Abstract
Arginine is an integral part of host defense when invading pathogens are encountered. The arginine metabolite nitric oxide (NO) confers antimicrobial properties, whereas the metabolite ornithine is utilized for polyamine synthesis. Polyamines are crucial to tissue repair and anti-inflammatory responses. iNOS/arginase balance can determine Th1/Th2 response. Furthermore, the host arginine pool and its metabolites are utilized as energy sources by various pathogens. Apart from its role as an immune modulator, recent studies have also highlighted the therapeutic effects of arginine. This article sheds light upon the roles of arginine metabolism during pathological conditions and its therapeutic potential.
Introduction
Arginine is a semi-essential amino acid which plays an important role during innate as well as adaptive immune responses [1••]. Arginine is a common substrate for four enzymes responsible for arginine catabolism in mammals: arginase, nitric oxide synthase (NOS), arginine decarboxylase (ADC) and arginine glycine amidinotransferase (AGAT). NOS is responsible for conversion of arginine to nitric oxide (NO) and citrulline. NO is a key player in innate immunity due to its antimicrobial potential. There are three isoforms of NOS – two constitutively expressed forms, neuronal NOS (NOS1) and endothelial NOS (NOS3), and inducible NOS (iNOS; NOS2), which is capable of high-output NO production. The rate-limiting step in NO production is the availability of arginine. The availability of arginine is determined by two factors, uptake into cells by cationic amino acid transporters (CATs) and the level of arginase [2]. Extracellular arginine is also known to increase iNOS expression at translational level by reducing the levels of phosphorylated eIF2α, eukaryotic translation initiation factor which regulates translation [3].
Arginase is a metalloenzyme which hydrolyzes L-arginine to ornithine and urea. The two isoforms of arginase exhibit differential subcellular localization and tissue distribution. Arginase I, a cytosolic enzyme, is predominantly expressed in hepatocytes. However, arginase II is a mitochondrial enzyme, and is expressed in brain, kidney, small intestine, monocytes and macrophages.
Arginine as an energy source during infection
Effective antimicrobial action in the intracellular environment in macrophages is brought about by molecules like nitric oxide (NO) and reactive oxygen species. The metabolism of arginine contributes to production of NO. The host cell maintains a basal level of free arginine in its cytoplasm. Intracellular pathogens like Salmonella typhimurium, Mycobacterium tuberculosis, etc. have the ability to utilize the host arginine pool. Arginine acts as a trigger for expression of various pathogenicity genes. The catabolism of arginine by the hydrolytic cleavage of arginine to ornithine and urea is well-studied in biological systems [4•]. Lately, extensive numbers of arginine utilization pathways have been discovered that highlight the importance of arginine as an energy source. The various pathways that operate in different microorganisms include the following: arginine to urea conversion pathway; arginine deaminase pathway; arginine succinyl transferase pathway; arginine transaminase, oxidase, and oxygenase pathways; and arginine decarboxylase pathways. The central enzyme to all these pathways is arginase [4•].
Agmatine is the product of the arginine decarboxylase pathway, which is efficiently utilized by bacteria as a source of energy [5••]. An example of a pathogen that utilizes its potential to metabolize arginine is Pseudomonas aeruginosa. It is an opportunistic pathogen, which colonizes the pulmonary system of the human body, especially the lungs. Agmatine has no deleterious effect on the pathogen. Further, agmatine is metabolized to putrescine, which acts as a source of ATP. A major cause of concern regarding Pseudomonas infection is its ability to form biofilms in the lungs. Biofilms are resistant to antibiotics and other antimicrobials owing to the rigid extracellular polymeric substance (EPS) [6]. Recently, it was shown that the presence of agmatine in the extracellular spaces of the lungs triggers biofilm formation. An alternate operon (agu2ABCA’) has been discovered, which detects the environmental agmatine in P. aeruginosa. In vitro experiments with the macrophage-like cell line, RAW 264.7, have also revealed the up-regulation of the arginine decarboxylase pathway in response to LPS and cytokines, resulting in increased agmatine concentrations [5••].
Leishmania genome includes an arginase encoding gene. Arginase-deficient Leishmania strain shows compromised intracellular survival in macrophages. In hosts, inhibition of arginase I and depletion of metabolites like agmatine result in decreased survival of Leishmania [7•]. These facts highlight the role of arginine metabolism during pathogenesis.
The importance of arginine metabolism has been reported in other pathogens like Salmonella Typhimurium, Helicobacter pylori and Mycobacterium tuberculosis [1•] as a source of energy and as a trigger for polyamine synthesis required for efficient pathogenesis (Figure 1). In the particular case of H. pylori infection, this bacterium possesses an arginase, encoded by the gene rocF, which can attenuate availability of arginine substrate for host cell iNOS, thus reducing production of NO and leading to evasion from its antibacterial effects [8•].
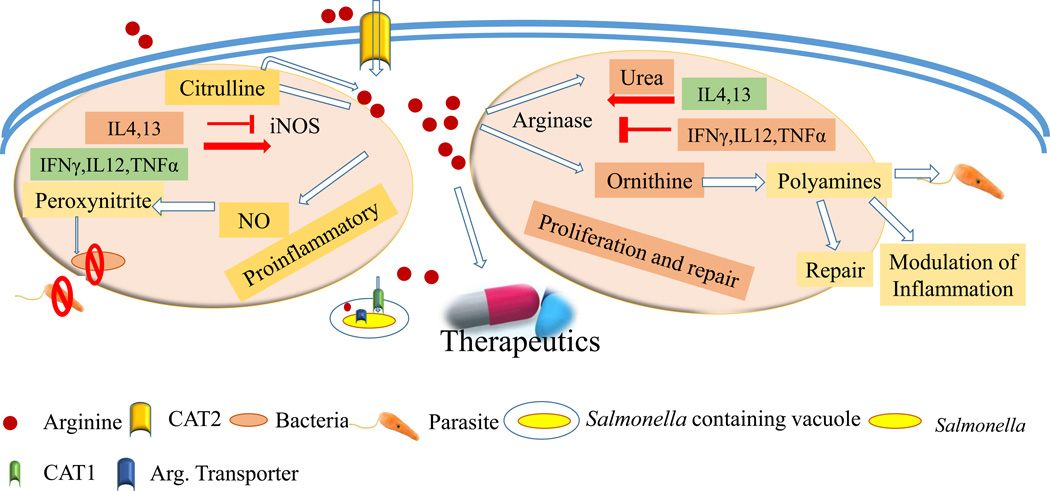
Figure 1. Overview of arginine metabolism and its regulation.
Arginine is transported via cationic amino acid transporter (CAT2) proteins in the host macrophages. Subsequently it is utilized in iNOS and arginase pathways. In the response to pathogens, iNOS is frequently upregulated and converts arginine to NO and citrulline. Further, citrulline can be utilized in arginine synthesis. NO and peroxynitrite exhibit antimicrobial activity. Arginase converts arginine to urea and ornithine. Ornithine contributes to polyamine synthesis, which is essential for tissue repair, serves as an energy source for various parasites, and can also modulate inflammation. Pro-inflammatory cytokines induce iNOS and repress arginase. Anti-inflammatory cytokines generally reverse the regulation of these enzymes. Arginine may have benefit as a therapeutic in conditions like acute kidney disorders and sepsis, as well as inflammatory bowel disease. The intracellular pathogen Salmonella recruits host arginine transporter (CAT1) to the Salmonella containing vacuole (SCV) to access the host cytosolic arginine pool. The bacterial arginine transporter (ArgT) imports arginine from the SCV lumen to the bacterial cytosol.
Host Sources of Arginine
In the host, arginine is transported via the y+, B0+, and b0+ transport systems. One such transporter system is the cationic amino acid transporter (CAT; also known as solute carrier 7A) family, which includes CAT1–4. Arginine is mostly transported by CAT1–3. CAT1 shows ubiquitous expression with the exception of liver. However, CAT2 has two splice variants CAT2A and B. CAT2A is a low affinity isoform primarily in the liver, and CAT2B is a high affinity transporter known to be abundant in macrophages. CAT3 is expressed in the brain and thymus [9•]. Murine macrophages, upon activation by IFN-γ, IL-4 or IL-10, upregulate CAT2, whereas CAT1 is constitutively expressed in these cells. This CAT2 upregulation leads to sustained production of NO in macrophages [10].
Citrulline, a byproduct of NO synthesis from arginine, is exported out of macrophages for arginine regeneration via the ornithine cycle [11]. Depletion of the arginine pool and pathogenic encounters cause metabolic switching from arginine to citrulline utilization for NO production. T cells have also been shown to utilize extracellular citrulline for continued proliferation during arginine starvation. This highlights the intercellular sharing of citrulline between immune cells for efficient NO production [12••].
Arginine as an immune modulator: A double-edged sword
Arginine metabolism is a deciding factor in innate immune response as arginine availability is rate-limiting in NO production. Besides its role in antimicrobial defense, arginine metabolism is crucial to M1 and M2 polarization effects. M1 macrophages are proinflammatory in nature, produce NO and NO-derived peroxynitrite, and can lead to a Th1 adaptive immune response. In contrast, heightened expression arginase is a hallmark of M2 differentiation, thus giving rise to an anti-inflammatory, anti-parasitic Th2 response. Type 1 cytokines (IFNγ, TNFα, IL-1, and IL-12) induce NO production and inhibit arginase I activity [13,14]. In contrast, cAMP and anti-inflammatory cytokines like IL-4, IL-13, and IL-10 can increase arginase I activity and have an inhibitory role in iNOS expression. [14,15] (Figure 1). Treatment of murine macrophages simultaneously with LPS and IFNγ induces iNOS expression. However, induction of arginase I in response to LPS treatment alone is also reported [16]. Depletion of the extracellular arginine level inhibits proinflammatory cytokine production in LPS-activated macrophages via ERK1/2 activation [17]. The level of extracellular arginine, as well as arginine uptake are key requirements for macrophage iNOS expression, which is regulated at the protein level in response to H. pylori infection [18••, 19]. Arginase plays a crucial role during infection-induced fibrosis and inflammation, along with parasite clearance. Different levels of arginine are also reported to affect T-cell proliferation. Apart from the role of arginase I [20], a recent study suggests that repression of arginase II is critical during dendritic cell maturation in order to promote T- cell proliferation.
Preterm neonates are more enriched in immunosuppressive CD71+ cells. These cells show high arginase II activity, and are thus responsible for increased susceptibility to infections. Premature newborns with low NO and plasma arginine levels are more susceptible to necrotizing enterocolitis [21•]. In rats, susceptibility to Toxoplasma gondii depends on iNOS/arginase I balance. High and low iNOS/arginase I ratio confers resistance, and susceptibility, respectively [22•].
Arginase II mRNA is a direct target of micro RNA-155 and its expression is highly upregulated in human and murine DCs as a response to maturation signals. This study also reveals that arginine depletion from the extracellular milieu impairs in vivo proliferation of CD4+ T-cells [23••]. Silencing of miR-155 in microglial cells leads to an increase in NO production and heightened TNF-α and IL-6 levels [24]. MicroRNA miR-223 positively regulates arginase-I expression and abrogates proinflammatory cytokine expression in bone marrow- and adipose tissue-derived macrophages, thereby committing macrophages towards an M2 phenotype [25]. IL-4 treatment and LPS induction leads to up-regulation and suppression of miR223 expression, respectively. Previously, the role of miR-124 in determining M2 phenotype and arginase expression has been documented during neuronal inflammation [26]. Another microRNA, miR-let-7c down-regulates the transcription factor C/EBP-δ. During LPS induction of alveolar macrophages, C/EBP-δ regulates proinflammatory response; hence, miR-let-7c contributes to M2 phenotype and arginase expression [27]. Due to this dichotomy, several pathogens employ an array of mechanisms to fine tune arginine metabolism.
Alteration of Arginine Metabolism by Pathogens
NO produced in granuloma macrophages is a key immune response to Mycobacterium infection. Earlier data suggests that BCG infection of macrophages results in induction of arginase in an IL-6- and IL-10-dependent manner [28]. In accordance, Mycobacterium tuberculosis infection in Arg1-deficient mice shows higher NO production, aggravated granuloma pathology and lower bacterial burden [15,28,29]. Arginase I expression in hypoxic granulomas restricts the L-arginine concentration and polyamine levels, subsequently decreasing T cell proliferation [29]. Importantly, in experimental M. tuberculosis infection, the demand for arginine substrate for iNOS can be met by back-conversion of citrulline to arginine by argininosuccinate synthase after uptake of extracellular arginine becomes rate-limiting [28].
A recent report using a tuberculosis meningitis model suggested that in response to Mycobacterium marinum infection, a macrophage-microglia network drives microglia polarization towards M1-type, with iNOS induction and arginase down-regulation [30]. Another intracellular pathogen, Salmonella typhimurium recruits the host cell cationic amino acid transporter (mCAT1) to the Salmonella-containing vacuole to gain access to the cytosolic arginine reservoir. Subsequently, the pathogen imports arginine from the vacuole by overexpressing the arginine transporter (ArgT) in order to support its growth [9] (Figure 1). Apart from intracellular bacteria, extracellular bacteria like Streptococcus pneumonia also modulate host arginase. S. pneumonia infection of alveolar macrophages induces arginase I expression in an IL-13-dependent manner, resulting in attenuated host defense against this pathogen during lung infection [31]. Similarly, Pseudomonas-infected lungs demonstrate a ~10-fold increase in arginase activity and a decrease in iNOS activity, leading to decreased production of NO [29].
The gastric pathogen H. pylori causes chronic active inflammation of the stomach and can lead to either peptic ulcer disease or gastric adenocarcinoma. It primarily interacts with the gastric epithelium as an extracellular pathogen, and it incites an innate and adaptive immune response. While it induces iNOS, it also induces host arginase II expression in mouse and human gastric tissue. Arg2−/− mice show lower bacterial burden and increased gastritis pathology as compared to WT mice, which is associated with an increased Th1/Th17 response [32]. H. pylori also causes apoptosis of host macrophages in order to evade host immune response; this apoptosis has been attributed to arginase activity, but also to downstream oxidation of polyamines, as the catabolism of spermine by the enzyme spermine oxidase generates H2O2 [33]. Additionally, the polyamine spermine not only inhibits expression of proinflammatory genes, but also results in reduction of iNOS protein expression in infected macrophages [34–36•]. However, during H. pylori infection, inhibition of polyamine synthesis with α-difluoromethylornithine (DFMO) reduces gastric inflammation and bacterial load in the mouse model [19], suggesting that this is a potential therapeutic agent.
Giardia lamblia is the etiological agent responsible for giardiasis. NO produced by iNOS is both cytostatic and cytopathic during Giardia infection [37,38]. Treatment with a Giardia extract induces arginase activity in host small intestine and in macrophages. However, infection of epithelial cells shows the reverse phenotype [39]. Interestingly, during infection by Giardia, macrophages expressing both arginase I and iNOS are recruited to the lamina propria. These double-positive cells were already reported to have an anti-inflammatory function in various infections [40]. In the case of Schistosoma mansoni infection, the eggs induce expression of IL-4 and IL-13, subsequently increasing arginase expression in lung and peritoneal macrophages [41•].
Arginine - A therapeutic approach
Agmatine, an intermediate metabolite in arginine metabolism, can activate α-1 adrenoreceptors and imidazolguanidine receptors. It has been shown to increase glomerular filtration and tubular reabsorption. This property of agmatine is being exploited to treat disorders related to renal dysfunction. These include chronic kidney disease (CKD), acute renal failure (ARF) and pre-eclampsia. In CKD, renal NO production decreases with declining renal function leading to infections. In ARF, changes in iNOS expression, along with increased levels of superoxide anion can result in increased peroxynitrite and nitrosative stress. Pre-eclampsia is reported to occur in approximately 5 to 10% of all pregnancies in the United States. It is major contributor to maternal and fetal morbidity and mortality. Gestational vasodilation is caused by elevated NO production. Exogenous administration of arginine in all these conditions can significantly reduce symptoms, improve renal function, and regulate NO production [42••].
During Plasmodium infection, Th1 response is critical for controlling parasite burden. Low arginine levels and endothelial dysfunction are common occurrences in malaria. Supplementation of L-arginine enhances NO production and decreases zygotes and ookinetes significantly [43•]. Notably, dietary arginine supplementation is a common practice as a preventive measure against sepsis in newborns [21]. Furthermore, arginine supplementation in mice has been reported to reduce experimental colitis in mice induced by either Citrobacter rodentium infection [44] or by an agent that causes epithelial injury, dextran sulfate sodium [45].
Conclusion
In this review article, the importance of arginine metabolism with respect to the host and pathogen has been discussed. The uptake of arginine is by cationic amino acid transporters (CATs), which are differentially expressed in different cell types. Following uptake, arginine is metabolized by the arginine deaminase pathway, wherein agmatine, an intermediate metabolite, serves as a connecting link between energy requirements and signaling. Moreover, the balance between arginase and iNOS expression dictates pro-inflammatory and anti-inflammatory responses. Hence, different pathogens employ strategies to counteract immune responses by interfering with host arginine metabolism. A fine balance between arginase and iNOS determines the host susceptibility to certain pathogens. Thus, arginine is shown to have potential in therapeutics, which involves the regulation of NO levels.
Arginine-tagged drug delivery systems can be engineered to target specific subcellular locations like Salmonella-containing vacuoles inside host cells. In the era of increasing antibiotic resistance, arginine may be a potent approach towards a next generation antimicrobial therapy.
Highlights.
Arginine as an energy source in the host and pathogen
Arginine-dependent NO production
Modulation of arginine metabolism- Strategy for immune evasion by pathogens
Bioengineered arginine tagged particles – Next generation antimicrobial therapy
Acknowledgements
This work was supported by the grant Life Science Research Board (LSRB0008) and DBT-IISc partnership program for advanced research in biological sciences and bioengineering to DC. Infrastructure support from ICMR (Center for Advanced Study in Molecular Medicine), DST (FIST), and UGC (special assistance) is acknowledged. MG is supported by a fellowship from IISc, India and AD is supported by a fellowship from Department of Biotechnology, India. DC received DAE SRC outstanding Investigator award and funds. KTW is supported by NIH grants R01DK053620, R01AT004821, R01CA190612, P01CA116087, and P01CA028842, a Department of Veterans Affairs Merit Review grant I01BX001453, the Thomas F. Frist Sr. Endowment, and the Vanderbilt Center for Mucosal Inflammation and Cancer.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• Of special interest
•• Of outstanding interest
- 1. Das P, Lahiri A, Lahiri A, Chakravortty D. Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathog. 2010;6:e1000899. doi: 10.1371/journal.ppat.1000899. ••The authors have discussed the role of arginase modulation during pathogenesis. It also addresses arginase as target for antimicrobial therapy.
- 2.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 3.Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci U S A. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cunin R, Glansdorff N, Pierard A, Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986;50:314–352. doi: 10.1128/mr.50.3.314-352.1986. •This classical article enlists the various pathways and for arginine metabolism in different bacteria.
- 5. Paulson NB, Gilbertsen AJ, Dalluge JJ, Welchlin CW, Hughes J, Han W, Blackwell TS, Laguna TA, Williams BJ. The arginine decarboxylase pathways of host and pathogen interact to impact inflammatory pathways in the lung. PLoS One. 2014;9:e111441. doi: 10.1371/journal.pone.0111441. •• The authors report the importance of arginine decarboxylase pathway and the role of agmatine in inflammatory pathways.
- 6.Malone JG. Role of small colony variants in persistence of Pseudomonas aeruginosa infections in cystic fibrosis lungs. Infect Drug Resist. 2015;8:237–247. doi: 10.2147/IDR.S68214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iniesta V, Carcelen J, Molano I, Peixoto PM, Redondo E, Parra P, Mangas M, Monroy I, Campo ML, Nieto CG, et al. Arginase I induction during Leishmania major infection mediates the development of disease. Infect Immun. 2005;73:6085–6090. doi: 10.1128/IAI.73.9.6085-6090.2005. • The article highlights the role of arginine in the pathogenesis and intracellular survival of Leishmania.
- 8. Gobert AP, McGee DJ, Akhtar M, Mendz GL, Newton JC, Cheng Y, Mobley HL, Wilson KT. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc Natl Acad Sci U S A. 2001;98:13844–13849. doi: 10.1073/pnas.241443798. • This study demonstrated that arginase expressed by H. pylori competes with macrophage iNOS for arginine substrate, thus reducing NO-dependent killing of the bacterium.
- 9. Das P, Lahiri A, Sen M, Iyer N, Kapoor N, Balaji KN, Chakravortty D. Cationic amino acid transporters and Salmonella Typhimurium ArgT collectively regulate arginine availability towards intracellular Salmonella growth. PLoS One. 2010;5:e15466. doi: 10.1371/journal.pone.0015466. • The authors have reported recruitment of host CAT to Salmonella containing vacoule to gain access to host arginine pool.
- 10.Yeramian A, Martin L, Arpa L, Bertran J, Soler C, McLeod C, Modolell M, Palacin M, Lloberas J, Celada A. Macrophages require distinct arginine catabolism and transport systems for proliferation and for activation. Eur J Immunol. 2006;36:1516–1526. doi: 10.1002/eji.200535694. [DOI] [PubMed] [Google Scholar]
- 11.Kornberg H. Krebs and his trinity of cycles. Nat Rev Mol Cell Biol. 2000;1:225–228. doi: 10.1038/35043073. [DOI] [PubMed] [Google Scholar]
- 12. Qualls JE, Subramanian C, Rafi W, Smith AM, Balouzian L, DeFreitas AA, Shirey KA, Reutterer B, Kernbauer E, Stockinger S, et al. Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host Microbe. 2012;12:313–323. doi: 10.1016/j.chom.2012.07.012. •• The authors have shown the alternative citrulline uptake in immune cells for NO production. The intercellular switching between arginine and citrulline utilization is also dicussed.
- 13.Chakravortty D, Hensel M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 2003;5:621–627. doi: 10.1016/s1286-4579(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 14.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 15.El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, Basaraba RJ, Konig T, Schleicher U, Koo MS, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonoki T, Nagasaki A, Gotoh T, Takiguchi M, Takeya M, Matsuzaki H, Mori M. Coinduction of nitric-oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysaccharide. J Biol Chem. 1997;272:3689–3693. doi: 10.1074/jbc.272.6.3689. [DOI] [PubMed] [Google Scholar]
- 17.Mieulet V, Yan L, Choisy C, Sully K, Procter J, Kouroumalis A, Krywawych S, Pende M, Ley SC, Moinard C, et al. TPL-2-mediated activation of MAPK downstream of TLR4 signaling is coupled to arginine availability. Sci Signal. 2010;3:ra61. doi: 10.1126/scisignal.2000934. [DOI] [PubMed] [Google Scholar]
- 18. Chaturvedi R, Asim M, Lewis ND, Algood HM, Cover TL, Kim PY, Wilson KT. L-arginine availability regulates inducible nitric oxide synthase-dependent host defense against Helicobacter pylori. Infect Immun. 2007;75:4305–4315. doi: 10.1128/IAI.00578-07. •• H. pylori induces expression of CAT2 and uptake of arginine, but this is impaired by spermine; treatment of mice with DFMO restores arginine uptake in gastric macrophages and NO production, and reduces both bacterial burden and inflammation in the stomach.
- 19.Chaturvedi R, Asim M, Hoge S, Lewis ND, Singh K, Barry DP, de Sablet T, Piazuelo MB, Sarvaria AR, Cheng Y, et al. Polyamines Impair Immunity to Helicobacter pylori by Inhibiting L-Arginine Uptake Required for Nitric Oxide Production. Gastroenterology. 2010;139:1686–1698. 1698, e1681–e1686. doi: 10.1053/j.gastro.2010.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schliehe C, Flynn EK, Vilagos B, Richson U, Swaminathan S, Bosnjak B, Bauer L, Kandasamy RK, Griesshammer IM, Kosack L, et al. The methyltransferase Setdb2 mediates virus-induced susceptibility to bacterial superinfection. Nat Immunol. 2015;16:67–74. doi: 10.1038/ni.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Badurdeen S, Mulongo M, Berkley JA. Arginine depletion increases susceptibility to serious infections in preterm newborns. Pediatr Res. 2015;77:290–297. doi: 10.1038/pr.2014.177. • The authors have discussed the immune supressive effects of arginase II in neonates along with protective effect of arginine in gut infections in preterm newborns.
- 22. Gao JM, Yi SQ, Wu MS, Geng GQ, Shen JL, Lu FL, Hide G, Lai DH, Lun ZR. Investigation of infectivity of neonates and adults from different rat strains to Toxoplasma gondii Prugniaud shows both variation which correlates with iNOS and Arginase-1 activity and increased susceptibility of neonates to infection. Exp Parasitol. 2015;149:47–53. doi: 10.1016/j.exppara.2014.12.008. • The authors have reported the relevance of iNOS/arginase balance in susceptibility to Toxoplasma gondii.
- 23. Dunand-Sauthier I, Irla M, Carnesecchi S, Seguin-Estevez Q, Vejnar CE, Zdobnov EM, Santiago-Raber ML, Reith W. Repression of arginase-2 expression in dendritic cells by microRNA-155 is critical for promoting T cell proliferation. J Immunol. 2014;193:1690–1700. doi: 10.4049/jimmunol.1301913. •• The authors have reported (1) micro RNA mediated repression of arginaseII (2) effect of extracllular arginine level on T cell proliferation (3) importance of arginase II repression for DC mediated Tcell proliferation.
- 24.Guedes J, Cardoso AL, Pedroso de Lima MC. Involvement of microRNA in microglia-mediated immune response. Clin Dev Immunol. 2013;2013:186872. doi: 10.1155/2013/186872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng ZX, Wang GX, Lin JD. A microRNA circuitry links macrophage polarization to metabolic homeostasis. Circulation. 2012;125:2815–2817. doi: 10.1161/CIRCULATIONAHA.112.111518. [DOI] [PubMed] [Google Scholar]
- 26.Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS One. 2013;8:e81774. doi: 10.1371/journal.pone.0081774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, Abraham E, Liu G. MicroRNA let-7c regulates macrophage polarization. J Immunol. 2013;190:6542–6549. doi: 10.4049/jimmunol.1202496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiber T, Ehlers S, Heitmann L, Rausch A, Mages J, Murray PJ, Lang R, Holscher C. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183:1301–1312. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grasemann H, Jaecklin T, Mehl A, Huang H, Rafii M, Pencharz P, Ratjen F. Multitracer stable isotope quantification of arginase and nitric oxide synthase activity in a mouse model of pseudomonas lung infection. Mediators Inflamm. 2014;2014:323526. doi: 10.1155/2014/323526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin Y, Sun X, Shao X, Cheng C, Feng J, Sun W, Gu D, Liu W, Xu F, Duan Y. Macrophage-Microglia Networks Drive M1 Microglia Polarization After Mycobacterium Infection. Inflammation. 2015;38:1609–1616. doi: 10.1007/s10753-015-0136-y. [DOI] [PubMed] [Google Scholar]
- 31.Knippenberg S, Brumshagen C, Aschenbrenner F, Welte T, Maus UA. Arginase 1 activity worsens lung-protective immunity against Streptococcus pneumoniae infection. Eur J Immunol. 2015;45:1716–1726. doi: 10.1002/eji.201445419. [DOI] [PubMed] [Google Scholar]
- 32.Lewis ND, Asim M, Barry DP, de Sablet T, Singh K, Piazuelo MB, Gobert AP, Chaturvedi R, Wilson KT. Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II. J Immunol. 2011;186:3632–3641. doi: 10.4049/jimmunol.1003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, Hacker A, Casero RA, Jr, Wilson KT. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161–40173. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- 34.Gobert AP, Cheng Y, Wang JY, Boucher JL, Iyer RK, Cederbaum SD, Casero RA, Jr, Newton JC, Wilson KT. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J Immunol. 2002;168:4692–4700. doi: 10.4049/jimmunol.168.9.4692. [DOI] [PubMed] [Google Scholar]
- 35.Moyat M, Velin D. Immune responses to Helicobacter pylori infection. World J Gastroenterol. 2014;20:5583–5593. doi: 10.3748/wjg.v20.i19.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bussiere FI, Chaturvedi R, Cheng Y, Gobert AP, Asim M, Blumberg DR, Xu H, Kim PY, Hacker A, Casero RA, Jr, et al. Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J Biol Chem. 2005;280:2409–2412. doi: 10.1074/jbc.C400498200. • The authors demonstrated that the polyamine spermine inhibits H. pylori-stimulated iNOS protein expression leading to loss of killing of this bacterium by macrophages.
- 37.Ropolo AS, Touz MC. A lesson in survival, by Giardia lamblia. Scientific World Journal. 2010;10:2019–2031. doi: 10.1100/tsw.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tako EA, Hassimi MF, Li E, Singer SM. Transcriptomic analysis of the host response to Giardia duodenalis infection reveals redundant mechanisms for parasite control. MBio. 2013;4:e00660–e00613. doi: 10.1128/mBio.00660-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stadelmann B, Hanevik K, Andersson MK, Bruserud O, Svard SG. The role of arginine and arginine-metabolizing enzymes during Giardia - host cell interactions in vitro. BMC Microbiol. 2013;13:256. doi: 10.1186/1471-2180-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maloney J, Keselman A, Li E, Singer SM. Macrophages expressing arginase 1 and nitric oxide synthase 2 accumulate in the small intestine during Giardia lamblia infection. Microbes Infect. 2015;17:462–467. doi: 10.1016/j.micinf.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. • The authors have reported how differential expression of NOS and arginase regulate granuloma pathology and inflammation.
- 42. Cherla G, Jaimes EA. Role of L-arginine in the pathogenesis and treatment of renal disease. J Nutr. 2004;134:2801S–2806S. doi: 10.1093/jn/134.10.2801S. discussion 2818S–2819S. •• The authors have reported the use of arginine as a therapeutic in preeclampsia and various disease conditions related to the renal system.
- 43. Zheng L, Pan Y, Feng Y, Cui L, Cao Y. L-Arginine supplementation in mice enhances NO production in spleen cells and inhibits Plasmodium yoelii transmission in mosquitoes. Parasit Vectors. 2015;8:326. doi: 10.1186/s13071-015-0940-0. • The authors have demonstrated L-arginine administration enhances the immunity to Plasmodium yoelii. Thus, suggesting therapeutic effects of L-arginine supplementation during malaria.
- 44.Gobert AP, Cheng Y, Akhtar M, Mersey BD, Blumberg DR, Cross RK, Chaturvedi R, Drachenberg CB, Boucher JL, Hacker A, et al. Protective role of arginase in a mouse model of colitis. J Immunol. 2004;173:2109–2117. doi: 10.4049/jimmunol.173.3.2109. [DOI] [PubMed] [Google Scholar]
- 45.Coburn LA, Gong X, Singh K, Asim M, Scull BP, Allaman MM, Williams CS, Rosen MJ, Washington MK, Barry DP, et al. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS One. 2012;7:e33546. doi: 10.1371/journal.pone.0033546. [DOI] [PMC free article] [PubMed] [Google Scholar]