Abstract
Two cases are presented using a two-stage approach and a custom antibiotic spacer placement. Temporomandibular reconstruction can be very demanding and accomplished with a variety of methods in preparation of a total joint and ramus reconstruction with total joint prostheses (TMJ Concepts, Ventura, CA). Three-dimensional reconstructions from diagnostic computed tomography were used to establish a virtually planned resection which included the entire condyle-ramus complex. From these data, digital designs were used to manufacture molds to facilitate intraoperative fabrication of precise custom anatomic spacers from rapidly setting antibiotic-impregnated polymethyl methacrylate. Molds were manufactured using vat polymerization (stereolithography) with a photopolymer in the first case and powder bed fusion (electron beam melting) with Ti6AL4V for the second. Surgical methodology and the use of molds for intraoperative spacer fabrication for each case are discussed.
Keywords: additive manufacturing, antibiotic spacer, joint resection, virtual surgery, 3D printing, TMJ Concepts
Osteomyelitis of the mandible is an uncommon but potentially devastating result of odontogenic infection, trauma, hardware failure, radiation or bisphosphonate-related osteonecrosis, and superinfection. Regardless of etiology, treatment typically involves long-term intravenous antibiotics and resection of affected bone and involved teeth. Delayed reconstruction after resolution of the infection is commonly employed with a two-stage approach to account for the unpredictability of resection margins as well as resolution of infection that would compromise any immediate autogenous or alloplastic graft. This treatment course combined with the use of implantable antibiotic infused beads has been well documented in the orthopedic literature for the treatment of infected joints.1 2
Strategies for maintaining anatomic relationships in cases of condylar resection and continuity defects include placement of a reconstruction bar with or without a condyle attachment and/or a polymethylmethacrylate (PMMA) spacer.3 PMMA has been used for more than 70 years in various surgical applications and antibiotic-eluting PMMA beads and spacers are currently a standard practice in orthopedic surgery. 4 5 Two cases are presented with an innovative use of virtual-assisted surgery, digital design, and additive manufacturing technologies (AMT) for immediate and morphologically accurate replacement of a resected condyle-ramus complex with an antibiotic-eluting spacer where staged reconstructions were necessitated.
Case 1
A 20-year-old man with an odontogenic infection resulting in right mandibular osteomyelitis confirmed with radiography and biopsy was admitted for initiation of long-term antibiotics and resection of the affected bone. Computed tomography (CT) imaging demonstrated cortical and medullary destruction of the lateral and medial ramus from the surface of the condyle to the inferior border immediately posterior to the second molar. Multiple pathologic fractures were evident in the ramus and condylar head (Fig. 1). A plan was developed guided by established protocol for chronic osteomyelitis as described by Wolford et al: (1) culture and microscopic exam with intraoral drain placement, start of empiric broad-spectrum long-term intravenous antibiotics with consult to the Infectious Diseases Service; (2) resection of the condyle/ramus complex with placement of antibiotic-impregnated PMMA spacer; and (3) secondary reconstruction with patient-fitted custom joint prosthesis.6
Fig. 1.
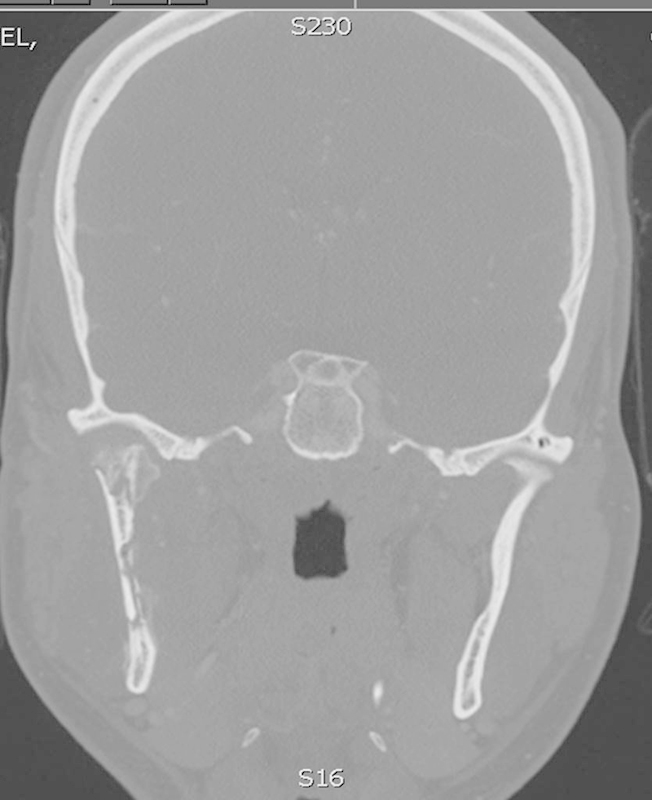
Coronal image of CT scan taken after patient reported mild continuing symptoms which failed to definitively resolve after two incision and drainage procedures. Image shows mottled bone of the ramus and condyle with early pathologic fracturing secondary to osteomyelitis. CT, computed tomography.
After a thorough discussion of the considerations regarding reconstruction with autologous materials and the options for alloplastic reconstruction, the patient elected to proceed with patient-fitted total joint reconstruction. The alloplast option was favored for its absence of donor site morbidity. As indicated by the plan, a PMMA spacer was to be fabricated using a digitally designed and manufactured mold. The initial step in virtual planning is to import and reconstruct the CT scan. The patient's CT was previously acquired using standard protocols at 1.4-mm-thick slices, for radiologic diagnosis purposes. These Digital Imaging and Communications in Medicine (DICOM) images were imported and a 3D reconstruction of the bony tissue was performed using MIMICS software (Materialise, Ann Arbor, MI). Manipulating the 3D digital model, the pathologic cortical defects and fractures were filled and the bony surface was smoothed. The area intended for resection, containing the areas of the osteomyelitis and an additional 5 mm margin, was then isolated and saved as a separate digital model. A digital mold was designed using the digital model of the planned resection, parting lines, free sculpted digital clay, a digital box, and multiple Boolean operations (Magics, Materialise and FreeForm Modeling Plus, SensAble Technologies, Woburn, MA).
The mold was processed on a build platform using Light Year (3D Systems, Rock Hill, SC) and manufactured from a class VI compliant resin (Acura ABS White SL 7810, 3D Systems) using a SLA 500 Stereolithography Apparatus (3D Systems) cavity side up to ensure the smoothest possible surface. Manual surface finishing was followed by post-curing (Post Curing Apparatus, 3D Systems). Ethylene oxide protocols were used for final sterilization.
Surgical approaches for this patient consisted of preauricular and intraoral incisions which were connected via dissection along the lateral ramus. The condyle/ramus complex was noted, consistent with imaging findings, to be severely degraded on exposure due to the infection and was resected as virtually planned. The mandible was resected anteriorly with a nerve-sparing sagittal split technique and the coronoid was removed. The margins were finally confirmed and adapted during the surgical exposure with direct visualization and intraoperative radiographs. During the exposure and removal of the condylar and mandibular segments, the spacer was prepared on the back table. The spacer design was planned for a slightly larger resection margin than anticipated to permit intraoperative adaptation of the spacer's anterior border to clinically clear margins displaying vital, bleeding bone. To assist with recovery and to prevent bonding of the uncured PMMA to the polymer of the impression mold, sterile surgical foil was adapted to the surface of the impression sides of the gas sterilized resin mold and the foil was then lubricated with a thin layer of petrolatum-based bacitracin ointment. The PMMA compound was prepared by mixing 40 g of Cobalt cement (Biomet Inc., Warsaw, IN) with 0.6 g tobramycin powder pressed into the mold, the mold was closed and the halves compressed for 20 minutes. The spacer was then recovered (Fig. 2), the excess and all anatomy not included in the resection, to include the duplicated coronoid process, was removed with a large acrylic bur. A medial longitudinal depression was made to accommodate the inferior alveolar nerve. After immersion in clindamycin solution, the spacer was then inserted with visualization of the condylar head into the fossa via the preauricular incision and aligned with the intact margin of remaining vital mandible. The inferior alveolar nerve was preserved and medialized along the prepared channel.
Fig. 2.
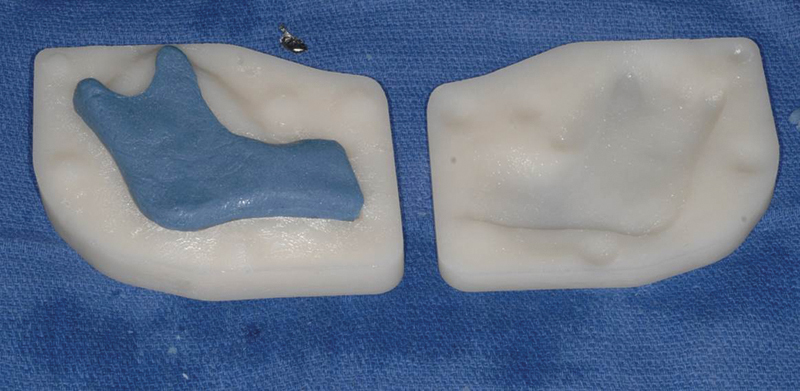
Resin spacer demonstrated immediately after fabrication before any intraoperative reduction, a sterile foil lining is required at fabrication to keep the PMMA from adhering to the resin. PMMA, polymethyl methacrylate.
The decision was made to avoid hardware placement into the site during the initial debridement and spacer placement. To prevent rotation of the segment, loading of the condyle, and changes in occlusion, the patient was placed into secure maxillomandibular fixation (MMF). The patient had excellent occlusion and no history of periodontal disease or clinical evidence of disease extension to the regions of the mandibular dentition; therefore, no teeth were included in the resection. Four standard fixation screws and simple 25-gauge wire loops were used to maintain MMF during surgery and in the planned period of immobilization (Fig. 3).
Fig. 3.
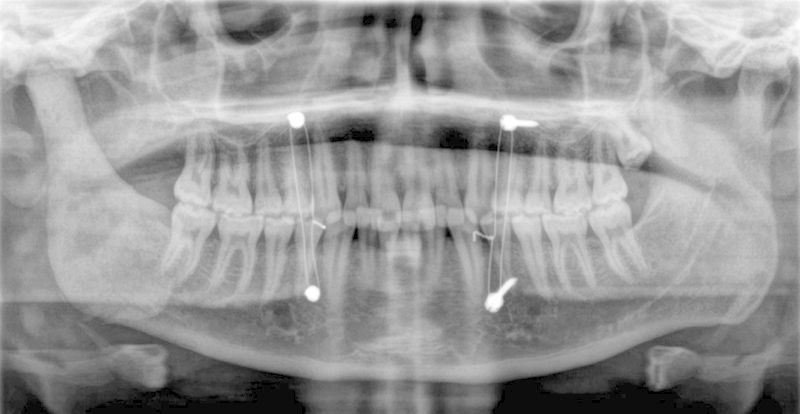
Panoramic radiograph of patient in Case 1 featured immediately postoperatively in maxillomandibular fixation. Note the lack of any fixation of the spacer as well as the excellent symmetry and condyle/fossa relationship when compared with the healthy condyle.
The patient tolerated the procedure well and was discharged the following day with a home intravenous (IV) antibiotic regimen and weekly clinic follow-up. A dehiscence of the intraoral closure was noted on the second week postoperatively due to rotation of the spacer superiorly. This was treated with suturing under local anesthetic but was noted again on week 3. Under IV sedation, the spacer was exposed through the previous intraoral incision and reduction of the anterior border of the ramus with repositioning of the spacer was performed. Placement of a temporary anchorage device screw to the mandibular body with wire fixation resolved the dehiscence and the tendency of the spacer to superior displacement. The intraoral wound was closed in layers and the patient healed without further issues.
Case 2
A 28-year-old woman with a history of central giant cell granuloma recurrences, having undergone previous failed autologous costochondral reconstruction of the temporomandibular complex, declined additional attempts at autologous reconstruction. Due to the retained reconstruction hardware, the decision was made to perform the resection of the failed reconstruction and alloplastic reconstruction in a staged fashion. By staging the procedure, any error that would be induced in the CT scans' DICOM data from the retained hardware would be eliminated. Accuracy of imaging used for the stereolithographic pattern of the final prosthesis would enable the most accurate and rapidly integrated prosthetic solution for this young, active patient (Fig. 4).
Fig. 4.
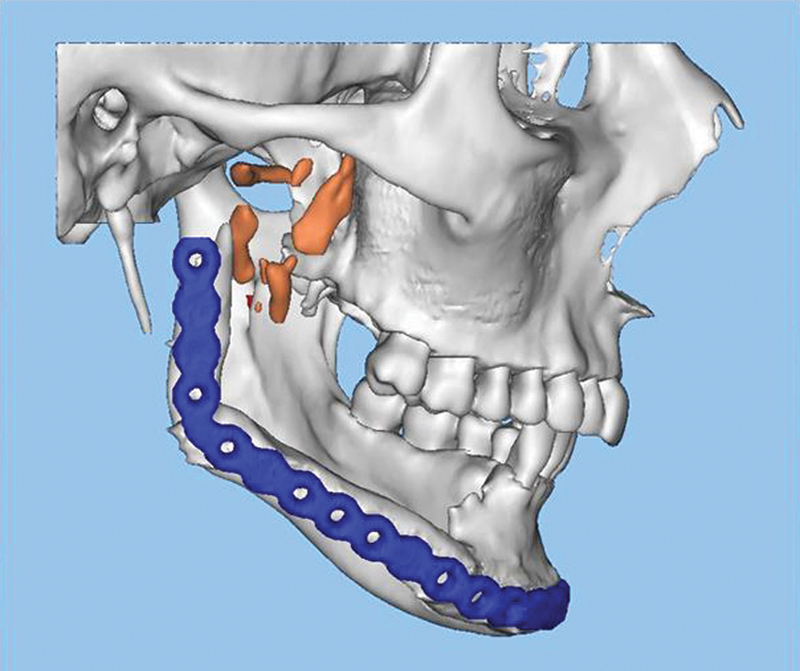
Preoperative 3D reconstruction from the cone beam CT scan of Case 2 demonstrating the retained hardware was well as multiple fragmented segments from the initial reconstruction. CT, computed tomography; 3D, three-dimensional.
The resection and reconstruction plans were performed in similar fashion to Case 1 with the exception of modifications which were gleaned from the first procedure. First, combined preauricular and submandibular approaches were used for both the resection and reconstruction. No intraoral incisions were made, due to the previous surgical removal of the native ramus and any need for a coronoidectomy as well as the presence of a pre-existing submandibular incision.
Modifications were made from the experiences of the previous case; the coronoid process was removed from the virtual model and the spacer mold design to eliminate intraoperative modification of the PMMA. To avoid some of the issue with the previous polymer mold, the mold was fabricated of Ti6AL4V with electron beam melting AMT (Figs. 5 and 6). The spacer was fabricated in the same manner as the previous case; however, no sterile foil was needed. Finally, the spacer was secured to the inferior border of the native mandible with a single surgical wire to prevent upward displacement and tissue breakdown from the pull of the pterygomasseteric sling on the spacer. As a result, this patient had no postoperative complications of spacer dehiscence before definitive reconstruction.
Fig. 5.
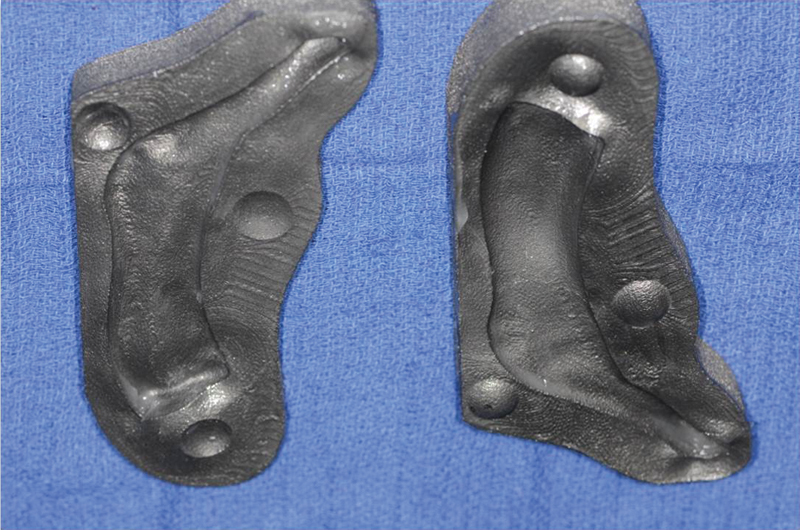
Titanium molds depicted from Case 2 which allowed for greater ease of spacer fabrication without the concern of binding of the spacer and mold. Note the missing coronoid in this model as compared with Fig. 3.
Fig. 6.
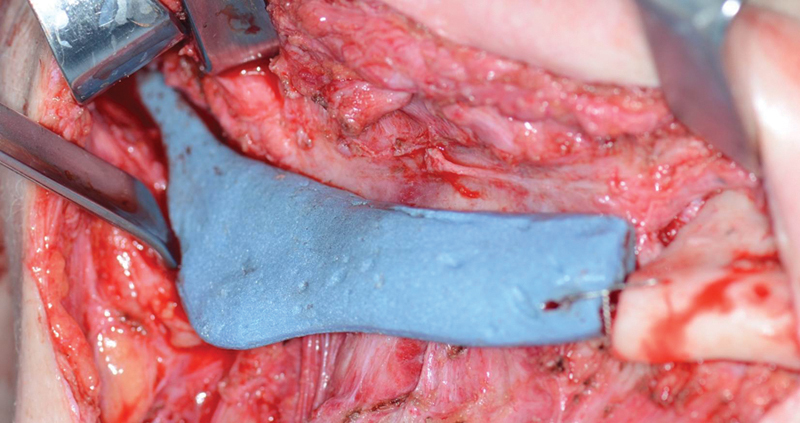
Spacer in place in Case 2 following the resection. The spacer was fixated in Case 2 with an inferior border wire.
Results
After 3 months, Patient 1 was taken to the main operating room for reconstruction with a TMJ Concepts (TMJ Concepts, Ventura, CA) custom alloplastic condyle/fossa/ramus implant. Classic submandibular and preauricular approaches were used. The spacer was partially encased in new bone formation along the medial border of the spacer up to the glenoid fossa, and this heterotopic bone was removed with the spacer. The periosteal envelope, now fibrous capsule, had a smooth and noninflamed appearance and the tissue was well perfused with no evidence of infection. In the joint space, the remaining unsalvageable disk and a small amount of interposing fibrous remodeled retrodiscal tissue was excised. No communication with the oral cavity occurred. The patient was placed in elastics postoperatively with interincisal opening of 25 mm at 3 weeks and 35 mm at 5 weeks. He maintained a soft diet for 4 weeks and then returned to normal diet and regular activities. Patient 2 underwent an uncomplicated healing interval of 8 weeks while the TMJ Concepts prosthesis was being fabricated. She was taken to the main operating room for reconstruction in a similar fashion to Patient 1.
Patient 2 was placed in elastics postoperatively with an initial incisal opening of 25 mm at 3 weeks and 35 mm at 5 weeks. She maintained a soft diet for 4 weeks and then returned to normal diet and regular activities. In both cases, a healthy appearing tissue envelope was found upon re-entry into the spacer capsule and there was notable growth of bone along the spacer (Fig. 7). No communication with the oral cavity occurred during either procedure. Both healed fibrous tissue/periosteal pockets accommodated the placement and fixation of the condylar/ramus implants without undue tension (Fig. 8).
Fig. 7.
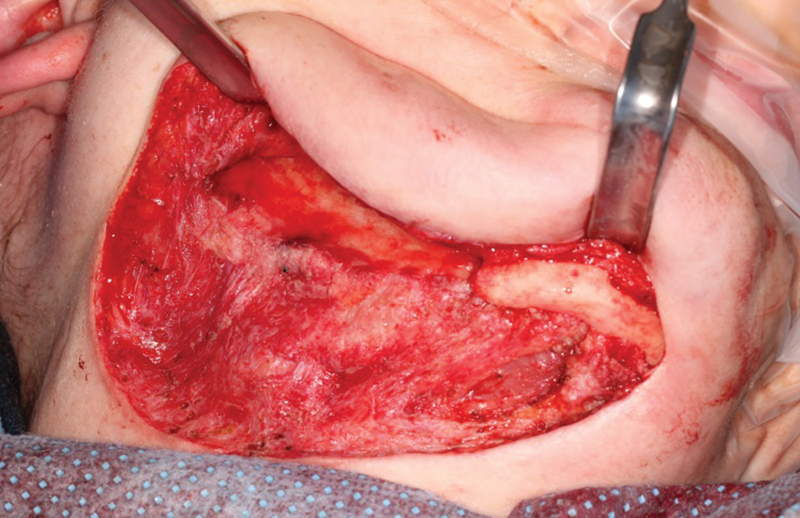
The healthy tissue bed in Case 2 following removal of the spacer and the excess bone at the time of the definitive reconstruction.
Fig. 8.
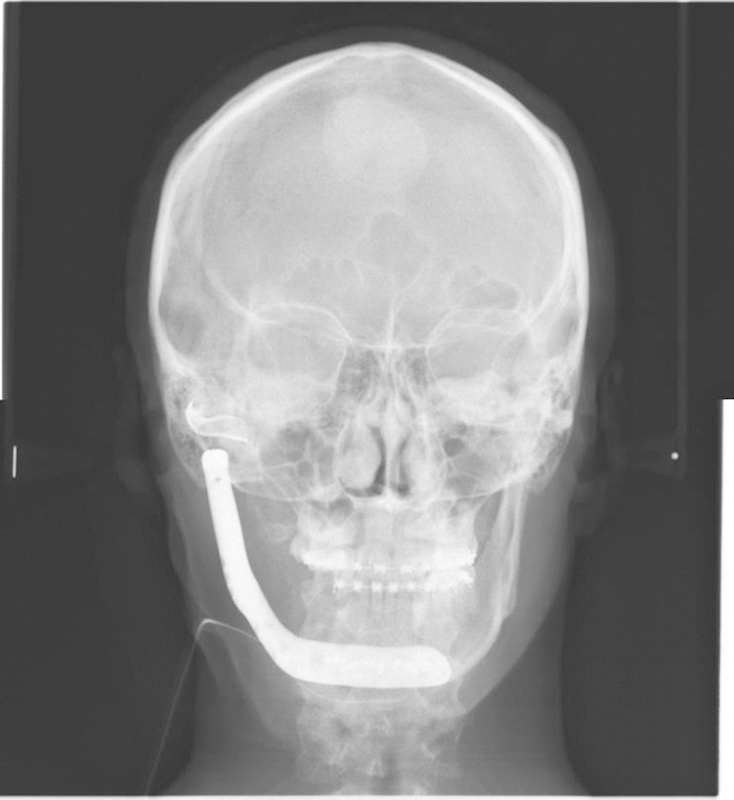
PA cephalogram of posttreatment Case 2.
Discussion
Temporary spacers offer significant advantages critical to the management of mandibular defects planned for reconstruction. Spacer placement at the time of resection: (1) maintains the original soft tissue envelope needed to accommodate the definitive implant or graft; (2) facilitates safe extra oral access via preservation of anatomic location of nerves and vessels that would otherwise be incorporated into the soft tissue contraction and scarring of the surgical site; (3) reduces the risk of perforation into the intraoral environment at the second surgery by maintaining periosteal planes; (4) retains preoperative facial esthetics and contour better than reconstruction bar alone; and (5) can serve as a vehicle for local high concentration antibiotic delivery in cases of deep infection.7 Disadvantages are the intraoperative time required for the added challenge of freehand replication of the anatomy of complex facial structures compared with that of the long bones for which it is routinely used, while addressing curing time and cooling of the materials. Consequently, an unnatural soft tissue drape over an imprecisely replicated skeletal structure will worsen cosmetic outcome. The use of digital design and fabrication techniques for the spacer are well suited to overcome these issues and, although originally custom, different size and curvature molds could be manufactured to produce spacer that can be customized for different cases.
The use of prefabricated molds for the temporomandibular spacers proved to decrease working time for fabrication and placement. Digital design for each of the cases followed the same methodology, however, comparing the two materials and AMT methods used to fabricate the molds for the spacers, the resin molds from vat polymerization techniques are less expensive and faster to fabricate than the titanium molds from electron beam melting technologies. Unfortunately, polymer molds require specialized gas sterilization, a foil liner for spacer release and protection from possible contamination of the PMMA from the polymer, and have a limited number of uses. In contrast, the titanium molds are easily sterilized with routine methods, require no foil liners, pose no issue with contamination of the PMMA, and can be reused indefinitely where a similar sized condylar replacement is required.
One technical insight gained from this case was that MMF alone was not sufficient to prevent superior rotation of the spacer. Securing the spacer with a temporary anchorage device, small screw or wire is recommended to stabilize the spacer before reconstruction. Another observation was that the blue color of the PMMA, contrasting sharply with the layers of new investing bone, was helpful as a reference in identifying the margins of new bone and that which was originally preserved in the resection. In comparing the cases, the increased amount of new bone deposition in Patient 1 was most likely due to the increased duration of MMF.
The capacity to regenerate condylar anatomy in children is well documented and has been suggested in adults.8 9 10 11 In this case, adult regenerative potential is suggested by the appearance of new bone extending from the resection border, intimately adhering to the spacer, superiorly to the fossa and overlying the medial aspect of the condyle spacer. At the time of reconstruction, aggressive bone removal with reciprocating saw and barrel bur was necessary to accommodate the custom implant fit.
The use of biodegradable antibiotic impregnated implants for the treatment of chronic infections has been described in the modern literature.12 13 Considering the extent of new bone formation seen in both patients, digital design and manufacturing of a resorb able antibiotic-eluting scaffold, or of a mold for its intraoperative fabrication, may be considered an option in the near future for young, otherwise healthy patients in whom the periosteum is preserved. This could in theory prevent the need for a second reconstructive surgery including donor site morbidity or the use of nonautogenous reconstructive materials.
Conclusion
The use of custom or semicustom spacers in the staged reconstruction of temporomandibular joints leads to a less complicated second surgery as well as a more accurate reconstruction, especially in the presence of existing hardware. If the fabrication technology is available, titanium molds are preferable since they can be easily sterilized and the PMMA is easily released. The conduction properties of metal permits the heat from the highly exothermic polymerization reaction of the PMMA to be transferred more quickly away via cold water bath or ambient air facilitating a fully polymerized spacer sooner and decreasing the risk of tissue heat damage form continued polymerization reactions. Titanium spacer molds can be maintained for use in future cases for a patient with reasonably similar anatomy or can be created to replicate larger or smaller condyle/ramus complexes as an off the shelf customizable product. The use of custom spacers has been an invaluable contribution to the reconstruction of the temporomandibular complex when staged reconstruction is indicated in the presence of osteomyelitis or when retained hardware from failed or previous reconstructions must be removed to facilitate planning of a custom alloplastic restoration.
References
- 1.Taggart T, Kerry R M, Norman P, Stockley I. The use of vancomycin-impregnated cement beads in the management of infection of prosthetic joints. J Bone Joint Surg Br. 2002;84(1):70–72. doi: 10.1302/0301-620x.84b1.11948. [DOI] [PubMed] [Google Scholar]
- 2.Kinik H, Karaduman M. Cierny-Mader Type III chronic osteomyelitis: the results of patients treated with debridement, irrigation, vancomycin beads and systemic antibiotics. Int Orthop. 2008;32(4):551–558. doi: 10.1007/s00264-007-0342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodger N M, Wang J, Smagalski G W, Hepworth B. Methylmethacrylate as a space maintainer in mandibular reconstruction. J Oral Maxillofac Surg. 2005;63(7):1048–1051. doi: 10.1016/j.joms.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Anagnostakos K, Wilmes P, Schmitt E, Kelm J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009;80(2):193–197. doi: 10.3109/17453670902884700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham A, Demarest G, Rosen P, DeCoster T A. Antibiotic bead production. Iowa Orthop J. 2000;20:31–35. [PMC free article] [PubMed] [Google Scholar]
- 6.Wolford L M, Rodrigues D B, McPhillips A. Management of the infected temporomandibular joint total joint prosthesis. J Oral Maxillofac Surg. 2010;68(11):2810–2823. doi: 10.1016/j.joms.2010.05.089. [DOI] [PubMed] [Google Scholar]
- 7.Goode R L, Reynolds B N. Tobramycin-impregnated methylmethacrylate for mandible reconstruction. Arch Otolaryngol Head Neck Surg. 1992;118(2):201–204. doi: 10.1001/archotol.1992.01880020107023. [DOI] [PubMed] [Google Scholar]
- 8.Mercuri L G Total joint reconstruction—autologous or alloplastic Oral Maxillofac Surg Clin North Am 2006183399–410., vii [DOI] [PubMed] [Google Scholar]
- 9.Mercuri L G, Edibam N R, Giobbie-Hurder A. Fourteen-year follow-up of a patient-fitted total temporomandibular joint reconstruction system. J Oral Maxillofac Surg. 2007;65(6):1140–1148. doi: 10.1016/j.joms.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P, Williams R, Monaghan A. Spontaneous mandibular regeneration: another option for mandibular reconstruction in children? Br J Oral Maxillofac Surg. 2013;51(5):e63–e66. doi: 10.1016/j.bjoms.2012.04.255. [DOI] [PubMed] [Google Scholar]
- 11.de Villa G H, Chen C T, Chen Y R. Spontaneous bone regeneration of the mandible in an elderly patient: a case report and review of the literature. Chang Gung Med J. 2003;26(5):363–369. [PubMed] [Google Scholar]
- 12.Liacouras P, Garnes J, Roman N, Petrich A, Grant G T. Designing and manufacturing an auricular prosthesis using computed tomography, 3-dimensional photographic imaging, and additive manufacturing: a clinical report. J Prosthet Dent. 2011;105(2):78–82. doi: 10.1016/S0022-3913(11)60002-4. [DOI] [PubMed] [Google Scholar]
- 13.Gitelis S, Brebach G T. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J Orthop Surg (Hong Kong) 2002;10(1):53–60. doi: 10.1177/230949900201000110. [DOI] [PubMed] [Google Scholar]


