Abstract
High strength extended release opioid products, which are indispensable tools in the management of pain, are associated with serious risks of unintentional and potentially fatal overdose, as well as of misuse and abuse that might lead to addiction. The issue of drug abuse becomes increasingly prominent when the dosage forms can be readily manipulated to release a high amount of opioid or to extract the drug in certain products or solvents. One approach to deter opioid drug abuse is by providing novel abuse deterrent formulations (ADF), with properties that may be viewed as barriers to abuse of the product. However, unlike regular extended release formulations, assessment of ADF technologies are challenging, in part due to the great variety of formulation designs available to achieve deterrence of abuse by oral, parenteral, nasal and respiratory routes. With limited prior history or literature information, and lack of compendial standards, evaluation and regulatory approval of these novel drug products become increasingly difficult. The present article describes a risk-based standardized in-vitro approach that can be utilized in general evaluation of abuse deterrent features for all ADF products.
Keywords: Abuse deterrent formulation, opioid analgesics, evaluation matrix, drug abuse, manipulation, mode of abuse, syringeability, injectability
Introduction
Opioid drugs are well known for their analgesic properties (as pain-relievers), for their ability to produce respiratory depression and their high potential for abuse. Consequently, while remaining as the leading therapeutic option for the treatment of both acute and chronic pain (Cheatle and Barker, 2014; Frank et al., 2014; Lusted et al., 2013), prescription opioids continue to be drugs of choice for those who abuse drugs. The number of opioid prescriptions dispensed in the United States (US) has seen a steady increase in the last decade, accompanied with a parallel escalation in the instances of abuse and/or misuse of these drugs (Budman et al., 2009; Services, 2013), and in many cases associated with serious consequences. It has been reported that the number of deaths related to prescription opioid drugs now exceeds the number of deaths involving all illicit drugs such as heroin and cocaine combined (CDC, 2014). In addition to morbidity and mortality, abuse of prescription opioid drugs is associated with high economic costs. The average direct health care costs for an opioid abuser is eight times higher than for non-abuser (White et al., 2005). According to the 2011 US National Survey of Substance Abuse Treatment Services (N-SSATS), around 1 million patients sought treatment for drug abuse resulting in significant loss of productivity (Administration, 2013). The total economic impact of prescription opioid drug abuse rose from $8.6 billion in 2001 to $55.7 billion in 2007 (Birnbaum et al., 2011; Meyer et al., 2014; Strassels, 2009).
Understanding the underlying factors that contribute to drug abuse is critical to formulate solutions to this multifaceted problem. Broadly speaking, the availability and cost of the prescription opioid drug products, social acceptability, and popularity among peers are common factors (Butler et al., 2010b; Hays, 2004). The majority of approved opioid drugs, currently available, are designed for oral administration, e.g., tablets, capsules, solutions etc. (Figure 1) making them easy targets of abuse. Indeed, a number of recent drug preference studies show oral tablets to be the major source of abuse/misuse of prescription opioids (Sellers et al., 2013; Sellers et al., 2006). The opioids with the most drug product approvals in the US market, i.e., oxycodone, hydrocodone, codeine and morphine, are also the most abused ones (Pergolizzi et al., 2012; Raffa et al., 2012). Beside the availability of drug products, properties such as onset and duration of action, the intensity of the effect, and the dependence potential of the active pharmaceutical ingredient (API) also contribute to the high incidence of prescription opioids abuse (Calderon and Klein, 2014).
Figure 1.
A select list of currently approved opioid analgesics drug products from Drugs@FDA database. Some of the products may not be available currently on the market. Left: number of approved products based on API; Middle: number of approved products based on route of administration; Right: List of dosage forms for oral route of administration. Last accessed on July 29th , 2014
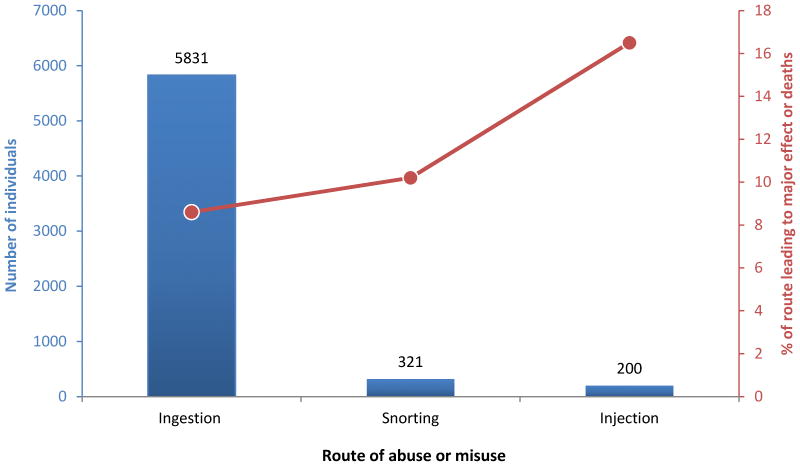
An abuser may choose to ingest multiple doses of a drug product or may manipulate (e.g., crush, cut, chew, grind, heat, and/or dissolve) the drug product to yield a high amount of opioid that could be easily abused via ingestion, snorting, inhaling, injection, or smoking. The preferred route of abuse is governed by a number of factors such as the type of abuser and their tolerance level, and varies based on the geographical location and demography of the abuser (Butler et al., 2010b; Hays, 2004; Katz et al., 2011). The more experienced abuser prefers injection route while the oral route is favored by non-experienced and occasional abusers (Katz et al., 2011; Katz et al., 2007; Pergolizzi et al., 2012). Overall, oral route is the route of choice followed by snorting and injection (Budman et al., 2009; Butler et al., 2010a; Hays, 2004; Katz et al., 2011; Katz et al., 2008; Sellers et al., 2013) (Figure 2). Yet, the highest mortality and severe morbidity rates are associated with the parenteral and nasal routes (Katz et al., 2011).
Figure 2.
Number of patients in treatment for substance abuse and percentage leading to major health effect or death, by routes of administration. Source: Data from 2006 Treatment Episode Data Set (TEDS) Highlights. (Katz et al., 2011)
Despite the sizable human and economic costs associated with the abuse of prescription opioid drugs, these medications are essential for improving the care and outcomes for millions of Americans living with chronic pain (APS, 2000). One way of providing safer prescription opioids while limiting their abuse, is to develop opioid formulations with design features that prevents or deters abuse, commonly refer to as abuse deterrent formulations (ADF). For example, a pentazocine drug product containing naloxone was approved by the US Food and Drug Administration (FDA) in 1982 (Raffa et al., 2012) but now discontinued. The naloxone component of this agonist-antagonist combination is designed to block the pentazocine’s positive effects when the product is abused. Various other design features have been used, including making it difficult to manipulate the drug product e.g., by making tablets very hard to prevent crushing, high viscosity of aqueous extracts to prevent injection, etc., or to make abuse of the manipulated product less attractive or rewarding, by slower drug release or by causing irritation during snorting, etc. A list of commercially available and under-development opioid drug products with abuse deterrent design features is provided in Table 1 (Alexander et al., 2014; Covvey, 2015; Pergolizzi et al., 2012; Raffa et al., 2012).
Table 1.
A selective list of drug products with abuse-deterrent design features*
| Product | Manufacture | API | Dosage form | Design feature | Product status | Reference |
|---|---|---|---|---|---|---|
| Talwin NX | Sanofi Aventis | Pentazocaine | Immediate release tablet | Contains Naloxone as an opioid antagonist | FDA approved 1982 | (Pergolizzi et al., 2012; Raffa et al., 2012) |
| Suboxone | Reckitt Benckiser Pharmaceuticals | Bupreorphine | Sublingual film | Contains Naloxone as an opioid antagonist | FDA approved 2002 | (Alexander et al., 2014; Pergolizzi et al., 2012; Raffa et al., 2012) |
| Embeda♯ | King pharmaceutics | Morphine | Extended release capsule | Contains Naloxone as an opioid antagonist | FDA approved 2009 | (Alexander et al., 2014; Pergolizzi et al., 2012; Raffa et al., 2012) |
| Oxycontin♯ | Purdue Pharma | Oxycodone | Extended release tablet | Polymer matrix that is hard to break and forms a gel upon water contact | FDA approved 2010 | (Alexander et al., 2014; Pergolizzi et al., 2012; Raffa et al., 2012) |
| Oxaydo | Egalet | Oxycodone | Immediate release tablet | Form a gel upon water contact and contain an aversive agent (Sodium lauryl sulfate) | FDA approved 2011 | (Alexander et al., 2014) |
| Opana ER | Endo Pharmaceuticals | Oxymorphone | Extended release tablet | Polymer matrix that is hard to break and form a gel upon water contact | FDA approved 2011 | (Alexander et al., 2014; Pergolizzi et al., 2012) |
| Nucynta | Ortho-McNiel-Jassen Pharmaceuticals | Tapentadol | Extended release tablet | Polymer matrix that is hard to break | FDA approved 2011 | (Alexander et al., 2014) |
| Targiniq ER♯ | Purdue Pharma | Oxycodone | Extended release tablet | Contains Naloxone as an opioid antagonist | FDA approved 2014 | (Alexander et al., 2014) |
| Hysingla ER♯ | Purdue Pharm | Hydrocodone | Extended release tablet | Tablet is difficult to crush, break or dissolve. It also forms a viscous hydrogel. | FDA approved 2014 | (Covvey, 2015) |
| Remoxy | Pfizer/King pharmaceutical | Oxycodone | Extended release capsule | Viscous liquid | In development | (Alexander et al., 2014; Pergolizzi et al., 2012; Raffa et al., 2012) |
| Xtampza ER | Collegium Pharmaceuticals | Oxycodone | Extended release capsule | Multi-particulate matrix with particles in a waxy base | FDA Tentative approval in 2015 | (Alexander et al., 2014; Pergolizzi et al., 2012) |
| COL-172 | Collegium Pharmaceuticals | Oxymorphone | Extended release capsule | Multi-particulate matrix with particles in a waxy base | In development | (Alexander et al., 2014; Pergolizzi et al., 2012) |
| NKTR-181 | Nektar Therapeutics | Polymer opioid conjugate that slowly get absorbed through the blood brain barrier | In development | (Alexander et al., 2014) |
This is not an exhaustive list, and not all products with ADF features are listed.
Labeling indicates the product contains abuse deterrent properties as per FDA guidance (FDA, 2015)
In an effort to address the opioid abuse/misuse problem while ensuring that patients in pain have appropriate access to opioid analgesics, FDA issued a draft guidance on Assessment of Abuse Potential of Drugs in 2010 (FDA, 2010). Another guidance on Abuse-Deterrent Opioid – Evaluation and Labeling was published in 2015 which focuses on evaluation of products with ADF technologies (FDA, 2015). These two guidances laid out the necessary foundation for more vigorous and science-based regulatory framework for evaluation of future ADF technologies. But challenges still remain. In particular, new standards may be required to assess the effectiveness of the ADF technologies in terms of deterring the abuse, especially for different drug applications, in addition to the typical safety and efficacy requirement (under intended use conditions). Ideally, the standards for evaluating the ADF features need to be based on a series of unified core principles. Furthermore, the standards need to be flexible enough to adapt to the needs of different types of drug applications and/or dosage forms. Positive confirmation of an abuse deterrent property of a new opioid drug product, regardless of the application type, can only be established when compared with a valid comparator. For instance, for a drug product in which ADF feature(s) is being introduced for the first time, the comparator could be a currently marketed drug product in same dosage form and similar pharmacokinetics (Tmax, Cmax, and AUC) with no ADF features. In cases where ADF features are being matched or improved in the currently marketed drug products, the comparator could be the currently marketed drug product with the ADF feature(s). In this regard, understanding mode of abuse and its relevance to ADF design feature(s) are critical from an evaluation point of view. In this article, the term mode of abuse is defined as a combination of method and route of administration an abuser may choose to alter the product performance with an intention to abuse, which may be as simple as cutting or crushing a tablet before ingestion, or may involve multiple steps such as grinding followed by extraction and injection. Commonly, opioid analgesics are altered in a number of ways before abused by the same or a different route of administration. For example, the product may be extracted from the intact dosage form or be physically or chemically tampered (e.g. cutting, splitting, grinding, heating, extraction, etc.) before ingesting (oral), snorting (nasal), smoking (respiratory), or injecting (parenteral) in the body. To evaluate ADF features against all possible combinations of product manipulation and or extraction, and the different route of administration would involve countless testing conditions (Katz et al., 2006). Furthermore, the exact method of abuse and/or even route of abuse may change over time. With our current understanding and use of the technology, it’s not a matter of “if” but “when” the abuser may match the advances in formulation technology with new ways to explore the weakness of the ADF feature. For these reasons, rather than focusing on specific alteration methods or individual routes of administration, the approach presented in this article focuses on understanding the function of an ADF feature in the context of mode of abuse and on designing appropriate tests that would evaluate ADF features covering a wide range of modes of abuse. Such an approach allows adaptation to the ever changing and increasing threat from the abuse of the opioids.
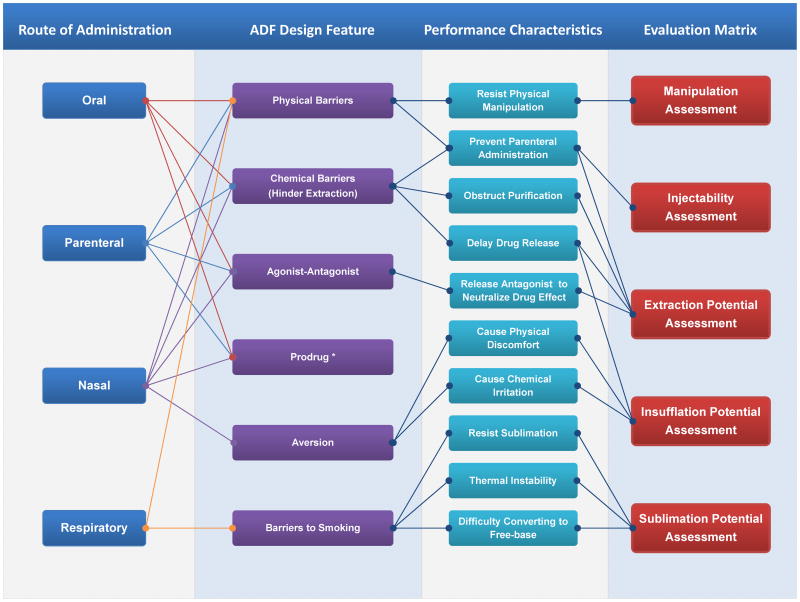
This article discusses scientific principles, and considerations of an approach aiming to standardize the evaluation matrix (Figure 3) that may allow a consistent standardized risk- and performance-based methodology for the in-vitro assessment of the ADF features of an opioid drug product. Rationale as well as the principles of the proposed evaluation matrix is first presented, followed by laboratory experiments to illustrate and validate the aforementioned approach. It should be noted that the approach discussed here represents the authors’ individual scientific observation and thinking on the issue, and does not in any way reflect the Agency’s position on this topic. Additionally, there are possibly other ways that the abuse deterrent features can be evaluated, e.g. by performing human in-vivo abuse liability assessment in which drug liking and other measures such as self-administration behavior are assessed under controlled conditions (Comer et al., 2012; Turk et al., 2012) or by performing in-vitro assessment under conditions that mimic all abuse conditions using an iterative model (Cone et al., 2013).
Figure 3.
Relationship between route of administration, ADF design features, product performance characteristics and matrices for evaluation. *Evaluation of the performance of prodrug should consider the mechanism and rate of the conversion under various conditions.
In this study, to develop the in-vitro standardization scheme, and in-vitro performance matrix, several formulations with different delivery systems of opioids would be needed. To avoid dealing with complexity of controlled substance license and its management, a model compound, sotalol that resembles the physicochemical properties of certain opioids, is used for demonstration of test methods and performance matrix. Selective and limited tests with actual ADF products were performed to demonstrate the utility of this approach.
Materials and Methods
Materials
Sotalol hydrochloride was obtained from Abblis Chemicals LLC (Houston, TX). Polyethylene oxide (POLYOX) WSR N-60K, WSR301, WSR303 (Colorcon, West point, PA), hypromellose K100M (Colorcon, West point, PA), polyethylene glycol 6000 (Fisher, Pittsburgh, PA), magnesium stearate (Sigma Aldrich, St. Louis, MO), and in-house purified and deionized water (Millipore, Bedford, MA) were used in this study. All other chemicals and reagents were of analytical grade.
Preparation of test tablets
Various techniques have been used to impart ADF features for the in-house prepared sotalol extended release formulations, one which was direct compression process (Formulation 1). Briefly, directly compression tablet involves blending of the formulation components in a Miniblend™ (Globe Pharma Inc., New Brunswick, NJ, USA), followed by compression (40 N) on a ten-station tablet press (Mini Press-1, Globe Pharma Inc., New Brunswick, NJ, USA) and curing (Mastropietro and Omidian, 2015b) at 80°C for 1 hour. Formulation 2 was prepared via another undisclosed process.
Risk- and performance-based in vitro assessment: five evaluation matrix
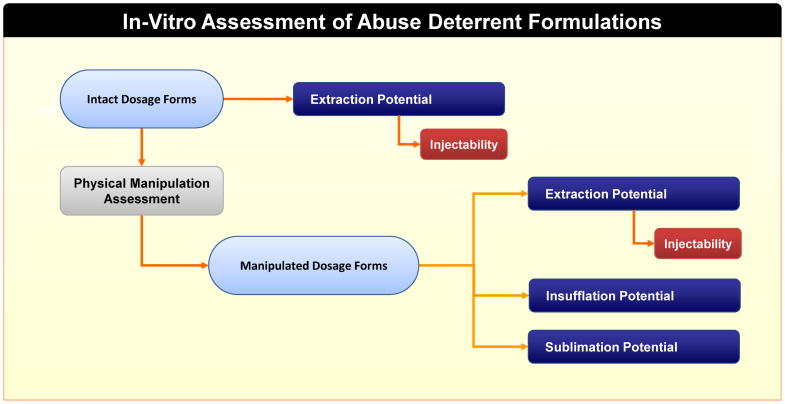
Based on a though risk-analysis of the mode of abuse and existing formulation design to impart the ADF features (Figure 3), the anticipated performance tests were grouped into five essential evaluation matrices, include: 1) Manipulation assessment; 2) Extraction potential assessment; 3) Injectability assessment; 4) Insufflation assessment; and 5) Sublimation potential assessment. In a broader scope, the term “manipulation” may sometimes be used to refer to all destructive methods aimed at retrieving drug from the products, including a variety of physical and/or chemical tampering methods. In this article, the term manipulation is used with a limited scope and refers to only physical alteration of the drug product. To utilize these assessments to determine if a product can possess potential to deter drug abuse, a stepwise approach was adopted. A key consideration in this approach is that it determines the apparent abuse-deterrent properties of a proposed opioid product when compared to its comparator under identical testing conditions. Methodologically, these studies were designed with the knowledge of the physicochemical properties of the proposed opioid product and its comparator, tampering methods commonly employed by abusers, route of administration unique to certain opioids, as well as those attributable to inadvertent misuse. To start, the intact dosage forms of both proposed new opioid drug product and its comparator underwent two simultaneous evaluations (Figure 4): extraction potential assessment and physical manipulation assessment. Injectability assessment for the intact dosage forms was only conducted if any of the extraction potential assessment studies indicated potential of substantial drug extraction. The goal of the physical manipulation assessment is to obtain suitable representative samples for further evaluation of extraction, injectability, insufflation, and sublimation. Similar to the intact dosage forms, evaluation of injectability for the manipulated dosage forms was only performed if any of the extraction tests exhibited substantial drug extraction. This helped eliminated redundancy in testing, as a product would less likely be susceptible for abuse by injection if only limited drug can be extracted in large quantity solvents. Two extreme forms of each drug product, i.e., the intact and the worst case of manipulated drug product were evaluated (if necessary, one or more intermediate manipulated dosage form(s) can also be assessed to ensure that the evaluation methods are discriminatory in nature). Note that the sublimation potential of a drug product is dependent on the inherent physicochemical properties of the API. Sotalol does not sublime. Hence, the test formulations in this study could not be assessed for sublimation potential. In addition to a risk-based approach, the following four principles can aid in the assessment of the ADF feature of a product: 1) Understand the design and feature of ADF with regard to mode of abuse; 2) Design appropriate performance tests to evaluate ADF features under all potential mode of abuse; 3) Base the tests on relative performance unique to the formulation design; 4) Use the same evaluation criteria for sameness consideration.
Figure 4.
Step-wise performance based in-vitro assessment of ADF features
Physical manipulation of test tablets
Various manipulation methods were evaluated to compromise/defeat the physical integrity of the sotalol test tablets. The idea is to evaluate the ability of crushing (mortar and pestle), chewing (simulating human bite force), grating, grinding, etc. and their impact on the integrity of the tablet. The appearance of the manipulated tablets after each test was recorded. In some cases, the particle size and size distribution was also determined, using sieve and high speed image analysis.
Particle size and distribution analysis
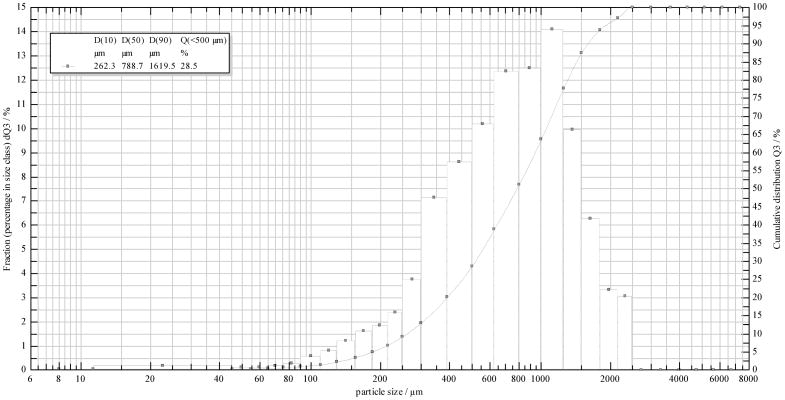
A Sympatec QICPIC high speed image analysis sensor equipped with dry gravity disperser (GRADIS/L) and vibratory feeder was used for the particle size and distribution analysis of the granules obtained for the manipulated tablets. Trigger condition was set at 0.1% optical concentration, and the feed rate was set at 50%. The instrument was set to M7 filter to analyze particles in the 10 to 3410 μm range. For sieve analysis, #10 (2000 μm) and #35 (500 μm) sieves were used to determine the percentage of particles below 2 mm and 500 μm.
Extraction by solvent
The ability of water and other solvents to extract the drug from the intact and manipulated tablets was evaluated. Table 2 provides a list of solvents that have been evaluated. The selection of the solvent is based on ease of availability and physicochemical properties of the solvent (e.g. acid/base, organic/aqueous, etc.). Similar to water, both Level 1 and Level 2 solvents are generally easily accessible in the household, with Level 1 solvents being more accessible than Level 2 solvents. For short term extraction studies, one test tablet or manipulated product (equivalent to one intact tablet) was transferred to a 100 mL beaker and 100 mL of selected solvents were added. Samples were withdrawn at 5, 15, 30, and 60 min to determine the amount of drug extracted. All samples were diluted 10 times (e.g. 100 μL diluted to 1 mL) before HPLC analysis. Effect of heat on the extraction was evaluated by repeating the study at elevated temperature. For aqueous solvent, the temperature was maintained at boiling condition of water (i.e. 100°C). For aqueous-alcohol solvents, water-bath temperature of 60°C was maintained. Due to safety concern, heat studies on strong acid/base and acetone were not conducted in this study. Effect of long term extraction was studied by soaking the products in the extraction solvents for overnight duration (20 h).
Table 2.
Solvent selection for extraction study
| Solvent Level | Cat. A (Alcohol) | Cat. B (Acidic) | Cat. C (Basic) | Cat. D (Others) |
|---|---|---|---|---|
| 0 | n/a | n/a | n/a | Water |
| 1 | 5 and 40% ethanol | 0.1 M Acetic acid | 0.1 M NaHCO3 | 0.9% NaCl |
| 2 | 70 and 100% isopropyl alcohol | 0.1 N HCl | 0.1N NaOH | Acetone |
Injectability assessment and syringeability test
Syringeability, which is part of the injectability assessment, was only performed when extraction study showed more than 50% of the drug extraction. A reduced solvent volume of 10 mL, instead of 100 mL, at the same temperature as those used in the extraction test was used. The ease of pulling the plunger of a 10 mL syringe fitted with an 18 gauge needle, and the volume of solution drawn into the syringe in 1 min were recorded. In the current study, the syringeability test was performed manually. To acquire more detailed understanding of the syringe process, a mechanical analyzer can also be used such that both pulling and push force can be quantified. The amount and concentration of the drug substance in the withdrawn solution was determined.
Viscosity determination
To aid the syringeability assessment, sample viscosity (throughout the time course of 30 min in small volume extraction media) was evaluated using a stress-controlled hybrid rheometer (DHR-3, TA Instruments, New Castle, Delaware, USA) equipped with a step-peltier stage (25°C) and a 25 mm sandblasted parallel plate. For each test, approximately 0.3 mL of sample was placed on the lower plate, before slowly lowering the upper plate to the preset trimming gap of 550 μm. After trimming excessive material, the geometry gap was set at 500 μm. Flow rate was increased from 1 to 100 s−1, and apparent viscosity was determined from power law equation: η=K·γn−1, where η is apparent viscosity, K is the consistency constant, γ is the shear rate, and n is the power law index.
Chromatographic conditions for analysis of sotalol HCl
The HPLC system consisted of a Hewlett Packard 1100 series (Agilent Technologies, Wilmington, DE, US) equipped with a quaternary pump, online degasser, column heater, autosampler and UV/Vis detector. Data collection and analysis were performed using ChemStation (Agilent Technologies). Separation was achieved on a Waters XBridge C18 3.5μm, 4.6×150 mm column protected using a XBridge C18 3.5 μm, 4.6×20mm security guard cartridge (Waters, Milford, MA, US). The elution was isocratic at 1.0 mL/min with a mobile phase of acetonatrile-0.5mM octanesulfonic acid (25:75, v/v, pH adjusted to 3.2). The column temperature was maintained at 30°C in a column oven, and auto-sampler was maintained at 20°C. The injection volume was 10 μL and detection was by UV at 235 nm. A calibration curve from 1–100 μg/mL was used to calculate the concentration of drug.
Results and Discussion
Sotalol hydrochloride was selected as a surrogate compound because it has similar physicochemical properties as those of opioid drugs (Table 3). The in-house prepared sotalol test formulations with ADF features were evaluated using the five evaluation matrix illustrated in Figure 3. In the next section, rationale as well as the analysis required to derive the evaluation matrix is reported and discussed in detail (risk assessment). Later, using surrogate ADF formulation as an example, a case study using a stepwise risk- and performance- based methodology is presented. However, due to the sensitive nature of the topic, only salient principles and results are discussed in detail below.
Table 3.
Physicochemical properties of various opioids and sotalol
| Drug | Strength (relative) | Half-life (hr) | pKa | logP | Aq. solubility (mg/L) a |
|---|---|---|---|---|---|
| Codeine | 1/10 | 3 | 9.19 | 1.2 | 577 |
| Propoxyphene | 1/10 | 6–12 | 9.52 | 4.1 | 4 |
| Meperidine | 1/8 | 3–4 | 8.16 | 2.9 | 1110 |
| Hydrocodone | 1 | 1–3 | 8.61 | 2.1 | 797 |
| Morphine | 1 | 2–4 | 9.12 | 1.0 | 10200 |
| Oxycodone | 1 to 2 | 3–4 | 8.21 | 1.0 | 5590 |
| Methadone | 3 to 4 | 15–30 | 9.12 | 4.1 | 6 |
| Hydromorphone | 5 | 2–3 | 8.59 | 1.7 | 4390 |
| Oxymorphone | 10 | 3–6 | 7.34 | 1.3 | 25600 |
| Fentanyl | 50 to 100 | 7 | 8.77 | 4.1 | 24 |
| Sotalol | N/A | 12 | 9.43 | 0.9 | 782 |
Predicted values from ALOGPS for the free-base
Evolution of standardizable evaluation matrix for ADF features
In evaluating the ADF feature of a product, a risk-based approach is most practical as most products would eventually fail by vigorous evaluation. Note that the risk refers to both the probability of occurrence of harm (in this case occurrence of abuse) and the severity of harm (ICH-Q9, 2006), which also depends on the nature of the product being evaluated. Two fundamental characteristics of this approach include: 1) An assessment of the ease of abuse, and 2) An assessment of the risk associated with a failure of an ADF feature. With this in mind, the ability to abuse the intact tablet, with minimal intervention, is the most appealing to the abuser and absence or a failure of the abuse deterrent feature at this stage would possess the highest risk. Similarly, use of multiple interventions, such as milling, heating, use of exotic solvents, etc., to abuse a product would present barriers that need to be overcome before abusing the product, implying some abuse deterrent feature in the formulation and may potentially lowering of the risk of abuse. To derive a set of evaluation matrix to assess these potential ADF features, a thorough analysis was conducted for various common routes of abuse. The analysis follows similar concepts as those used in a cause and effect analysis, as the goal was to link the various ADF design features in the context of mode of abuse with common routes of abuse. The section below provides two examples of this analysis, focused on abuse by oral ingestion and nasal insufflation.
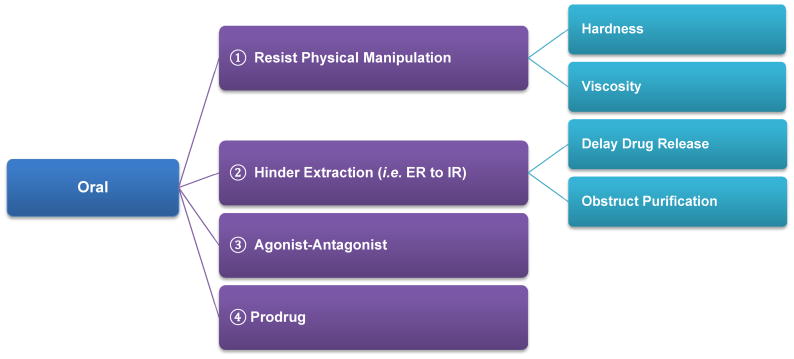
1) Risk analysis on ADF to deter oral abuse
As shown in Figure 5, the ADF feature for orally ingested formulations could be a product design element that imparts resistance to physical manipulation, e.g., by formulating tablets of extended release (ER) formulations with superior physical strength (Mastropietro and Omidian, 2015a, b) that resist crushing, cutting, etc thus preventing the abuser from achieving an instant high due to rapid release of the drug. Evaluation of such an ADF feature would therefore involve assessment of various physical manipulation methodologies. Some of the questions that the evaluation should answer include: What is the degree of difficulty of the manipulation? How successful is each manipulation technique in achieving its goal? If the structure of tablet is compromised, what is the size and size distribution of the resulting particles? These are important questions to understand the efficiency of the manipulation techniques and the risks associated with a failure of the ADF feature. In addition to hardness, drug product could be in a very viscous dosage form (e.g. liquid filled capsules) that has extended release properties.
Figure 5.
Risk analysis of ADF design features in relationship to oral route of abuse
Another most commonly employed option is to design ER formulation that maintains the ER feature after physical manipulation and or prevents the extraction of drug in various solvents. This ADF feature is relevant not only to abuse by oral route, but also for abuse by other routes, such as parenteral, nasal, etc. as it deters abuse by not only slowing down the release of drug (i.e. increase Tmax, decrease Cmax and AUC); but also by creating barrier for the abuser to obtain pure drug substance (i.e. for parenteral injection, or for snorting). Evaluation of such an ADF feature would involve extraction from intact and manipulated drug product in a variety of solvents under different conditions such as time, temperature, agitation speed, etc.
The third possible option would be agonist-antagonist combination, which may be used to significantly reduce the drug’s psychoactive effect in CNS when abused. Under normal use conditions, only opioid drug substance is released from these formulations. But when manipulated, the sequestered antagonist is released and competitively binds to the opioid receptors to neutralize the drug effect in the body (Johnson et al., 2012). Evaluation of this type of ADF feature would require assessment of the release (extraction) characteristics of agonist and antagonist under both normal and abuse conditions (Johnson et al., 2010; Mastropietro and Omidian, 2015b; Setnik et al., 2015).
Formulation using pro-drug approach is another option to deter drug abuse (Alexander et al., 2014; Jasinski and Krishnan, 2009). In such formulations, the pro-drug would only convert into the active drug form in its intended use condition (e.g. converted by specific enzymes in gastrointestinal tract). Administration of the product, either intact or manipulated, by other route of administration would render it ineffective. Evaluation of such type of ADF feature needs to take into consideration the conversion rate of prodrug under various conditions (e.g. pH, temperature, enzyme concentration etc.). Use of this approach may be beneficial in preventing abuse by oral, nasal and injection routes.
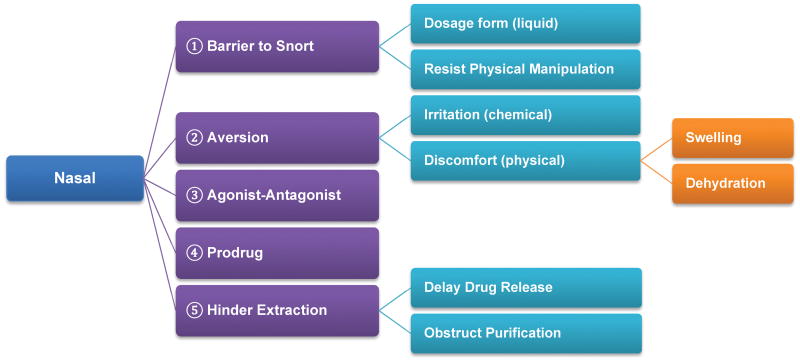
2) Risk analysis on ADF to deter nasal insufflation
As shown in Figure 6, barriers to snorting may be created by virtue of dosage form itself (e.g. viscous liquid filled capsules) or by use of physical barriers that make it difficult to convert the dosage form into powder suitable for snorting (e.g. Figure 7b). To assess the potential of the manipulated powder to be snorted, particles size and size distribution would need to be determined. It has been reported that the narrowest nasal passage diameter is approximately 4 to 8 mm (Swift and Kesavanthan, 1996). Hence, particles larger than this range may pose little risk in terms of abuse by nasal route. On the other hand, particles smaller than 20μm (ultra-fine powder) may escape the filtration by nasal hairs reaching the lungs (Swift and Kesavanthan, 1996) leading to chronic exposure safety risk. In addition to size, other particle aerodynamic properties such as density, shape, etc. may also influence the abuse potential of the drug product by nasal route.
Figure 6.
Risk analysis of ADF design features in relationship to nasal route of abuse
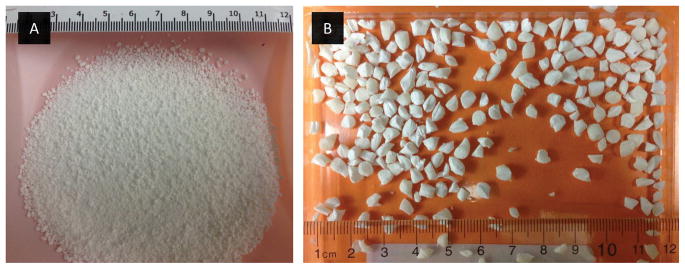
Figure 7.
Product obtained after manipulation of the sotalol test tablets
Other option may include creating discomfort or irritation in the nasal passage when the manipulated dosage form is snorted. This can be achieved through chemical and physical approaches. For example, use of chemical irritant to interact with mucus to induce reflexes leading to sinus congestion (Shusterman, 2003). It is also possible to create discomfort by physical means such as rapid swelling of polymer accompanied by localized dehydration effect. Evaluation of these types of ADF features may require different in-vitro testing procedures, with a focus on both performance and in-vivo relevance. It should also be noted that the aversion effect caused by an irritant may not necessarily be linearly dose-dependent. Consequently, unnecessary increase in the irritant level may do more harm to patients than overall benefit.
Similar analysis has been conducted for other routes (refer to supplemental materials). For example, barrier to smoking is mostly governed by the inherent physicochemical properties of the API as well as its interaction with the formulation components (e.g. polymers or complexation agents). If the drug can sublime then the risk associated with abuse by smoking becomes a function of thermal stability of the drug and/or its free base when burned. Accordingly, evaluation of sublimation potential of such APIs within a dosage form (after manipulation) should be studied and the concentration and amount of API in the vapor phase should be determined.
Based on the above analyses, it is evident that similar ADF feature may be applicable to multiple routes and can be evaluated on similar performance characteristics (Figure 3). Fundamentally it is not the route that differentiates various ADF features, but rather the function and performance of the dosage form or delivery systems. If the properties of the excipient and design of the dosage form is understood, the appropriate performance matrix can be determined. Accordingly, to reduce unnecessary repetitive tests, ADF features can be evaluated using performance matrices based on design similarity. The knowledge gained from a single performance matrix can be applicable across different routes of abuse. For instance, the assessment of the resistance to physical manipulation under a variety of manipulation techniques could provide information on the robustness of the physical barriers that have been incorporated in the product design. The relevant portions of this information can then be used to determine the abuse-potential by different routes of administration.
Risk- and performance-based in vitro assessment: A case study using Sotalol
A risk-based in-vitro assessment of ADF feature(s) of a drug product needs to be based on a comparison of its performance against a valid comparator. The two extreme forms of each drug product, i.e., the intact and the worst case of manipulated drug product could be evaluated (if necessary, one or more intermediate manipulated dosage form(s) can also be assessed to ensure that the evaluation methods are discriminatory in nature). The risk-based, stepwise approach of assessment of abuse deterrent formulation involves (Figure 4): 1) Manipulation assessment for the intact dosage form; 2) Extraction potential assessment from the intact, and one or more manipulated forms of the drug product; 3) Injectability potential assessment of the intact and the manipulated forms, where applicable; 4) Insufflation potential assessment of the manipulated dosage form(s); and 5) Sublimation potential assessment of the manipulated dosage form(s)
1) Manipulation assessment
Based on formulation design and processing conditions used to impart the ADF features, ADF drug products may differ greatly in the ease of manipulation. For instance, the current OxyContin tablets are designed to have higher resistance to crushing and breaking as compared to the earlier and now withdrawn OxyContin ER product. A comprehensive evaluation of various manipulation techniques, with different operation principles (e.g. cutting with a knife, crushing with a mortar/pestle, or milling with a coffee grinder), can be performed to determine their effectiveness in compromising the physical integrity of these tablets. The goal of this assessment is to quantify the work-effort required to produce a given change. For manipulation that involves particle size reduction (e.g. splitting, crushing, grinding, etc.), particle size and size distribution of the resulting manipulated drug products can be determined. For example, sotalol test formulation 1 could easily be manipulated, using one step manipulation process, into smaller particles out of which over 20% were below 500 μm (Figure 7a and Figure 8). On the other hand, sotalol test formulation 2 showed relatively high resistance to physical tampering, even when multiple physical manipulations were performed, and was very difficult to break into size smaller than 3 mm. (Figure 7b). Accordingly, a comparison between formulations 1 and 2 would suggest the latter possesses a higher degree of resistance to physical manipulation. This may potentially prorogate to a higher degree of resistance to extraction too, assuming smaller particles can lead to faster and/or greater extraction.
Figure 8.
Particle size distribution of manipulated sotalol tablets shown in Figure 7a (n=3)
2) Extraction potential assessment
Extraction refers to the process of using solvents/chemicals to retrieve drug from the products, including intact and/or manipulated dosage forms. Among many commonly used house-hold solvents, water is the most accessible one, and hence easy extraction of the drug in water implies the highest abuse potential. Accordingly, the extraction potential of the intact and the manipulated drug product in water was evaluated first before in other solvents. Table 2 provides a list of solvents that may be evaluated as part of the extraction studies after water extraction studies. The selection of the solvent is based on ease of availability and physicochemical properties of the solvent (e.g. acid/base, organic/aqueous, etc.). Note that the list does not in any way suggest the order of probability of extraction in these solvents, but based on relative risks. It should also be noted that this is a select rather than comprehensive list. If the drug is known to be soluble in oil-based systems, extraction studies in representative oil-based systems could also be performed. Some of the factors that may affect extraction of the drug include:
Solubility of the drug in the extraction solvent
Particle size and surface area of the sample
Volume and viscosity of the extraction solvent
Temperature of the extraction solvent
Agitation condition
Time
Solubility of the API in the extraction solvent is the most important chemical attribute governing the extraction potential of a drug product. A complete solubility profile may be helpful in establishing appropriate test conditions for these studies. Typically, particle size affects both the release and purity with finer particles producing higher and faster release. Temperature also plays an important role in extraction. Extraction under sustained heat (e.g. up to 60 min) at/or near boiling temperature of the solvents, if can be safely performed, can be evaluated. The rate and type of agitation can also greatly impact the outcome of the extraction studies so sampling at multiple time point (e.g. 5, 15, 30, 60 min), as shown in Table 4 is also useful. The extraction study results showing in Table 4 can also be used to interpret the drug release behavior. Indeed, a comparison of the dissolution data (not shown) and the extraction results (in aqueous solvents using intact dosage form for this formulation) confirmed that percentage released and extracted were very similar within the first hour. With respect to the ADF features, for this particular formulation, physical manipulation resulted in significant increase in the level of drug extraction, particularly in water. Solvents in which substantial amount (e.g. >50%) of drug can be extracted in a relatively short period of time (e.g. 30 min) were further evaluated in lower solvent volume (5–10 mL) under similar conditions in injectability assessment.
Table 4.
Extraction of intact and manipulated sotalol test formulation 1 in 100 mL of water and different solvent at room temperature. (n=3)
| Type of sample | Solvent Level | Solvent |
Extraction (%)
|
|||
|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 60 min | |||
| Intact dosage form | Level 0 | H2O | 5.0 ± 0.4 | 4.4 ± 0.5 | 14.4 ± 0.4 | 23.8 ± 3.1 |
|
| ||||||
| Level 1 | 5% EtOH | 8.9 ± 0.9 | 7.8 ± 0.2 | 11.8 ± 1.2 | 18.7 ± 0.4 | |
| 40% EtOH | 2.2 ± 0.4 | 5.5 ± 0.4 | 7.6 ± 1.4 | 12.2 ± 1.9 | ||
| 0.1 M Acetic Acid | 5.0 ± 1.6 | 9.6 ± 0.9 | 15.1 ± 0.8 | 23.7 ± 1.2 | ||
| 0.1 M NaHCO3 | 4.0 ± 0.9 | 7.5 ± 0.4 | 13.0 ± 0.4 | 19.0 ± 0.3 | ||
| 0.9% NaCl | 2.9 ± 0.2 | 6.3 ± 1.1 | 9.9 ± 1.9 | 16.2 ± 1.8 | ||
|
| ||||||
| Level 2 | 70% IPA | 0.8 ± 0.1 | 1.9 ± 0.1 | 3.3 ± 0.5 | 6.0 ± 0.9 | |
| 100% IPA | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.6 ± 0.1 | 1.0 ± 0.1 | ||
| 0.1 N HCl | 3.4 ± 0.2 | 7.8 ± 0.9 | 11.1 ± 0.4 | 18.0 ± 1.4 | ||
| 0.1 N NaOH | 3.5 ± 0.4 | 7.8 ± 0.2 | 12.9 ± 1.8 | 18.9 ± 1.2 | ||
| Acetone | 0.6 ± 0.1 | 1.3 ± 0.2 | 2.2 ± 0.3 | 3.5 ± 0.5 | ||
|
| ||||||
| Manipulated dosage form | Level 0 | H2O | 45.1 ± 15.8 | 54.3 ± 6.9 | 68.2 ± 5.6 | 76.5 ± 4.7 |
| Level 1 | 5% EtOH | 38.0 ± 2.6 | 52.7 ± 2.3 | 63.2 ± 1.8 | 75.2 ± 2.3 | |
| 40% EtOH | 27.5 ± 1.4 | 49.5 ± 3.0 | 63.7 ± 2.8 | 73.8 ± 1.6 | ||
| 0.1 M Acetic Acid | 34.2 ± 3.4 | 55.5 ± 2.8 | 67.1 ± 3.2 | 75.4 ± 3.3 | ||
| 0.1 M NaHCO3 | 22.5 ± 1.8 | 40.0 ± 1.1 | 57.1 ± 2.1 | 68.7 ± 0.8 | ||
| 0.9% NaCl | 19.9 ± 1.8 | 41.8 ± 2.6 | 55.1 ± 3.3 | 69.0 ± 2.3 | ||
| Level 2 | 70% IPA | 22.7 ± 1.3 | 49.1 ± 10.4 | 63.6 ± 3.5 | 75.6 ± 5.4 | |
| 100% IPA | 19.1 ± 1.8 | 39.0 ± 2.1 | 58.0 ± 1.3 | 69.9 ± 3.1 | ||
| 0.1 N HCl | 37.5 ± 4.8 | 44.5 ± 4.4 | 51.4 ± 5.3 | 55.5 ± 4.5 | ||
| 0.1 N NaOH | 21.5 ± 2.2 | 42.5 ± 3.0 | 64.4 ± 4.9 | 86.3 ± 7.4 | ||
| Acetone | 45.1 ± 15.8 | 54.3 ± 6.9 | 68.2 ± 5.6 | 76.5 ± 4.7 | ||
3) Injectability assessment
Abuse by parenteral route requires not only extraction of the API from the intact and manipulated drug product in a relative small volume (i.e. extractability) but also the suitability of the extraction solution to be injected (i.e. parenteral administrable) and the ability of the extract to be syringed and pushed through an injection needle (i.e. syringeability). In this article, the term injectability is used to refer to the ability of a drug to be injected, which includes the feasibility of being both parenteral administrable and being syringeable.
The extractability of drug in solvent(s) was already evaluated in the extraction potential Assessment; hence, the injectability assessment (using a smaller volume) was performed only when the amount extracted was equal to or greater than 50% in 30 min under a certain condition. Even though most organic solvents are not parenterally administrable, pure drug substance may still be obtained after evaporation and may be reconstituted for parenteral administration. For this reason, extraction in selected small volume organic solvents followed by evaporation and reconstitution with water should also be conducted.
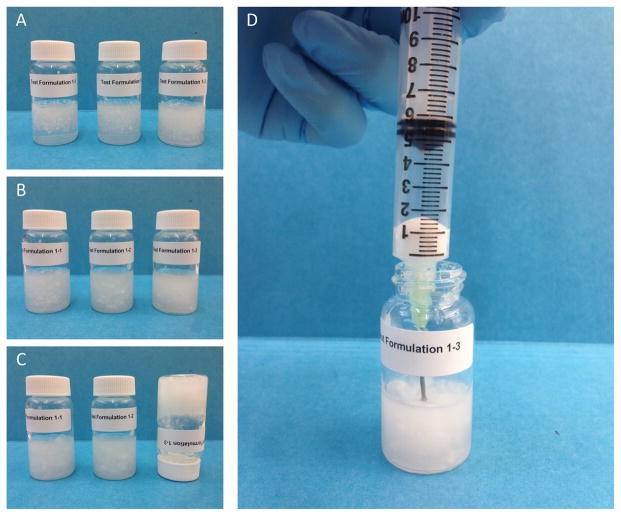
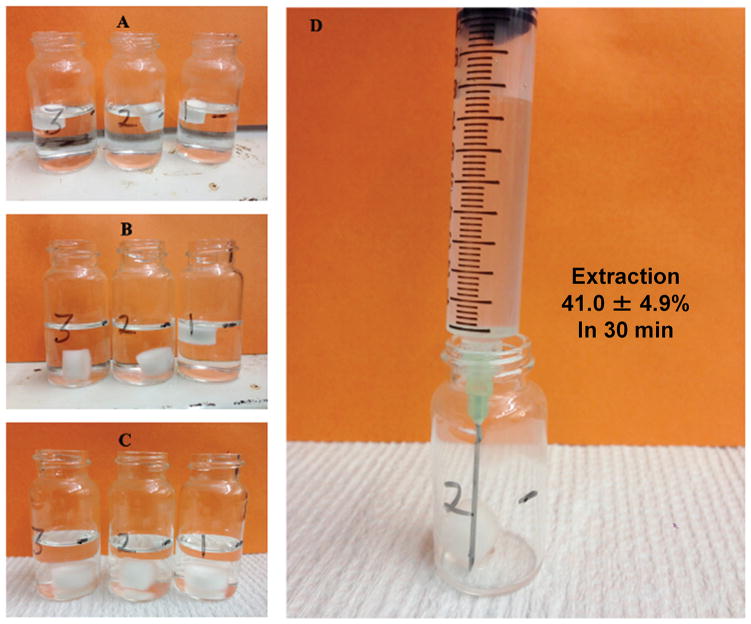
Figures 9–10 illustrate two examples demonstrating a typical syringeability test. As shown in Figure 9, significant swelling of the powder, accompanied by an increase in the solution viscosity, was observed almost immediately after addition of the manipulated drug product. At 1 min, the solution apparent viscosity was determined to be 0.2 Pa·s with n=0.7. Within 30 min, the solution had transformed into a gel (10.5 Pa·s with n=0.4) that was difficult to pull into a syringe fitted with an 18 gauge needle (Figure 9d). In contrast, when an intact dosage form of one test formulation was subjected to a certain solvent condition (details omitted), the tablet matrix remained intact and the solution viscosity stayed low (Figure 10). HPLC analysis confirmed that nearly half of the drug (41.0 ± 4.9%) was extracted into the 7.8 mL syringed solution. Such a condition would possibly lead to a high risk for parenteral abuse. It should be noted that in a real-world setting the abuse-by-injection route typically involves the use of 1–3 mL syringe and hypodermic needles (23–29 gauges). The smaller the size of the syringe and the narrower the diameter of the needle, the more difficult it will be to withdraw/expel viscous solution. Accordingly, the selection of 10 mL syringe and 18 gauge needle in this study represents a “worse-case” scenario for in vitro assessment of syringeability. In addition to pulling the plunger to assess the syringeability, force required to push out the withdrawn liquid can also be used to benchmark the difficulty of syringe.
Figure 9.
Syringeability test of manipulated test formulation 1 in 10 mL water at 25°C. (A) 1 min after addition of manipulated tablets; (B) 10 min after addition of manipulated tablets; (C) 30 min after addition of manipulated tablets; and (D) difficulty withdrawing using a 10 mL syringe with an 18 gauge needle.
Figure 10.
Syringeability test of manipulated test formulation 1 under a different extraction condition. (A) 1 min after addition of manipulated tablets; (B) 10 min after addition of manipulated tablets; (C) 30 min after addition of manipulated tablets; and (D) withdrawing using a 10 mL syringe with an 18 gauge needle.
4) Insufflation potential assessment
Abuse by insufflation requires the drug product to be available in particles suitable for snorting. The information gained during manipulation assessment may provide knowledge about the ease of converting the drug product into a form suitable for snorting. For example, sotalol test formulation 2 could not be manipulated into particles smaller than 3 mm. Hence this formulation shows negligible risk for abuse by nasal route. On the other hand, sotalol test formulation 1 could easily be reduced into particles less than 2 mm (Figure 7a and 8). Hence, additional assessment is needed on the manipulated forms of this formulation to determine its release characteristics (from extraction studies) and aversion potential to abuse by insufflation. The mechanism of aversion could be due to physical discomfort or chemical irritation. Selective tests could be performed to evaluate the manipulated drug product for both aversion mechanisms. Additionally, the gelation tendency of the product may also be a good predictor for prevention of nasal abuse. Aversion assessment of the test formulations was beyond the scope of the present study.
Single point vs. statistical comparison
One practical challenge during extraction assessment, particularly for comparison of different products, is that relatively low amounts of drug can be extracted in most testing conditions, significantly less than 20% in 30 min in most cases. Considering the inherent variations associated with the test itself, it is difficult to make direct comparisons of two products based on a single point data (e.g. Reference=3.5±0.5% drug dissolved while Test=4.8±0.5% at 30 min). In such cases, the results of an extraction study could be used to create a drug release profile under different extraction conditions. These profiles can then be used for statistical comparison as is done for the Similarity (f2) test for dissolution studies.(Shah et al., 1998)
In vitro ADF evaluation: a challenge in relative comparison
A product utilizing ADF technology may not completely eliminate abuse. Rather, it needs to demonstrate apparent advantages over non-ADF products, both in vitro and in vivo, by reducing the probability of abuse (risk-based approach). Positive confirmation of an abuse deterrent property of a new opioid drug product, regardless of the application type, can only be established when compare to a suitable comparator. The basis of comparison could be one or more of the following approaches: 1) Proposed label claim for the NDA with first-time or improved ADF feature; or, the label claim of the RLD for the ANDA; 2) Selective evaluation of the critical ADF features based on formulation design; 3) Exhaustive evaluation for all possible ADF features; or 4) Assessment against a standardized evaluation matrix. In the first approach, evaluations regarding the approved label claim of abuse deterrence are typically required. However, it should be cautioned that this approach may be limited by the uncertainty associated with the label claim. For a product being developed to contain an ADF feature for the first time, the label may not include claims for all ADF features until significant post-marketing data is obtained, (FDA, 2015) and changes to the label claims may occur throughout the lifecycle of the product. The second approach focuses on the drug product formulation design and ensures that products are compared based on design and performance. It should be noted that this approach requires individual standards for evaluating different formulation design and technologies (e.g., osmotic pump, liquid filled capsule, matrix tablets, etc.). These standards would also need to be constantly revised as new formulation design and technology becomes available. Third approach provides the most assurance of the quality of the ADF feature of the product. However, the sheer amount of the evaluation studies required may sometimes be unnecessary and burdensome. The method and results described in the current study is one example and attempt to the fourth approach: standardized evaluation matrix.
Conclusions
Abuse deterrent products are relatively new formulations, and are complicated because of a lack of methodologies for assessing the abuse deterrent properties. There are no standardized methods for in-vitro evaluation of abuse deterrent properties. In this study, a performance matrix approach for evaluation of the abuse deterrence features of opioids drug products was proposed. Determination of the ADF feature(s) is to be based on totality of various factors encompassing product design evaluation, the ease of abuse, performance of the ADF feature, and the risk associated with the failure of an ADF feature. Furthermore, the comparison of potential superiority of ADF feature(s) is to be based on the relative performance by identical methods of evaluation, and independent of the product application type. One distinct advantage of using a standardized performance matrix approach is that the knowledge gained from a single evaluation study (e.g. extraction) can be applied across abuse routes of administration, and thus reducing the testing burden. For instance, the amount and concentration of the API extracted under different conditions as part of the extraction potential studies can provide information about the abuse potential by oral and parenteral routes. The ability to recover pure or almost pure API from these extracts can also provide information on the abuse potential by insufflation. An increase in difficulty or complexity of extraction process and/or a decrease in the purity of the extract would result in reducing the drug product’s appeal to the abuser. Furthermore, a standardized approach would allow comparison of ADF performance across different formulation designs and modes of abuse, which in turn can facilitate the development of future ADF formulations with improved features.
Supplementary Material
Supplemental Figure 1. Risk analysis of ADF design features in relationship to parenteral route of abuse
Supplemental Figure 2. Risk analysis of ADF design features in relationship to pulmonary route of abuse
Footnotes
Disclaimer: This article reflects the views of the author and should not be construed to represent FDA’s views or policies.
References
- Administration, S.A.a.M.H.S. National Survey of Substance Abuse Treatment Services (N-SSATS): 2012. Data on Substance Abuse Treatment Facilities 2013 [Google Scholar]
- Alexander L, Mannion RO, Weingarten B, Fanelli RJ, Stiles GL. Development and impact of prescription opioid abuse deterrent formulation technologies. Drug Alcohol Depend. 2014;138:1–6. doi: 10.1016/j.drugalcdep.2014.02.006. [DOI] [PubMed] [Google Scholar]
- APS. A position statement from the American Pain Society, Case Manager. 2000. Pain assessment and treatment in the managed care environment; pp. 50–53. [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- Budman SH, Grimes Serrano JM, Butler SF. Can abuse deterrent formulations make a difference? Expectation and speculation. Harm Reduct J. 2009;6:8. doi: 10.1186/1477-7517-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Black R, Grimes Serrano JM, Folensbee L, Chang A, Katz N. Estimating attractiveness for abuse of a not-yet-marketed “abuse-deterrent” prescription opioid formulation. Pain Med. 2010a;11:81–91. doi: 10.1111/j.1526-4637.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- Butler SF, Fernandez KC, Chang A, Benoit C, Morey LC, Black R, Katz N. Measuring attractiveness for abuse of prescription opioids. Pain Med. 2010b;11:67–80. doi: 10.1111/j.1526-4637.2009.00736.x. [DOI] [PubMed] [Google Scholar]
- Calderon SN, Klein M. A regulatory perspective on the abuse potential evaluation of novel stimulant drugs in the United States. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- CDC. Vital Signs: Overdoses of Prescription Opioid Pain Relievers-United States, 1999–2008. MMWR 2011. 2014;60:1–6. [PubMed] [Google Scholar]
- Cheatle MD, Barker C. Improving opioid prescription practices and reducing patient risk in the primary care setting. J Pain Res. 2014;7:301–311. doi: 10.2147/JPR.S37306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Zacny JP, Dworkin RH, Turk DC, Bigelow GE, Foltin RW, Jasinski DR, Sellers EM, Adams EH, Balster R, Burke LB, Cerny I, Colucci RD, Cone E, Cowan P, Farrar JT, Haddox JD, Haythornthwaite JA, Hertz S, Jay GW, Johanson CE, Junor R, Katz NP, Klein M, Kopecky EA, Leiderman DB, McDermott MP, O'Brien C, O'Connor AB, Palmer PP, Raja SN, Rappaport BA, Rauschkolb C, Rowbotham MC, Sampaio C, Setnik B, Sokolowska M, Stauffer JW, Walsh SL. Core outcome measures for opioid abuse liability laboratory assessment studies in humans: IMMPACT recommendations. Pain. 2012;153:2315–2324. doi: 10.1016/j.pain.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone EJ, Giordano J, Weingarten B. An iterative model for in vitro laboratory assessment of tamper deterrent formulations. Drug Alcohol Depend. 2013;131:100–105. doi: 10.1016/j.drugalcdep.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Covvey JR. Recent developments toward the safer use of opioids in the USA, with a focus on hydrocodone. Research in Social and Administrative Pharmacy; 2015. In Press ( http://dx.doi.org/10.1016/j.sapharm.2015.02.001) [DOI] [PubMed] [Google Scholar]
- FDA. Draft guidance on Assessment of Abuse Potential of Drugs. 2010 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM198650.pdf.
- FDA. Guidance on Abuse-Deterrent Opioid - Evaluation and Labeling. 2015 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM334743.pdf.
- Frank JW, Bair MJ, Becker WC, Krebs EE, Liebschutz JM, Alford DP. Update in pain medicine for primary care providers: a narrative review, 2010–2012. Pain Med. 2014;15:425–431. doi: 10.1111/pme.12337. [DOI] [PubMed] [Google Scholar]
- Hays LR. Journal of Addictive Diseases. Routledge: 2004. A Profile of OxyContin Addiction; pp. 1–9. [DOI] [PubMed] [Google Scholar]
- ICH-Q9. Quality risk management. 2006 http://www.fda.gov/downloads/Drugs/.../Guidances/ucm073511.pdf.
- Jasinski DR, Krishnan S. Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse. J Psychopharmacol. 2009;23:419–427. doi: 10.1177/0269881109103113. [DOI] [PubMed] [Google Scholar]
- Johnson FK, Ciric S, Boudriau S, Kisicki J, Stauffer J. Effects of Alcohol on the Pharmacokinetics of Morphine Sulfate and Naltrexone Hydrochloride Extended Release Capsules. The Journal of Clinical Pharmacology. 2012;52:747–756. doi: 10.1177/0091270011403740. [DOI] [PubMed] [Google Scholar]
- Johnson FK, Stark JG, Bieberdorf FA, Stauffer J. Relative oral bioavailability of morphine and naltrexone derived from crushed morphine sulfate and naltrexone hydrochloride extended-release capsules versus intact product and versus naltrexone solution: a single-dose, randomized-sequence, open-label, three-way crossover trial in healthy volunteers. Clinical therapeutics. 2010;32:1149–1164. doi: 10.1016/j.clinthera.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Katz N, Dart RC, Bailey E, Trudeau J, Osgood E, Paillard F. Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37:205–217. doi: 10.3109/00952990.2011.569623. [DOI] [PubMed] [Google Scholar]
- Katz N, Fernandez K, Chang A, Benoit C, Butler SF. Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain. 2008;24:528–535. doi: 10.1097/AJP.0b013e318167a087. [DOI] [PubMed] [Google Scholar]
- Katz NP, Adams EH, Chilcoat H, Colucci RD, Comer SD, Goliber P, Grudzinskas C, Jasinski D, Lande SD, Passik SD, Schnoll SH, Sellers E, Travers D, Weiss R. Challenges in the Development of Prescription Opioid Abuse-deterrent Formulations. The Clinical Journal of Pain. 2007:23. doi: 10.1097/AJP.0b013e318125c5e8. [DOI] [PubMed] [Google Scholar]
- Katz NP, Buse DC, Budman SH, Wing Venuti S, Fernandez KC, Benoit C, Bianchi R, Cooper D, Jasinski DR, Smith DE, Butler SF. Development and Preliminary Experience with an Ease of Extractability Rating System for Prescription Opioids, Drug Development and Industrial Pharmacy. Informa Healthcare. 2006:727–746. doi: 10.1080/03639040500529093. [DOI] [PubMed] [Google Scholar]
- Lusted A, Roerecke M, Goldner E, Rehm J, Fischer B. Prevalence of pain among nonmedical prescription opioid users in substance use treatment populations: systematic review and meta-analyses. Pain Physician. 2013;16:E671–E684. [PubMed] [Google Scholar]
- Mastropietro DJ, Omidian H. Abuse-deterrent formulations: part 1 – development of a formulation-based classification system. Expert Opinion on Drug Metabolism & Toxicology. 2015a;11:193–204. doi: 10.1517/17425255.2015.979786. [DOI] [PubMed] [Google Scholar]
- Mastropietro DJ, Omidian H. Abuse-deterrent formulations: Part 2: commercial products and proprietary technologies. Expert Opinion on Pharmacotherapy. 2015b;16:305–323. doi: 10.1517/14656566.2014.970175. [DOI] [PubMed] [Google Scholar]
- Meyer R, Patel AM, Rattana SK, Quock TP, Mody SH. Prescription Opioid Abuse: A Literature Review of the Clinical and Economic Burden in the United States. Popul Health Manag. 2014 doi: 10.1089/pop.2013.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi JV, Raffa RB, Pergolizzi JS, Taylor R. Non-analgesic effects of opioids: factors relevant to opioid abuse and abuse- deterrent formulations. Curr Pharm Des. 2012;18:6109–6115. doi: 10.2174/138161212803582379. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Pergolizzi JV, Jr, Muniz E, Taylor R, Jr, Pergolizzi J. Designing opioids that deter abuse. Pain Res Treat. 2012:282981. doi: 10.1155/2012/282981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers EM, Perrino PJ, Colucci SV, Harris SC. Attractiveness of reformulated OxyContin(R) tablets: assessing comparative preferences and tampering potential. J Psychopharmacol. 2013;27:808–816. doi: 10.1177/0269881113493364. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Schuller R, Romach MK, Horbay GL. Relative abuse potential of opioid formulations in Canada: a structured field study. J Opioid Manag. 2006;2:219–227. doi: 10.5055/jom.2006.0034. [DOI] [PubMed] [Google Scholar]
- Services, U.S.D.o.H.a.H. Addressing Prescription Drug Abuse in the United States: Current Activities and Future Opportunities. 2013. [Google Scholar]
- Setnik B, Bramson C, Bass A, Levy-Cooperman N, Malhotra B, Matschke K, Sommerville KW, Wolfram G, Geoffroy P. Intranasal administration of crushed ALO-02 (extended-release oxycodone with sequestered naltrexone): A randomized, controlled abuse-potential study in nondependent recreational opioid users. Journal of clinical pharmacology. 2015 doi: 10.1002/jcph.552. [DOI] [PubMed] [Google Scholar]
- Shah VP, Tsong Y, Sathe P, Liu JP. In Vitro Dissolution Profile Comparison-Statistics and Analysis of the Similarity Factor, f2, Pharmaceutical Research. Kluwer Academic Publishers-Plenum Publishers; 1998. pp. 889–896. [DOI] [PubMed] [Google Scholar]
- Shusterman D. Current Allergy and Asthma Reports. Current Medicine Group; 2003. Toxicology of nasal irritants; pp. 258–265. [DOI] [PubMed] [Google Scholar]
- Strassels SA. Economic burden of prescription opioid misuse and abuse. J Manag Care Pharm. 2009;15:556–562. doi: 10.18553/jmcp.2009.15.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift D, Kesavanthan J. The Anterior Human Nasal Passage as A Fibrous Filter for Particles. Chemical Engineering Communications. Taylor & Francis; 1996. pp. 65–78. [Google Scholar]
- Turk DC, O'Connor AB, Dworkin RH, Chaudhry A, Katz NP, Adams EH, Brownstein JS, Comer SD, Dart R, Dasgupta N, Denisco RA, Klein M, Leiderman DB, Lubran R, Rappaport BA, Zacny JP, Ahdieh H, Burke LB, Cowan P, Jacobs P, Malamut R, Markman J, Michna E, Palmer P, Peirce-Sandner S, Potter JS, Raja SN, Rauschkolb C, Roland CL, Webster LR, Weiss RD, Wolf K. Research design considerations for clinical studies of abuse-deterrent opioid analgesics: IMMPACT recommendations. Pain. 2012;153:1997–2008. doi: 10.1016/j.pain.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AG, Birnbaum HG, Mareva MN, Daher M, Vallow S, Schein J, Katz N. Direct costs of opioid abuse in an insured population in the United States. J Manag Care Pharm. 2005;11:469–479. doi: 10.18553/jmcp.2005.11.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Risk analysis of ADF design features in relationship to parenteral route of abuse
Supplemental Figure 2. Risk analysis of ADF design features in relationship to pulmonary route of abuse