Abstract
Purpose
Pre-clinical studies suggest that single nucleotide polymorphisms (SNPs) in the Fcγ receptor (FCGR) genes influence response to rituximab, but the clinical relevance of this is uncertain.
Experimental Design
We prospectively obtained specimens for genotyping in the RESORT study, where 408 previously untreated, low tumor burden follicular lymphoma (FL) patients were treated with single agent rituximab. Patients received rituximab in 4 weekly doses and responders were randomized to rituximab re-treatment (RR) upon progression versus maintenance rituximab (MR). SNP genotyping was performed in 321 consenting patients.
Results
Response rates to initial therapy and response duration were correlated with the FCGR3A SNP at position 158 (rs396991) and the FCGR2A SNP at position 131 (rs1801274). The response rate to initial rituximab was 71%. No FCGR genotypes or grouping of genotypes were predictive of initial response. 289 patients were randomized to RR (n = 143) or to MR (n = 146). With a median follow up of 5.5 years, the 3-yr response duration in the RR arm and the MR arm was 50% and 78%, respectively. Genotyping was available in 235 of 289 randomized patients. In patients receiving RR (n = 115) or MR (n =120), response duration was not associated with any FCGR genotypes or genotype combinations.
Conclusions
Based on this analysis of treatment-naïve, low tumor burden FL, we conclude that the FCGR3A and FCGR2A SNPs do not confer differential responsiveness to rituximab.
Keywords: rituximab, lymphoma, Fc gamma receptor, polymorphism
Introduction
Follicular lymphoma (FL) patients who are asymptomatic and with low tumor burden are candidates for a watch and wait strategy, as early treatment has not been shown to improve survival.(1, 2) However, single agent rituximab is often administered to these patients, with a goal of delaying the need for chemotherapy. (3–7) Rituximab, an IgG1 subclass monoclonal antibody to CD20, has revolutionized therapy of FL by improving response rates, duration of responses, and overall survival.(5, 8–13) One postulated mechanism of action is antibody-dependent cell-mediated cytotoxicity. In this process, binding of the Fcγ receptor (FCγR) on macrophages and NK cells to the Fc portion of the rituximab antibody induces phagocytosis of the target cell, to which the antibody is bound.(14)
In vitro and animal studies have suggested that variation in specific single nucleotide polymorphisms (SNPs) in the FCGR sequence might confer variable responses to rituximab, due to the efficacy of Fc binding and triggering of antibody dependent cellular cytotoxicity (ADCC).(15, 16) The FCγRIIIA receptor (CD16a) is present on NK cells, monocytes, and macrophages.(17) A valine/phenylalanine (V/F) polymorphism at amino acid position 158 of FCGR3A (rs396991) has been identified in humans, with the valine allele demonstrating higher affinity to human IgG1 than phenylalanine, resulting in enhanced antibody-dependent cellular cytotoxicity (ADCC).(18) The FCγRIIA receptor is present on monocytes and macrophages, but not NK cells.(17) A histidine/arginine (H/R) polymorphism at position 131 of FCGR2A (rs1801274) affects binding affinity of IgG2, with the histidine allele binding more strongly.
The first clinical study in FL examining the influence of these SNPs in FL suggested the FCGR3A VV genotype, (but not the FCGR2A HH genotype) was associated with improved response rates to single agent rituximab.(3) A retrospective analysis from Stanford University then reported improved response rates and time to progression in patients with a FCGR3A valine/valine (VV) genotype, and in patients with a FCGR2A histidine/histidine (HH) genotype.(19) A prospective multicenter trial of single agent rituximab for FL found the FCGR3A VV genotype was associated with event-free survival but not response rate.(4) These three studies support the hypothesis that ADCC plays in important role in cell killing and that certain polymorphisms in FCGR3A and FCGR2A may influence ADCC. However, each study had different findings on the relative importance of FCGR3A vs. FCGR2A and different findings regarding the influence on response rate and/or response duration. Using patient derived samples from E4402, the Rituximab Extended Schedule or Retreatment Trial (RESORT), we conducted a correlative analysis, evaluating the impact of these candidate SNPs in the FCGR3A and FCGR2A genes. The goal was to definitively determine the clinical significance of these two polymorphisms.
Methods
Patients
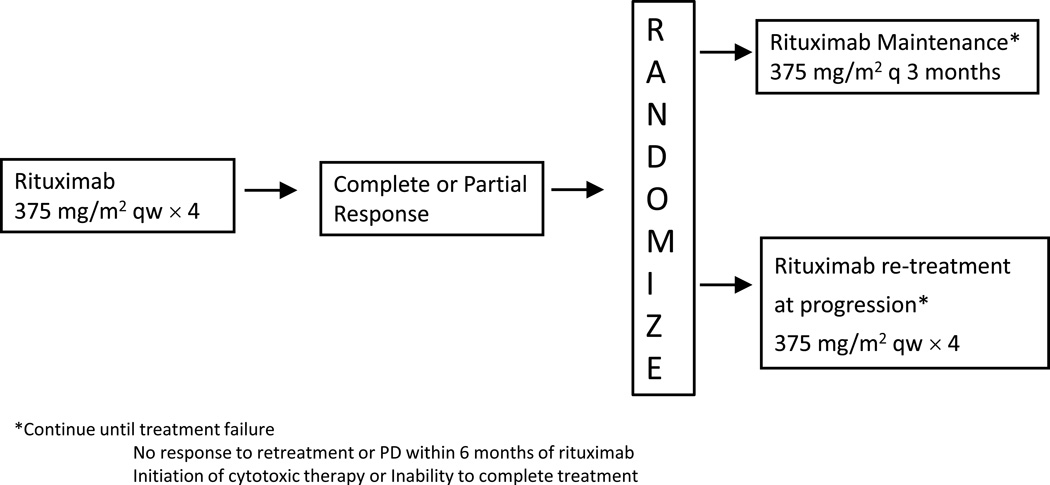
RESORT was a multi-center randomized trial that enrolled 408 FL patients between November 2003 and September 2008.(20) Patients received single agent rituximab (375mg/m2) in 4 weekly doses, followed by randomization for responders to re-treatment with rituximab upon progression (375mg/m2 × 4 weekly doses) versus maintenance rituximab (375mg/m2 once q12 weeks) (figure 1).
Figure 1.
Treatment schema for E4402 (RESORT) trial
DNA extraction and genotyping
SNP genotyping was performed to assess the FCGR3A SNP genotype for rs396991 [valine (V) or phenylalanine (F)] and the FCGR2A SNP genotype at rs1801274 [histidine (H) or arginine (R)]. Using banked peripheral blood mononuclear cells (PBMCs) (N = 212) or formalin fixed paraffin embedded tumor tissue (FFPE) (N = 109), DNA was extracted using an automated platform (AutoGen FlexStar Qiagen chemistries), followed by quantification by UV absorbance and quality control by 260/280 OD ratio and PicoGreen, and then storage in TE buffer. For SNP genotyping, samples were plated into 96 well plates and genotyped on the Taqman platform (Applied Biosystems 7900HT Fast RealTime PCR System). Sequence data and assay conditions for rs396991 (FCGR3A) and rs1801274 (FCGR2A) are provided at SNP500 (http://snp500cancer.nci.nihh.gov). Genotype data was analyzed using Applied Biosystems SDS 2.3 analysis software. Quality control samples included study replicates (5%); FCGR2A and FCGR3A known wild-type homozygotes, heterozygotes, homozygote variants; and a DNA negative control. We summarized the call rates per SNP as well as per sample, examining samples which failed in 5% or more of SNPs. SNPs which failed in 5% or more of individuals were evaluated by Sanger sequencing. Likewise, if the error rate in the subjects for whom repeated genotypes was judged to be unacceptably high (>2%), genotyping was repeated until consistent results were achieved.
The FCGR3A rs396991 SNP has been reported to be difficult to accurately genotype, due to high copy number variation (CNV) and high homology (97%) between FCGR3A and FCGR3B genes. FCGR3B carries an invariant G at the position that corresponds to FCGR3A 4985T>G and co-amplification of FCGR3A and FCGR3B can lead to SNP miscalls. Transgenomics, Inc., Omaha, NE developed primers and probes specific for genotyping the FCGR3A that does not co-amplify the FCGR3B. To test the Transgenomics proprietary technology, cases with available PBMC DNA (N = 212) were run in duplicate using both the commercial TaqMan platform and the Transgenomics platform. All PBMC cases were also subjected to Sanger sequencing, with the sequencing result used as the reference gold standard to determine assay accuracy.
Statistical Analysis
Fisher’s exact test was used to compare response rates amongst the different genotypes. A logistic regression model was employed to evaluate the polymorphism effect on response rate accounting for other patient characteristics. Duration of response (DOR) was defined as the time from documented response to documented progression and estimated using the Kaplan and Meier method. Logrank test (one-sided significance level of 0.05) was used to compare DOR. A Cox proportional hazards regression model was used to evaluate the significance of polymorphism effect on DOR after adjusting for other patient characteristics.
Results
A total of 408 FL patients were enrolled in RESORT. Of these, 321 underwent SNP genotyping, while unavailable clinical material precluded genotyping in 87 patients. There were no major differences in the baseline characteristics when comparing the genotyped population to the entire population (Table 1). PBMCs were the DNA source in 212 cases and FFPE tissue was the DNA source in 109 cases. PBMC DNA was subjected to genotyping by both commercially available Taqman (TM) technology and Transgenomics (TG) proprietary pyrosequencing technology. Using TM for genotyping the FCGR3A SNP, three cases could not be genotyped and 18 cases were miscalled (using sequencing as the gold standard) for an accuracy rate of 90% (191/212). Using TG genotyping for the FCGR3A SNP, there were 9 cases that could not be genotyped and 1 case miscalled for an accuracy rate of 95% (202/212). Using TM for genotyping the FCGR2A SNP, five cases could not be genotyped and three cases were miscalled for an accuracy rate of 96% (204/212). Using TG for genotyping the FCGR2A SNP, there were no failures to genotype and there was 1 miscall for an accuracy rate of 99% (211/212). Including the 109 FFPE cases and the 212 PBMC cases, and after adjudicating discrepant PBMC cases by sequencing, the final FCGR3A and FCGR2A genotype frequencies were VV 14%, VF 45%, FF 40% and HH 28%, HR 47%, RR 22%, respectively.
Table 1.
Patient characteristics for patients from RESORT trial as well as subset who had SNP sequencing
| Patient characteristics | All follicular lymphoma patients on RESORT study (n = 408) |
Follicular lymphoma patients on study with SNP sequencing (n = 321) |
|---|---|---|
| % | % | |
| Age (Median, range) | 58 (25–86) | 60 (25–86) |
| Gender (M/F) | 48/52 | 46/54 |
| PS (0/1) | 85/15 | 86/14 |
| Stage | ||
| III | 50 | 49 |
| IV | 49 | 50 |
| FLIPI | ||
| 0–1 | 18 | 17 |
| 2 | 46 | 48 |
| 3–5 | 36 | 35 |
| B2M elevated | 41 | 42 |
The overall response rate (ORR) to initial rituximab was 71%. The likelihood of obtaining a complete response or any response was not correlated with FCGR3A genotype (VV vs. VF vs. FF, or VV vs. F carrier) (Table 2). Similarly, the likelihood of obtaining a complete response or any response was not correlated with FCGR2A genotype (HH vs. HR vs. RR, or HH vs. R carrier) (Table 3). Additionally, no combination of genotypes (e.g., VV/HH vs. FF/RR) was associated with complete response or overall response rates (data not shown).
Table 2.
Induction response by FCGR3A genotype. 3 out of 321 samples have no FCGR3A data
| VV (n = 43) | VF (n = 145) | FF (n = 130) | p value | |
|---|---|---|---|---|
| CR | 4 (9%) | 19 (13%) | 15 (11%) | 0.78 |
| ORR | 33 (76%) | 106 (73%) | 97 (74%) | 0.88 |
| SD | 6 (14%) | 33 (23%) | 25 (19%) | |
| PD | 2 (5%) | 3 (2%) | 3 (2%) |
Table 3.
Induction response by FCGR2A genotype. 6 out of 321 samples have no FCGR2A data
| HH (n = 91) | HR (n = 153) | RR (n = 71) | p value | |
|---|---|---|---|---|
| CR | 9 (10%) | 18 (12%) | 11 (16%) | 0.54 |
| ORR | 65 (72%) | 113 (74%) | 53 (76%) | 0.86 |
| SD | 17 (19%) | 33 (22%) | 13 (19%) | |
| PD | 5 (6%) | 2 (1%) | 1 (1%) |
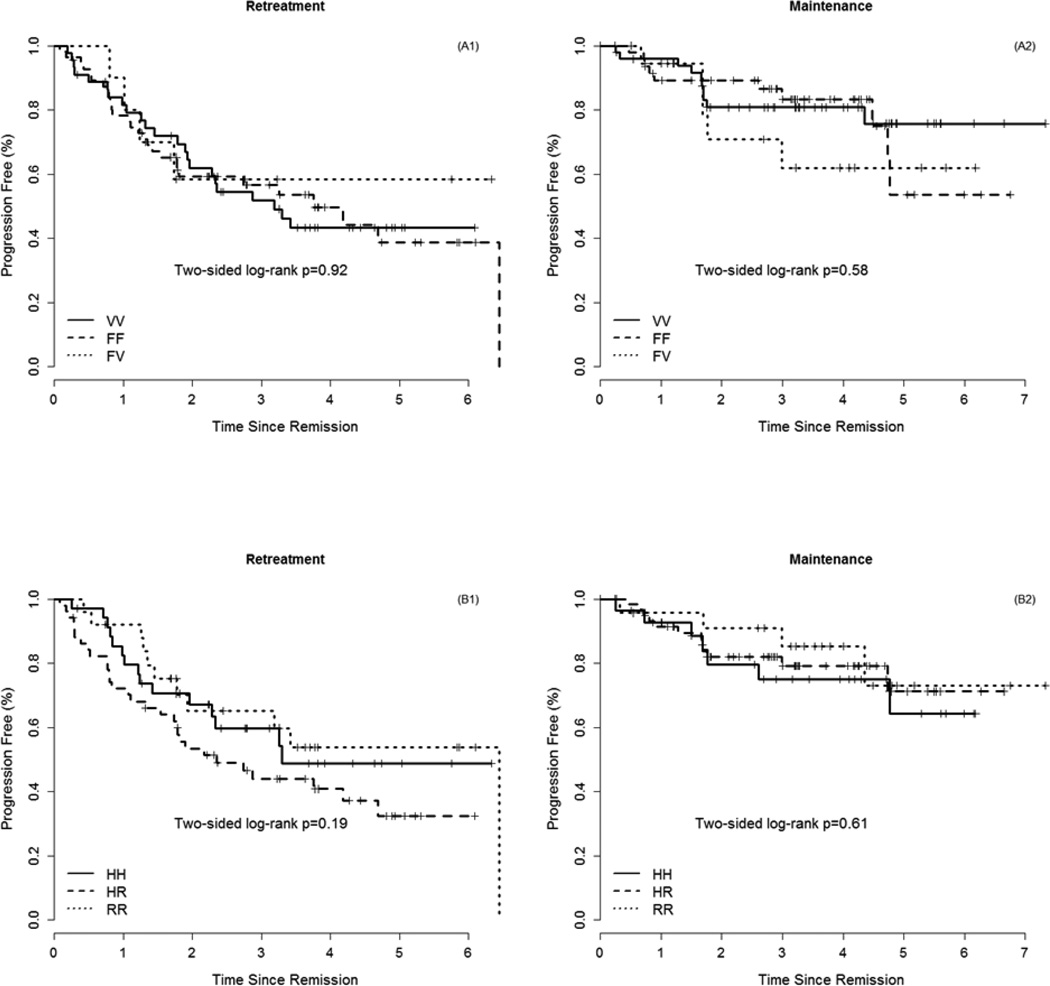
Genotyping was performed in 235 of 289 randomized patients, RR (n = 115) or to MR (N = 120). With a median follow up of 5.5 years, the 3-yr response duration in the RR arm and the MR arm was 50% and 78%, respectively.. The FCGR3A genotype was not associated with response duration in the RR (p = 0.92) or the MR (p = 0.58) treatment arms (Fig 2A). Similarly, the FCGR2A genotype was not associated with response duration in the RR (p = 0.19) or the MR (p = 0.61) treatment arms (Fig 2B). Additionally, no combination of genotypes (e.g., VV/HH vs. FF/RR) influenced duration of response or survival (data not shown).
Figure 2.
Response duration by FCGR3A genotype on RR arm (A1) and MR arm (A2) and by FCGR2A genotype on RR arm (B1) and MR arm (B2)
Discussion
Based on this analysis of a treatment-naïve population of low tumor burden FL, we conclude that the two candidate missense SNPs in FCGR3A (rs396991) and FCGR2A (rs1801274), alone or in combination, does not predict the likelihood or the durability of response to single agent rituximab. These data are in contrast to three early reports examining this question and suggesting these polymorphisms impact the efficacy of rituximab.
In a study with a patient population similar to ours, Cartron et aldetermined the FCGR3A and FCGR2A genotypes in 49 patients receiving rituximab.(3) The objective response rates at 12 months were 90% for VV patients and 51% for F carriers (P=0.03). PFS at 3 years was 56% for VV patients and 35% for F carriers (p = NS). There was no impact of the FCGR2A polymorphism on outcome. A retrospective analysis of 87 FL patients receiving single agent rituximab at Stanford University demonstrated that FCGR3A V/V patients (n = 13) and FCGR2A H/H patients (n = 20) experienced higher response rates and more durable remissions compared to FCGR3A F carriers and FCGR2A H carriers, respectively.(19)
Finally, a retrospective analysis from the SAKK (Swiss group for Clinical Cancer Research), in which patients with follicular and mantle cell lymphoma were treated with single agent rituximab, followed by no further treatment or maintenance rituximab, the FCGR3A V/V genotype was associated with superior event-free survival.(4) Because these studies were retrospective, relatively small with a heterogeneous FL population, the true impact of these polymorphisms remained unclear. Analysis of the RESORT patients was an ideal opportunity to evaluate these findings in a large prospective study of homogeneous FL patients. The findings from the present work should be considered definitive and highlight the importance of prospective studies in homogenous populations and with adequate power to identify predictive biomarkers.
Consistent with our findings, another large prospective study evaluating these polymorphisms in FL patients receiving single agent rituximab found no association with response rate or response duration.(21) This United Kingdom sponsored intergroup trial randomized low tumor burden and asymptomatic FL to “watch and wait” versus rituximab induction alone versus rituximab induction followed by rituximab maintenance. No difference was seen in the 257 patients for whom FCGR genotyping was available with respect to CR rate, time to next treatment, or progression free survival.
Analyses of previously untreated high tumor burden follicular lymphoma patients treated with combination rituximab-chemotherapy, similarly found no impact of these SNPs on response rate, progression, or overall survival.(22, 23) The PRIMA study,(24) which showed an improvement in progression-free survival (PFS) after maintenance rituximab in patients who were treated with immunochemotherapy induction, was designed with pre-specified objectives to clarify the role of FCGR polymorphisms in this context. The PRIMA investigators also found no impact of different FCGR genotypes on response rates to initial immunochemotherapy or maintenance rituximab, nor any difference in PFS at any timepoint.(22)
Our results were somewhat unexpected given the strong preclinical data suggesting the FCGR3A 158V/F polymorphism positively influences both the binding of IgG as well as the expression of CD16 by NK cells. (18, 19) It is possible that the models do not accurately recapitulate the human tumor environment or it is possible that ADCC is not the major mechanism of cell killing after rituximab therapy. Rituximab’s precise mechanism of action remains ill-defined and could include components of ADCC, complement mediated cytotoxicity, and direct killing. (13, 15), (13, 25, 26) Direct effects attributed to rituximab include inhibition of cell proliferation, induction of phosphatidylserine translocation, cell signaling via increased phosphorylation, and induction of apoptosis.(27)
Many factors can influence FL response to rituximab, including biologic heterogeneity, clonal evolution, tumor bulk, prior treatments, and host factors. This analysis attempted to minimize many of these factors, by evaluating a relatively homogeneous FL population with low tumor burden, no prior therapy, and protocolized treatment and follow-up of patients. Despite this, we were unable to find a differential response based on FCGR genotype at the two candidate loci, as hypothesized by pre-clinical and small clinical studies. While this does not preclude a role of other genetic variation in these genes in treatment response and outcomes, none has been identified to date. Given these definitive results, we conclude that the selection of patients for single agent rituximab therapy should not be based upon these FCGR3A or FCGR2A genotype status and these data raise additional questions regarding the mechanism of rituximab cytotoxicity.
Translational Relevance.
Presented here are results of a correlative science study from the E4402 (RESORT) study, which indicate a lack of predictive value of Fc gamma receptor polymorphisms in determining rituximab response in patients with previously untreated, low tumor burden follicular lymphoma. Prior studies have indicated differential responses to rituximab based on these polymorphisms. However, the prior studies tended to be small, retrospective analyses with heterogeneous patient populations. The submitted study was a prospectively planned evaluation of a large, homogeneous follicular lymphoma population, all treated with single agent rituximab. We were unable to find a differential response based on FCGR genotype or any combination of genotype. This manuscript presents definitive results, concluding that FCGR3A and 2A should not be used to select patients for single agent rituximab therapy.
Acknowledgments
Financial Support: This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180820, CA180794, CA180799, CA180790, CA180847, CA180816 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. This work was supported by a grant from the Lymphoma Research Foundation and the University of Wisconsin Forward Lymphoma Fund.
Footnotes
Conflict of Interest disclosure: MEW has research funding from Genentech, RDG consults for Genentech/Roche, Celgene, Janssen, Seattle Genetics and is part of speakers bureau of Seattle Genetics, SJH is employed by Genentech/Roche, BSK consults for Genentech/Roche. The remaining authors declare no competing financial interests.
References
- 1.Ardeshna KM, Smith P, Norton A, Hancock BW, Hoskin PJ, MacLennan KA, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet. 2003 Aug 16;362(9383):516–522. doi: 10.1016/s0140-6736(03)14110-4. PubMed PMID: 12932382. [DOI] [PubMed] [Google Scholar]
- 2.Brice P, Bastion Y, Lepage E, Brousse N, Haioun C, Moreau P, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997 Mar;15(3):1110–1117. doi: 10.1200/JCO.1997.15.3.1110. PubMed PMID: 9060552. [DOI] [PubMed] [Google Scholar]
- 3.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002 Feb 1;99(3):754–758. doi: 10.1182/blood.v99.3.754. PubMed PMID: 11806974. [DOI] [PubMed] [Google Scholar]
- 4.Ghielmini M, Rufibach K, Salles G, Leoncini-Franscini L, Leger-Falandry C, Cogliatti S, et al. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2005 Oct;16(10):1675–1682. doi: 10.1093/annonc/mdi320. PubMed PMID: 16030029. [DOI] [PubMed] [Google Scholar]
- 5.Hainsworth JD, Burris HA, 3rd, Morrissey LH, Litchy S, Scullin DC, Jr, Bearden JD, 3rd, et al. Rituximab monoclonal antibody as initial systemic therapy for patients with low-grade non-Hodgkin lymphoma. Blood. 2000 May 15;95(10):3052–3056. PubMed PMID: 10807768. [PubMed] [Google Scholar]
- 6.Friedberg JW, Taylor MD, Cerhan JR, Flowers CR, Dillon H, Farber CM, et al. Follicular lymphoma in the United States: first report of the national LymphoCare study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Mar 10;27(8):1202–1208. doi: 10.1200/JCO.2008.18.1495. PubMed PMID: 19204203. Pubmed Central PMCID: 2738614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardeshna KM, Qian W, Smith P, Braganca N, Lowry L, Patrick P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. The lancet oncology. 2014 Apr;15(4):424–435. doi: 10.1016/S1470-2045(14)70027-0. PubMed PMID: 24602760. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998 Aug;16(8):2825–2833. doi: 10.1200/JCO.1998.16.8.2825. PubMed PMID: 9704735. [DOI] [PubMed] [Google Scholar]
- 9.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997 Sep 15;90(6):2188–2195. PubMed PMID: 9310469. [PubMed] [Google Scholar]
- 10.Colombat P, Salles G, Brousse N, Eftekhari P, Soubeyran P, Delwail V, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001 Jan 1;97(1):101–106. doi: 10.1182/blood.v97.1.101. PubMed PMID: 11133748. [DOI] [PubMed] [Google Scholar]
- 11.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998 Sep 15;92(6):1927–1932. PubMed PMID: 9731049. [PubMed] [Google Scholar]
- 12.Foran JM, Rohatiner AZ, Cunningham D, Popescu RA, Solal-Celigny P, Ghielmini M, et al. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000 Jan;18(2):317–324. doi: 10.1200/JCO.2000.18.2.317. PubMed PMID: 10637245. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DR, Grillo-Lopez A, Varns C, Chambers KS, Hanna N. Targeted anti-cancer therapy using rituximab, a chimaeric anti-CD20 antibody (IDEC-C2B8) in the treatment of non-Hodgkin's B-cell lymphoma. Biochemical Society transactions. 1997 May;25(2):705–708. doi: 10.1042/bst0250705. PubMed PMID: 9191187. [DOI] [PubMed] [Google Scholar]
- 14.Maloney DG, Smith B, Rose A. Rituximab: mechanism of action and resistance. Seminars in oncology. 2002 Feb;29(1) Suppl 2:2–9. PubMed PMID: 11842383. [PubMed] [Google Scholar]
- 15.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nature medicine. 2000 Apr;6(4):443–446. doi: 10.1038/74704. PubMed PMID: 10742152. [DOI] [PubMed] [Google Scholar]
- 16.Hatjiharissi E, Hansen M, Santos DD, Xu L, Leleu X, Dimmock EW, et al. Genetic linkage of Fc gamma RIIa and Fc gamma RIIIa and implications for their use in predicting clinical responses to CD20-directed monoclonal antibody therapy. Clinical lymphoma & myeloma. 2007 Jan;7(4):286–290. doi: 10.3816/clm.2007.n.004. PubMed PMID: 17324336. [DOI] [PubMed] [Google Scholar]
- 17.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004 Nov 1;104(9):2635–2642. doi: 10.1182/blood-2004-03-1110. PubMed PMID: 15226177. [DOI] [PubMed] [Google Scholar]
- 18.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997 Aug 1;90(3):1109–1114. PubMed PMID: 9242542. [PubMed] [Google Scholar]
- 19.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. Journal of clinical oncology. 2003 Nov 1;21(21):3940–3947. doi: 10.1200/JCO.2003.05.013. PubMed PMID: 12975461. [DOI] [PubMed] [Google Scholar]
- 20.Kahl BS, Hong F, Williams ME, Gascoyne RD, Wagner LI, Krauss JC, et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: eastern cooperative oncology group protocol e4402. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Oct 1;32(28):3096–3102. doi: 10.1200/JCO.2014.56.5853. PubMed PMID: 25154829. Pubmed Central PMCID: 4171355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry LPM, Ardeshna K, et al., editors. International Conference on Malignant Lymphoma. Lugano, Switzerland: Annals of Oncology; 2011. FCgR polymorphisms do not influence response to rituximab in asymptomatic, non-bulky follicular lymphoma; results from the intergroup trial of rituximab vs "watch and wait". [Google Scholar]
- 22.Ghesquieres H, Cartron G, Seymour JF, Delfau-Larue MH, Offner F, Soubeyran P, et al. Clinical outcome of patients with follicular lymphoma receiving chemoimmunotherapy in the PRIMA study is not affected by FCGR3A and FCGR2A polymorphisms. Blood. 2012 Sep 27;120(13):2650–2657. doi: 10.1182/blood-2012-05-431825. PubMed PMID: 22885164. [DOI] [PubMed] [Google Scholar]
- 23.Carlotti E, Palumbo GA, Oldani E, Tibullo D, Salmoiraghi S, Rossi A, et al. FcgammaRIIIA and FcgammaRIIA polymorphisms do not predict clinical outcome of follicular non-Hodgkin's lymphoma patients treated with sequential CHOP and rituximab. Haematologica. 2007 Aug;92(8):1127–1130. doi: 10.3324/haematol.11288. PubMed PMID: 17650444. [DOI] [PubMed] [Google Scholar]
- 24.Salles G, Seymour JF, Offner F, Lopez-Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011 Jan 1;377(9759):42–51. doi: 10.1016/S0140-6736(10)62175-7. PubMed PMID: 21176949. [DOI] [PubMed] [Google Scholar]
- 25.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994 Jan 15;83(2):435–445. PubMed PMID: 7506951. [PubMed] [Google Scholar]
- 26.Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000 Jun 15;95(12):3900–3908. PubMed PMID: 10845926. [PubMed] [Google Scholar]
- 27.Maloney DG. Mechanism of action of rituximab. Anti-cancer drugs. 2001 Jun;12(Suppl 2):S1–S4. PubMed PMID: 11508930. [PubMed] [Google Scholar]