic orofacial clefting and contribute risk toward isolated cleft lip and palate (1/700 live births). Rare variants in IRF6 can lead to Van der Woude Syndrome (1/35,000 live births) and Popliteal Pterygium Syndrome (1/300,000 live births). Furthermore, IRF6 regulates GRHL3 and rare variants in this downstream target can also lead to Van der Woude Syndrome. In addition, a common variant (rs642961) in the IRF6 locus is found in 30% of the world’s population and contributes risk for isolated orofacial clefting. Biochemical studies revealed that rs642961 abrogates one of four AP-2alpha binding sites. Like IRF6 and GRHL3, rare variants in TFAP2A can also lead to syndromic orofacial clefting with lip pits (Branchio-oculo-facial Syndrome). The literature suggests that AP-2alpha, IRF6 and GRHL3 are part of a pathway that is essential for lip and palate development. In addition to updating the pathways, players and pursuits, this review will highlight some of the current questions in the study of orofacial clefting.
Keywords: IRF6, TFAP2A, GRHL3, Van der Woude Syndrome, Popliteal Pterygium Syndrome, Branchio-Oculo-Facial Syndrome, Cleft lip and palate, embryonic development, mouse models
Historical Introduction
According to high-resolution imaging of Egyptian mummies, orofacial clefts have been a part of the human condition for over 3000 years (Hoffman and Hudgins, 2002). In 1845, Demarquay described orofacial clefts and lip pits as a “very rare” congenital malformation (as cited by Cervenka et al. (Cervenka J, 1967). Lip pits, or indentations, occurring with cleft lip and palate were subsequently described in five generations of an affected family (Test and Falls, 1947). Van der Woude reviewed the literature in 1954 and concluded that this entity traversed generations in an autosomal dominant pattern (Van Der Woude, 1954). As a result of this study, lip pits along with orofacial clefting became known as Van der Woude Syndrome (VWS OMIM #119300). In 1962, Levy highlighted the presence of symmetrically located lip pits on the medial edge of lower lips, now a nearly pathognomonic feature of VWS or “Lip Pit Syndrome” (Levy, 1962). The earliest recorded survey of VWS prevalence among all forms of cleft lip and palate took place in 1971 when Dronamraju reported that eight of 260 clefting families (3%) had VWS (Dronamraju, 1971). The incidence of VWS has been directly estimated at 3.6/100,000 live births (Burdick, 1986). Today, VWS is recognized as the most common syndromic orofacial clefting disorder.
Current clinical context and impact of orofacial clefting
Orofacial clefting results from defective palate and lip closure between the 6th and 12th week of human gestation. Current standard of care for children born with a cleft lip and plate includes surgical closure of a cleft lip by 4 months and closure of the palate by 12 months. Closure of alveolar clefts with bone grafts should be complete by 11 years of age, correction of residual abnormalities by 12 years of age and final nasal contours and treatment of breathing problems by 17 years of age. In addition to these surgeries, children born with CLP need to undergo speech therapy until the age of 11. According to the American Society of Plastic Surgeons, children born with CLP require a multi-disciplinary team to receive appropriate care. This team includes a pediatrician, pediatric dentist, otolaryngologist, auditory specialist, speech pathologist, genetic counselor and a social worker. Costs include surgical procedure, nursing, hospital stay, anesthesia, medication, devices and clinical tests. In total, each individual born with a cleft lip and/or palate (CLP) will require $200,000 for medical treatment (Wehby and Cassell, 2010). However, this extensive treatment regimen is not sufficient to prevent physical, psychological, social and neurological sequelae (Nopoulos et al., 2007b; Manna et al., 2009).
Despite interdisciplinary medical treatment and the enormous cost, there are numerous complications. These commonly include bleeding, infection, irregular healing of scars and puckering of tissues (contractures), asymmetries, remaining deformities, anesthesia risks, allergies to suture material and glue, damage to deep structures, such as blood vessels, nerves and muscles, and the possibility of surgery revision. In addition, changes in nose shape and teeth alignment may result after or from cleft repair. Teeth abnormalities associated with CLP may require additional repair (Aizenbud et al., 2011). Finally, because the mouth and palate are integral tissues, CLP morbidity also includes poor feeding, growth retardation and repeated ear infections.
Considering the complications and the number of healthcare providers needed for treatment, there has been a shift of CLP repair from the primary care office to teaching hospitals and an associated increase in cost (Basseri et al., 2011). While ongoing clinical investigation in CLP treatment has led to a dramatic decrease in the associated morbidity (Mulliken, 2004), the challenges are even greater for developing countries (Furr et al., 2010). In addition, individuals born with a CLP have an increased risk for cancer (Taioli et al., 2010) and neurological ((Shriver et al., 2006; Boes et al., 2007; Calzolari et al., 2007; Nopoulos et al., 2007b; Conrad et al., 2008; Conrad et al., 2009; Conrad et al., 2010; Nopoulos et al., 2010; Rosen et al., 2011), musculoskeletal and cardiovascular diseases (Calzolari et al., 2007), surgical complications (Jones et al., 2010) and an increased risk of mortality between birth and 55 years of age (Christensen et al., 2004). However, we already know that syndromic clefting is responsive to in utero stimuli. For example, reduced maternal folate, alcohol consumption and maternal smoking increase clefting risk (Wu et al., 2010; Blanton et al., 2011). It follows that if the phenotype can be exacerbated, it can be ameliorated. These challenges highlight the emphasis on prevention, rather than treatment of CLP.
Variability of VWS suggests a personalized diagnostic and therapeutic intervention for iCLP
Variable expressivity and incomplete penetrance in VWS suggests unidentified genetic and/or environmental modifiers for isolated cleft lip and palate (iCLP, OMIM # 119530). Analysis of a single large VWS pedigree with multiple affected individuals showed the presence of lip pits with or without clefting, suggesting variable expressivity of a single rare variant (Baker, 1964). Janku et al. reported a penetrance of 96.7%, with lip pits present in 88% of affected individuals and clefting in 21% (Janku et al., 1980). Burdick et al. examined 864 individuals from 164 families and found that cleft lip and palate occurred more commonly than isolated cleft palate (Burdick et al., 1985). VWS is also associated with hypodontia, bifid uvula, hypernasal voice, lip mounds that secrete mucus, Hirshsprung disease, congenital heart defects, popliteal webs, limb anomalies and accessory nipples (Van Der Woude, 1954; Janku et al., 1980; Shprintzen et al., 1980; Nopoulos et al., 2007a; Nopoulos et al., 2007b). However, these associated anomalies are rare and 12–15% of individuals with VWS will present with orofacial clefting as an isolated clinical finding. As such, these individuals are phenotypically indistinguishable from the clinical diagnosis of isolated cleft lip and palate (iCLP). However, in contrast to the genetically simple, ‘one gene, one phenotype’ model of syndromes, iCLP is a common, complex disease with multiple genetic and environmental factors (Cobourne, 2004). Considering the phenotypic and clinical overlap, Murray et al. hypothesized that discovering the etiology of VWS would provide insights into iCLP (Murray et al., 1990).
While the phenotype suggests VWS can be a clinical model for iCLP, is there evidence that VWS can be a genetic model for iCLP? As a Mendelian disorder, familial studies are a critical component in VWS research (Cervenka et al., 1967; Vignale et al., 1998; Kondo et al., 2002). Houdayer et al. (2001) used a family design to ask if VWS and iCLP were associated in a parametric linkage analysis and transmission disequilibrium tests (TDT) (Houdayer et al., 2001). Although parametric linkage was not supportive, TDT provided evidence for a genetic link between VWS and iCLP (Houdayer et al., 2001). TDT measures the over-transmitted allele from parents to affected offspring and as such is robust to population structure, e.g., population stratification. However, there is no correlation between sex and phenotype in VWS, whereas males with iCLP tend to be more severely affected than females (Burdick et al., 1985; Calzolari et al., 2007; Huang et al., 2007).
Variable expressivity in VWS suggests that genetic and environmental modifiers could be leveraged to reduce disease burden. Known phenotypic modifiers within the VWS spectrum include locus heterogeneity (Peyrard-Janvid et al., 2014), different types (mis-sense vs. truncation) (de Lima et al., 2009) and location of mutations (DNA Binding Domain vs. Protein Interaction Domain vs. Activation Domain) (Knight et al., 2006) and regulation of gene expression (enhancer, promoter) (Fakhouri et al., 2014). Significantly, a search for genetic modifiers using common variants at candidate loci did not yield a formally significant association (Leslie et al., 2013). However, prior work on a single pedigree identified a genetic modifier at 17p11.2–11.1 (Sertie et al., 1999). This suggests that a personalized, ‘all of the above approach’, will be necessary to map the road(s) to pathology. In contrast, at the cellular and biochemical levels, little is known about how IRF6 protein location (e.g. sequestration in sub-cellular organelle and exocytosis), activity and stability (e.g. resistance to degradation/turnover) contributes to the clinical spectrum of VWS.
Mutations in IRF6 cause VWS and PPS
The first study into the etiology of VWS used the “red blood cell antigen” for genetic linkage as well as several biochemical assays, including electrophoretic studies of glucose-6-phosphate-dehydrogenase, haptoglobin, phosphoglucomutase, and hemoglobin (Schneider, 1973). An interstitial deletion of chromosome 1 at q32-q41 in a 41-month-old girl with lip pits refined the VWS locus (Bocian and Walker, 1987). A candidate-gene-and-region approach successfully identified linkage with the renin gene and the D1S65 locus in 1q using restriction fragment length polymorphisms (Murray et al., 1990). A microdeletion refined the VWS locus to 4.1 mega base pairs within 1q32-q41 (Sander et al., 1994). Cloning of the critical region allowed the production of a single YAC clone with an 850 kb segment containing the microdeletion and later a 900 kb gene map (Schutte et al., 1996; Schutte et al., 2000).
Several important twin studies have contributed to our understanding of VWS (Neuman and Shulman, 1961). Dizygotic twins discordant for VWS were described by Levy et al. (Levy, 1962). Cervenka et al. (1967) characterized the first published description of twins with lip pits, who were then identified as “probably monozygotic” based on facial features and blood typing (Cervenka et al., 1967). However, only one had a unilateral cleft lip. Monozygotic twins who are concordant for VWS were reported 30 years later when Hersh and Verdi (1992) showed siblings with unilateral cleft lip and palate, and lip pits (Hersh and Verdi, 1992). Currently, four monozygotic twins concordant for VWS have been reported (Tokat et al., 2005; Jobling et al., 2011). One of these reported on monozygotic twins who had VWS with varying levels of disease severity; i.e. one only had lip pits while the other had lip pits, a cleft lip and a cleft palate (Jobling et al., 2011). These findings are significant because they suggest monochorionic, diamniotic twins with VWS can have variable expressivity as a result of somatic mutations in IRF6, variants in the interacting genetic network, placentation or stochastic features.
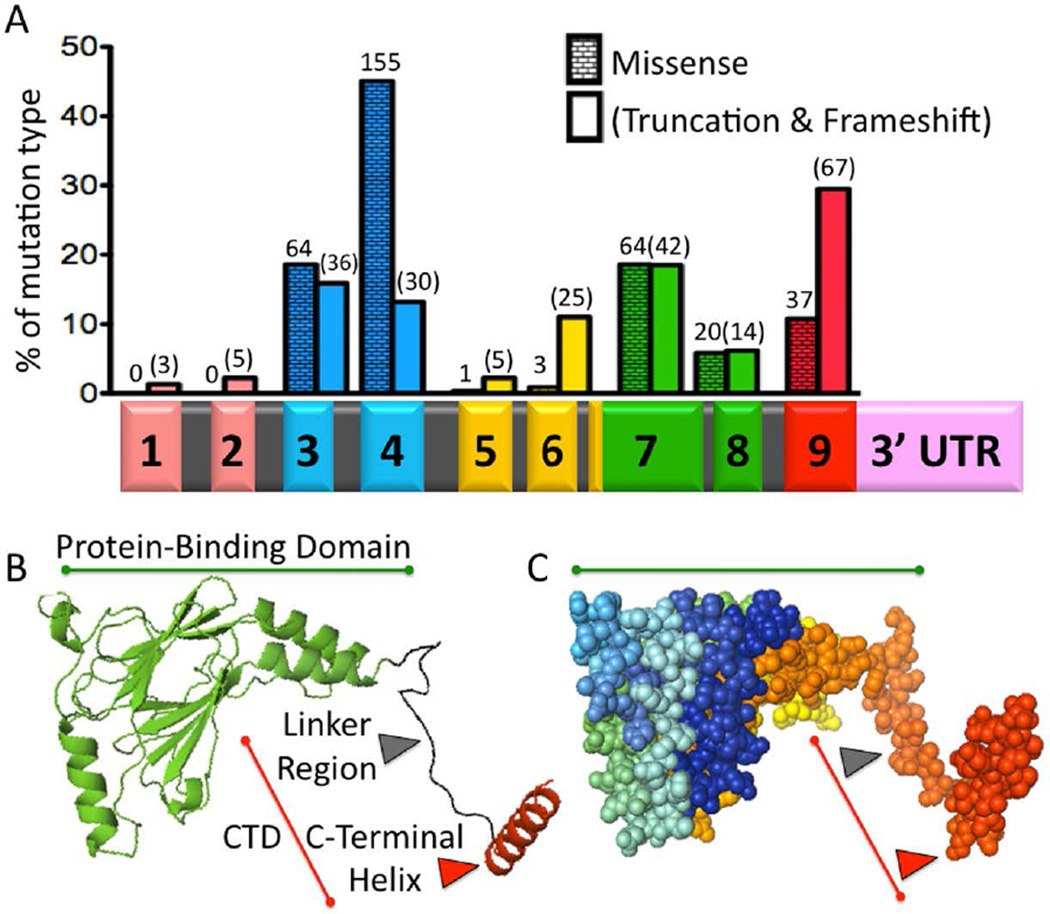
Nearly 150 years after Demarquay first described a clefting syndrome with lip pits, Kondo et al. (2002) used discordant monozygotic twins to discover Interferon Regulatory Factor 6 (IRF6) as the VWS gene (Kondo et al., 2002). Aided by advancing technology and a genetic map, targeted sequencing showed an IRF6 mutation in the affected individual but not the unaffected sibling. Prevalence screening then showed that 68% of families with VWS had a mutation in IRF6 (de Lima et al., 2009) (Fig. 1). With that discovery, the authors turned their attention to popliteal pterygium syndrome (PPS OMIM #119500), a rarer form of syndromic clefting that had been reported in VWS pedigrees. PPS, like VWS, can include orofacial clefting, lip pits, hypodontia and skin anomalies. However, PPS is a more severe phenotype because it also includes webbing in the back of the knee (popliteal fossa), genital anomalies (hypoplasia of the labia majora, cryptorchidism or bifid scrotum), webbing between toes or fingers (syndactyly), triangular folds of skin over nails and tissue connecting the upper and lower eyelids (ankyloblepharon) (Bixler et al., 1973; Escobar and Weaver, 1978; Hammer et al., 1989). Furthermore, prior analysis of three families with PPS had already shown linkage to the Van der Woude Syndrome locus, at 1q32-q41 (Lees et al., 1999; Wong and Gustafsson, 2000). With linkage and phenotypic similarity, sequencing by Kondo et al. (2002) revealed that all 13 families with PPS had an IRF6 mutation (Kondo et al., 2002). In contrast to VWS, more severely affected individuals who were diagnosed with PPS had a preponderance of single nucleotide substitution in exons 3 or 4, the DNA binding domain (Fig. 1) (Kondo et al., 2002; Leslie et al., 2012).
Figure 1. Syndromic Mutations and the Structure of IRF6.
A) IRF6 Mutations in VWS and PPS. Histogram showing the percent of either missense (running bond pattern) or truncation/frameshift (blank pattern) mutations contained in each exon. Number of probands is shown above each bar, for a total of 571 (Leslie et al., 2012; Leslie et al., 2015). Beneath the histogram is the IRF6 primary transcript with a bar graph color code to indicate functional protein regions. Exons 1 and 2 (peach color) constitute the 5’ Untranslated Region. Exons 3 and 4 are the DNA binding domain (blue). Exons 5 and 6 are less conserved (yellow). The majority of exon 7 and all of exon 8 are the Protein-Binding Domain (also known as the Interferon Association Domain) (green). Exon 9 contains the C-Terminal Domain (CTD) (red). The 3’Untranslated Region is also illustrated (pink). Introns are not drawn to scale. B) Protein Structure. A cartoon of IRF6 Protein-Binding Domain (PBD) (green) and C-Terminal Domain (CTD) (red), based on the structure of IRF5. The PBD consists of two beta-pleated sheets that form a central pore and are surrounded by three helices. The CTD consists of the Linker Region (black) and the C-Terminal Helix (red), which controls dimerization and activation/repression of IRF5. C) A sphere model of IRF6 in the same orientation as the cartoon model. The PBD is highlighted with a green line, while the CTD is highlighted with a red line. Different regions of the protein are highlighted using a spectrum to label the carbon backbone. Both the cartoon and sphere models were created using PyMol.
Since discovery of IRF6, case reports and case series from a wide geographical distribution have expanded the phenotypic spectrum of VWS (Kayano et al., 2003; Kim et al., 2003; Shotelersuk et al., 2003; Wang et al., 2003; Gatta et al., 2004; Ghassibe et al., 2004; Matsuzawa et al., 2004; Ghassibe et al., 2005; Item et al., 2005; Wang et al., 2005; Ye et al., 2005; Du et al., 2006a; Du et al., 2006b; Brosch et al., 2007; Paranaiba et al., 2008; Tan et al., 2008; de Lima et al., 2009; Yeetong et al., 2009; Malik et al., 2010; Scioletti et al., 2010; Birkeland et al., 2011; Minones-Suarez et al., 2011; Salahshourifar et al., 2011). While most studies of VWS have identified point mutations in IRF6, deletions as large as 2.98 Mb, involving 25 genes, have also been reported (Sander et al., 1994; Schutte et al., 1999; Salahshourifar et al., 2011). Considering that genomic deletions and premature stop codons can lead to VWS, the most likely etiology is genetic haplosufficiency. More severe phenotypes are associated with large deletions relative to point mutations, which suggests that additional genes within this locus are interacting with IRF6. de Lima et al. (2009) conducted a comprehensive study of IRF6 mutations leading to VWS and found that while truncating mutations occurred throughout the open reading frame, point mutations are significantly over-represented in exons 3, 4, 7 and 8 (de Lima et al., 2009). From this, the authors concluded that exons 3 and 4 (DNA binding domain) and exons 7 and 8 (protein binding domain) coded for the functional machinery of the protein (Fig. 1). Mutations leading to PPS were more frequently found at residues predicted to bind DNA within exons 3 and 4 (discussed in “IRF6 structure-function in development and disease”). While this genotype-phenotype correlation was strong, it was not absolute because a single IRF6 mutation can be associated with VWS or PPS within a family.
For families with VWS who did not have a mutation in IRF6, recent efforts have focused on finding targets of IRF6. In that regard, Grhl3 was found to be downstream of IRF6 in zebrafish superficial epithelium and murine epidermis (de la Garza et al., 2013). Furthermore, prior analysis in a large Finnish family with VWS showed linkage to 1p34-p36, which contains the GRHL3 locus (Koillinen et al., 2001). Exome sequencing in this family and targeted sequencing in others showed that 5% of families with VWS have mutations in GRHL3. Despite sequencing the open reading frames of both IRF6 and GRHL3, the etiology of VWS is unknown in the remaining 27% of affected families. Mutations in regulatory regions (Fakhouri et al., 2014), additional loci and/or combinations of mutations might be contributing to pathology in remaining families.
Variants within IRF6 are associated with iCLP
Given that 10–15% of VWS appears as isolated Cleft Lip and Palate (iCLP) and that mutations in IRF6 lead to VWS, it was predicted that common variants in IRF6 could be contributing to the multifactorial risk of isolated, or non-syndromic orofacial clefting. Consistent with this rationale, three recent studies found a strong association between IRF6 and isolated orofacial clefting (Zucchero et al., 2004; Rahimov et al., 2008; Beaty et al., 2010). The first, by Zucchero et al. (2004), showed that a non-synonymous substitution (V274I) within IRF6 is associated with 12% of all orofacial clefting (Zucchero et al., 2004). Considering that clefting can be lethal in non-human primates and the ancestral allele confers risk, the association seemed counter-intuitive. However, despite the change in amino acid sequence, a substitution from valine to isoleucine is fairly conservative, i.e. is not predicted to alter protein structure/function. Instead, the authors predicted the ancestral allele was in linkage disequilibrium (LD) with the etiologic variant, i.e., the ancestral allele is on the same haplotype block as a disease predisposing, derived variant.
Consistent with this hypothesis, sequencing of highly conserved regions within the LD block that contains IRF6 (140 kb in length) revealed an association to a non-coding variant (rs642961) 9.7 kb upstream of the IRF6 transcription start site (Rahimov et al., 2008). Importantly, the disease-associated allele at rs642961 is derived. Furthermore, rs642961 lies within a 608 bp sequence (MCS9.7) that is highly conserved and has enhancer activity that recapitulates endogenous IRF6 expression in vivo (Fakhouri et al., 2012) (functional significance in “Transcriptional regulation of IRF6 expression”). Importantly, rs642961 is also associated with the severity of iCLP (Kerameddin et al., 2015). While maternal and environmental factors did not modify the risk at rs642961, prenatal multi-vitamin supplementation reduces the risk of orofacial clefting for individuals carrying two additional IRF6 variants (rs2076153 and rs17015218) (Wu et al., 2010). These data further support the role of IRF6 in iCLP and suggest that personalized intervention is on the horizon. With identification, genetic and environmental factors that alter IRF6 expression or function may be leveraged to alter disease penetrance and/or expressivity in VWS and iCLP.
Murine Irf6 alleles
Murine alleles have provided a wealth of information about the function of IRF6. A gene trap allele (Irfgt/+), inserted 36 base pairs into intron 1, has several splice donor/acceptor sites and a stop codon that prematurely terminates IRF6 translation (Ingraham et al., 2006). Another allele (R84C) was made by targeted insertion of a human IRF6 mutation that disrupts the DNA binding domain and leads to PPS (IrfR84C/+) (Richardson et al., 2006). A more recent allele (Irf6clft1/+) resulted from a forward genetic screen using N-ethyl-N-Nitrosourea (ENU) mutagenesis (Stottmann et al., 2010). Interestingly, affected embryos had a mutation at proline-39, which was previously reported in a VWS pedigree (Kondo et al., 2002). With both deletions and human mutations, currently available murine models provide both fidelity and facility for understanding and intervening in this network.
The murine phenotypic spectrum includes cleft palate, microcephaly, tongue and mandibular defects, clubbed limbs, syndactyly, a bifid xiphoid and a shortened fused tail (Ingraham et al., 2006; Richardson et al., 2006; Boell et al., 2013; Goudy et al., 2013). Considering the expression pattern, affect in knockout mice and role in palatal development, the role of IRF6 in epithelium is a focus of many ongoing studies. Epithelial abnormalities include a hyperproliferative epidermis that fails to differentiate, a permeable skin barrier, esophageal adhesions and pervasive oral adhesions. Intraoral adhesions prevent palatal elevation and result in a cleft palate. It is currently unclear whether the pathological affects of adhesions are physical, e.g. restraining the shelves, and/or biological, e.g. downstream signaling. Loss of IRF6 also leads to evagination of tooth epithelium while a hypomorphic allele does not (Blackburn et al., 2012). Finally, over-expressing IRF6 using the Krt14 promoter leads to absence of the skull and an open eye in 22% of embryos but rescues palatal defects caused by loss of Tgfbr2 signaling (Iwata et al., 2013).
IRF6 structure-function in development and disease
Interferon Regulatory Factor 6 is a member of the IRF family of transcription factors, which share a high degree of sequence identity and have a common helix-turn-helix DNA binding motif (Taniguchi et al., 2001). The IRF family plays an important role in host defense by regulating the innate and/or adaptive immune systems (Honda and Taniguchi, 2006). In contrast, Irf6 regulates embryonic development, including orofacial, skin, limb, tongue and brain morphogenesis (Ingraham et al., 2006; Biggs et al., 2011; Aerts et al., 2013; Goudy et al., 2013). IRF6 is composed of nine exons, with a start codon in exon 3 and a stop codon in exon 9 (Fig. 1) (Bailey et al., 2005). Kondo et al. (2002) detected two Irf6 transcripts from whole mouse embryos from E4.5 to E18.5 (Kondo et al., 2002). The smaller transcript (4.4 kb) is most common and includes an unspliced intron in the 3’UTR. The size and significance of a larger transcript is currently unknown. IRF6 expression was found in the brain, eyes, heart, liver, lung, placenta, skin, testes and tongue but not the spleen (Kondo et al., 2002). IRF6 is made up of 467 amino acids and Western blotting showed a band at 59 kDa (Bailey et al., 2005; Knight et al., 2006). Phosphorylation is required to activate IRF6 in cell culture and this form of the protein has been detected in murine mammary epithelium (Bailey et al., 2005; Bailey et al., 2009). Phosphorylation results in a second western blot band at 63 kDa (Bailey et al., 2005). Based on sequence identity and structure of IRF5 (Chen et al., 2008), IRF6 likely forms a dimer to function.
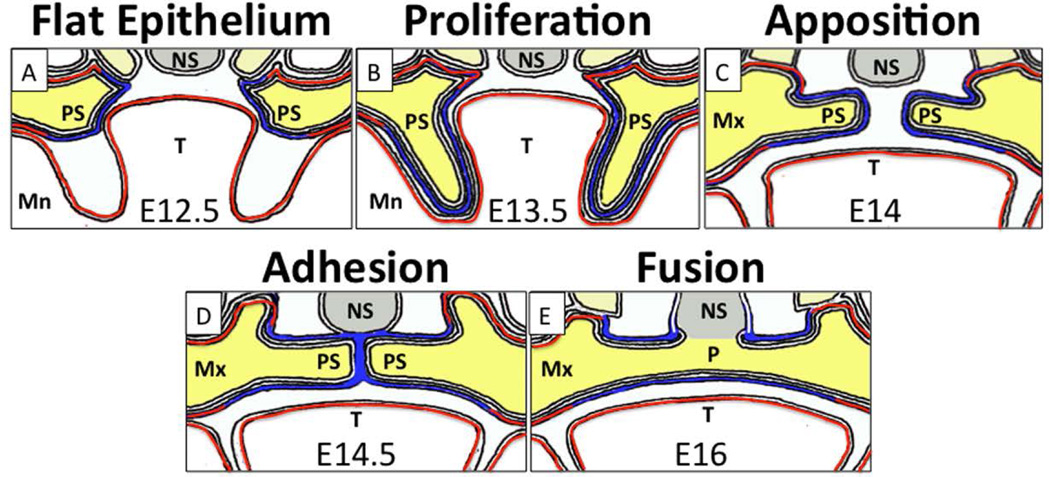
Consistent with human development, IRF6 expression is seen in murine oral epithelium (Fig. 2). In palatal epithelium, IRF6 expression is observed in both periderm and basal cells from E12.5 to E17.5 (Fig. 2). Periderm, marked by intermediate filaments KRT6 and KRT17, is a flat, squamous monolayer that coats the entire embryo. Periderm prevents pathological interepithelial adhesions between adjacent structures, including the palate, tongue and mandible (Richardson et al., 2014). Basal cells are cuboidal and are marked by intermediate filaments KRT5 and KRT14. Basal cells give rise to and anchor the periderm to the basal lamina (Richardson et al., 2009). At E12.5, palatal shelves start as mesenchymal buds covered by periderm and basal cells. During the next 24 hours, the palatal shelves, including both the mesenchyme and epithelium, proliferate and expand, to inhabit the space between the tongue and mandible bilaterally. Loss of IRF6 arrests palatal development starting here, with the shelves wedged between the tongue and mandible until birth (Richardson et al., 2009). At E13.5, the palatal shelves elevate and pivot toward midline, ultimately apposing above the tongue. By E14.5, periderm cells are lost along the medial surface of the palatal shelves. Periderm cells that lack IRF6 have abnormal morphology and expression of KRT6 and KRT17 (Richardson et al., 2009; Peyrard-Janvid et al., 2014). At E14.5, the underlying basal cells adhere to each other and interdigitate to form the medial edge seam. Dissolution of this seam, in part via cell death, is paramount in fusion of palatal shelves. Without IRF6, basal cells continuously proliferate and express KRT14 (Ingraham et al., 2006). Between E15.5 and E17.5, the palate forms when a complete mesenchymal bridge separates the nasal and oral cavities (Knight et al., 2006). Irf6 expression is also seen at the fusion point of the lateral and medial nasal processes and the maxillary processes, which fuse at E11.5 to form the upper lip (Knight et al., 2006). Loss of IRF6 in the mouse is not associated with a cleft lip (Ingraham et al., 2006; Richardson et al., 2006).
Figure 2. IRF6 expression during stages of palatal development.
A) Palatal development begins at E12.5 with a flat palatal epithelium (blue) that expresses Irf6, and an underlying mesenchyme (yellow). B) A period of rapid proliferation leads to formation of palatal shelves alongside the tongue (T) and mandible (Mn). Irf6 is also expressed in mandibular, lingual and maxillary epithelium (red). C) Reorientation of the palatal shelves leads to a midline pivot and a horizontal suspension above the tongue. D) Apposition of the palatal shelves, followed by loss of periderm, allows adhesion, or interdigitation of the epithelial cells to produce the medial edge seam. Irf6 is expressed in both periderm and basal cells and is required for their function. E) Dissolution of the medial edge seam, which is composed of basal cells, leads to fusion of the shelves. After fusion, a mesenchymal bridge separates the nasal cavity from the oral cavity. Mn: mandible; Mx: Maxilla; NS: nasal septum; P: palate; PS: palatal shelves; T: tongue.
Based on the crystal structure of IRF1, IRF6 appears to contain a highly conserved penta-tryptophan winged-helix-loop-helix DNA binding domain in exons 3 and 4 (Kondo et al., 2002; Little et al., 2009). IRF6 is structurally characterized as transcription factor but it is mainly detected in the cytoplasm and rarely visualized in the nucleus with various antibodies. However, several lines of evidence suggest that IRF6 binds DNA and transcriptionally regulates gene expression in critical developmental pathways. First, injection of cDNA containing the IRF6 DNA binding domain (dominant negative construct) leads to more severe developmental defects than knocking down the transcript with a morpholino in zebrafish and xenopus embryos (Sabel et al., 2009; de la Garza et al., 2013). In humans, a mutation in the DNA binding domain, R84C, is associated with more severe developmental defects, including Popliteal Pterygium Syndrome (de Lima et al., 2009). Furthermore, R84C heterozygous embryos have more pervasive oral adhesions than embryos heterozygous for a gene trap (null) allele (Ingraham et al., 2006; Richardson et al., 2006). Biochemically, R84C appears to reduce IRF6 DNA-binding affinity (Little et al., 2009) with a concomitant reduction in transactivation of a luciferase reporter (Su, 2011). Together, these data suggest that R84C leads to gain-of-function during dimerization (protein-protein interaction) by sequestering protein from the other allele. Furthermore, these data suggest that transcriptional activity by IRF6 is fundamental for function.
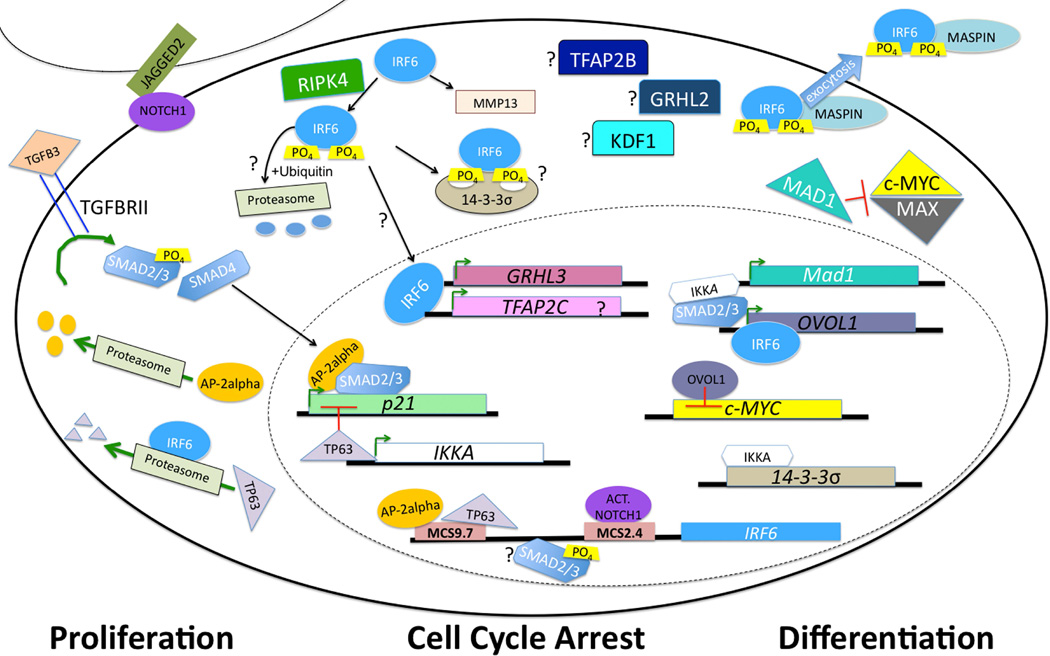
In support of these data, Irf6 transcriptionally regulates grhl3 via a highly conserved binding element. Furthermore, grhl3 mRNA partially rescues zebrafish embryos injected with a dominant negative Irf6 (de la Garza et al., 2013) (Fig. 3). In primary human keratinocytes, a genome wide screen showed that IRF6 binds within this highly conserved GRHL3 element and that knocking down IRF6 leads to a reduction of GRHL3 expression (Botti et al., 2011). During palatal development, IRF6 is required for GRHL3 expression in the epithelium and oral periderm (de la Garza et al., 2013). Like Irf6, loss of Grhl3 leads to bilateral oral adhesions and palatal clefting (Peyrard-Janvid et al., 2014). IRF6 also transcriptionally regulates OVOL1, a transcription factor regulating epithelial differentiation and a repressor of the oncogenic protein c-MYC (Nair et al., 2006; Botti et al., 2011). Importantly, the MYC locus (8q24) is a major factor in nonsyndromic cleft lip and palate (Birnbaum et al., 2009). Together, these data suggest that IRF6 transcriptionally regulates several epithelial factors.
Figure 3. An Orofacial Gene Regulatory Network.
IRF6 regulates the switch between proliferation and differentiation, and loss of function mutations lead to orofacial clefting. Current data support interaction with TGFB, NOTCH and OVOL1, among other factors. However, many questions remain, including how IRF6 is targeted to the proteasome, whether it regulates TFAP2A, TFAP2B or GRHL2, and where KDF1 fits in this pathway.
However, perhaps the predominantly cytoplasmic expression pattern of IRF6 suggests a limited number of transcriptional targets. An illustrative comparison can be made with TP63, which is co-expressed with IRF6 throughout epithelial development and almost exclusively localized to the nucleus. TP63 binds over 7,500 genes and 1,213 (16%) of these targets are differentially expressed with knockdown studies (McDade et al., 2012). In contrast, a genome-wide survey showed that IRF6 binds nearly 2,200 genes and that only 2.6% (56/2177) of these putative targets were affected in a knockdown assay (Botti et al., 2011). Therefore, IRF6 binds fewer transcriptional targets and has an affect on a minority of these. Considering the importance of IRF6 in embryonic development, it follows that both transcriptional and post-translational targets are critical.
An important post-translational target of IRF6 is TP63. In humans, mutations in TP63 can lead to Ectrodactyly, Ectodermal Dysplasia (EEC), which includes cleft lip and palate (OMIM #604292). While TP63 drives IRF6 expression, IRF6 post-translationally targets TP63 for degradation via the proteasome (Moretti et al., 2010). This negative feedback loop is critical for palatal development (Thomason et al., 2010). In the mouse, embryos doubly heterozygous for Trp63 and Irf6 can develop a cleft palate. Considering cytoplasmic localization and regulation of TP63 protein stability, post-translational regulation seems critical. Protein-protein interactions by IRF6 that results in degradation of TP63 are most likely mediated by the protein-binding domain (exons 7 and 8) (Bailey et al., 2005) (Fig. 1). Another important protein target of IRF6 is the Mammary Serine Protease Inhibitor (Maspin, SERPINB5) (Bailey et al., 2005). In contrast to TP63, IRF6 cooperatively binds to Maspin to regulate differentiation in mammary epithelium. Transient re-expression of IRF6 reduced breast cancer invasiveness and loss of IRF6 in skin is associated with squamous cell carcinoma (Botti et al., 2011; Stransky et al., 2011). Mutations in IRF6 are also found in 5% of patients with head and neck squamous cell carcinoma (HNSCC) (Stransky et al., 2011). Regulatory partners and pathway of IRF6 in HNSCC are currently undetermined but an epithelial origin suggests a similar pathway. Aside from TP63 and Maspin, little is known about protein-protein interactions mediated by IRF6. Important targets for future work include identifying the E3 ubiquitin ligases that regulate and are regulated by IRF6.
The C-terminal domain (CTD) consists of a linker region and an α-helix encoded by exon 9, which appears to harbor the regulatory domain for IRF6 in mammary epithelium (Bailey et al., 2005) (Fig. 1). Important clues regarding function of the C-terminus can be gleaned from IRF5, which is most similar to IRF6. The C-terminal domain in IRF5 contains an activation/repression switch that is regulated by phosphorylation and is highly sensitive to mutagenesis (Chen et al., 2008). In human keratinocytes, converting serine and threonine to aspartic acid leads to constitutive activation and nuclear localization of IRF6 (Su, 2011). The factors regulating the on/off switch at this C-terminal domain are currently unknown.
Exons 5 and 6 appear to encode a less conserved proline-rich region. In contrast to frequent point mutations in the DNA binding domain, 30/34 disease-causing mutations in exons 5 and 6 are protein truncations (Fig. 1). Underrepresentation of missense mutations along with less conservation suggests that most coding changes in exons 5 and 6 are either innocuous or are associated with a different disease process. In support of the latter, whole-exome sequencing revealed an exon 5 mutation in a patient with Pierre Robin Sequence (Pengelly et al., 2014).
Biochemically, rapid turnover of IRF6 via the proteasome (Bailey et al., 2008) might be a way to regulate transcriptional and post-translational activity. Phosphorylated (active) IRF6 is also secreted by mammary epithelium into milk (Bailey et al., 2009). Within mammary ducts, alveolar cells use exocytosis to secrete proteins, which may contribute to sequestration or cytoplasmic localization. Considering presence in topologically distinct compartments (cytosol and extracellular medium) (Arnoys and Wang, 2007), IRF6 exhibits ‘dual localization’. Fibroblast growth factor 3 (FGF3), among others in the FGF signaling pathway, also exhibits dual localization and contributes risk for orofacial clefting (Arnoys and Wang, 2007; Riley et al., 2007). A recent bioinformatic analysis shows IRF6 is related to several FGF pathway genes, among other important orofacial targets (Dai et al., 2015), and perhaps this dual localization is an explanation. Examining IRF6 expression in multiple tissues and time points along with inhibition of nuclear export with Leptomycin B may further elucidate the targets and functions of IRF6.
Transcriptional regulation of IRF6 expression
IRF6 expression is implicated in multiple disease processes, including clefting and cancer. Therefore, understanding how this gene is regulated is critical. An enhancer (MCS9.7) recapitulates endogenous IRF6 expression in skin and oral epithelium (Fakhouri et al., 2012). A common DNA variant (rs642961) in this enhancer is associated with isolated cleft lip and palate (CLP) but not cleft palate only (Rahimov et al., 2008). Biochemically, rs642961 abrogates one of four AP-2alpha binding sites within MCS9.7. The Transcription Factor Activating Protein 2 (AP-2) family of transcription factors is composed of five members that homo or heterodimerize to repress or activate gene expression through a common, conserved binding element. Like IRF6, mutations in TFAP2A can also lead to syndromic orofacial clefting with lip pits, known as Branchio-Oculo-Facial Syndrome (BOFS OMIM # 113620). BOFS is dominantly inherited and can present with malformation of the eyes, ears and skin (Li et al., 2013). Similar to Irf6 knockout embryos (discussed above in “Murine Irf6 alleles”), loss of AP-2alpha leads to severe craniofacial, limb and skin defects (Schorle et al., 1996; Zhang et al., 1996). However, Tfap2a knockout embryos are unique in the biomedical literature for absence of a thoracic and abdominal body wall as well as neural tube defects. Facial clefting, which results from failed neural tube closure, precludes analysis of palatal development in Tfap2a−/− embryos. However, tissue-specific deletion of Tfap2a supports a role in palatal development that is independent of neural tube closure (Brewer et al., 2004).
Recent work confirms that AP-2alpha binds to the MCS9.7 enhancer and regulates IRF6 expression (McDade et al., 2012), consistent with a functional role for rs642961. As such, rs642961 may reduce AP-2alpha trans-activation of IRF6 expression, contributing to orofacial clefting risk. Alternatively, it is interesting to consider how loss of IRF6 might be contributing to the phenotypic spectrum of BOFS. In fact, numerous individuals with BOFS have mutations in the DNA binding domain of AP-2alpha, suggesting the importance of downstream transcriptional targets. Likewise, TP63 binds MCS9.7 and regulates IRF6 expression in primary keratinocytes (McDade et al., 2012) (Fig. 3). It is unknown whether IRF6 has a feedback function on AP-2alpha, as it has on TP63. However, recent data suggest that IRF6 binds upstream of TFAP2C, but not TFAP2A (Botti et al., 2011). MCS9.7 also contains binding sites for MAFB, which was recently associated with CLP (Beaty et al., 2010; Moretti et al., 2010; Thomason et al., 2010). More recent work suggests additional IRF6 enhancers are regulated by Notch signaling in keratinocytes (Restivo et al., 2011).
From morphology to molecule: Locus heterogeneity and the genotype-phenotype correlation in the mouse
The Irf6 knockout phenotype is reproduced by knocking out genes at four additional loci (i.e., locus heterogeneity); Stratifin (14-3-3σ), Ikka, Kdf1, and, to a lesser extent, Ripk4 (Herron et al., 2005; Rountree et al., 2010; Song et al., 2010; Lee et al., 2013). 14-3-3σ is a tumor suppressor protein that interacts with TP53 via a positive feedback loop to regulate the G2/M cell cycle checkpoint (Hermeking et al., 1997; Yang et al., 2003). 14-3-3σ also enhances Protein Kinase C activity and contains a Pleckstrin homology domain, critical in protein-protein interaction with serine/threonine phosphorylation (Dellambra et al., 1995; Yaffe et al., 1997; Rittinger et al., 1999). Irf6 genetically interacts with 14-3-3σ in skin, limb, craniofacial and oral cavity development. If the genetic interaction is direct, 14-3-3σ may be involved in phosphorylation and post-translational activation of IRF6 (Fig. 3). Mutations in 14-3-3σ have not been associated with syndromic human disease. However, hypermethylation of a CpG regulatory island reduced 14-3-3σ expression in 91% of breast carcinoma cells (Ferguson et al., 2000), and likely constitutes an early oncogenic event (Umbricht et al., 2001).
In humans, homozygous recessive mutations in Nuclear Factor Kappa-B Kinase subunit alpha (IKKA) leads to Severe Fetal Encasement Malformation, also called Cocoon Syndrome (OMIM # 613630). Cocoon Syndrome includes body wall, skin, limb and neural tube defects (Lahtela et al., 2010). IKKA, also known as CHUK, is a serine/threonine protein kinase that regulates the activation of NF-kB by marking its repressors (IkB Kinase) for ubiquitin-mediated degradation. In the skin, IKKA is a tumor suppressor protein and functions independently of NF-kB and IkB Kinase (Hu et al., 2001). IKKA is downstream of Transforming Growth Factor-β (TGFB) signaling in a complex with Smad2/3, which allows nuclear translocation independent of Smad4 (Descargues et al., 2008). Likewise, TGFB signaling regulates IRF6 expression (Le et al., 2012; Iwata et al., 2013). Downstream, both IKKA and IRF6 regulate OVOL1 expression (Descargues et al., 2008; Botti et al., 2011). In the palate, TGFB signaling regulates Irf6 through SMAD4 (Iwata et al., 2013) but the molecular context of IKKA function in this tissue is less clearly delineated. Despite the phenotypic similarity in skin and palate, and the common upstream and downstream molecular targets, preliminary work does not support a genetic interaction between Ikka and Irf6 (Richardson et al., 2006). Unfortunately, testing epistasis in the mouse is highly specific but not sensitive, i.e. absence of proof is not proof of absence. Additional clues may emerge from an analysis of IRF6 expression in Ikka knockout murine skin. As such, IKKA may be upstream of IRF6 in skin and palate development but our current assays have not been sufficiently sensitive.
Like 14-3-3σ, the Receptor-Interacting serine/threonine Protein Kinase 4 (RIPK4) regulates keratinocytes differentiation and interacts with Protein Kinase C (Chen et al., 2001). Like IKKA, RIPK4 activates NF-kB (Meylan et al., 2002). However, Ripk4 knockout embryos appear to be the least severely affected of the cohort (Holland et al., 2002). In contrast, human mutations in RIPK4 can lead to a lethal type of Popliteal Pterygium Syndrome, called Bartsocas-Papas Syndrome (BPS OMIM # 263650). Like PPS, caused by mutation in IRF6, BPS is associated with popliteal webbing, ankyloblepharon, cleft lip and palate and syndactyly (Mitchell et al., 2011; Kalay et al., 2012). Like Cocoon Syndrome (OMIM #613630), caused by mutations in IKKA, BPS is associated with severe craniofacial defects, leading to superficial visualization of the nasal cavity, in what may be a form of facial clefting. In vivo assays of epistasis between Ripk4 and Irf6, Ikka or 14-3-3σ have not been reported. However, recent work shows that RIPK4 activates IRF6 (Kwa et al., 2015). A recently discovered gene, Keratinocytes Differentiation Factor 1 (Kdf1), like Irf6, appears to interact with Trp63 and 14-3-3σ in skin, limb and craniofacial development (Lee et al., 2013). Cytoplasmic localization and association with the cellular membrane suggest a signaling function for KDF1.
Is there also evidence for locus heterogeneity in humans? Like IRF6, mutations in RIPK4 can lead to PPS. Like RIPK4, mutations in IKKA can lead to BPS (Leslie et al., 2015). Therefore, for the 27% of families without a known genetic mutation leading to VWS, 14-3-3σ, IKKA, KDF1 and RIPK4 appear to be good candidate genes. For the remaining 73% of families with either a mutation in IRF6 or GRHL3, common or rare variants in 14-3-3σ, IKKA, KDF1 and RIPK4 may be acting as genetic modifiers. In addition to cellular and biochemical assays, a systematic analysis for epistasis in the mouse, including double and triple mutants, may contribute to our knowledge of this complex and seemingly redundant network.
Translating Therapies
Ongoing preventative efforts have focused on folate and multi-vitamin supplementation to reduce birth defects (van Rooij et al., 2004; Wu et al., 2010). Associations between orofacial clefting and variants in genes of folate metabolism provide a context for this approach (Mills et al., 2008; Blanton et al., 2011). A recent review suggests folate supplementation affects gene expression through methylation reactions (Blom et al., 2006). Bisulfite sequencing showed a ~300 bp CpG island in the IRF6 promoter was methylated. Methylation of the IRF6 promoter reduced expression and increased risk for squamous cell carcinoma (Botti et al., 2011). If supplementation affects methylation and epigenetic regulation at the IRF6 promoter, a therapeutic intervention might be possible by titrating folate and IRF6 levels based on personal risk. However, if folate intake increases methylation and reduces IRF6 expression, mass fortification may not reduce risk for everyone. For Grhl3, while folate had no affect, inositol supplementation rescued neural tube defects (Ting et al., 2003). The oral cavity was not evaluated with inositol supplementation.
Given widespread epithelial pathology, several studies predicted rescuing epidermal cells would prevent the knockout phenotype. Consistent with this rationale, using the KRT14 promoter to drive Ikka in basal epithelial cells of Ikka knockout embryos led to rescue of skin, skeletal and limb defects (Sil et al., 2004). However, the pups did not feed and died due to persistent esophageal adhesions that occluded the gastrointestinal tract. In contrast to wildtype embryos (Vassar et al., 1989; Takahashi et al., 1995), the KRT14 promoter was inactive in the esophagus of Ikka knockout embryos. Skeletal and limb rescue is intriguing because it involves both cartilaginous and bony structures that lie beneath the epidermal cells, strongly suggesting non-cell autonomous function. Unlike skin and limb rescue, a curled tail persisted, suggesting additional cell autonomous function for Ikka in neural tube development. In an analogous experiment using the KRT5 promoter, only a super-physiological dose of IKKA completely rescued tail development (Liu et al., 2011). This is intriguing for two reasons. First, it suggests IKKA and epidermal cells are necessary for tail development. Second, it suggests a sufficiently high dose of epithelial IKKA can compensate for extra-epithelial IKKA. The compensation might be through non-cell autonomous signaling or direct action of IKKA at distant cells.
Similarly, using the KRT14 promoter to drive Ripk4 in Ripk4 knockout pups rescued cutaneous defects. As seen with Ikka, KRT14 spatio-temporal regulation of Ripk4 was not sufficient to rescue esophageal adhesions (Rountree et al., 2010). In a test for epistasis, epithelial expression of Ripk4 using the KRT14 promoter did not rescue Ikka and 14-3-3σ knockout embryos. Considering less severely affected knockout embryos and failure to rescue loss of Ikka and 14-3-3σ, RIPK4 may be in a parallel, but converging pathway or require both IKKA and 14-3-3σ for function. Rescue of Irf6 and Kdf1 knockout embryos using the KRT14-Ripk4 transgene has not been reported.
In addition to genetic rescue, experimental embryonic gene therapy protocols to prevent disease in animal models have been developed for cystic fibrosis (Keswani et al., 2011), Duchenne muscular dystrophy (Koppanati et al., 2010), Herlitz junctional epidermolysis bullosa (Muhle et al., 2006; Endo et al., 2011), thrombotic thrombocytopenic purpura (Niiya et al., 2009) and congenital blindness (Dejneka et al., 2004). Likewise, gene delivery to oral epithelium and developing epidermis is possible during development (Wu et al., 2012). In mature skin, epithelial stratification (cornified layer) and keratin secretion forms a physical barrier that prevents entry by viral and bacterial pathogens. However, during early embryonic development, a cornified layer is not present, leaving the tissue highly susceptible to transduction. As such, intra-aminotic injection of a viral vector with epithelial tropism may provide robust targeting. Like developing epidermis, oral epithelium can be transduced. Circulation of amniotic fluid in and through the embryo also ensures delivery of viral vectors into the oral cavity. As lip and palate development occurs between the 6th and 10th week of human gestation, ultrasound may be used to visualize fetal anatomy. Considering mouse models discussed here, in vivo assays seem feasible. Furthermore, transduction of periderm, a cell type lost before birth, limits untoward long-term affects. Finally, the immune-privileged status of amniotic fluid limits innate and adaptive blunting of gene delivery. Like many orofacial clefting genes, Irf6-related pathogenesis results from insufficient epithelial expression. As such, in utero gene delivery may provide a therapeutic modality for single gene clefting disorders.
Future Directions
Orofacial clefting is a common birth defect, with multiple genetic and environmental factors. Among these, IRF6 variants result in two orofacial clefting syndromes (VWS and PPS) and increase risk for isolated cleft lip and palate. Crucially, the genes that regulate and are regulated by IRF6 also cause syndromic orofacial clefts (TFAP2A and BOFS, GRHL3 and VWS, TP63 and EEC). Furthermore, recent literature suggests an emerging phenotypic spectrum from mutations in IRF6, RIPK4 and IKKA and locus heterogeneity for PPS, BPS and Cocoon Syndrome, all of which confer significant morbidity and mortality and involve epidermal development. More broadly still, variants within IRF6 are associated with squamous cell carcinoma. Currently available murine models include a genetrap, human mutations, a hypomorph and an over-expresser, providing robust resources for additional investigation. This network is critical, the tools are available and the system appears to be amenable to interventions.
Currently, the cause of VWS is unknown in more than 25% of affected families. Determining the etiology in these families may reveal additional risk factors in isolated orofacial clefting. Considering locus heterogeneity and evolving phenotypic spectrums, identifying environmental and genetic modifiers seems critical in understanding, and perhaps one day managing, risk. Work thus far suggests that a personalized approach will be necessary. It is also not clear how specific cellular and biochemical processes contribute to disease penetrance and severity. In that regard, identifying the post-translational targets of and E3 ligases that regulate and are deployed by IRF6 seems critical. Molecularly, describing transport of IRF6 in and out of the nucleus may aid in designing small-molecule targets. Despite locus heterogeneity in mice, Ikka and Irf6 do not interact and either our in vivo assay is not sufficiently sensitive or there are two, or more, converging pathways leading to the same phenotype. While the former is simpler, the latter is supported by interaction between Irf6 and 14-3-3σ. Furthermore, Ripk4 expression did not rescue Ikka and 14-3-3σ knockout mice. As such, it will be important to test for epistasis among Irf6, 14-3-3σ, Ikka, Kdf1 and Ripk4. Finally, identification of GRHL3 mutations in families with VWS and inositol rescue of a birth defect in Grhl3 knockout mice suggests that testing the role of supplements may be reasonable in orofacial clefts.
ACKNOWLEDGEMENTS
Financial support to YAK (F31DE022696-01) came from the NIH National Institute of Dental and Craniofacial Research. Additional support to BCS was provided from NIH (DE13513) and Michigan State University grants. We thank Dr. John Wang and Dr. Patrick Venta for critical review of this manuscript. We also thank Nicole Patel for illustrating the stages of palatal development.
Footnotes
The authors have no financial or personal relationships with organizations that may constitute a conflict of interest. The authors have reviewed the journal’s policy on disclosure of potential conflicts of interest.
References
- Aerts A, DeVolder I, Weinberg SM, Thedens D, Dunnwald M, Schutte BC, Nopoulos P. Haploinsufficiency of interferon regulatory factor 6 alters brain morphology in the mouse. Am J Med Genet A. 2013;164A:655–660. doi: 10.1002/ajmg.a.36333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenbud D, Coval M, Hazan-Molina H, Harari D. Isolated soft tissue cleft lip: epidemiology and associated dental anomalies. Oral Dis. 2011;17:221–231. doi: 10.1111/j.1601-0825.2010.01729.x. [DOI] [PubMed] [Google Scholar]
- Arnoys EJ, Wang JL. Dual localization: proteins in extracellular and intracellular compartments. Acta Histochem. 2007;109:89–110. doi: 10.1016/j.acthis.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Bailey CM, Abbott DE, Margaryan NV, Khalkhali-Ellis Z, Hendrix MJ. Interferon regulatory factor 6 promotes cell cycle arrest and is regulated by the proteasome in a cell cycle-dependent manner. Mol Cell Biol. 2008;28:2235–2243. doi: 10.1128/MCB.01866-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CM, Khalkhali-Ellis Z, Kondo S, Margaryan NV, Seftor RE, Wheaton WW, Amir S, Pins MR, Schutte BC, Hendrix MJ. Mammary serine protease inhibitor (Maspin) binds directly to interferon regulatory factor 6: identification of a novel serpin partnership. J Biol Chem. 2005;280:34210–34217. doi: 10.1074/jbc.M503523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CM, Margaryan NV, Abbott DE, Schutte BC, Yang B, Khalkhali-Ellis Z, Hendrix MJ. Temporal and spatial expression patterns for the tumor suppressor Maspin and its binding partner interferon regulatory factor 6 during breast development. Dev Growth Differ. 2009;51:473–481. doi: 10.1111/j.1440-169X.2009.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BR. A Family with Bilateral Congenital Pits of the Inferior Lip. Oral Surg Oral Med Oral Pathol. 1964;18:494–497. doi: 10.1016/0030-4220(64)90398-6. [DOI] [PubMed] [Google Scholar]
- Basseri B, Kianmahd BD, Roostaeian J, Kohan E, Wasson KL, Basseri RJ, Bradley JP. Current national incidence, trends, and health care resource utilization of cleft lip-cleft palate. Plast Reconstr Surg. 2011;127:1255–1262. doi: 10.1097/PRS.0b013e3182043af6. [DOI] [PubMed] [Google Scholar]
- Beaty TH, Murray JC, Marazita ML, Munger RG, Ruczinski I, Hetmanski JB, Liang KY, Wu T, Murray T, Fallin MD, Redett RA, Raymond G, Schwender H, Jin SC, Cooper ME, Dunnwald M, Mansilla MA, Leslie E, Bullard S, Lidral AC, Moreno LM, Menezes R, Vieira AR, Petrin A, Wilcox AJ, Lie RT, Jabs EW, Wu-Chou YH, Chen PK, Wang H, Ye X, Huang S, Yeow V, Chong SS, Jee SH, Shi B, Christensen K, Melbye M, Doheny KF, Pugh EW, Ling H, Castilla EE, Czeizel AE, Ma L, Field LL, Brody L, Pangilinan F, Mills JL, Molloy AM, Kirke PN, Scott JM, Arcos-Burgos M, Scott AF. A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4. Nat Genet. 2010;42:525–529. doi: 10.1038/ng.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs LC, Rhea L, Schutte BC, Dunnwald M. Interferon Regulatory Factor 6 Is Necessary, but Not Sufficient, for Keratinocyte Differentiation. J Invest Dermatol. 2011;132:50–58. doi: 10.1038/jid.2011.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeland AC, Larrabee Y, Kent DT, Flores C, Su GH, Lee JH, Haddad J., Jr Novel IRF6 mutations in Honduran Van der Woude syndrome patients. Mol Med Report. 2011;4:237–241. doi: 10.3892/mmr.2011.423. [DOI] [PubMed] [Google Scholar]
- Birnbaum S, Ludwig KU, Reutter H, Herms S, Steffens M, Rubini M, Baluardo C, Ferrian M, Almeida de Assis N, Alblas MA, Barth S, Freudenberg J, Lauster C, Schmidt G, Scheer M, Braumann B, Berge SJ, Reich RH, Schiefke F, Hemprich A, Potzsch S, Steegers-Theunissen RP, Potzsch B, Moebus S, Horsthemke B, Kramer FJ, Wienker TF, Mossey PA, Propping P, Cichon S, Hoffmann P, Knapp M, Nothen MM, Mangold E. Key susceptibility locus for nonsyndromic cleft lip with or without cleft palate on chromosome 8q24. Nat Genet. 2009;41:473–477. doi: 10.1038/ng.333. [DOI] [PubMed] [Google Scholar]
- Bixler D, Poland C, Nance WE. Phenotypic variation in the popliteal pterygium syndrome. Clin Genet. 1973;4:220–228. doi: 10.1111/j.1399-0004.1973.tb01146.x. [DOI] [PubMed] [Google Scholar]
- Blackburn J, Ohazama A, Kawasaki K, Otsuka-Tanaka Y, Liu B, Honda K, Rountree RB, Hu Y, Kawasaki M, Birchmeier W, Schmidt-Ullrich R, Kinoshita A, Schutte BC, Hammond NL, Dixon MJ, Sharpe PT. The role of Irf6 in tooth epithelial invagination. Dev Biol. 2012;365:61–70. doi: 10.1016/j.ydbio.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton SH, Henry RR, Yuan Q, Mulliken JB, Stal S, Finnell RH, Hecht JT. Folate pathway and nonsyndromic cleft lip and palate. Birth Defects Res A Clin Mol Teratol. 2011;91:50–60. doi: 10.1002/bdra.20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nat Rev Neurosci. 2006;7:724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocian M, Walker AP. Lip pits and deletion 1q32–41. Am J Med Genet. 1987;26:437–443. doi: 10.1002/ajmg.1320260223. [DOI] [PubMed] [Google Scholar]
- Boell L, Pallares LF, Brodski C, Chen Y, Christian JL, Kousa YA, Kuss P, Nelsen S, Novikov O, Schutte BC, Wang Y, Tautz D. Exploring the effects of gene dosage on mandible shape in mice as a model for studying the genetic basis of natural variation. Dev Genes Evol. 2013;223:279–287. doi: 10.1007/s00427-013-0443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, Murko V, Wood JL, Langbehn DR, Canady J, Richman L, Nopoulos P. Social function in boys with cleft lip and palate: relationship to ventral frontal cortex morphology. Behav Brain Res. 2007;181:224–231. doi: 10.1016/j.bbr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botti E, Spallone G, Moretti F, Marinari B, Pinetti V, Galanti S, De Meo PD, De Nicola F, Ganci F, Castrignano T, Pesole G, Chimenti S, Guerrini L, Fanciulli M, Blandino G, Karin M, Costanzo A. Developmental factor IRF6 exhibits tumor suppressor activity in squamous cell carcinomas. Proc Natl Acad Sci U S A. 2011;108:13710–13715. doi: 10.1073/pnas.1110931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer S, Feng W, Huang J, Sullivan S, Williams T. Wnt1-Cre-mediated deletion of AP-2alpha causes multiple neural crest-related defects. Dev Biol. 2004;267:135–152. doi: 10.1016/j.ydbio.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Brosch S, Baur M, Blin N, Reinert S, Pfister M. A novel IRF6 nonsense mutation (Y67X) in a German family with Van der Woude syndrome. Int J Mol Med. 2007;20:85–89. [PubMed] [Google Scholar]
- Burdick AB. Genetic epidemiology and control of genetic expression in van der Woude syndrome. J Craniofac Genet Dev Biol. 1986;(Suppl 2):99–105. [PubMed] [Google Scholar]
- Burdick AB, Bixler D, Puckett CL. Genetic analysis in families with van der Woude syndrome. J Craniofac Genet Dev Biol. 1985;5:181–208. [PubMed] [Google Scholar]
- Calzolari E, Pierini A, Astolfi G, Bianchi F, Neville AJ, Rivieri F. Associated anomalies in multi-malformed infants with cleft lip and palate: An epidemiologic study of nearly 6 million births in 23 EUROCAT registries. Am J Med Genet A. 2007;143:528–537. doi: 10.1002/ajmg.a.31447. [DOI] [PubMed] [Google Scholar]
- Cervenka JGR, Anderson VE. The syndrome of pits of the lower lip and cleft lip and/or palate. Genetic considerations. Am J Hum Genet. 1967;19:416–432. [PMC free article] [PubMed] [Google Scholar]
- Chen L, Haider K, Ponda M, Cariappa A, Rowitch D, Pillai S. Protein kinase C-associated kinase (PKK), a novel membrane-associated, ankyrin repeat-containing protein kinase. J Biol Chem. 2001;276:21737–21744. doi: 10.1074/jbc.M008069200. [DOI] [PubMed] [Google Scholar]
- Chen W, Lam SS, Srinath H, Jiang Z, Correia JJ, Schiffer CA, Fitzgerald KA, Lin K, Royer WE., Jr Insights into interferon regulatory factor activation from the crystal structure of dimeric IRF5. Nat Struct Mol Biol. 2008;15:1213–1220. doi: 10.1038/nsmb.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Juel K, Herskind AM, Murray JC. Long term follow up study of survival associated with cleft lip and palate at birth. BMJ. 2004;328:1405. doi: 10.1136/bmj.38106.559120.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobourne MT. The complex genetics of cleft lip and palate. Eur J Orthod. 2004;26:7–16. doi: 10.1093/ejo/26.1.7. [DOI] [PubMed] [Google Scholar]
- Conrad AL, Canady J, Richman L, Nopoulos P. Incidence of neurological soft signs in children with isolated cleft of the lip or palate. Percept Mot Skills. 2008;106:197–206. doi: 10.2466/pms.106.1.197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad AL, Dailey S, Richman L, Canady J, Karnell MP, Axelson E, Nopoulos P. Cerebellum Structure Differences and Relationship to Speech in Boys and Girls With Nonsyndromic Cleft of the Lip and/or Palate. Cleft Palate Craniofac J. 2010;47:469–475. doi: 10.1597/08-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad AL, Richman L, Nopoulos P, Dailey S. Neuropsychological functioning in children with non-syndromic cleft of the lip and/or palate. Child Neuropsychol. 2009;15:471–484. doi: 10.1080/09297040802691120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Yu H, Si J, Fang B, Shen SG. Irf6-Related Gene Regulatory Network Involved in Palate and Lip Development. J Craniofac Surg. 2015;26:1600–1605. doi: 10.1097/SCS.0000000000001526. [DOI] [PubMed] [Google Scholar]
- de la Garza G, Schleiffarth JR, Dunnwald M, Mankad A, Weirather JL, Bonde G, Butcher S, Mansour TA, Kousa YA, Fukazawa CF, Houston DW, Manak JR, Schutte BC, Wagner DS, Cornell RA. Interferon regulatory factor 6 promotes differentiation of the periderm by activating expression of grainyhead-like 3. J Invest Dermatol. 2013;133:68–77. doi: 10.1038/jid.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima RL, Hoper SA, Ghassibe M, Cooper ME, Rorick NK, Kondo S, Katz L, Marazita ML, Compton J, Bale S, Hehr U, Dixon MJ, Daack-Hirsch S, Boute O, Bayet B, Revencu N, Verellen-Dumoulin C, Vikkula M, Richieri-Costa A, Moretti-Ferreira D, Murray JC, Schutte BC. Prevalence and nonrandom distribution of exonic mutations in interferon regulatory factor 6 in 307 families with Van der Woude syndrome and 37 families with popliteal pterygium syndrome. Genet Med. 2009;11:241–247. doi: 10.1097/GIM.0b013e318197a49a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejneka NS, Surace EM, Aleman TS, Cideciyan AV, Lyubarsky A, Savchenko A, Redmond TM, Tang W, Wei Z, Rex TS, Glover E, Maguire AM, Pugh EN, Jr, Jacobson SG, Bennett J. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther. 2004;9:182–188. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Dellambra E, Patrone M, Sparatore B, Negri A, Ceciliani F, Bondanza S, Molina F, Cancedda FD, De Luca M. Stratifin, a keratinocyte specific 14-3-3 protein, harbors a pleckstrin homology (PH) domain and enhances protein kinase C activity. J Cell Sci. 1995;108(Pt 11):3569–3579. doi: 10.1242/jcs.108.11.3569. [DOI] [PubMed] [Google Scholar]
- Descargues P, Sil AK, Sano Y, Korchynskyi O, Han G, Owens P, Wang XJ, Karin M. IKKalpha is a critical coregulator of a Smad4-independent TGFbeta-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:2487–2492. doi: 10.1073/pnas.0712044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronamraju KR. Genetic studies of a cleft palate clinic population. Birth Defects Orig Artic Ser. 1971;7:54–57. [PubMed] [Google Scholar]
- Du X, Tang W, Tian W, Li S, Li X, Liu L, Zheng X, Chen X, Lin Y, Tang Y. Novel IRF6 mutations in Chinese patients with Van der Woude syndrome. J Dent Res. 2006a;85:937–940. doi: 10.1177/154405910608501013. [DOI] [PubMed] [Google Scholar]
- Du XY, Tang W, Tian WD, Li XY, Liu L, Zheng XH. [Identification of three novel mutations of IRF6 in Chinese families with Van der Woude syndrome] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2006b;23:82–83. [PubMed] [Google Scholar]
- Endo M, Zoltick PW, Radu A, Qiujie J, Matsui C, Marinkovich PM, McGrath J, Tamai K, Uitto J, Flake AW. Early intra-amniotic gene transfer using lentiviral vector improves skin blistering phenotype in a murine model of Herlitz junctional epidermolysis bullosa. Gene Ther. 2011;19:561–569. doi: 10.1038/gt.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar V, Weaver D. Popliteal pterygium syndrome: a phenotypic and genetic analysis. J Med Genet. 1978;15:35–42. doi: 10.1136/jmg.15.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri WD, Rahimov F, Attanasio C, Kouwenhoven EN, Ferreira De Lima RL, Felix TM, Nitschke L, Huver D, Barrons J, Kousa YA, Leslie E, Pennacchio LA, Van Bokhoven H, Visel A, Zhou H, Murray JC, Schutte BC. An etiologic regulatory mutation in IRF6 with loss- and gain-of-function effects. Hum Mol Genet. 2014;23:2711–2720. doi: 10.1093/hmg/ddt664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri WD, Rhea L, Du T, Sweezer E, Morrison H, Fitzpatrick D, Yang B, Dunnwald M, Schutte BC. MCS9.7 enhancer activity is highly, but not completely, associated with expression of Irf6 and p63. Dev Dyn. 2012;241:340–349. doi: 10.1002/dvdy.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AT, Evron E, Umbricht CB, Pandita TK, Chan TA, Hermeking H, Marks JR, Lambers AR, Futreal PA, Stampfer MR, Sukumar S. High frequency of hypermethylation at the 14-3-3 sigma locus leads to gene silencing in breast cancer. Proc Natl Acad Sci U S A. 2000;97:6049–6054. doi: 10.1073/pnas.100566997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr MC, Larkin E, Blakeley R, Albert TW, Tsugawa L, Weber SM. Extending multidisciplinary management of cleft palate to the developing world. J Oral Maxillofac Surg. 2010;69:237–241. doi: 10.1016/j.joms.2010.06.214. [DOI] [PubMed] [Google Scholar]
- Gatta V, Scarciolla O, Cupaioli M, Palka C, Chiesa PL, Stuppia L. A novel mutation of the IRF6 gene in an Italian family with Van der Woude syndrome. Mutat Res. 2004;547:49–53. doi: 10.1016/j.mrfmmm.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Ghassibe M, Bayet B, Revencu N, Verellen-Dumoulin C, Gillerot Y, Vanwijck R, Vikkula M. Interferon regulatory factor-6: a gene predisposing to isolated cleft lip with or without cleft palate in the Belgian population. Eur J Hum Genet. 2005;13:1239–1242. doi: 10.1038/sj.ejhg.5201486. [DOI] [PubMed] [Google Scholar]
- Ghassibe M, Revencu N, Bayet B, Gillerot Y, Vanwijck R, Verellen-Dumoulin C, Vikkula M. Six families with van der Woude and/or popliteal pterygium syndrome: all with a mutation in the IRF6 gene. J Med Genet. 2004;41:e15. doi: 10.1136/jmg.2003.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudy S, Angel P, Jacobs B, Hill C, Mainini V, Smith AL, Kousa YA, Caprioli R, Prince LS, Baldwin S, Schutte BC. Cell-autonomous and non-cell-autonomous roles for IRF6 during development of the tongue. PLoS One. 2013;8:e56270. doi: 10.1371/journal.pone.0056270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer J, Klausler M, Schinzel A. The popliteal pterygium syndrome: distinct phenotypic variation in two families. Helv Paediatr Acta. 1989;43:507–514. [PubMed] [Google Scholar]
- Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- Herron BJ, Liddell RA, Parker A, Grant S, Kinne J, Fisher JK, Siracusa LD. A mutation in stratifin is responsible for the repeated epilation (Er) phenotype in mice. Nat Genet. 2005;37:1210–1212. doi: 10.1038/ng1652. [DOI] [PubMed] [Google Scholar]
- Hersh JH, Verdi GD. Natal teeth in monozygotic twins with Van der Woude syndrome. Cleft Palate Craniofac J. 1992;29:279–281. doi: 10.1597/1545-1569_1992_029_0279_ntimtw_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Hoffman H, Hudgins PA. Head and skull base features of nine Egyptian mummies: evaluation with high-resolution CT and reformation techniques. AJR Am J Roentgenol. 2002;178:1367–1376. doi: 10.2214/ajr.178.6.1781367. [DOI] [PubMed] [Google Scholar]
- Holland P, Willis C, Kanaly S, Glaccum M, Warren A, Charrier K, Murison J, Derry J, Virca G, Bird T, Peschon J. RIP4 is an ankyrin repeat-containing kinase essential for keratinocyte differentiation. Curr Biol. 2002;12:1424–1428. doi: 10.1016/s0960-9822(02)01075-8. [DOI] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. Toll-like receptor signaling and IRF transcription factors. IUBMB Life. 2006;58:290–295. doi: 10.1080/15216540600702206. [DOI] [PubMed] [Google Scholar]
- Houdayer C, Bonaiti-Pellie C, Erguy C, Soupre V, Dondon MG, Burglen L, Cougoureux E, Couderc R, Vazquez MP, Bahuau M. Possible relationship between the van der Woude syndrome (vWS) locus and nonsyndromic cleft lip with or without cleft palate (NSCL/P) Am J Med Genet. 2001;104:86–92. doi: 10.1002/1096-8628(20011115)104:1<86::aid-ajmg10053>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- Huang JJ, Hou JW, Tan YC, Chen KT, Lo LJ, Chen YR. Van der Woude syndrome: clinical presentation in 64 patients. Cleft Palate Craniofac J. 2007;44:649–652. doi: 10.1597/06-094.1. [DOI] [PubMed] [Google Scholar]
- Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, Murray JC, Schutte BC. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38:1335–1340. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Item CB, Turhani D, Thurnher D, Yerit K, Sinko K, Wittwer G, Adeyemo WL, Frei K, Erginel-Unaltuna N, Watzinger F, Ewers R. Van Der Woude syndrome: variable penetrance of a novel mutation (p.Arg 84Gly) of the IRF6 gene in a Turkish family. Int J Mol Med. 2005;15:247–251. [PubMed] [Google Scholar]
- Iwata J, Suzuki A, Pelikan RC, Ho TV, Sanchez-Lara PA, Urata M, Dixon MJ, Chai Y. Smad4-Irf6 genetic interaction and TGFbeta-mediated IRF6 signaling cascade are crucial for palatal fusion in mice. Development. 2013;140:1220–1230. doi: 10.1242/dev.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku P, Robinow M, Kelly T, Bralley R, Baynes A, Edgerton MT. The van der Woude syndrome in a large kindred: variability, penetrance, genetic risks. Am J Med Genet. 1980;5:117–123. doi: 10.1002/ajmg.1320050203. [DOI] [PubMed] [Google Scholar]
- Jobling R, Ferrier RA, McLeod R, Petrin AL, Murray JC, Thomas MA. Monozygotic twins with variable expression of Van der Woude syndrome. Am J Med Genet A. 2011;155A:2008–2010. doi: 10.1002/ajmg.a.34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Canady JW, Brookes JT, Wehby GL, L’Heureux J, Schutte BC, Murray JC, Dunnwald M. Wound complications after cleft repair in children with Van der Woude syndrome. J Craniofac Surg. 2010;21:1350–1353. doi: 10.1097/SCS.0b013e3181ec6aad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalay E, Sezgin O, Chellappa V, Mutlu M, Morsy H, Kayserili H, Kreiger E, Cansu A, Toraman B, Abdalla EM, Aslan Y, Pillai S, Akarsu NA. Mutations in RIPK4 cause the autosomal-recessive form of popliteal pterygium syndrome. Am J Hum Genet. 2012;90:76–85. doi: 10.1016/j.ajhg.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano S, Kure S, Suzuki Y, Kanno K, Aoki Y, Kondo S, Schutte BC, Murray JC, Yamada A, Matsubara Y. Novel IRF6 mutations in Japanese patients with Van der Woude syndrome: two missense mutations (R45Q and P396S) and a 17-kb deletion. J Hum Genet. 2003;48:622–628. doi: 10.1007/s10038-003-0089-0. [DOI] [PubMed] [Google Scholar]
- Kerameddin S, Namipashaki A, Ebrahimi S, Ansari-Pour N. IRF6 Is a Marker of Severity in Nonsyndromic Cleft Lip/Palate. J Dent Res: SAGE. 2015:1S–7S. doi: 10.1177/0022034515581013. [DOI] [PubMed] [Google Scholar]
- Keswani SG, Le LD, Morris LM, Lim FY, Katz AB, Ghobril N, Habli M, Frischer JS, Crombleholme TM. Submucosal gland development in the human fetal trachea xenograft model: implications for fetal gene therapy. J Pediatr Surg. 2011;46:33–38. doi: 10.1016/j.jpedsurg.2010.09.064. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park JY, Lee TJ, Yoo HW. Identification of two novel mutations of IRF6 in Korean families affected with Van der Woude syndrome. Int J Mol Med. 2003;12:465–468. [PubMed] [Google Scholar]
- Knight AS, Schutte BC, Jiang R, Dixon MJ. Developmental expression analysis of the mouse and chick orthologues of IRF6: the gene mutated in Van der Woude syndrome. Dev Dyn. 2006;235:1441–1447. doi: 10.1002/dvdy.20598. [DOI] [PubMed] [Google Scholar]
- Koillinen H, Wong FK, Rautio J, Ollikainen V, Karsten A, Larson O, Teh BT, Huggare J, Lahermo P, Larsson C, Kere J. Mapping of the second locus for the Van der Woude syndrome to chromosome 1p34. Eur J Hum Genet. 2001;9:747–752. doi: 10.1038/sj.ejhg.5200713. [DOI] [PubMed] [Google Scholar]
- Kondo S, Schutte BC, Richardson RJ, Bjork BC, Knight AS, Watanabe Y, Howard E, de Lima RL, Daack-Hirsch S, Sander A, McDonald-McGinn DM, Zackai EH, Lammer EJ, Aylsworth AS, Ardinger HH, Lidral AC, Pober BR, Moreno L, Arcos-Burgos M, Valencia C, Houdayer C, Bahuau M, Moretti-Ferreira D, Richieri-Costa A, Dixon MJ, Murray JC. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet. 2002;32:285–289. doi: 10.1038/ng985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppanati BM, Li J, Reay DP, Wang B, Daood M, Zheng H, Xiao X, Watchko JF, Clemens PR. Improvement of the mdx mouse dystrophic phenotype by systemic in utero AAV8 delivery of a minidystrophin gene. Gene Ther. 2010;17:1355–1362. doi: 10.1038/gt.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa MQ, Huynh J, Reynolds EC, Hamilton JA, Scholz GM. Disease-associated mutations in IRF6 and RIPK4 dysregulate their signalling functions. Cell Signal. 2015;27:1509–1516. doi: 10.1016/j.cellsig.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Lahtela J, Nousiainen HO, Stefanovic V, Tallila J, Viskari H, Karikoski R, Gentile M, Saloranta C, Varilo T, Salonen R, Kestila M. Mutant CHUK and severe fetal encasement malformation. N Engl J Med. 2010;363:1631–1637. doi: 10.1056/NEJMoa0911698. [DOI] [PubMed] [Google Scholar]
- Le M, Naridze R, Morrison J, Biggs LC, Rhea L, Schutte BC, Kaartinen V, Dunnwald M. Transforming growth factor Beta 3 is required for excisional wound repair in vivo. PLoS One. 2012;7:e48040. doi: 10.1371/journal.pone.0048040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kong Y, Weatherbee SD. Forward genetics identifies Kdf1/1810019J16Rik as an essential regulator of the proliferation-differentiation decision in epidermal progenitor cells. Dev Biol. 2013;383:201–213. doi: 10.1016/j.ydbio.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees MM, Winter RM, Malcolm S, Saal HM, Chitty L. Popliteal pterygium syndrome: a clinical study of three families and report of linkage to the Van der Woude syndrome locus on 1q32. J Med Genet. 1999;36:888–892. [PMC free article] [PubMed] [Google Scholar]
- Leslie EJ, Mancuso JL, Schutte BC, Cooper ME, Durda KM, L’Heureux J, Zucchero TM, Marazita ML, Murray JC. Search for genetic modifiers of IRF6 and genotype-phenotype correlations in Van der Woude and popliteal pterygium syndromes. Am J Med Genet A. 2013;161:2535–2544. doi: 10.1002/ajmg.a.36133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EJ, O’Sullivan J, Cunningham ML, Singh A, Goudy SL, Ababneh F, Alsubaie L, Ch’ng GS, van der Laar IM, Hoogeboom AJ, Dunnwald M, Kapoor S, Jiramongkolchai P, Standley J, Manak JR, Murray JC, Dixon MJ. Expanding the genetic and phenotypic spectrum of popliteal pterygium disorders. Am J Med Genet A. 2015;167A:545–552. doi: 10.1002/ajmg.a.36896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EJ, Standley J, Compton J, Bale S, Schutte BC, Murray JC. Comparative analysis of IRF6 variants in families with Van der Woude syndrome and popliteal pterygium syndrome using public whole-exome databases. Genet Med. 2012;15:338–344. doi: 10.1038/gim.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. [Twins in a family with lower lip malformation] Acta Genet Stat Med. 1962;12:33–40. [PubMed] [Google Scholar]
- Li H, Sheridan R, Williams T. Analysis of TFAP2A mutations in Branchio-Oculo-Facial Syndrome indicates functional complexity within the AP-2alpha DNA-binding domain. Hum Mol Genet. 2013;22:3195–3206. doi: 10.1093/hmg/ddt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ, Rorick NK, Su LI, Baldock C, Malhotra S, Jowitt T, Gakhar L, Subramanian R, Schutte BC, Dixon MJ, Shore P. Missense mutations that cause Van der Woude syndrome and popliteal pterygium syndrome affect the DNA-binding and transcriptional activation functions of IRF6. Hum Mol Genet. 2009;18:535–545. doi: 10.1093/hmg/ddn381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Willette-Brown J, Liu S, Chen X, Fischer SM, Hu Y. IKKalpha represses a network of inflammation and proliferation pathways and elevates c-Myc antagonists and differentiation in a dose-dependent manner in the skin. Cell Death Differ. 2011;18:1854–1864. doi: 10.1038/cdd.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Kakar N, Hasnain S, Ahmad J, Wilcox ER, Naz S. Epidemiology of Van der Woude syndrome from mutational analyses in affected patients from Pakistan. Clin Genet. 2010;78:247–256. doi: 10.1111/j.1399-0004.2010.01375.x. [DOI] [PubMed] [Google Scholar]
- Manna F, Pensiero S, Clarich G, Guarneri GF, Parodi PC. Cleft lip and palate: current status from the literature and our experience. J Craniofac Surg. 2009;20:1383–1387. doi: 10.1097/SCS.0b013e3181b0daa3. [DOI] [PubMed] [Google Scholar]
- Matsuzawa N, Yoshiura K, Machida J, Nakamura T, Niimi T, Furukawa H, Toyoda T, Natsume N, Shimozato K, Niikawa N. Two missense mutations in the IRF6 gene in two Japanese families with Van der Woude syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:414–417. doi: 10.1016/j.tripleo.2003.12.034. [DOI] [PubMed] [Google Scholar]
- McDade SS, Henry AE, Pivato GP, Kozarewa I, Mitsopoulos C, Fenwick K, Assiotis I, Hakas J, Zvelebil M, Orr N, Lord CJ, Patel D, Ashworth A, McCance DJ. Genome-wide analysis of p63 binding sites identifies AP-2 factors as co-regulators of epidermal differentiation. Nucleic Acids Res. 2012;40:7190–7206. doi: 10.1093/nar/gks389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Martinon F, Thome M, Gschwendt M, Tschopp J. RIP4 (DIK/PKK), a novel member of the RIP kinase family, activates NF-kappa B and is processed during apoptosis. EMBO Rep. 2002;3:1201–1208. doi: 10.1093/embo-reports/kvf236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JL, Molloy AM, Parle-McDermott A, Troendle JF, Brody LC, Conley MR, Cox C, Pangilinan F, Orr DJ, Earley M, McKiernan E, Lynn EC, Doyle A, Scott JM, Kirke PN. Folate-related gene polymorphisms as risk factors for cleft lip and cleft palate. Birth Defects Res A Clin Mol Teratol. 2008;82:636–643. doi: 10.1002/bdra.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minones-Suarez L, Mas-Vidal A, Fernandez-Toral J, Llano-Rivas I, Gonzalez-Garcia M. A novel mutation in the IRF6 gene associated with facial asymmetry in a family affected with Van der Woude Syndrome. Pediatr Dermatol. 2011;29:768–770. doi: 10.1111/j.1525-1470.2011.01575.x. [DOI] [PubMed] [Google Scholar]
- Mitchell K, O’Sullivan J, Missero C, Blair E, Richardson R, Anderson B, Antonini D, Murray JC, Shanske AL, Schutte BC, Romano RA, Sinha S, Bhaskar SS, Black GC, Dixon J, Dixon MJ. Exome sequence identifies RIPK4 as the Bartsocas-Papas syndrome locus. Am J Hum Genet. 2011;90:69–75. doi: 10.1016/j.ajhg.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti F, Marinari B, Lo Iacono N, Botti E, Giunta A, Spallone G, Garaffo G, Vernersson-Lindahl E, Merlo G, Mills AA, Ballaro C, Alema S, Chimenti S, Guerrini L, Costanzo A. A regulatory feedback loop involving p63 and IRF6 links the pathogenesis of 2 genetically different human ectodermal dysplasias. J Clin Invest. 2010;120:1570–1577. doi: 10.1172/JCI40267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle C, Neuner A, Park J, Pacho F, Jiang Q, Waddington SN, Schneider H. Evaluation of prenatal intra-amniotic LAMB3 gene delivery in a mouse model of Herlitz disease. Gene Ther. 2006;13:1665–1676. doi: 10.1038/sj.gt.3302832. [DOI] [PubMed] [Google Scholar]
- Mulliken JB. The changing faces of children with cleft lip and palate. N Engl J Med. 2004;351:745–747. doi: 10.1056/NEJMp048157. [DOI] [PubMed] [Google Scholar]
- Murray JC, Nishimura DY, Buetow KH, Ardinger HH, Spence MA, Sparkes RS, Falk RE, Falk PM, Gardner RJ, Harkness EM, et al. Linkage of an autosomal dominant clefting syndrome (Van der Woude) to loci on chromosome Iq. Am J Hum Genet. 1990;46:486–491. [PMC free article] [PubMed] [Google Scholar]
- Nair M, Teng A, Bilanchone V, Agrawal A, Li B, Dai X. Ovol1 regulates the growth arrest of embryonic epidermal progenitor cells and represses c-myc transcription. J Cell Biol. 2006;173:253–264. doi: 10.1083/jcb.200508196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman Z, Shulman J. Congenital sinuses of the lower lip. Oral Surg Oral Med Oral Pathol. 1961;14:1415–1420. doi: 10.1016/0030-4220(61)90243-2. [DOI] [PubMed] [Google Scholar]
- Niiya M, Endo M, Shang D, Zoltick PW, Muvarak NE, Cao W, Jin SY, Skipwith CG, Motto DG, Flake AW, Zheng XL. Correction of ADAMTS13 deficiency by in utero gene transfer of lentiviral vector encoding ADAMTS13 genes. Mol Ther. 2009;17:34–41. doi: 10.1038/mt.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Boes AD, Jabines A, Conrad AL, Canady J, Richman L, Dawson JD. Hyperactivity, impulsivity, and inattention in boys with cleft lip and palate: relationship to ventromedial prefrontal cortex morphology. J Neurodev Disord. 2010;2:235–242. doi: 10.1007/s11689-010-9060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Richman L, Andreasen N, Murray JC, Schutte B. Cognitive dysfunction in adults with Van der Woude syndrome. Genet Med. 2007a;9:213–218. doi: 10.1097/gim.0b013e3180335abd. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Richman L, Andreasen NC, Murray JC, Schutte B. Abnormal brain structure in adults with Van der Woude syndrome. Clin Genet. 2007b;71:511–517. doi: 10.1111/j.1399-0004.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- Paranaiba LM, Martelli-Junior H, Oliveira Swerts MS, Line SR, Coletta RD. Novel mutations in the IRF6 gene in Brazilian families with Van der Woude syndrome. Int J Mol Med. 2008;22:507–511. [PubMed] [Google Scholar]
- Pengelly RJ, Upstill-Goddard R, Arias L, Martinez J, Gibson J, Knut M, Collins AL, Ennis S, Collins A, Briceno I. Resolving clinical diagnoses for syndromic cleft lip and/or palate phenotypes using whole-exome sequencing. Clin Genet: John Wiley & Sons Ltd; 2014. pp. 1–9. [DOI] [PubMed] [Google Scholar]
- Peyrard-Janvid M, Leslie EJ, Kousa YA, Smith TL, Dunnwald M, Magnusson M, Lentz BA, Unneberg P, Fransson I, Koillinen HK, Rautio J, Pegelow M, Karsten A, Basel-Vanagaite L, Gordon W, Andersen B, Svensson T, Murray JC, Cornell RA, Kere J, Schutte BC. Dominant mutations in GRHL3 cause Van der Woude Syndrome and disrupt oral periderm development. Am J Hum Genet. 2014;94:23–32. doi: 10.1016/j.ajhg.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimov F, Marazita ML, Visel A, Cooper ME, Hitchler MJ, Rubini M, Domann FE, Govil M, Christensen K, Bille C, Melbye M, Jugessur A, Lie RT, Wilcox AJ, Fitzpatrick DR, Green ED, Mossey PA, Little J, Steegers-Theunissen RP, Pennacchio LA, Schutte BC, Murray JC. Disruption of an AP-2alpha binding site in an IRF6 enhancer is associated with cleft lip. Nat Genet. 2008;40:1341–1347. doi: 10.1038/ng.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo G, Nguyen BC, Dziunycz P, Ristorcelli E, Ryan RJ, Ozuysal OY, Di Piazza M, Radtke F, Dixon MJ, Hofbauer GF, Lefort K, Dotto GP. IRF6 is a mediator of Notch pro-differentiation and tumour suppressive function in keratinocytes. EMBO J. 2011;30:4571–4585. doi: 10.1038/emboj.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Jiang R, Dixon MJ. Integration of IRF6 and Jagged2 signalling is essential for controlling palatal adhesion and fusion competence. Hum Mol Genet. 2009;18:2632–2642. doi: 10.1093/hmg/ddp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Malhotra S, Hardman MJ, Knowles L, Boot-Handford RP, Shore P, Whitmarsh A, Dixon MJ. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006;38:1329–1334. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Hammond NL, Coulombe PA, Saloranta C, Nousiainen HO, Salonen R, Berry A, Hanley N, Headon D, Karikoski R, Dixon MJ. Periderm prevents pathological epithelial adhesions during embryogenesis. J Clin Invest. 2014;124:3891–3900. doi: 10.1172/JCI71946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BM, Mansilla MA, Ma J, Daack-Hirsch S, Maher BS, Raffensperger LM, Russo ET, Vieira AR, Dode C, Mohammadi M, Marazita ML, Murray JC. Impaired FGF signaling contributes to cleft lip and palate. Proc Natl Acad Sci U S A. 2007;104:4512–4517. doi: 10.1073/pnas.0607956104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittinger K, Budman J, Xu J, Volinia S, Cantley LC, Smerdon SJ, Gamblin SJ, Yaffe MB. Structural analysis of 14-3-3 phosphopeptide complexes identifies a dual role for the nuclear export signal of 14-3-3 in ligand binding. Mol Cell. 1999;4:153–166. doi: 10.1016/s1097-2765(00)80363-9. [DOI] [PubMed] [Google Scholar]
- Rosen H, Chiou GJ, Stoler JM, Mulliken JB, Tarui T, Meara JG, Estroff JA. Magnetic Resonance Imaging for Detection of Brain Abnormalities in Fetuses With Cleft Lip and/or Cleft Palate. Cleft Palate Craniofac J. 2011;48:619–622. doi: 10.1597/09-262. [DOI] [PubMed] [Google Scholar]
- Rountree RB, Willis CR, Dinh H, Blumberg H, Bailey K, Dean C, Jr, Peschon JJ, Holland PM. RIP4 regulates epidermal differentiation and cutaneous inflammation. J Invest Dermatol. 2010;130:102–112. doi: 10.1038/jid.2009.223. [DOI] [PubMed] [Google Scholar]
- Sabel JL, d’Alencon C, O’Brien EK, Van Otterloo E, Lutz K, Cuykendall TN, Schutte BC, Houston DW, Cornell RA. Maternal Interferon Regulatory Factor 6 is required for the differentiation of primary superficial epithelia in Danio and Xenopus embryos. Dev Biol. 2009;325:249–262. doi: 10.1016/j.ydbio.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahshourifar I, Halim AS, Sulaiman WA, Ariffin R, Naili Muhamad Nor N, Zilfalil BA. De novo interstitial deletion of 1q32.2-q32.3 including the entire IRF6 gene in a patient with oral cleft and other dysmorphic features. Cytogenet Genome Res. 2011;134:83–87. doi: 10.1159/000325541. [DOI] [PubMed] [Google Scholar]
- Sander A, Schmelzle R, Murray J. Evidence for a microdeletion in 1q32–41 involving the gene responsible for Van der Woude syndrome. Hum Mol Genet. 1994;3:575–578. doi: 10.1093/hmg/3.4.575. [DOI] [PubMed] [Google Scholar]
- Schneider EL. Lip pits and congenital absence of second premolars: varied expression of the Lip Pits syndrome. J Med Genet. 1973;10:346–349. doi: 10.1136/jmg.10.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature. 1996;381:235–238. doi: 10.1038/381235a0. [DOI] [PubMed] [Google Scholar]
- Schutte BC, Basart AM, Watanabe Y, Laffin JJ, Coppage K, Bjork BC, Daack-Hirsch S, Patil S, Dixon MJ, Murray JC. Microdeletions at chromosome bands 1q32-q41 as a cause of Van der Woude syndrome. Am J Med Genet. 1999;84:145–150. doi: 10.1002/(sici)1096-8628(19990521)84:2<145::aid-ajmg11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Schutte BC, Bjork BC, Coppage KB, Malik MI, Gregory SG, Scott DJ, Brentzell LM, Watanabe Y, Dixon MJ, Murray JC. A preliminary gene map for the Van der Woude syndrome critical region derived from 900 kb of genomic sequence at 1q32-q41. Genome Res. 2000;10:81–94. [PMC free article] [PubMed] [Google Scholar]
- Schutte BC, Sander A, Malik M, Murray JC. Refinement of the Van der Woude gene location and construction of a 3.5-Mb YAC contig and STS map spanning the critical region in 1q32-q41. Genomics. 1996;36:507–514. doi: 10.1006/geno.1996.0496. [DOI] [PubMed] [Google Scholar]
- Scioletti AP, Brancati F, Gatta V, Antonucci I, Peissel B, Pizzuti A, Mortellaro C, Tete S, Gherlone E, Palka G, Stuppia L. Two novel mutations affecting splicing in the IRF6 gene associated with van der Woude syndrome. J Craniofac Surg. 2010;21:1654–1656. doi: 10.1097/SCS.0b013e3181ef69ef. [DOI] [PubMed] [Google Scholar]
- Sertie AL, Sousa AV, Steman S, Pavanello RC, Passos-Bueno MR. Linkage analysis in a large Brazilian family with van der Woude syndrome suggests the existence of a susceptibility locus for cleft palate at 17p11.2–11.1. Am J Hum Genet. 1999;65:433–440. doi: 10.1086/302491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotelersuk V, Srichomthong C, Yoshiura K, Niikawa N. A novel mutation, 1234del(C), of the IRF6 in a Thai family with Van der Woude syndrome. Int J Mol Med. 2003;11:505–507. [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Sidoti EJ. The penetrance and variable expression of the Van der Woude syndrome: implications for genetic counseling. Cleft Palate J. 1980;17:52–57. [PubMed] [Google Scholar]
- Shriver AS, Canady J, Richman L, Andreasen NC, Nopoulos P. Structure and function of the superior temporal plane in adult males with cleft lip and palate: pathologic enlargement with no relationship to childhood hearing deficits. J Child Psychol Psychiatry. 2006;47:994–1002. doi: 10.1111/j.1469-7610.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- Song L, Dong W, Gao M, Li J, Hu M, Guo N, Huang C. A novel role of IKKalpha in the mediation of UVB-induced G0/G1 cell cycle arrest response by suppressing Cyclin D1 expression. Biochim Biophys Acta. 2010;1803:323–332. doi: 10.1016/j.bbamcr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottmann RW, Bjork BC, Doyle JB, Beier DR. Identification of a Van der Woude syndrome mutation in the cleft palate 1 mutant mouse. Genesis. 2010;48:303–308. doi: 10.1002/dvg.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D, Cortes ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC, Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L, Rangel-Escareno C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M, Lander ES, Getz G, Golub TR, Garraway LA, Grandis JR. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L-I. Faculty of Life Science. Manchester: University of Manchester; 2011. The effects of disease mutations on the transcription factor Interferon Regulatory Factor 6; p. 231. [Google Scholar]
- Taioli E, Ragin C, Robertson L, Linkov F, Thurman NE, Vieira AR. Cleft lip and palate in family members of cancer survivors. Cancer Invest. 2010;28:958–962. doi: 10.3109/07357907.2010.483510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Shikata N, Senzaki H, Shintaku M, Tsubura A. Immunohistochemical staining patterns of keratins in normal oesophageal epithelium and carcinoma of the oesophagus. Histopathology. 1995;26:45–50. doi: 10.1111/j.1365-2559.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Tan EC, Lim EC, Yap SH, Lee ST, Cheng J, Por YC, Yeow V. Identification of IRF6 gene variants in three families with Van der Woude syndrome. Int J Mol Med. 2008;21:747–751. [PubMed] [Google Scholar]
- Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- Test AR, Falls HF. Dominant inheritance of cleft lip and palate in five generations. J Oral Surg (Chic) 1947;5:292–297. [PubMed] [Google Scholar]
- Thomason HA, Zhou H, Kouwenhoven EN, Dotto GP, Restivo G, Nguyen BC, Little H, Dixon MJ, van Bokhoven H, Dixon J. Cooperation between the transcription factors p63 and IRF6 is essential to prevent cleft palate in mice. J Clin Invest. 2010;120:1561–1569. doi: 10.1172/JCI40266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting SB, Wilanowski T, Auden A, Hall M, Voss AK, Thomas T, Parekh V, Cunningham JM, Jane SM. Inositol- and folate-resistant neural tube defects in mice lacking the epithelial-specific factor Grhl-3. Nat Med. 2003;9:1513–1519. doi: 10.1038/nm961. [DOI] [PubMed] [Google Scholar]
- Tokat C, Bilkay U, Songur E, Akin Y. Van der Woude syndrome in twins. J Craniofac Surg. 2005;16:936–939. doi: 10.1097/01.scs.0000168777.01851.cd. [DOI] [PubMed] [Google Scholar]
- Umbricht CB, Evron E, Gabrielson E, Ferguson A, Marks J, Sukumar S. Hypermethylation of 14-3-3 sigma (stratifin) is an early event in breast cancer. Oncogene. 2001;20:3348–3353. doi: 10.1038/sj.onc.1204438. [DOI] [PubMed] [Google Scholar]
- Van Der Woude A. Fistula labii inferioris congenita and its association with cleft lip and palate. Am J Hum Genet. 1954;6:244–256. [PMC free article] [PubMed] [Google Scholar]
- van Rooij IA, Ocke MC, Straatman H, Zielhuis GA, Merkus HM, Steegers-Theunissen RP. Periconceptional folate intake by supplement and food reduces the risk of nonsyndromic cleft lip with or without cleft palate. Prev Med. 2004;39:689–694. doi: 10.1016/j.ypmed.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci U S A. 1989;86:1563–1567. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignale R, Araujo J, Pascal G, Reissenweber N, Abulafia J, Quadrelli R, Vaglio A, Larrandaburo M, Reyno S. Van der Woude syndrome. A case report. Pediatr Dermatol. 1998;15:459–463. doi: 10.1046/j.1525-1470.1998.1998015459.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu J, Zhang H, Xiao M, Li J, Yang C, Lin X, Wu Z, Hu L, Kong X. Novel mutations in the IRF6 gene for Van der Woude syndrome. Hum Genet. 2003;113:382–386. doi: 10.1007/s00439-003-0989-2. [DOI] [PubMed] [Google Scholar]
- Wang XF, Xiao MZ, Shi JN, Zhang HB, Hu LD, Kong XY. [IRF6 gene mutation analysis in a van Der Woude syndrome family in Henan province] Shanghai Kou Qiang Yi Xue. 2005;14:234–237. [PubMed] [Google Scholar]
- Wehby GL, Cassell CH. The impact of orofacial clefts on quality of life and healthcare use and costs. Oral Dis. 2010;16:3–10. doi: 10.1111/j.1601-0825.2009.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong FK, Gustafsson B. Popliteal pterygium syndrome in a Swedish family--clinical findings and genetic analysis with the van der Woude syndrome locus at 1q32-q41. Acta Odontol Scand. 2000;58:85–88. doi: 10.1080/000163500429334. [DOI] [PubMed] [Google Scholar]
- Wu C, Endo M, Yang BH, Radecki MA, Davis PF, Zoltick PW, Spivak RM, Flake AW, Kirschner RE, Nah HD. Intra-amniotic transient transduction of the periderm with a viral vector encoding TGFbeta3 prevents cleft palate in Tgfbeta3(−/−) mouse embryos. Mol Ther. 2012;21:8–17. doi: 10.1038/mt.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Liang KY, Hetmanski JB, Ruczinski I, Fallin MD, Ingersoll RG, Wang H, Huang S, Ye X, Wu-Chou YH, Chen PK, Jabs EW, Shi B, Redett R, Scott AF, Beaty TH. Evidence of gene-environment interaction for the IRF6 gene and maternal multivitamin supplementation in controlling the risk of cleft lip with/without cleft palate. Hum Genet. 2010;128:401–410. doi: 10.1007/s00439-010-0863-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell. 1997;91:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- Yang HY, Wen YY, Chen CH, Lozano G, Lee MH. 14-3-3 sigma positively regulates p53 and suppresses tumor growth. Mol Cell Biol. 2003;23:7096–7107. doi: 10.1128/MCB.23.20.7096-7107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XQ, Jin HX, Shi LS, Fan MW, Song GT, Fan HL, Bian Z. Identification of novel mutations of IRF6 gene in Chinese families with Van der Woude syndrome. Int J Mol Med. 2005;16:851–856. [PubMed] [Google Scholar]
- Yeetong P, Mahatumarat C, Siriwan P, Rojvachiranonda N, Suphapeetiporn K, Shotelersuk V. Three novel mutations of the IRF6 gene with one associated with an unusual feature in Van der Woude syndrome. Am J Med Genet A. 2009;149A:2489–2492. doi: 10.1002/ajmg.a.33048. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature. 1996;381:238–241. doi: 10.1038/381238a0. [DOI] [PubMed] [Google Scholar]
- Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, Ribeiro L, Caprau D, Christensen K, Suzuki Y, Machida J, Natsume N, Yoshiura K, Vieira AR, Orioli IM, Castilla EE, Moreno L, Arcos-Burgos M, Lidral AC, Field LL, Liu YE, Ray A, Goldstein TH, Schultz RE, Shi M, Johnson MK, Kondo S, Schutte BC, Marazita ML, Murray JC. Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med. 2004;351:769–780. doi: 10.1056/NEJMoa032909. [DOI] [PubMed] [Google Scholar]