Abstract
Oxidative stress contributes substantially to the pathophysiology of diabetic nephropathy (DN). Consumption of an antioxidant-fortified (AO) diet from an early age prevents or delays later development of DN in the Zucker rat female with type 2 diabetes. We hypothesize this is due to effects on mesangial matrix and renal nitric oxide synthase (NOS) distribution and to sex-specific differences in NOS responses in the diabetic kidney. Total glomerular tuft area (GTA) and PAS-positive tuft area (PTA), endothelial (e), neuronal (n) and inducible (i) NOS were quantified in males and females on AO or regular (REG) diet at 6 and 20 weeks of age. eNOS was observed in glomeruli and tubules. nNOS predominantly localized to tubular epithelium in both cortex and medulla. iNOS was expressed in proximal and distal tubules and collecting ducts. Sex, diabetes duration and AO diet affected the distribution of the three isoforms. GTA and PTA increased with duration of hyperglycemia and showed a negative correlation with renal levels of all NOS isoforms. AO diet in both genders was associated with less PAS-positive staining and less mesangial expansion than the REG diet, an early increase in cortical iNOS in males, and sex-specific changes in cortical eNOS at 20 weeks. These effects of AO diet may contribute to sex-specific preservation of renal function in females.
Keywords: Diabetic nephropathy, obesity, antioxidant diet, sex difference, nitric oxide synthases, mesangial matrix
Introduction
Diabetic nephropathy (DN) develops in up to 40 % of patients with type 2 diabetes mellitus (T2DM) and is the leading cause of end stage renal disease (ESRD) in the United States. Obesity is a contributing factor to the debilitating pathologies associated with T2DM (Collins et al., 2009, Harvey, 2003, Schena and Gesualdo, 2005, Sego, 2007), including DN. DN that develops in individuals with both obesity and diabetes (T2bDM) may have pathological features not seen with diabetes alone.
On the structural level, DN is characterized by progressive expansion of the mesangial matrix associated with glomerular hypertrophy, thickening of glomerular basement membrane with later mesangiolysis and formation of Kimmelstiel-Wilson nodules (Abrass, 1995, Kanetsuna et al., 2007, Nakagawa et al., 2007). Progressive expansion of the mesangium ultimately occludes the glomerular capillaries, a central mechanism in the development of ESRD (Steffes et al., 1989). DN is also characterized by tubular dysfunction and primary tubulointerstitial injury may play a role in initiating loss of renal function (Bonventre, 2012, Najafian et al., 2011, Phillips and Steadman, 2002). High glucose and production of advanced glycation end products stimulate proinflammatory cytokines and these, in turn, contribute to increases in intracellular reactive oxygen species (ROS) in renal tubular epithelial cells (Han et al., 2005, Tang et al., 2011). This has considerable impact as the tubulointerstitium accounts for more than 90% of kidney volume (Bonventre, 2012).
Multiple mechanisms contribute to the development of DN. One that is supported by numerous studies is the role of impaired nitric oxide (NO) synthesis in the development of renal dysfunction(Baylis, 2008, Komers and Anderson, 2003). In the normal kidney, this vasodilator helps regulate renal hemodynamics and maintain diuresis and natriuresis (Eppel et al., 2003, Majid and Navar, 2001, Mount and Power, 2006, Wilcox, 1998).
The obese Zucker rat (fa/fa) model of DN is characterized by a gene mutation (fa/fa) that results in lack of leptin receptors and development of T2bDM with DN (Chander et al., 2004, Coimbra et al., 2000, Ionescu et al., 1985, Zucker and Antoniades, 1972). At 6 weeks of age hyperglycemia develops and by 20 weeks the rats exhibit impaired renal function and glomerulosclerosis (Chander, Gealekman, 2004, Coimbra, Janssen, 2000, Ionescu, Sauter, 1985). Sexual dimorphism in development of DN has been described in patients (Baylis, 2008b, 2009, Denton and Baylis, 2007) and has also been described in the obese diabetic Zucker rat (Slyvka et al., 2009).
We have previously shown that an antioxidant-fortified (AO) diet is associated with preservation of renal function in the obese female Zucker rat and that this correlates with effects on protein levels of endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS) (Slyvka, Inman, 2009, Slyvka et al., 2011), the three NOS isoforms that are responsible for NO production in kidney (Alderton et al., 2001). In addition, multiple metabolic parameters were studied in obese diabetic Zucker rats and compared between those on regular (REG) and AO-fortified diets. Beneficial differences that were found include: lower body weight of males at 6 weeks of age (AO < REG), higher glomerular filtration rate (GFR), with less glomerular and tubulo-interstitial pathology in females at 20 weeks, and lower blood glucose of females at 6 and 13 weeks (AO < REG). However the relationship between glomerular matrix expansion and NO synthase (NOS) isoform expression and distribution in both glomeruli and tubules has not previously been examined. .
The goal of the present study is to measure glomerular mesangial matrix proliferation and observe the distribution of eNOS, nNOS, and iNOS in the kidney cortex and medulla of obese Zucker rats and to characterize the effects of sex, age and AO diet on these parameters. The findings will be interpreted in light of previously reported effects of the AO diet on renal function and structure, metabolic profile and NO levels in these rats (Slyvka, Inman, 2009, Slyvka, Wang, 2011) and will contribute to our understanding of the effects of sex and AO on the pathophysiology of DN in T2bDM.
2. Materials and Methods
2.1 Animals and Diets
Studies were conducted on obese (fa/fa) male (n=24) and female (n=22) Zucker rats (Table 1) (Harlan-Sprague Dawley, Indianapolis, IN) obtained at four weeks of age. Animals were housed under controlled conditions of lighting, temperature and humidity. Rats were fed ad libitum the REG rat diet 5012 or AO fortified diet, (Purina Mills, Inc., St. Louis, MO). The REG diet contained 32 IU α-tocopherol, 0.23 ppm selenium, 71 ppm zinc, 12 ppm copper, 69 ppm manganese, and 4.3 ppm β-carotene per kg food, and plain tap water. The AO diet consisted of rat diet 5012 supplemented with increased levels of several substances with known antioxidant effects (Vega-Lopez et al., 2004, Wiernsperger, 2003): 160 IU α-tocopherol/kg food, 1.15 ppm selenium, 150 ppm zinc, 60 ppm copper, 150 ppm manganese and 21.5 ppm β-carotene per kg food, and ascorbic acid fortified water, 1000 U/l.
Table 1.
Distribution of Zucker rats according to the experimental groups.
| Age | Male (n) | Female (n) | ||
|---|---|---|---|---|
| REG diet | AO diet | REG diet | AO diet | |
| 6 weeks | 6 | 6 | 6 | 6 |
| 20 weeks | 6 | 6 | 4 | 6 |
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Care and Use Committee of Ohio University (Permit Number: 27-2956). Rats were euthanized with 100 mg/kg i.v. pentobarbital and all efforts were made to minimize suffering. Kidneys were harvested at two time points: 6 weeks of age when hyperglycemia is first evident, and 20 weeks of age when there is significant nephropathy (Coimbra, Janssen, 2000, Ionescu, Sauter, 1985, Slyvka, Inman, 2009).
2.2 Tissue preparation
The kidneys were rapidly removed and half of one kidney was fixed in 10% buffered formalin overnight at 4C, and embedded in paraffin. Samples were prepared from 8 groups of animals as detailed in Table 1.
2.3 Mesangial matrix
To quantify the glomerular mesangial matrix area, 4-μm thick sections were prepared, PAS-stained and examined at 400× magnification. Fifteen randomly selected consecutive glomeruli of good quality with centralized polarity were analyzed in each kidney section. All sections were examined and scored by two independent observers with excellent correlation between the two observers. Image-Pro Plus 5.1 software was used to measure the area of the glomerular tuft and quantify the PAS positive area within the tuft. The level of mesangium expansion was assessed blindly by two independent observers. The ratio of PAS-positive tuft area (PTA) (μm2) to the total glomerular tuft area (GTA) (μm2), PTA/GTA (%) was calculated.
2.4 Immunohistochemistry (IHC)
For IHC, sections of 4-μm thickness were pretreated for 60 min at 60C after de-paraffination, and hydration antigen retrieval was performed in a crock pot at 90C for 40 min in 10 mM sodium citrate buffer, pH 6.0. Slides were cooled to room temperature, then treated with 3% H2O2 in PBS (Fisher Scientific, Pittsburg, PA), pH 7.4, for 30 min to block endogenous peroxidase. To block nonspecific binding, sections were incubated for 30 min at room temperature in 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) in PBS, pH 7.4, followed by streptavidin and biotin blocking according to the manufacturer’s protocol (#SP-2002, Vector Laboratories, Burlingame CA). Sections were incubated overnight at 4C with one of the following isoform specific primary rabbit polyclonal IgG antibodies: anti-eNOS 1.5 μg/ml, SC-654 (Santa Cruz Biotech, Santa Cruz, CA), anti-nNOS 1 μg/ml, #7155, (Sigma-Aldrich) and anti-iNOS 2 μg/ml, ab3523, (Abcam Inc., Cambridge, MA). All primary antibodies were validated by their manufacturers for use in rats and were certified by the manufacturers to exhibit no cross reactivity among the different isoforms. Isotype negative controls were performed on a consecutive section using isotypic normal rabbit IgG at equivalent concentration for each primary antibody (#10500C, Invitrogen Corporation, Camarillo, CA). Slides were washed and 0.5 μg/ml of the biotinylated secondary goat anti-rabbit antibody (# B-2770, Thermo Fisher, Rockford, IL) was added for 1 hour at room temperature. Immunohistochemical reactivity was localized by reaction with diaminobenzidine (Sigma-Aldrich, St. Louis, MO) using DAB Enhanced Liquid Substrate System for Immunohistochemistry (D 3939, Sigma Aldrich). Following deposition of the oxidized insoluble brown DAB end-product, the immunostained sections were counterstained with hematoxylin for 15 sec, dehydrated and mounted.
Slides from both cortex and medulla from each animal were examined blindly by light microscopy at 200-fold magnification. The numbers of NOS isoform positive stained tubules and percentage of positively stained glomeruli (glomerular score) were counted for objective quantification in each of 5 randomly selected consecutive fields. The samples were also evaluated subjectively for extent of stained cells and staining intensity to determine an IHC score on a scale of 0-7, according to the protocol developed by Tugcu et al. (Tugcu et al., 2008). The obtained values were then assigned a rank order using Kruskal-Wallis non-parametric statistics. These values are reported in Tables 2, 3 and 4.
Table 2.
Quantification of eNOS expression in Zucker rat kidney presented as rank order.
| Animal Group and Age |
Tubules (#) | IHC score | Glomerular score % |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| Cortex | Medulla | Cortex | Medulla | |||
| REG Female | 6 w | 22.08 | 11.00 | 26.50 | 14.67 | 17.33 |
|
|
||||||
| REG Female | 20 w | 9.00 # | 8.25 | 6.70 # | 3.50 | 26.70 |
|
|
||||||
| REG Male | 6 w | 32.00 θ | 13.88 | 29.00 | 16.50 | 19.42 |
|
|
||||||
| REG Male | 20 w | 37.67 θ | 16.75 | 35.42 θ | 20.50 | 41.75 #θ |
|
|
||||||
| AO Female | 6 w | 13.17 | 7.83 | 14.00 * | 8.25 | 13.42 |
|
|
||||||
| AO Female | 20 w | 25.88 * | 2.00 | 21.50 * | 3.50 | 21.25 |
|
|
||||||
| AO Male | 6 w | 34.75 θ | 19.80 θ | 36.50 θ | 20.00 θ | 19.17 |
|
|
||||||
| AO Male | 20 w | 8.08 #θ * | 5.75 | 11.17 # * | 11.75 | 25.00 θ |
Rank order values are based on non-parametric analysis using the Kruskal-Wallis test. *, θ, # significantly different, p < 0.05,
- REG vs AO,
- Female vs Male,
- 6 vs 20 weeks.
Table 3.
Quantification of nNOS expression in Zucker rat kidney presented as rank order.
| Animal Group | Age | Tubules (#) | IHC score | ||
|---|---|---|---|---|---|
| Cortex | Medulla | Cortex | Medulla | ||
| REG Female | 6 w | 21.58 | 7.50 | 21.83 | 12.00 |
| REG Female | 20 w | 12.25 | 9.00 | 12.00 | 7.50 |
| REG Male | 6 w | 38.00 θ | 21.50θ | 36.42 θ | 22.83 |
| REG Male | 20 w | 9.58 # | 8.33 # | 17.50 # | 12.50 # |
| AO Female | 6 w | 14.50 | 8.83 | 17.50 | 12.75 |
| AO Female | 20 w | 20.00 | 5.00 | 18.50 | 7.50 |
| AO Male | 6 w | 34.17 θ | 18.40 | 32.33 θ | 20.92 |
| AO Male | 20 w | 25.67 * | 16.67 | 19.08 | 22.83 |
Rank order values are based on non-parametric analysis using the Kruskal-Wallis test. *, θ, # significantly different, p < 0.05,
- REG vs AO,
- Female vs Male,
- 6 vs 20 weeks. nNOS immunostaining was not observed in glomeruli.
Table 4.
Quantification of iNOS expression in Zucker rat kidney presented as rank order.
| Animal Group | Age | Tubules (#) | IHC score | ||
|---|---|---|---|---|---|
| Cortex | Medulla | Cortex | Medulla | ||
| REG Female | 6 w | 27.67 | 19.00 | 33.83 | 13.50 |
| REG Female | 20 w | 21.00 | 9.50 | 21.38 | 10.50 |
| REG Male | 6 w | 26.00 | 6.93 | 23.38 | 12.21 |
| REG Male | 20 w | 12.67 | 12.25 | 16.42 | 10.50 |
| AO Female | 6 w | 17.00 | 16.83 | 24.33 | 18.83 |
| AO Female | 20 w | 26.50 | 28.00 | 27.75 | 31.50 |
| AO Male | 6 w | 41.17 θ * | 23.83 * | 25.83 | 23.83 * |
| AO Male | 20 w | 15.33 # | 13.00# | 15.83 | 14.75 |
Rank order values are based on non-parametric analysis using the Kruskal-Wallis test. *, θ, # significantly different, p < 0.05,
- REG vs AO,
- Female vs Male,
- 6 vs 20 weeks. iNOS immunostaining was not observed in glomeruli.
2.5 Statistical Analysis
GTA and PTA were analyzed using the General Linear Model ANOVA with multiple comparisons with Sidak adjustment. NOS isoform measures were compared by rank order using the nonparametric Kruskal-Wallis test (Zar, 1984). Overall correlations between measures were analyzed by both Spearman and Pearson methods. The 0.05 level of probability was used as the criterion of significance. Statistical analysis was performed using SPSS, version 15.0 for Windows.
3. Results
3.1 Mesangial Matrix
The rats in this study exhibited hyperglycemia at 6 weeks of age. On a REG diet the fasting blood glucose was 444 ± 75.6 mg/dL (n = 9) in females and 328 ± 85.5 mg/dL (n = 13) in males. It remained elevated at 20 weeks: 443 ± 29.3 mg/dL (n = 13) in females and 439 ± 48.8 mg/dL (n = 11) in males. In the non- diabetic Zucker rat, random blood glucose averages between 114-136 mg/dL over the same age range (Yokoi et al., 2013).
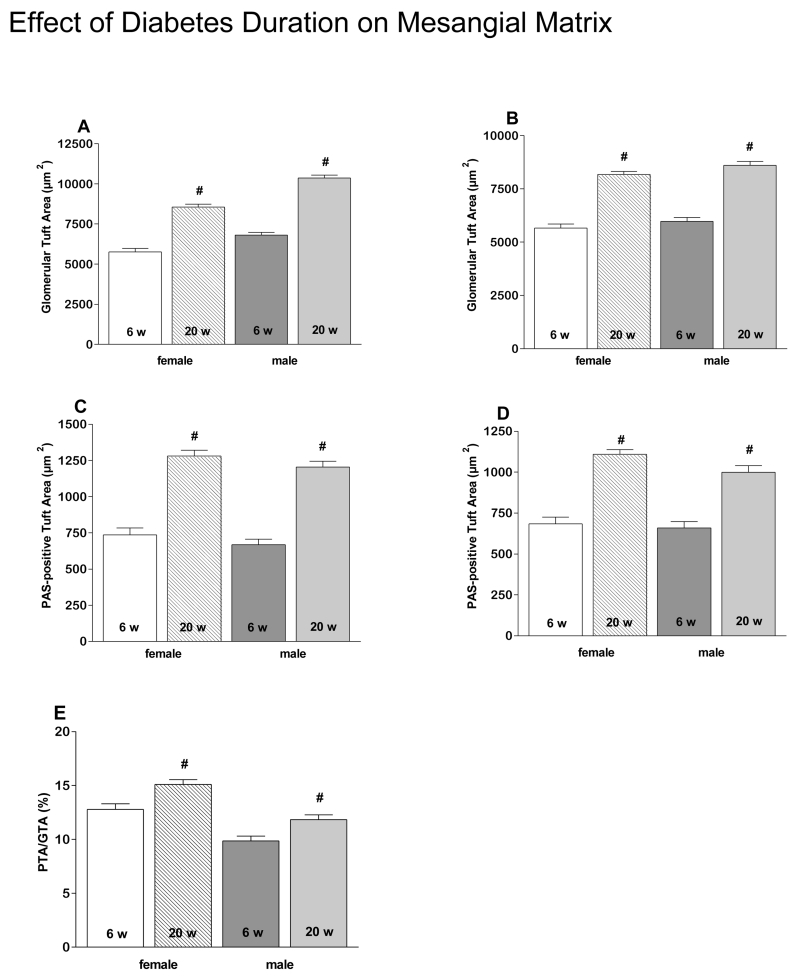
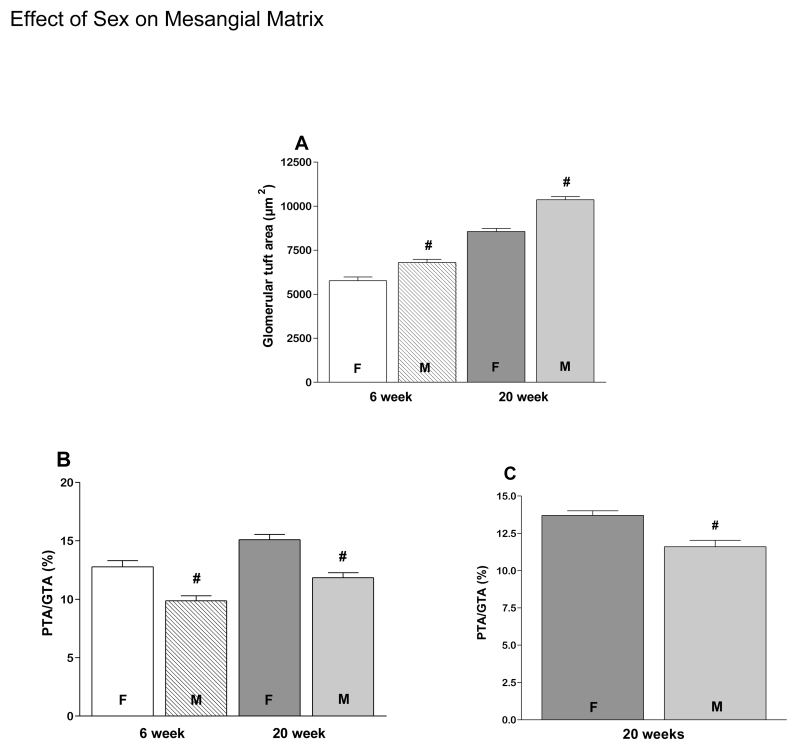
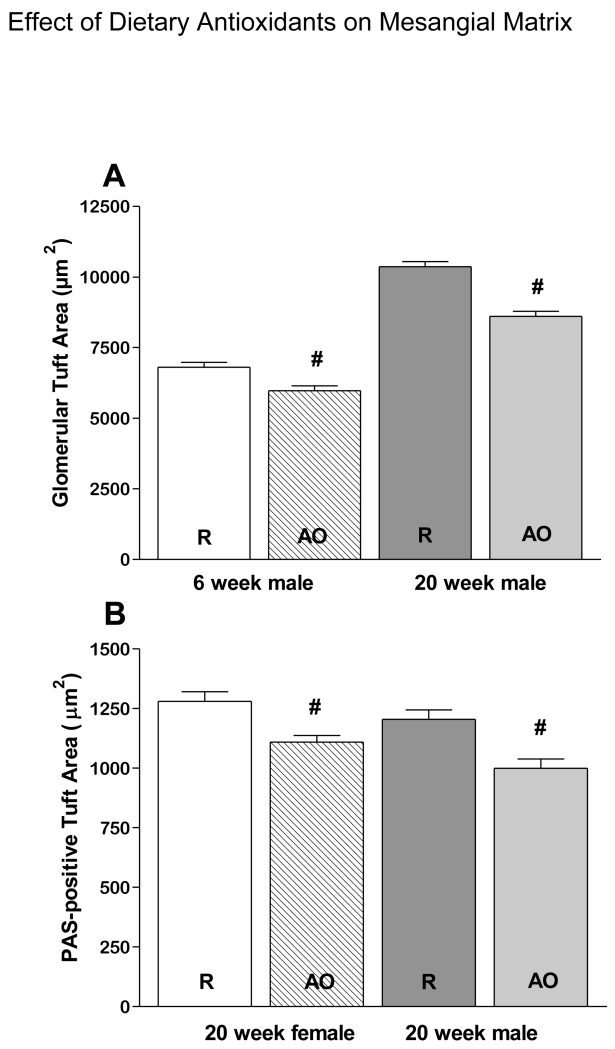
As expected, GTA and PTA increased in all groups between 6 and 20 weeks of age (Fig. 1A-D). However PTA/GTA increased only in REG diet animals (Fig. 1E), but not AO diet animals (data not shown). Also as expected, on the REG diet males had larger GTA at both 6 and 20 weeks (Fig. 2A) than females, resulting in a lower PTA/GTA ratio in the males (Fig 2B. PTA/GTA was also lower in males at 20 weeks on the AO diet (Fig. 2C). GTA was smaller in males on the AO diet compared to males on the REG diet at 6 and 20 weeks (Fig. 3A). PTA was less at 20 weeks in both males and females on the AO diet (Fig. 3B). There were no diet related differences in PTA/GTA.
Figure 1.
Glomerular structure changes with duration of diabetes in the Zucker rat. GTA increases with age in both males and females on both REG (A) and AO (B) diets. Increases in PTA are also observed in both genders on both REG (C) and AO (D) diets. PTA/GTA ratio is significantly increased in REG diet groups, both males and females (E).
# - significantly different 6 vs. 20 weeks, P < 0.05. 6w- 6 weeks, 20w- 20 weeks.
Figure 2.
Gender affects glomerular structure. GTA is larger in males than in females at both 6 and 20 weeks on the REG diet (A). PTA/GTA in females is higher at 6 weeks on the REG diet (B) and at 20 weeks on both REG (B) and AO diets (C).
# - significantly different females vs. males, P < 0.05. F- female, M- male.
Figure 3.
Differences in glomerular structure are associated with dietary antioxidants. GTA in males at 6 and 20 weeks of age (A) and PAS-positive Tuft Area at 20 weeks in both males and females (B) are lower in animals on the AO-fortified diet.
# - significantly different REG vs. AO diet, P < 0.05. R- regular diet, AO- antioxidant diet.
3.2 eNOS
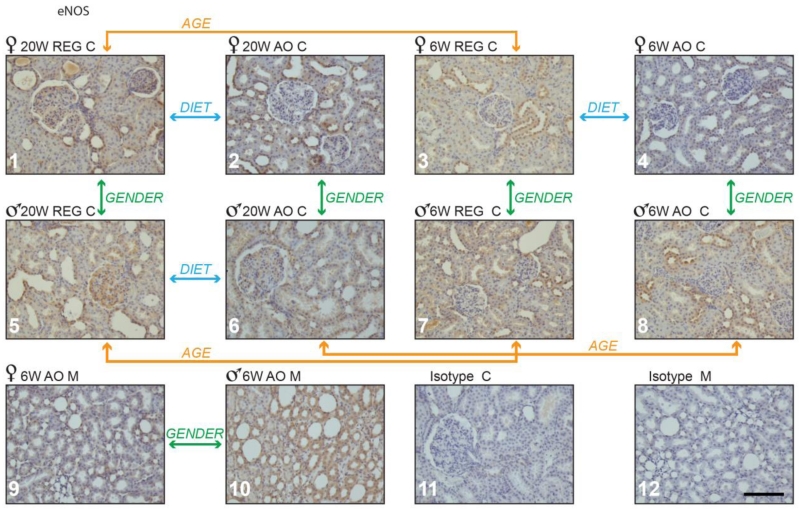
eNOS was the only isoform observed in glomeruli. Males on both diets had higher glomerular scores than females (Table 2). Based on tubular morphology the majority of eNOS positive tubules were distal, with less staining in proximal tubules. Although a lower IHC score was observed in the cortex of females on the AO diet (14) vs. REG diet (26.5) at 6 weeks (Table 2 and Fig. 4, panel 3 vs. 4), no difference was seen in males. By 20 weeks (16 weeks on the AO diet), eNOS expression in cortex showed a higher number of positively stained tubules and IHC score (Fig. 4, panel 1 vs. 2 in females). In contrast, males at 20 weeks showed lower numbers of positive tubules, and lower values for IHC and glomerular scores with AO supplementation (Fig. 4, panel 5 vs. 6).
Figure 4.
eNOS positive staining in obese Zucker rat kidney cortex and medulla. Panels 1 through 10: Zucker rat kidney stained immuno-histochemically for eNOS; representative samples are shown from groups demonstrating statistically significant differences in staining. Panels 11 and 12 show representative Zucker rat kidney sections treated with non-immune serum (isotype control). M - medulla; C-cortex; 6 w- 6 weeks, 20 w- 20 weeks. Magnification: 200×, bar = 100μm.
Sex differences of eNOS expression were also observed. Males had more eNOS positive stained tubules in cortex than females at 6 and 20 weeks (Fig. 4, panel 5 vs.1 and panel 7 vs. 3). At 20 weeks, this was associated with higher IHC and glomerular scores in cortex (Table 2). eNOS was also higher in males on the AO diet at 6 weeks, with a larger number of stained tubules and higher IHC score in both cortex and medulla (Table 2 and Fig. 4, panel 8 vs. 4 and panel 10 vs. 9). However, by 20 weeks on this diet the number of eNOS positive tubules was significantly higher for females in the cortex (Table 2 and Fig. 4, panel 2 vs. 6).
In cortex the number of eNOS positive tubules and IHC scores decreased with increasing age in females, but not males on REG diet (Table 2 and Fig. 4, panel 3 vs. 1). A decrease in these parameters was also seen in males on AO diet with age (Table 2 and Fig. 4, panel 8 vs. 6), while females maintained eNOS expression in cortical tubules. The glomerular score increased with age in males on REG diet (Table 2 and Fig. 4 panel 5 vs. 7), but not in females. On the REG diet at 20 weeks eNOS staining was also detected in the tubulo-interstitium.
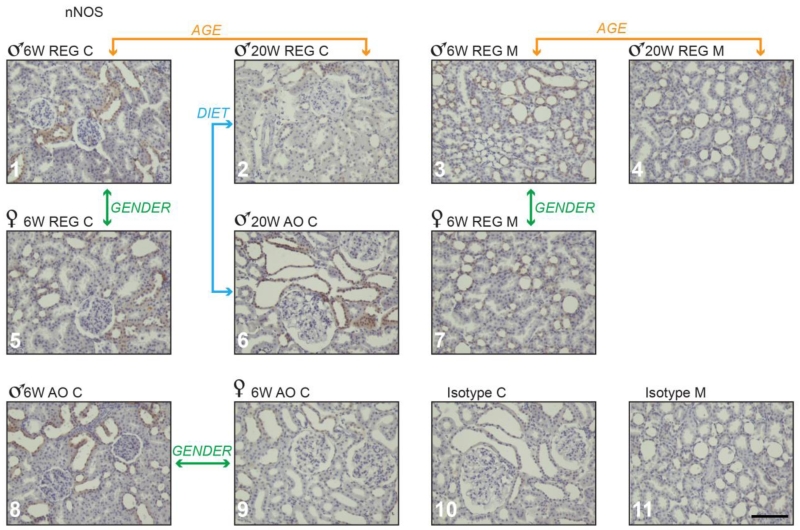
3.3 nNOS
In the present study, as shown by others (Maeda et al., 2003, Shin et al., 2000, Yabuki et al., 2006), nNOS was detected in tubules in both cortex and medulla of diabetic rodents, but not in glomeruli. We also detected nNOS in some collecting ducts. At 20 weeks males on the AO diet had higher numbers of nNOS positively stained tubules in cortex when compared with males on the REG diet (Table 3 and Fig. 5, panel 2 vs. 6). No diet differences were observed in females. At 6 weeks, males on both diets had more nNOS in kidneys than their female counterparts. They had greater numbers of nNOS positive stained tubules in cortex and medulla and higher IHC scores in cortex. (Table 3 and Fig. 5, panels 1 vs. 5, 3 vs. 7 and 8 vs. 9). Males on REG diet at 20 weeks showed an age related decrease in both nNOS positive tubular score and IHC score in both cortex and medulla compared to REG diet males at 6 weeks (Table 3 and Fig. 5, panels 1 vs. 2 and 3 vs. 4).
Figure 5.
nNOS positive staining in obese Zucker rat kidney cortex and medulla. Panels 1 through 9: Zucker rat kidney stained immune-histochemically for nNOS; representative samples are shown from groups demonstrating statistically significant differences in staining. Panels 10 and 11 show representative Zucker rat kidney sections treated with non-immune serum (isotype control). M-medulla; C-cortex; 6 w- 6 weeks, 20 w- 20 weeks. Magnification: 200×, bar = 100μm.
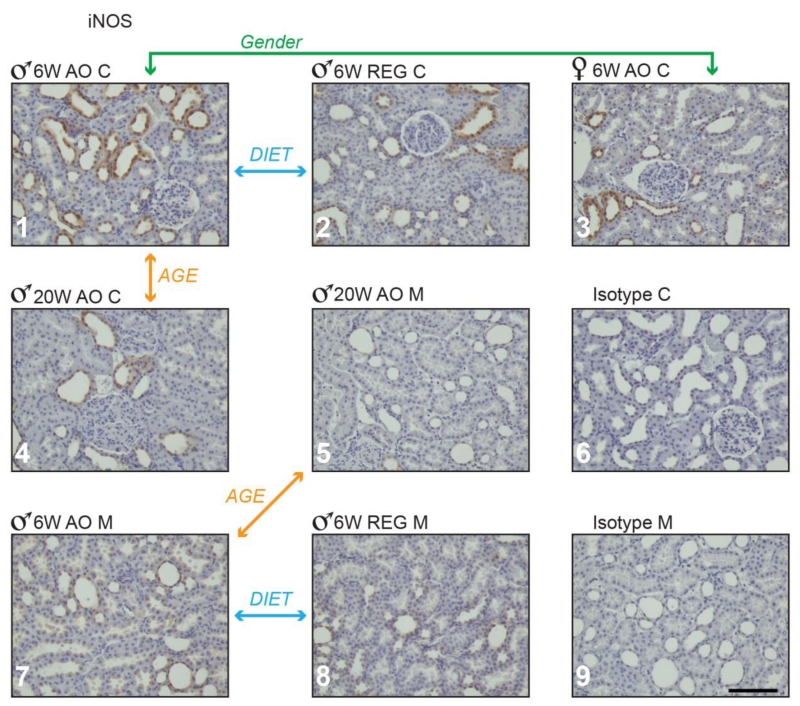
3.4 iNOS
In agreement with the results of others (Cosenzi et al., 2002, Fujihara et al., 2002, Hohenstein et al., 2008, Kuloglu and Aydin, 2014, Ptilovanciv et al., 2013), iNOS was detected in renal tubules in both cortex and medulla (Figure 6). A greater number of iNOS positive tubules was detected in 6 week males on AO diet than on the REG diet in both cortex and medulla. Total IHC score was also higher in medulla (Table 4 and Fig. 6, panels 1 vs. 2 and 7 vs. 8). No diet differences were observed in females. At 6 weeks males on the AO diet had more iNOS-positive stained tubules in cortex than females (Table 4 and Fig. 6, panel 1 vs. 3). Scores for iNOS positively stained tubules decreased in males on AO diet between 6 and 20 weeks, in both cortex and medulla (Table 4 and Fig. 6, panels 1 vs. 4 and 7 vs. 5).
Figure 6.
iNOS positive staining in obese Zucker rat kidney cortex and medulla. Panels 1 through 7: Zucker rat kidney stained immune-histochemically for iNOS; representative samples are shown from groups demonstrating statistically significant differences in staining. Panels 8 and 9 show representative Zucker rat kidney sections treated with non-immune serum (isotype control). M-medulla; C-cortex; 6 w- 6 weeks, 20 w- 20 weeks. Magnification: 200×, bar = 100μm.
3.5 Correlation analysis
The results obtained with Pearson and Spearman analyses were comparable. Spearman correlations with correlation coefficients ≥ |0.5| and p values ≤ 0.05 are presented in Supplementary Table 1. Degree of PAS staining in glomeruli (PTA) showed a positive correlation with GTA (Table S1, N 1). A positive correlation was observed between numbers of immune-positive tubules and degree of staining in cortex and medulla for eNOS (Table S1, N 2 and 3), nNOS (Table S1, N 4 and 5) and iNOS (Table S1, N 6). Also a positive correlation was seen between numbers of tubules immune-positive for iNOS and nNOS in cortex (Table S1, N 7).
Mesangial matrix PAS staining and glomerular tuft expansion showed a number of negative correlations with the immunostaining for NOS isoforms. For eNOS in medulla, a negative correlation was observed with GTA (Table S1, N 8). A negative correlation was observed between nNOS in medulla and both PTA (Table S1, N 12) and PTA/GTA (Table S1, N 16 and 17) and for nNOS in cortex with GTA (Table S1, N 9), PTA (Table S1, N 11 and 13), and PTA/GTA (Table S1, N 15). A similar negative correlation was observed between iNOS in cortex and both GTA and PTA (Table S1, N 10 and 14).
Please see Table S1 in the section on supplementary data given at the end of this article for details.
4. Discussion
4.1 Glomerular staining and morphology
Mesangial matrix expansion, glomerular basement membrane thickening and glomerular hypertrophy are key histological findings in early DN (Abrass, 1995, Couser and Johnson, 1994, Kasiske et al., 1985, Schena and Gesualdo, 2005), followed later by glomerular contraction. In the present study, increases in both GTA and PTA were observed in both male and female obese diabetic Zucker rats with age and both GTA and PTA were less in rats on the AO diet indicating a correspondence between diet and matrix proliferation. As mesangial cells are important in regulation of glomerular filtration, this may be related to the improved GFR in 20 week females on the AO diet observed by Slyvka et al. (Slyvka, Inman, 2009). Treatment of STZ-induced diabetic rats (Wistar) with an NO donor has been shown to alleviate extracellular matrix proliferation (ECM) (Hsu et al., 2015), consistent with our finding that the AO-fortified diet also decreases the ECM in diabetic rats.
4.2 eNOS
Pathologic changes in the kidney are accompanied by changes in the expression and localization of constitutive and inducible NOS isoforms. In animal models of DN, endothelial damage caused by increased inflammation and excess production of ROS coupled with ineffective NO action leads to compensatory increases in constitutive eNOS and nNOS as well as iNOS (Prabhakar et al., 2007, Tan et al., 2007), eventually compounding and accelerating the damage.
In the current experiment, levels of eNOS protein increased with duration of hyperglycemia in glomerular and vascular endothelium, in agreement with the findings of others (Veelken et al., 2000). It is known that eNOS levels are increased in glomerular endothelial cells of patients with DN (Hohenstein, Hugo, 2008). An increase in glomerular eNOS has also been seen in diabetic patients with microalbuminuria (Hiragushi et al., 2001). We observed that treatment with an AO-fortified diet resulted in a decrease in eNOS staining in glomeruli in 3 groups of animals: females at 6 and 20 weeks of age and males at 20 weeks, possibly reflecting decreased inflammation or increased NO effectiveness.
Few studies have been done comparing renal eNOS expression in the diabetic kidney of males and females. Male gender has been associated with more rapid progression of kidney disease (Baylis, 2009, Denton and Baylis, 2007, Neugarten and Silbiger, 1995) and overall, in the present study, levels of eNOS were higher in the kidneys of diabetic males than females. At 20 weeks females on the AO diet had lower glomerular scores for eNOS, but higher numbers of eNOS positive stained tubules and IHC scores in cortex. This group had better preservation of renal function as reported previously (Slyvka, Inman, 2009).
Under physiological conditions renal NO is derived from constitutive eNOS and nNOS (Raij and Baylis, 1995). In healthy kidneys, eNOS is localized in the endothelium of glomeruli and afferent and efferent arterioles where it generates vasodilatory NO. It is not detected in other cell types or in the tubular epithelial cells (Bachmann et al., 1995, Furusu et al., 1998, Ishii et al., 2001, Jarry et al., 2003). We found eNOS expressed in distal and to a smaller degree in proximal tubular epithelial cells in the Zucker obese diabetic rat, and although others have found increased peroxynitrite in renal tubules with DM (Hsu, Lee, 2015), the functional significance of tubular expression of eNOS remains to be established. Clearly, additional studies are needed regarding the role of eNOS in specific renal cell types as it relates to glomerular and tubular nephropathy.
4.3 nNOS
In the normal kidney, nNOS contributes to the constitutive generation of NO (Raij and Baylis, 1995). nNOS is found in the macula densa where it plays a role in tubule-glomerular feedback (Bachmann, Bosse, 1995, Ishii, Patel, 2001, Welch et al., 1999). It is also observed in efferent arterioles and in tubular epithelial cells all along the nephron with higher levels in the cortex and outer medulla than in the inner medulla (Bachmann, Bosse, 1995, Jarry, Renaudin, 2003). As mentioned above, renal damage in DN leads to compensatory increases in nNOS (Prabhakar, Starnes, 2007, Tan, Forbes, 2007). In the present study, nNOS was predominantly localized to the cortex (Table 3). It was also detected in epithelial cells of the thick ascending tubules, inner medullary collecting ducts and to a lesser extent the parietal epithelium of Bowman’s capsule. These findings are in agreement with those of others in the diabetic kidney (Fujihara, Mattar, 2002, Jarry, Renaudin, 2003, Komers et al., 2000, Yabuki, Tahara, 2006). The diminished expression of nNOS in epithelial cells of tubules observed here in the diabetic male rat on a REG diet with age may be related to pathological changes in renal hemodynamics and electrolyte and glucose transport regulation. Maintenance of nNOS in the 20 week males on the AO diet may indicate an effect of the diet to maintain compensatory NO production; unfortunately this did not correspond to augmentation of renal function in this group (Slyvka, Inman, 2009).
Although higher levels of nNOS have been reported in medulla than in cortex in rodent models of T1DN (Shin, Lai, 2000), cortical levels were higher in the present study in the obese Zucker rat as well as in a study by Yabuki et al. in the Long Evans Tokushima Fatty rat (Yabuki, Tahara, 2006). This may reflect pathophysiological differences specific to the type of diabetes or to the comorbid obesity, or an inability of the Zucker rat to mount an adequate compensatory nNOS response in the medulla. Li et al. previously reported that this rat model can exhibit downregulation of nNOS in the setting of tubular damage preceding frank diabetes and renal failure (Li et al., 2005).
Few studies have been done on the effects of diabetes on nNOS in kidneys of female rats. In the present study, overall nNOS expression was lower in females than in males. This may be indicative of a sex difference in the degree of tubular inflammation and damage or due to the known effects of sex steroids on the pathogenesis of renal disease, including cell proliferation, synthesis and degradation of collagen and proteoglycans and oxidative balance (Neugarten et al., 1997). Androgens can increase levels of damaging superoxide in the kidney (Iliescu et al., 2007). Estrogens increase endogenous antioxidant activity and suppress collagen synthesis in glomerular mesangial cells (Kwan et al., 1996). These hormonal effects may evoke a more modest induction of nNOS in females than that seen in males.
4.4 iNOS
iNOS is not found in significant amounts in healthy kidney (Jarry, Renaudin, 2003, Romagnani et al., 1999). However, as shown here, iNOS is expressed in aging or damaged kidneys, including DN, in proximal and distal tubules and both cortical and medullary collecting ducts (Cosenzi, Bernobich, 2002, Fujihara, Mattar, 2002, Liang et al., 2010, Ptilovanciv, Fernandes, 2013, Veelken, Hilgers, 2000). iNOS has also been observed in glomerular epithelial and mesangial cells of diabetic rats and humans and the intensity and extent of staining increase with severity of nephropathy (Hohenstein, Hugo, 2008, Jeong et al., 2009, Moon et al., 2011). iNOS null mice demonstrate increased mesangial hypercellularity and expansion as well as more prominent tubulo-interstitial fibrosis (Trachtman et al., 2002). However, iNOS was not detected in glomeruli in the present study, possibly due to differences in species, strain or treatment.
There were no sex differences in iNOS in rats on a REG diet. However, provision of an AO-fortified diet resulted in increased iNOS in kidney of diabetic Zucker male rats at 6 weeks, but not in female rats. The effects of iNOS in the injured kidney remain controversial. Low levels of compensatory NO production by iNOS may be beneficial, especially early in the disease process, by increasing vasodilatory [NO] and inducing Cu/Zn superoxide dismutase (Alderton, Cooper, 2001, Pfeilschifter et al., 2003). With continued exposure to NO generated by iNOS and ROS generation by NOS monomers, which increase in DM due to dissociation of dimers (Slyvka, Wang, 2011), NO chemistry shifts towards harmful effects such as nitrosylation, nitration and oxidation (Alderton, Cooper, 2001, Brune, 2002, Pfeilschifter et al., 2001). ROS and reactive nitrogen species (RNS) are harmful to the kidney (Droge, 2002, Tan, Forbes, 2007, van der Vliet et al., 1996) and increased nitrosative and oxidative stress is one of the leading mechanisms in development of DN (Komers and Anderson, 2003, Lee et al., 2007, Lee, 1999, Prabhakar, Starnes, 2007, Tan, Forbes, 2007).
The increases in iNOS on the AO diet seen here in males were no longer evident at 20 weeks. Administration of antioxidants can downregulate iNOS and decrease both oxidative and nitrosative stress in T2DM (Brigelius-Flohe, 2007, Douillet et al., 1996, Hamilton et al., 2007, Montonen et al., 2004, Slyvka, Inman, 2009, Slyvka, Wang, 2011, Sun et al., 2008, Tetsuka et al., 1996), but this may vary with the total NO and NOS balance at different stages of disease.
4.5 Correlations
Evaluation of simultaneous expression of the three NOS isoforms in both cortex and medulla reveals several positive correlations in their expression, consistent with a coordinated response to the pathological changes taking place in the diabetic kidney. The negative relationship of cortical nNOS and iNOS with PTA and GTA (Table S1, N9, 10, 11, 13, and 14) may indicate that these isoforms are protective against glomerular tuft and mesangial matrix expansion and is consistent with previous studies. NO inhibits proliferation of mesangial cells (Raij and Shultz, 1993, Rupprecht et al., 2000), adhesion of mesangial cells to ECM proteins (Yao et al., 1998), expression of intracellular adhesion molecule-1 (Ikeda et al., 1996) and synthesis of the matrix proteins collagen and fibronectin (Trachtman et al., 1995). It also down-regulates connective tissue growth factor (Keil et al., 2002) and promotes mesangial cell apoptosis (Brune, 2002). Altogether, this results in inhibition of mesangium proliferation and reduced matrix production. The large number of negative correlations between iNOS or nNOS and PTA or GTA may also indicate an interaction between glomerular filtration and perfusion and subsequent effects of the filtrate on the tubules that decrease expression of iNOS and nNOS. Or it may reflect two independent responses to one common mediator. However further studies are needed to define the exact mechanism.REG diet males had larger GTA associated with higher levels of IHC staining of constitutive eNOS and nNOS, but not inducible iNOS. At 20 weeks, males on the AO diet had lower PTA/GTA associated with less intensive total eNOS staining in cortex, while females in this category had higher levels of eNOS protein and eNOS dimers and better preservation of renal function (Slyvka, Inman, 2009, Slyvka, Wang, 2011).
5. Summary
The development of DN in obese Zucker rat is characterized by progressive increases in GTA and PTA that are negatively associated with levels of all three NOS isoforms in kidney. Overall, males have higher levels of eNOS in glomeruli than females and lower values for PTA/GTA. eNOS, nNOS and iNOS are detected in tubules in both cortex and medulla consistent with a potential role for all three isoforms in the progression of diabetic tubulopathy. Age-related worsening of mesangial tuft expansion and deposition of PAS-positive material in the obese diabetic Zucker rat is ameliorated by an AO-fortified diet in both genders, supporting a role for an anti-oxidant fortified diet, in combination with other therapies, as a preventive measure to reduce glomerular pathology in T2DM patients at high risk for the development of diabetic nephropathy.
Supplementary Material
Highlights.
Effects of antioxidant (AO) diet, sex and age are examined in diabetic rat kidney
AO diet interacts with sex on distribution of NOS isoforms and mesangium structure
These findings may underlie better preservation of GFR in females
Acknowledgements
This study was supported by the National Institutes of Health (NIH) NIDDK Award R15DK073066 to SRI and FVN. JD was supported by an OU-HCOM Summer Undergraduate Research Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- AO
antioxidant
- DN
diabetic nephropathy
- ECM
extracellular matrix
- Enos
endothelial nitric oxide synthase
- ESRD
end stage renal disease
- GTA
glomerular tuft area
- IHC
immunohistochemistry
- iNOS
inducible nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- PAS
periodic acid Schiff stain
- PTA
PAS-positive glomerular tuft area
- T2DM
type 2 diabetes mellitus
- T2bDM
type 2 diabetes associated with obesity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abrass CK. Diabetic nephropathy. Mechanisms of mesangial matrix expansion. The Western journal of medicine. 1995;162:318–21. [PMC free article] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. The American journal of physiology. 1995;268:F885–98. doi: 10.1152/ajprenal.1995.268.5.F885. [DOI] [PubMed] [Google Scholar]
- Baylis C. Nitric oxide deficiency in chronic kidney disease. American journal of physiology Renal physiology. 2008;294:F1–9. doi: 10.1152/ajprenal.00424.2007. [DOI] [PubMed] [Google Scholar]
- Baylis C. Sexual dimorphism of the aging kidney: role of nitric oxide deficiency. Physiology (Bethesda) 2008b;23:142–50. doi: 10.1152/physiol.00001.2008. [DOI] [PubMed] [Google Scholar]
- Baylis C. Sexual dimorphism in the aging kidney:differences in the nitric oxide system. Nat Rev Nephrol. 2009;5:384–96. doi: 10.1038/nrneph.2009.90. [DOI] [PubMed] [Google Scholar]
- Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol. 2012;32:452–62. doi: 10.1016/j.semnephrol.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohe R. Adverse effects of vitamin E by induction of drug metabolism. Genes Nutr. 2007;2:249–56. doi: 10.1007/s12263-007-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune B. Nitric oxide and apoptosis in mesangial cells. Kidney international. 2002;61:786–9. doi: 10.1046/j.1523-1755.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Chander PN, Gealekman O, Brodsky SV, Elitok S, Tojo A, Crabtree M, et al. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen. J Am Soc Nephrol. 2004;15:2391–403. doi: 10.1097/01.ASN.0000135971.88164.2C. [DOI] [PubMed] [Google Scholar]
- Coimbra TM, Janssen U, Grone HJ, Ostendorf T, Kunter U, Schmidt H, et al. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000;57:167–82. doi: 10.1046/j.1523-1755.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- Collins AJ, Foley RN. United States Renal Data System 2008 Annual Data Report. Am J Kidney Disease. 2009;53:S1–S374. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Cosenzi A, Bernobich E, Bonavita M, Trevisan R, Bellini G, Campanacci L. Early effects of diabetes on inducible nitric oxide synthase in the kidney. Acta Diabetol. 2002;39:91–6. doi: 10.1007/s005920200019. [DOI] [PubMed] [Google Scholar]
- Couser WG, Johnson RJ. Mechanisms of progressive renal disease in glomerulonephritis. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1994;23:193–8. doi: 10.1016/s0272-6386(12)80971-1. [DOI] [PubMed] [Google Scholar]
- Denton K, Baylis C. Physiological and molecular mechanisms governing sexual dimorphism of kidney, cardiac, and vascular function. Am J Physiol Regul Integr Comp Physiol. 2007;292:R697–9. doi: 10.1152/ajpregu.00766.2006. [DOI] [PubMed] [Google Scholar]
- Douillet C, Tabib A, Bost M, Accominotti M, Borson-Chazot F, Ciavatti M. A selenium supplement associated or not with vitamin E delays early renal lesions in experimental diabetes in rats. Proc Soc Exp Biol Med. 1996;211:323–31. doi: 10.3181/00379727-211-43976. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Eppel GA, Denton KM, Malpas SC, Evans RG. Nitric oxide in responses of regional kidney perfusion to renal nerve stimulation and renal ischaemia. Pflugers Arch. 2003;447:205–13. doi: 10.1007/s00424-003-1149-1. [DOI] [PubMed] [Google Scholar]
- Fujihara CK, Mattar AL, Vieira JM, Jr., Malheiros DM, Noronha Ide L, Goncalves AR, et al. Evidence for the existence of two distinct functions for the inducible NO synthase in the rat kidney: effect of aminoguanidine in rats with 5/6 ablation. Journal of the American Society of Nephrology: JASN. 2002;13:2278–87. doi: 10.1097/01.asn.0000027354.12330.f4. [DOI] [PubMed] [Google Scholar]
- Furusu A, Miyazaki M, Abe K, Tsukasaki S, Shioshita K, Sasaki O, et al. Expression of endothelial and inducible nitric oxide synthase in human glomerulonephritis. Kidney international. 1998;53:1760–8. doi: 10.1046/j.1523-1755.1998.00907.x. [DOI] [PubMed] [Google Scholar]
- Hamilton SJ, Chew GT, Watts GF. Therapeutic regulation of endothelial dysfunction in type 2 diabetes mellitus. Diab Vasc Dis Res. 2007;4:89–102. doi: 10.3132/dvdr.2007.026. [DOI] [PubMed] [Google Scholar]
- Han HJ, Lee YJ, Park SH, Lee JH, Taub M. High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. Am J Physiol Renal Physiol. 2005;288:F988–96. doi: 10.1152/ajprenal.00327.2004. [DOI] [PubMed] [Google Scholar]
- Harvey JN. Trends in the prevalence of diabetic nephropathy in type 1 and type 2 diabetes. Curr Opin Nephrol Hypertens. 2003;12:317–22. doi: 10.1097/00041552-200305000-00015. [DOI] [PubMed] [Google Scholar]
- Hiragushi K, Sugimoto H, Shikata K, Yamashita T, Miyatake N, Shikata Y, et al. Nitric oxide system is involved in glomerular hyperfiltration in Japanese normo- and micro- albuminuric patients with type 2 diabetes. Diabetes Res Clin Pract. 2001;53:149–59. doi: 10.1016/s0168-8227(01)00260-1. [DOI] [PubMed] [Google Scholar]
- Hohenstein B, Hugo CP, Hausknecht B, Boehmer KP, Riess RH, Schmieder RE. Analysis of NO-synthase expression and clinical risk factors in human diabetic nephropathy. Nephrol Dial Transplant. 2008;23:1346–54. doi: 10.1093/ndt/gfm797. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Lee PH, Lei CC, Ho C, Shih YH, Lin CL. Nitric oxide donors rescue diabetic nephropathy through oxidative-stress-and nitrosative-stress-mediated Wnt signaling pathways. Journal of diabetes investigation. 2015;6:24–34. doi: 10.1111/jdi.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Ikeda U, Takahashi M, Shimada K, Minota S, Kano S. Nitric oxide inhibits intracellular adhesion molecule-1 expression in rat mesangial cells. J Am Soc Nephrol. 1996;7:2213–8. doi: 10.1681/ASN.V7102213. [DOI] [PubMed] [Google Scholar]
- Iliescu R, Cucchiarelli VE, Yanes LL, Iles JW, Reckelhoff JF. Impact of androgen- induced oxidative stress on hypertension in male SHR. American journal of physiology Regulatory, integrative and comparative physiology. 2007;292:R731–5. doi: 10.1152/ajpregu.00353.2006. [DOI] [PubMed] [Google Scholar]
- Ionescu E, Sauter JF, Jeanrenaud B. Abnormal oral glucose tolerance in genetically obese (fa/fa) rats. Am J Physiol. 1985;248:E500–6. doi: 10.1152/ajpendo.1985.248.5.E500. [DOI] [PubMed] [Google Scholar]
- Ishii N, Patel KP, Lane PH, Taylor T, Bian K, Murad F, et al. Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. Journal of the American Society of Nephrology: JASN. 2001;12:1630–9. doi: 10.1681/ASN.V1281630. [DOI] [PubMed] [Google Scholar]
- Jarry A, Renaudin K, Denis MG, Robard M, Buffin-Meyer B, Karam G, et al. Expression of NOS1 and soluble guanylyl cyclase by human kidney epithelial cells: morphological evidence for an autocrine/paracrine action of nitric oxide. Kidney international. 2003;64:170–80. doi: 10.1046/j.1523-1755.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- Jeong KH, Lee TW, Ihm CG, Lee SH, Moon JY, Lim SJ. Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin-induced diabetic rats. Am J Nephrol. 2009;29:274–82. doi: 10.1159/000158635. [DOI] [PubMed] [Google Scholar]
- Kanetsuna Y, Takahashi K, Nagata M, Gannon MA, Breyer MD, Harris RC, et al. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol. 2007;170:1473–84. doi: 10.2353/ajpath.2007.060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiske BL, Cleary MP, O’Donnell MP, Keane WF. Effects of genetic obesity on renal structure and function in the Zucker rat. J Lab Clin Med. 1985;106:598–604. [PubMed] [Google Scholar]
- Keil A, Blom IE, Goldschmeding R, Rupprecht HD. Nitric oxide down-regulates connective tissue growth factor in rat mesangial cells. Kidney Int. 2002;62:401–11. doi: 10.1046/j.1523-1755.2002.00462.x. [DOI] [PubMed] [Google Scholar]
- Komers R, Anderson S. Paradoxes of nitric oxide in the diabetic kidney. Am J Physiol Renal Physiol. 2003;284:F1121–37. doi: 10.1152/ajprenal.00265.2002. [DOI] [PubMed] [Google Scholar]
- Komers R, Lindsley JN, Oyama TT, Allison KM, Anderson S. Role of neuronal nitric oxide synthase (NOS1) in the pathogenesis of renal hemodynamic changes in diabetes. American journal of physiology Renal physiology. 2000;279:F573–83. doi: 10.1152/ajprenal.2000.279.3.F573. [DOI] [PubMed] [Google Scholar]
- Kuloglu T, Aydin S. Immunohistochemical expressions of adropin and inducible nitric oxide synthase in renal tissues of rats with streptozotocin-induced experimental diabetes. Biotechnic & histochemistry: official publication of the Biological Stain Commission. 2014;89:104–10. doi: 10.3109/10520295.2013.821713. [DOI] [PubMed] [Google Scholar]
- Kwan G, Neugarten J, Sherman M, Ding Q, Fotadar U, Lei J, et al. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney international. 1996;50:1173–9. doi: 10.1038/ki.1996.425. [DOI] [PubMed] [Google Scholar]
- Lee HB, Seo JY, Yu MR, Uh ST, Ha H. Radical approach to diabetic nephropathy. Kidney Int Suppl. 2007:S67–70. doi: 10.1038/sj.ki.5002389. [DOI] [PubMed] [Google Scholar]
- Lee HS. Oxidized LDL, glomerular mesangial cells and collagen. Diabetes Res Clin Pract. 1999;45:117–22. doi: 10.1016/s0168-8227(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Li Z, Rodriguez-Iturbe B, Ni Z, Shahkarami A, Sepassi L, Vaziri ND. Effect of hereditary obesity on renal expressions of NO synthase, caveolin-1, AKt, guanylate cyclase, and calmodulin. Kidney Int. 2005;68:2766–72. doi: 10.1111/j.1523-1755.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- Liang JH, Li YN, Qi JS, Jia XX. Peroxynitrite-induced protein nitration is responsible for renal mitochondrial damage in diabetic rat. Journal of endocrinological investigation. 2010;33:140–6. doi: 10.1007/BF03346572. [DOI] [PubMed] [Google Scholar]
- Maeda M, Yabuki A, Suzuki S, Matsumoto M, Taniguchi K, Nishinakagawa H. Renal lesions in spontaneous insulin-dependent diabetes mellitus in the nonobese diabetic mouse: acute phase of diabetes. Veterinary pathology. 2003;40:187–95. doi: 10.1354/vp.40-2-187. [DOI] [PubMed] [Google Scholar]
- Majid DS, Navar LG. Nitric oxide in the control of renal hemodynamics and excretory function. Am J Hypertens. 2001;14:74S–82S. doi: 10.1016/s0895-7061(01)02073-8. [DOI] [PubMed] [Google Scholar]
- Montonen J, Knekt P, Jarvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004;27:362–6. doi: 10.2337/diacare.27.2.362. [DOI] [PubMed] [Google Scholar]
- Moon JY, Tanimoto M, Gohda T, Hagiwara S, Yamazaki T, Ohara I, et al. Attenuating effect of angiotensin-(1-7) on angiotensin II-mediated NAD(P)H oxidase activation in type 2 diabetic nephropathy of KK-A(y)/Ta mice. Am J Physiol Renal Physiol. 2011;300:F1271–82. doi: 10.1152/ajprenal.00065.2010. [DOI] [PubMed] [Google Scholar]
- Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 2006;187:433–46. doi: 10.1111/j.1748-1716.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- Najafian B, Alpers CE, Fogo AB. Pathology of human diabetic nephropathy. Contributions to nephrology. 2011;170:36–47. doi: 10.1159/000324942. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–50. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Ding Q, Friedman A, Lei J, Silbiger S. Sex hormones and renal nitric oxide synthases. Journal of the American Society of Nephrology: JASN. 1997;8:1240–6. doi: 10.1681/ASN.V881240. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Silbiger SR. Effects of sex hormones on mesangial cells. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1995;26:147–51. doi: 10.1016/0272-6386(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Eberhardt W, Beck KF. Regulation of gene expression by nitric oxide. Pflugers Archiv: European journal of physiology. 2001;442:479–86. doi: 10.1007/s004240100586. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Eberhardt W, Beck KF, Huwiler A. Redox signaling in mesangial cells. Nephron Experimental nephrology. 2003;93:e23–6. doi: 10.1159/000066652. [DOI] [PubMed] [Google Scholar]
- Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histology and histopathology. 2002;17:247–52. doi: 10.14670/HH-17.247. [DOI] [PubMed] [Google Scholar]
- Prabhakar S, Starnes J, Shi S, Lonis B, Tran R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol. 2007;18:2945–52. doi: 10.1681/ASN.2006080895. [DOI] [PubMed] [Google Scholar]
- Ptilovanciv EO, Fernandes GS, Teixeira LC, Reis LA, Pessoa EA, Convento MB, et al. Heme oxygenase 1 improves glucoses metabolism and kidney histological alterations in diabetic rats. Diabetology & metabolic syndrome. 2013;5:3. doi: 10.1186/1758-5996-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij L, Baylis C. Glomerular actions of nitric oxide. Kidney International. 1995;48:20–32. doi: 10.1038/ki.1995.262. [DOI] [PubMed] [Google Scholar]
- Raij L, Shultz PJ. Endothelium-derived relaxing factor, nitric oxide: effects on and production by mesangial cells and the glomerulus. J Am Soc Nephrol. 1993;3:1435–41. doi: 10.1681/ASN.V381435. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Pupilli C, Lasagni L, Baccari MC, Bellini F, Amorosi A, et al. Inducible nitric oxide synthase expression in vascular and glomerular structures of human chronic allograft nephropathy. J Pathol. 1999;187:345–50. doi: 10.1002/(SICI)1096-9896(199902)187:3<345::AID-PATH239>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Rupprecht HD, Akagi Y, Keil A, Hofer G. Nitric oxide inhibits growth of glomerular mesangial cells: role of the transcription factor EGR-1. Kidney Int. 2000;57:70–82. doi: 10.1046/j.1523-1755.2000.00828.x. [DOI] [PubMed] [Google Scholar]
- Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol. 2005;16(Suppl 1):S30–3. doi: 10.1681/asn.2004110970. [DOI] [PubMed] [Google Scholar]
- Sego S. Pathophysiology of diabetic nephropathy. Nephrol Nurs J. 2007;34:631–3. [PubMed] [Google Scholar]
- Shin SJ, Lai FJ, Wen JD, Hsiao PJ, Hsieh MC, Tzeng TF, et al. Neuronal and endothelial nitric oxide synthase expression in outer medulla of streptozotocin-induced diabetic rat kidney. Diabetologia. 2000;43:649–59. doi: 10.1007/s001250051354. [DOI] [PubMed] [Google Scholar]
- Slyvka Y, Inman SR, Malgor R, Jackson EJ, Yee J, Oshogwemoh O, et al. Protective effects of antioxidant fortified diet on renal function and metabolic profile in obese Zucker rat. Endocrine. 2009;39:89–100. doi: 10.1007/s12020-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyvka Y, Wang Z, Yee J, Inman SR, Nowak FV. Antioxidant diet, gender and age affect renal expression of nitric oxide synthases in obese diabetic rats. Nitric oxide: biology and chemistry / official journal of the Nitric Oxide Society. 2011;24:50–60. doi: 10.1016/j.niox.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Steffes MW, Osterby R, Chavers B, Mauer SM. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes. 1989;38:1077–81. doi: 10.2337/diab.38.9.1077. [DOI] [PubMed] [Google Scholar]
- Sun J, Druhan LJ, Zweier JL. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys. 2008;471:126–33. doi: 10.1016/j.abb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AL, Forbes JM, Cooper ME. AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol. 2007;27:130–43. doi: 10.1016/j.semnephrol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Tang SC, Leung JC, Lai KN. Diabetic tubulopathy: an emerging entity. Contributions to nephrology. 2011;170:124–34. doi: 10.1159/000325647. [DOI] [PubMed] [Google Scholar]
- Tetsuka T, Baier LD, Morrison AR. Antioxidants inhibit interleukin-1-induced cyclooxygenase and nitric-oxide synthase expression in rat mesangial cells. Evidence for post-transcriptional regulation. The Journal of biological chemistry. 1996;271:11689–93. doi: 10.1074/jbc.271.20.11689. [DOI] [PubMed] [Google Scholar]
- Trachtman H, Futterweit S, Pine E, Mann J, Valderrama E. Chronic diabetic nephropathy: role of inducible nitric oxide synthase. Pediatric nephrology. 2002;17:20–9. doi: 10.1007/s004670200004. [DOI] [PubMed] [Google Scholar]
- Trachtman H, Futterweit S, Singhal P. Nitric oxide modulates the synthesis of extracellular matrix proteins in cultured rat mesangial cells. Biochem Biophys Res Commun. 1995;207:120–5. doi: 10.1006/bbrc.1995.1161. [DOI] [PubMed] [Google Scholar]
- Tugcu V, Bas M, Ozbek E, Kemahli E, Arinci YV, Tuhri M, et al. Pyrolidium dithiocarbamate prevents shockwave lithotripsy-induced renal injury through inhibition of nuclear factor-kappa B and inducible nitric oxide synthase activity in rats. J Endourol. 2008;22:559–66. doi: 10.1089/end.2007.0295. [DOI] [PubMed] [Google Scholar]
- van der Vliet A, Eiserich JP, Kaur H, Cross CE, Halliwell B. Nitrotyrosine as biomarker for reactive nitrogen species. Methods Enzymol. 1996;269:175–84. doi: 10.1016/s0076-6879(96)69019-3. [DOI] [PubMed] [Google Scholar]
- Veelken R, Hilgers KF, Hartner A, Haas A, Bohmer KP, Sterzel RB. Nitric oxide synthase isoforms and glomerular hyperfiltration in early diabetic nephropathy. J Am Soc Nephrol. 2000;11:71–9. doi: 10.1681/ASN.V11171. [DOI] [PubMed] [Google Scholar]
- Vega-Lopez S, Devaraj S, Jialal I. Oxidative stress and antioxidant supplementation in the management of diabetic cardiovascular disease. J Investig Med. 2004;52:24–32. doi: 10.1136/jim-52-01-23. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Wilcox CS, Thomson SC. Nitric oxide and tubuloglomerular feedback. Seminars in nephrology. 1999;19:251–62. [PubMed] [Google Scholar]
- Wiernsperger NF. Oxidative stress: the special case of diabetes. Biofactors. 2003;19:11–8. doi: 10.1002/biof.5520190103. [DOI] [PubMed] [Google Scholar]
- Wilcox CS. Role of macula densa NOS in tubuloglomerular feedback. Curr Opin Nephrol Hypertens. 1998;7:443–9. doi: 10.1097/00041552-199807000-00016. [DOI] [PubMed] [Google Scholar]
- Yabuki A, Tahara T, Taniguchi K, Matsumoto M, Suzuki S. Neuronal nitric oxide synthase and cyclooxygenase-2 in diabetic nephropathy of type 2 diabetic OLETF rats. Exp Anim. 2006;55:17–25. doi: 10.1538/expanim.55.17. [DOI] [PubMed] [Google Scholar]
- Yao J, Schoecklmann HO, Prols F, Gauer S, Sterzel RB. Exogenous nitric oxide inhibits mesangial cell adhesion to extracellular matrix components. Kidney Int. 1998;53:598–608. doi: 10.1046/j.1523-1755.1998.00793.x. [DOI] [PubMed] [Google Scholar]
- Yokoi N, Hoshino M, Hidaka S, Yoshida E, Beppu M, Hoshikawa R, et al. A Novel Rat Model of Type 2 Diabetes: The Zucker Fatty Diabetes Mellitus ZFDM Rat. Journal of diabetes research. 2013;2013:103731. doi: 10.1155/2013/103731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Multicentral hypothesis: the analysis of variance. Biostatistical Analysis. 2nd Prentice Hall; Englewood Cliffs, NJ: 1984. [Google Scholar]
- Zucker LM, Antoniades HN. Insulin and obesity in the Zucker genetically obese rat “fatty”. Endocrinology. 1972;90:1320–30. doi: 10.1210/endo-90-5-1320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.