Abstract
Oxidative stress is involved in activating photoreceptor death in several retinal degenerations. Docosahexaenoic acid (DHA), the major polyunsaturated fatty acid in the retina, protects cultured retina photoreceptors from apoptosis induced by oxidative stress and promotes photoreceptor differentiation. Here we investigated whether eicosapentaenoic acid (EPA), a metabolic precursor to DHA, had similar effects and whether retinal neurons could metabolize EPA to DHA. Adding EPA to rat retina neuronal cultures increased opsin expression and protected photoreceptors from apoptosis induced by the oxidants paraquat (PQ) and hydrogen peroxide (H2O2). Palmitic, oleic, and arachidonic acids had no protective effect, showing the specificity for DHA. We found that EPA supplementation significantly increased DHA percentage in retinal neurons, but not EPA percentage. Photoreceptors and glial cells expressed Δ6 desaturase (FADS2), which introduces the last double bond in DHA biosynthetic pathway. Pre-treatment of neuronal cultures with CP-24879 hydrochloride, a Δ5/Δ6 desaturase inhibitor, prevented EPA-induced increase in DHA percentage and completely blocked EPA protection and its effect on photoreceptor differentiation. These results suggest that EPA promoted photoreceptor differentiation and rescued photoreceptors from oxidative stress-induced apoptosis through its elongation and desaturation to DHA. Our data show, for the first time, that isolated retinal neurons can synthesize DHA in culture.
Keywords: polyunsaturated fatty acid, apoptosis, omega-3 fatty acids, differentiation, retina, fatty acid desaturase
INTRODUCTION
Omega 3 (n-3) long-chain fatty acids, such as eicosapentaenoic acid (20:5 n-3, EPA) and docosahexaenoic acid (22:6 n-3, DHA), are neuroactive lipids that provide multiple health benefits, ranging from promoting proper fetal development and healthy aging (Krauss-Etschmann et al. 2007; Su et al. 2008; Smith et al. 2009) to modulation of anti-inflammatory processes, neuro-inflammation, synaptic plasticity, and neuroprotection (Okabe et al. 2011; Lu et al. 2010; Kawashima et al. 2010; Crupi et al. 2013; Sinn et al. 2012; Dyall and Michael-Titus 2008; Patten et al. 2013; Trépanier et al. 2015).
DHA is the most abundant polyunsaturated fatty acid (PUFA) in the brain and the retina. In parallel with retinal development, levels of DHA increase in rat retinas during the first month of postnatal life. Twenty-five percent of total fatty acids in retinal tissue and over 50% of those fatty acids esterified in the phospholipids that form the outer segments of photoreceptors are DHA (Anderson and Maude 1972; Tinoco 1982; Fliesler and Anderson 1983). This PUFA is obtained from the diet or is synthesized in the liver from α-linolenic acid (18:3 n-3, ALA) and longer chain precursors, and is delivered to the brain and retina (Scott and Bazan 1989; Bazan et al. 2011). However, the enzymes required for DHA synthesis are present in the eye. Though retinal pigment epithelium cells are more effective in synthesizing DHA, retinal cells can also manufacture this fatty acid (Wang and Anderson 1993; Rotstein et al. 1996b). Furthermore, intravitreal injection with radioactive DHA precursors leads to the accrual and recovery of radioactive DHA in retinal lipids (Bazan et al. 1982; Wetzel et al. 1991; Alvarez et al. 1994; Stinson et al. 1991), which is very rapid when [14C]EPA is used as a precursor (Bazan et al., 1982). In rat brains, astrocytes can convert ALA to DHA, although brain or cerebellar neurons cannot do so (Moore et al. 1991). It is still unclear, however, whether neurons or glial cells are responsible for DHA biosynthesis in the retina, since both cell types are present in the isolated retinas used for the abovementioned studies.
DHA has long been recognized for its essential structural functions in the retina. It plays a crucial role in mediating proper visual function (Wheeler et al. 1975; Neuringer et al. 1984; Uauy et al. 1990) as it is essential for conformational changes in rhodopsin and for optimizing the early steps of visual signal transduction (Jeffrey et al. 2001; Litman et al. 2001; Mitchell et al. 1998; 2012). Interestingly, DHA plays dual roles in retinal physiology and pathology. The high PUFA content in the retina has been associated with the sensitivity of retinal tissue to oxidative damage, which is involved in photoreceptor death in retinal neurodegenerative diseases, including retinitis pigmentosa and age-related macular degeneration (AMD) (Carmody et al. 1999; Lohr et al. 2006; Beatty et al. 1999; Hollyfield et al. 2008). DHA is oxidized upon light exposure, increasing retinal vulnerability to photo-oxidative damage (Tanito et al. 2005; Tanito et al. 2009). A DHA oxidation fragment has been proposed to form adducts that contribute to the development of AMD (Hollyfield et al. 2008). Noteworthy, products of 5-lipoxygenase oxidation of DHA can be protective in oxygen-induced retinopathies (Sapieha et al. 2011). DHA also plays a protective role in photoreceptors; in these cells, it promotes survival, advances differentiation in vitro (Rotstein et al. 1996b; Rotstein et al. 1998; Rotstein et al. 1997), and prevents apoptosis induced by oxidative stress (Rotstein et al. 2003; German et al. 2006; German et al. 2013).
EPA, a metabolic precursor of DHA, is a minor structural component in several tissues, including the retina. It has numerous beneficial effects, including ameliorating neuroinflammation and cognitive impairment (Labrousse et al. 2012; Taepavarapruk and Song 2010), diminishing the progression of experimental autoimmune encephalitis in vivo (Unoda et al. 2013), and offering neuroprotection in in vitro models of Parkinson’s disease (Luchtman et al. 2013). Increasing n-3 PUFA tissue levels in animal models, by dietary or genetic means, decreases retinal lesions and pathological angiogenesis (Connor et al. 2007; Tuo et al. 2009). Serum levels of both EPA and DHA have been significantly associated with lower risk for neovascular AMD, geographic atrophy and retinopathy of prematurity (Merle et al. 2014; Reynolds et al. 2013; Fu et al. 2015).
Diverse model in vivo systems support the protective effects of EPA and DHA and elucidate their mechanisms of actions; however, few have investigated the potency of EPA. Both PUFA have shown similar effects in animal and cell models, but this is not always the case. DHA protects rat retinas from N-methyl-N-nitrosaurea-induced degeneration, but EPA does not (Moriguchi et al. 2003). The aims of this study were to determine whether EPA, like DHA, promoted the survival and differentiation of retina photoreceptors in culture and whether retina neurons could synthesize DHA using EPA as a precursor. The availability of primary cultures of pure retinal neurons allowed us to investigate these questions, which are difficult to respond in a more complex, in vivo system. As different oxidants affect cell viability and antioxidant protection in different ways (Lu et al. 2006), we have evaluated EPA’s protective effect upon oxidative stress induced by paraquat (methyl viologen dichloride hydrate, PQ) and hydrogen peroxide (H2O2). PQ induces anion superoxide generation and promotes photoreceptor apoptosis (Rotstein et al. 2003). H2O2, by itself a reactive oxygen species (ROS) and a physiological mediator of oxidative stress-induced apoptosis, has been widely used to produce oxidative damage in several cell types, including photoreceptors (Yamashita et al. 1992; Lu et al. 2006; Hoyt et al. 1997; Chucair et al. 2007; German et al. 2013). Our results show that supplementation with EPA protected photoreceptors from oxidative stress-induced apoptosis and promoted their differentiation, simultaneously increasing DHA levels in neuronal lipids. Inhibiting DHA synthesis not only prevented this increase, but also blocked EPA’s effects on survival and differentiation. These results demonstrate, for the first time, that retinal neurons can elongate and desaturate EPA to synthesize DHA, and that this synthesis of DHA is required for the neuroprotective effects of EPA on photoreceptors.
MATERIALS AND METHODS
Materials
In all experiments, we used 1-to-2-day-old albino Wistar rats bred in our own colony. At this early time of development, we used eyes from male and female pups indistinctly to obtain retina cells, which were pooled before seeding them. All procedures concerning animal use were carried out in accordance with the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals, incorporated in the Institute for Laboratory Animal Research (ILAR) Guide for Care and Use of Laboratory Animals, the ARRIVE Guidelines and were approved by the Institutional Review Committee for Laboratory Animal Research of the Universidad Nacional del Sur. Plastic 35-mm diameter culture dishes (CellStar) were from Greiner Bio-One (Frickenhausen, Germany). Dulbecco’s modified Eagle medium (DMEM), trypsin, insulin, and gentamicin were purchased from Invitrogen (Carlsbad, CA). EPA was obtained from Nu-Chek Prep (Elysian, MN). Bovine serum albumin (BSA, Fraction V; fatty acid-free; low endotoxin, tissue culture tested), trypsin inhibitor, transferrin, hydrocortisone, putrescine, insulin, poly-L-ornithine, gentamycin, 4,6-diamino-2-phenylindole (DAPI), paraquat dichloride (methyl viologen, 1,10-dimethyl-4,40-bipyridinium dichloride, PQ), fluorescein-conjugated secondary antibodies, paraformaldehyde, CP-24879 hydrochloride, and monoclonal anti-syntaxin antibody (HPC-1) were procured from Sigma (St Louis, MO, USA). Monoclonal antibody against BrdU (clone G3G4) was purchased from Developmental Studies Hybridoma Bank (DSHB), developed under the auspices of the NICHD, and maintained by The University of Iowa, Department of Biological Sciences (Iowa City, IA, USA). Hydrogen peroxide (H2O2) 30% was obtained from Baker (Argentina). Rabbit polyclonal antibody against Δ6 desaturase (FADS2) was procured from Abgent (San Diego, CA). Secondary antibody, Cy2-conjugated goat anti–rabbit, was from Jackson Immuno Research (West Grove, PA). MitoTracker Red CMXRos, propidium iodide (PI), recombinant 5-bromo-2-deoxyuridine-5-triphosphate (BrdUTP), TOPRO-3 (TOPRO) and terminal deoxy-nucleotidyl transferase (TdT) buffer were purchased from Invitrogen (Argentina). Monoclonal antibody anti–opsin (Rho4D2) was a generous gift from Robert Molday (University of British Columbia, Canada). Quick-ZOl was obtained from Kalium Technologies (Argentina), enzyme M-MLV RT from Promega (Madison, WI), and SYBR fast universal mix from KAPA Biosystems (Wilmington, Massachusetts). Primers for PCR were purchased from Biodynamics (Buenos Aires, Argentina).
Solvents were HPLC grade. All other reagents were analytical grade.
Cell cultures
Purified cultures of rat retinal neurons were prepared as previously described (Rotstein et al. 1996a; Rotstein et al. 1997). In brief, retinas from two-day-old rat pups were dissected and dissociated by trypsin digestion, which was followed by mechanical dissociation. Cells were then re-suspended in a chemically defined medium that lacked fatty acids and had none of the trophic factors required for photoreceptors (Rotstein et al. 1996a). We seeded approximately 0.5×106 cells per dish on 35-mm diameter dishes that had previously been sequentially treated with poly-ornithine or poly-lysine and Schwannoma conditioned medium (Adler 1982). Cultures were incubated at 36°C in a humidified atmosphere of 5% CO2. Dissociated retinal cells grown in these conditions give rise to pure neuronal cultures, mainly comprised of photoreceptors and amacrine cells, in which photoreceptors start degenerating through an apoptotic pathway after 4 days in vitro (Rotstein et al. 1996a). Mixed cultures of Müller glial cells and neurons were prepared from rat retinas, following previously described protocols (Politi et al. 1996; Politi et al. 2001a; Abrahan et al. 2009). Cells were then resuspended in DMEM containing 10% fetal calf serum (FCS) and seeded at a density of 2.0 × 106 cells per dish in 35-mm diameter plastic dishes, with no previous treatment. ARPE-19 cell line cultures were prepared as described (German et al. 2008).
Fatty acid supplementation
Neurons, cultured in fatty acid-free, chemically defined media, were supplemented with fatty acids complexed with bovine serum albumin (BSA) in a 2:1 (fatty acid:BSA) molar ratio added at day one in vitro (Rotstein et al. 1996a). The same volume of a BSA solution was added to the control cultures. To evaluate whether EPA protected photoreceptors exposed to oxidative stress, we used EPA concentrations ranging from 1 to 6 μM. A 3 μM concentration was chosen for subsequent experiments.
To ascertain the specificity of the effect of EPA addition and exclude both a nonspecific effect of fatty acids in a fatty acid-lacking media, and a general, non-specific lipid effect, palmitic (PAM), oleic (OLA), and arachidonic (ARA) acids complexed with BSA were added at 4 μM concentration (Rotstein et al. 1996a, 2003). This concentration is slightly higher than that used for EPA because different fatty acids vary in the optimal concentration required to achieve a certain effect and we had previously established that 4 μM PAM, OLA and ARA concentrations are not deleterious (Rotstein et al., 1996; 1997; 2003).
H2O2 and Paraquat (PQ) treatments
Cultures were treated with H2O2, as previously described (Chucair et al. 2007), with slight modifications. On day 3, cultures were incubated for 30 min at 36°C with 10 μM H2O2. The medium was replaced with 2.5 mL fresh neuronal medium, and cells were incubated for 5.5h and then fixed.
On day 3, 48 μM of PQ (final concentration in the incubation medium, in Ca2+-Mg-free Hank’s solution) was added to cultures. Neurons were then incubated for 24 h before fixation.
Inhibition of DHA synthesis
To evaluate whether retinal neurons metabolized EPA to DHA, on day 1 cultures were supplemented with or without CP-24879 hydrochloride (CP), an inhibitor of Δ5/Δ6 fatty acyl desaturases (FADS1, FADS2) solubilized in DMSO (5μM final concentration in culture), 1 h before EPA addition (Levin et al. 2002; Obukowicz et al. 1998). The same volume of DMSO was added to control cultures. On day 3, cultures were treated with H2O2. Cells were scraped for lipid analysis on day 4 or fixed to evaluate differentiation on day 6.
Immunocytochemical methods
Neurons were fixed with 2% paraformaldehyde (PF) in phosphate-buffered saline solution (PBS, 0.9% NaCl in 0.01 M NaH2PO4; pH 7.4) for at least 1 h, followed by permeation with Triton X-100 (0.1% in PBS). Neuronal cell types were identified by their morphology using phase contrast microscopy, and with immunocytochemistry using monoclonal antibodies Rho4D2 and syntaxin (HPC-1), which selectively recognize photoreceptors and amacrine neurons, respectively (Barnstable 1980; Kljavin et al. 1994; Hicks and Barnstable 1987). Controls for immunocytochemistry were performed by omitting either the primary or the secondary antibody. Morphological scoring was performed according to the following criteria. Photoreceptors usually display at least three of the following characteristics: a) a small round cell body (3–5 μm); b) a single neurite at one end, which usually ends in a conspicuous synaptic “spherule”; c) sometimes they display a connecting cilium at the opposite end, but they fail to develop their characteristic outer segments; d) opsin is diffusely distributed over their cell body; e) their cell body is usually darker than that of amacrine neurons. Amacrine neurons are larger than photoreceptors (7–20 μm) and have multiple neurites.
Cell Viability and Apoptosis
Cell viability was determined by the exclusion of propidium iodide (PI). For PI staining, cultures were incubated before fixation for 20 min in 0.5μg/mL PI solution (Jordán et al. 1997).
Apoptosis was determined by Terminal Deoxynucleotide Transferase dUTP Nick End Labeling assay (TUNEL). Cells were fixed on day 4 with 2% PF for 15 min, and then stored in 70% ethanol for 48 h at −20°C. Before the enzymatic reaction, cells were washed twice with PBS, for 5 min each time, at room temperature. Cells were pre-incubated with 1X TdT buffer for 15 min, and then incubated with the TdT reaction mixture (0.05mM BrdUTP, 0.3U/μL TdT in TdT buffer) at 37°C in a humidified atmosphere for 1 h. The reaction was stopped by 15-min incubation with stop buffer (300mM NaCl, 30mM sodium citrate; pH 7.4) at room temperature. Negative controls were prepared by omitting TdT. The presence of BrdU was determined with an anti-BrdU monoclonal antibody, according to standard immunocytochemical techniques.
Since changes in nuclei integrity are a hallmark of apoptosis, we determined the amount of cells with pyknotic or fragmented nuclei by incubating cells with DAPI for 20 min after fixation.
Evaluation of mitochondrial membrane potential
To assess the percentage of cells with preserved mitochondrial membrane potential following oxidative stress, the cultures were incubated before fixation for 20 min with the fluorescent probe MitoTracker (0.1 μg/mL), which labels with a bright red fluorescence active mitochondria. The number of photoreceptor cells displaying fluorescent mitochondria was determined and quantified.
Evaluation of Photoreceptor Differentiation
Opsin expression was evaluated by immunocytochemistry, using Rho4D2 (Barnstable 1980; Hicks and Barnstable 1987), followed by a Cy2-conjugated goat anti-mouse secondary antibody and by RT-PCR, as described below.
Evaluation of Fatty acyl Δ6 Desaturase Expression
Expression of FADS2 in neuronal cultures was determined by immunocytochemistry, and RT-PCR, after fixation or lysis of the cultures.
For immunocytochemical analysis, once fixed, photoreceptors in neuronal cultures were labeled with Rho4D2 and glial cells in mixed neuro-glial primary cultures were labeled with an anti-vimentin antibody. FADS2 expression in neuronal, neuro-glial, and ARPE-19 cell cultures was determined with an anti-FADS2 antibody. Nuclei were stained with TOPRO or DAPI.
Evaluation of Opsin and FADS2 Transcription
Opsin and FADS2 transcription levels were analyzed by quantitative RT-PCR. Total RNA from cells isolated from pure neuronal cultures, pure glial cultures, and brain tissues from 10 day old rats, was extracted using Quick-ZOl (Kalium Technologies,) according to the manufacturer’s instructions. cDNA was made with enzyme M-MLV RT (Promega) using random hexamer primers. Quantitative PCR was performed using SYBR fast universal mix (KAPA Biosystems) in a Corbette RG 6000 thermal cycler.
The primers used in this study were: TBP, FTGGGATTGTACCACAGCTCCA and RCTCATGATGACTGCAGCAAACC; Opsin, F CACCCTTGGAGGTGAAATCGG and RTGATTCTCCCCAAAGCGGAA; FADS2, F CATCGGACACTATTCGGGAGA and RCCCTGAAGTCCTCGGTGATC. All primers were designed to span introns with an efficiency above 90%.
Fatty acid analysis
Day 4 cultured neurons were washed with ice-cold PBS, scraped, and transferred to glass tubes. After a 10-min centrifugation at 1000 rpm, the supernatant was removed, the cell pellets were extracted with chloroform:methanol (2:1, vol/vol) and the extracts were partitioned (Folch et al. 1957). All samples were kept in an N2 atmosphere. To analyze the fatty acid composition of neuronal phospholipids, lipids were separated by thin-layer chromatography (TLC), using hexane-ether-acetic acid (80:20:1, vol/vol) (Rotstein et al. 1997). Phospholipids were scraped into screw cap glass tubes with nonstick coating, and extracted with chloroform:methanol:water (5:5:1, vol/vol). To prepare the methyl ester derivatives of their fatty acids, methanolys was performed with NaOH 0.5 N in methanol at 50°C for 10 min. Methyl esters were extracted twice with chloroform and were partitioned with methanol:hexane:water (1:1:1, vol/vol) to remove impurities. Analysis of methyl esters was performed by gas-liquid chromatography, using 10% SP2330 glass columns on an 80-to-100-mesh high-density, white diatomite support (Chromosorb WAW; Supelco, Bellefonte, PA). A gas chromatograph with flame ionization detector was used (model 3200; Varian; Sunnyvale, CA). The runs were temperature-programmed, 5°C/min, with l65°C and 225°C as initial and final temperatures, respectively. Nitrogen was the carrier gas, at a flow rate of 30 ml/min.
Statistical analysis
The results represent the average of at least three separate experiments (± SD), and each experiment was performed in triplicate. For cytochemical studies, 10 fields per sample were analyzed in each case and scoring was done in a blinded manner. Statistical significance was determined either by one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test or by Student’s t-test, as indicated in each figure legend.
RESULTS
The addition of EPA prevented oxidative stress-induced apoptosis of photoreceptors
DHA protects cultured photoreceptors from oxidative stress-induced apoptosis (Rotstein et al. 2003; German et al. 2013). To evaluate whether EPA had a similar protective effect, we supplemented cultured retinal neurons with 3 μM EPA on day 1 and induced oxidative stress on day 3 with 48 μM PQ for 24 h. On day 4, cultures with or without EPA had few TUNEL-positive cells (Fig. 1a, b, e, f), and pyknotic nuclei (Fig. 1q, r, u, v). Both TUNEL-positive cells (Fig. 1c, g, arrowheads) and cells having pyknotic nuclei (Fig. 1k, s, w, thin arrows), including photoreceptors (Fig. 1k, o, s, w, wide white arrow) increased after PQ treatment. Supplementation with EPA before PQ treatment prevented the increase in TUNEL-positive cells (Fig. 1d, h, arrowheads) and in cells with pyknotic nuclei (Fig. 1l, p, t, x).
Figure 1. Addition of EPA protected photoreceptors from Paraquat-induced apoptosis.
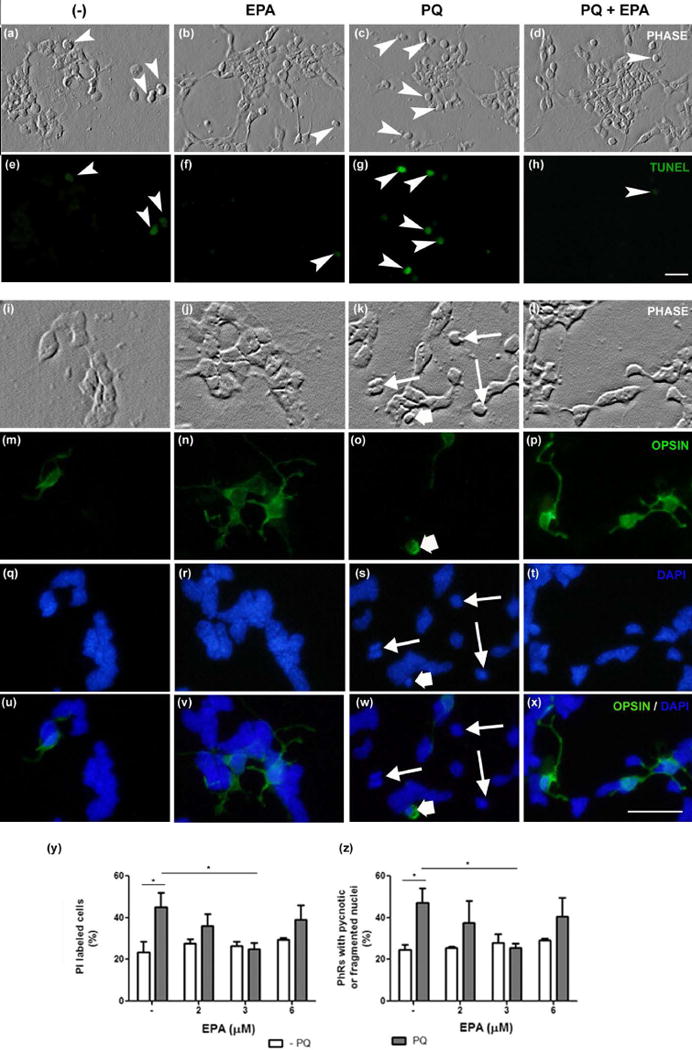
Rat retina neuronal cultures were supplemented with 3 μM eicosapentaenoic acid (EPA) or with its vehicle, a bovine serum albumin (BSA) solution (−) at day 1 and treated with 48 μM Paraquat (PQ) at day 3. Cultures were fixed 24 h later. Phase contrast (a–d, i–l) and fluorescence photomicrographs (e–h, m–x) show the effect of EPA addition on apoptosis, evaluated by TUNEL assay (e–h) and by DAPI labeling (q-t; merge in u-x, blue labeling), to evidence pyknotic nuclei. Photoreceptors were identified by immunocytochemistry, using Rho4D2, anti-opsin, monoclonal antibody (m–p; merge in u–x, green labeling). Note that the amount of TUNEL-labeled, apoptotic cells (arrowheads in e–h) and cells showing pyknotic nuclei (s, w, thin arrows), including opsin-labeled photoreceptors (o, s, w, wide white arrow), increased in PQ-treated cultures (c, g, k, o, s, w) compared to controls and EPA-supplemented cultures. EPA addition prevented these increases (d, h, l, p, t, x) upon PQ treatment. Bars depict the effect of different EPA concentrations on cell viability (y), determined by propidium iodide (PI) labeling and apoptosis of photoreceptors (PhRs) (z), determined by analyzing the amount of pyknotic or fragmented nuclei. Three separate experiments with three dishes for each condition were analyzed and 10 fields per dish were counted blindly in all experiments. Bar, 20 μm. *p≤0.05; statistically significant differences determined by a one way-ANOVA test, followed by a Tukey’s post hoc test.
We then investigated which EPA concentration was the most effective for neuroprotection. The percentage of PI-labeled cells was slightly above 20% in control and EPA-supplemented cultures, and doubled upon PQ treatment (Fig. 1y). The addition of 2 μM EPA slightly reduced the percentage of PI-labeled cells in PQ-treated cultures, though not significantly, whereas 3 μM EPA completely prevented its increase. Addition of 6 μM EPA increased the number of PI-labeled cells upon PQ treatment, suggesting that high amounts of EPA could lead to cell death. Treatment with 3 μM EPA prevented photoreceptor apoptosis and reduced the percentage of photoreceptors with fragmented or pyknotic nuclei to levels near those of controls, despite PQ treatment (Fig. 1z). Hence, we used 3 μM EPA for further experiments.
To evaluate whether EPA supplementation could protect photoreceptors from oxidative damage induced by a different oxidant, we treated retina neurons in culture with H2O2 (Chucair et al. 2007; Abrahan et al. 2009). Controls and EPA-supplemented cultures had few TUNEL-labeled cells (Fig. 2 a, b, e, f, large arrowheads); most photoreceptors showed intact nuclei (Fig. 1i, j, q, r, thin arrows) and preserved their mitochondrial membrane potentials, as determined by MitoTracker labeling (Fig. 2 m, n, small arrowheads, u). H2O2 increased the number of TUNEL-positive cells (Fig. 2 c, g, large arrowheads) and the number of cells with pyknotic and fragmented nuclei (Fig. 2k, s, thin arrows; v), and induced mitochondrial membrane depolarization, as evidenced by the loss of red fluorescent mitochondria (Fig. 2o, u). Pretreatment of the cultured retinal neurons with EPA prevented the increase in TUNEL-positive cells (Fig. 2d, h) and the number of photoreceptors with fragmented nuclei (Fig. 2l, t, thin arrows, v) induced by H2O2, preserving their mitochondrial membrane potentials (Fig. 2p, u). Together, our data suggest that supplementation with EPA protected photoreceptors from the oxidative stress induced by both PQ and H2O2.
Figure 2. EPA addition protected photoreceptors from H2O2-induced apoptosis.
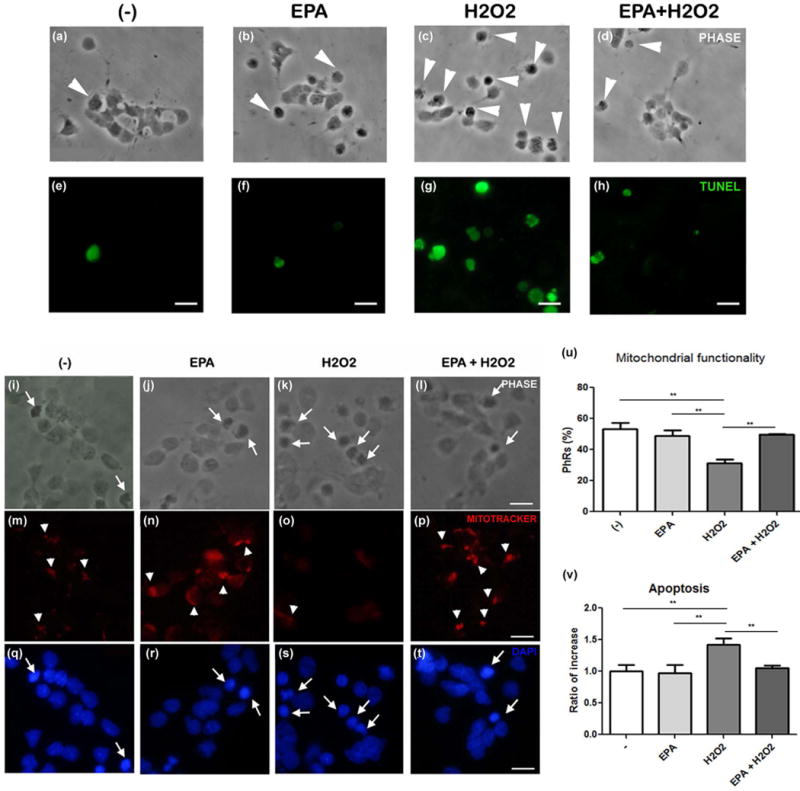
Phase contrast (a–d, i–l) and fluorescence photomicrographs (e–h, m–t) of neuronal cultures supplemented with vehicle (−) or 3 μm EPA at day 1 in culture and treated at day 3 with 10 μM H2O2. Apoptosis was evaluated by TUNEL assay (e–h); large arrowheads (a–d) indicate the corresponding TUNEL-labeled (apoptotic) cells in e–h. Mitochondrial functionality (m–p) was determined with Mitotracker; small arrowheads show photoreceptors preserving their mitochondrial membrane polarization. Pyknotic or fragmented nuclei (q–t) were determined by DAPI labeling; arrows indicate apoptotic photoreceptors. Bars, 15 μm. Bars depict mean ± SD of the percentage of photoreceptors preserving their mitochondrial membrane polarization (u) and the ratio of increase in the number of apoptotic photoreceptors (v). Three different experiments with three dishes for each condition were analyzed and 10 fields per dish were counted blindly in all experiments. ** p≤0.01; statistically significant differences determined by a one way-ANOVA test followed by a Tukey’s test.
To determine whether other major retinal fatty acids are able to provide this protection, we assessed the effect of fatty acids, such as palmitic (PAM), oleic (OLA), and arachidonic (ARA) acids, with chain lengths or unsaturation different from those of EPA, on photoreceptor viability in PQ-treated cultures. PQ doubled the percentage of PI-positive cells, compared with controls; the addition of 4 μM PAM, OLA, or ARA did not prevent this increase (Fig. 3a). Only supplementation with 3 μM EPA reduced the number of PI-positive cells. Similarly, the percentage of photoreceptors with pyknotic or fragmented nuclei was the same in PQ-treated cultures, with or without PAM, OLA, or ARA (Fig. 3b), whereas the addition of EPA reduced PQ-induced apoptosis of photoreceptors.
Figure 3. Oxidative stress-induced apoptosis of photoreceptors was prevented only by EPA addition.
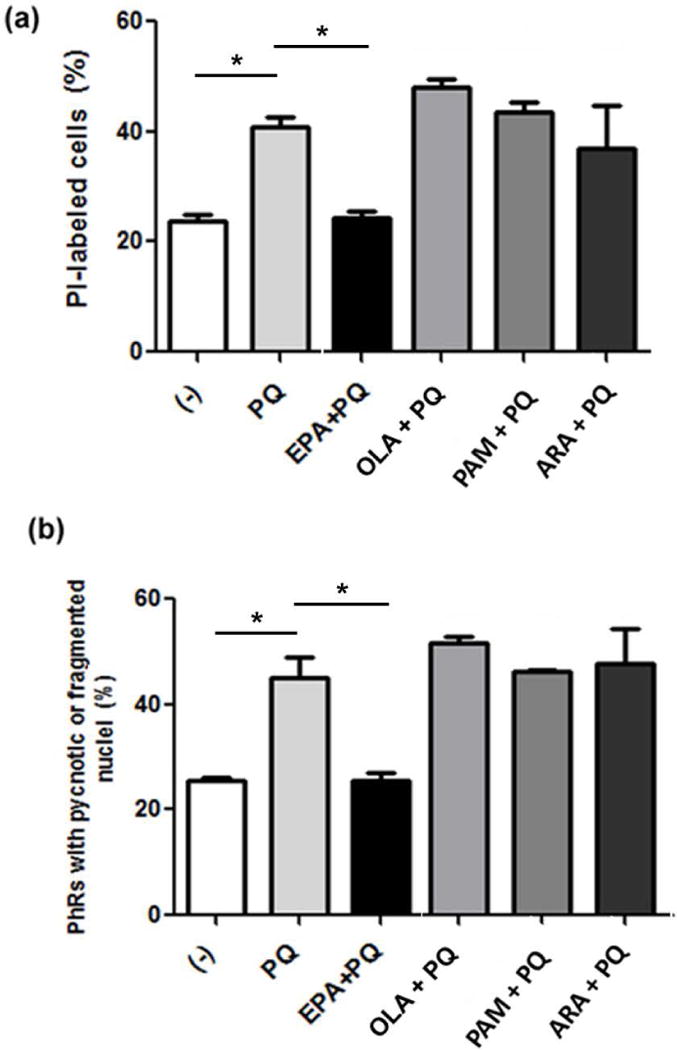
Neuronal cultures were supplemented at day 1 with either 3 μM EPA, or 4 μM oleic (OLA), palmitic (PAM), or arachidonic (ARA) acid, or vehicle (−) and treated with PQ at day 3. Bars depict the percentage of PI-labeled cells (a) and of photoreceptors with fragmented or pyknotic (apoptotic) nuclei (b). Three experiments with three dishes for each condition were analyzed and 10 fields per dish were counted blindly in all experiments. *p≤0.05, statistically significant differences determined by a one-way ANOVA test followed by a Tukey’s test.
EPA enhances opsin expression in photoreceptors
Differentiation of photoreceptors is restricted in our culture conditions; after their final mitosis, most of the photoreceptors remain as round cells and very few express opsin or develop apical processes resembling the characteristic outer segments of mature photoreceptors (Rotstein et al. 1998). The addition of DHA increases opsin levels and promotes the development of apical processes (Garelli et al. 2006). Supplementation with EPA had similar effects on photoreceptor differentiation. EPA-supplemented cultures had more opsin-labeled photoreceptors than did controls (Fig. 4a–d, arrows). The fold increase in opsin-positive photoreceptors was nearly 3 and 1.75 after 4 and 6 days in culture, respectively (Fig. 4e). RT-qPCR analysis confirmed that EPA addition increased opsin expression in 6-day cultures (Fig. 4f).
Figure 4. EPA addition stimulated opsin expression in photoreceptors.
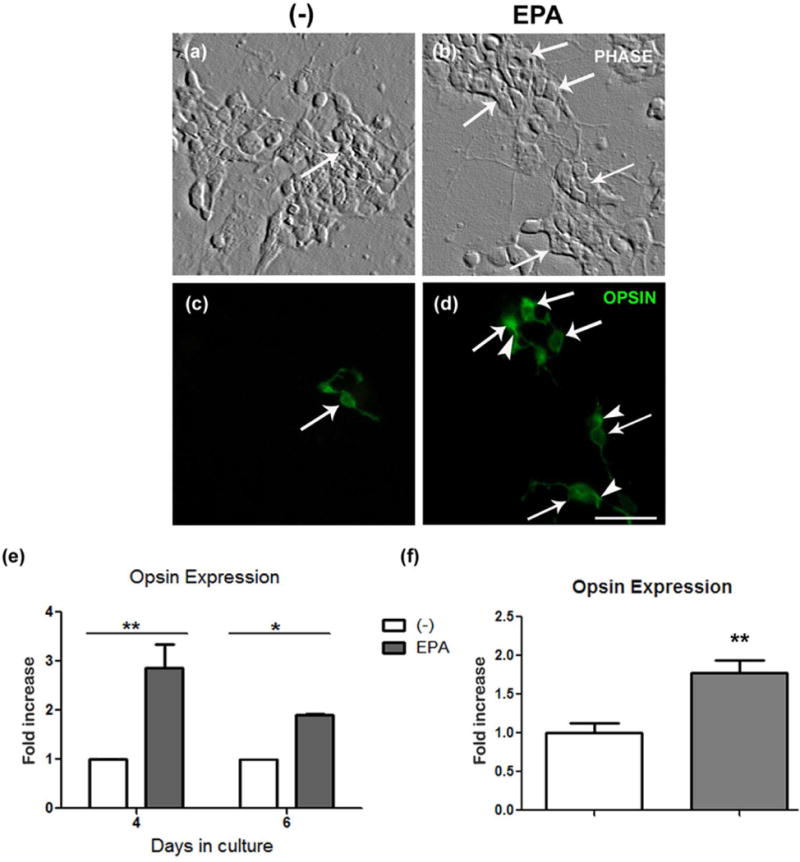
Phase contrast (a, b) and fluorescence (c, d) photomicrographs of control (a, c) and 3 μM EPA-supplemented (b, d) 6 day neuronal cultures, show opsin expression (c, d) determined by immunocytochemistry. Note that EPA supplementation increased the number of opsin-expressing photoreceptors (arrows) and led to the formation of apical processes (arrowheads in d). Bar, 10 μm. Bars (e) depict the fold-change in the amount of photoreceptors expressing opsin in EPA-supplemented, 4 and 6 day cultures compared to controls, determined by immunocytochemistry. Bars (f) depict the fold-change in the level of opsin mRNA, determined by qRT-PCR in 6-day cultures supplemented with EPA compared to controls. Three experiments with three dishes for each condition were analyzed and 10 fields per sample were counted blindly in all experiments. *p≤0.05; ** p≤0.01, statistically significant differences compared to controls, determined by a Student’s t-test.
The addition of EPA also promoted the formation of apical processes; photoreceptors in controls had diffuse opsin labeling on their cell bodies and lacked apical processes (Fig. 4c), whereas EPA supplementation induced the development of these processes, which showed an intense opsin labeling (Fig. 4d, arrowheads).
EPA supplementation increased DHA levels in neuronal phospholipids
Adding DHA to neuronal cultures leads to a rapid accretion of this fatty acid in neuronal lipids; however, adding fatty acids, such as PAM, OLA, and ARA, does not modify their levels in neuronal lipids, even though these fatty acids are esterified in these lipids (Rotstein et al. 1996b; Rotstein et al. 1998). Analysis of the fatty acid composition of neuronal lipids revealed that the (mole) percentage of EPA was very low in neuronal phospholipids in controls, and no significant increase occurred upon EPA supplementation (Fig. 5a). In contrast, the percentage of DHA increased significantly, almost doubling in EPA-supplemented cultures compared with controls, whereas the percentage of docosapentaenoic acid, n-3 (n-3 DPA), an EPA immediate elongation product (Fig. 5b) had a slight, non-significant, rise (Fig. 5a). This finding suggests that isolated retinal neurons took up EPA and elongated and desaturated it to synthesize DHA.
Figure 5. Addition of EPA increased docosahexaenoic acid (DHA) levels in retina neurons.
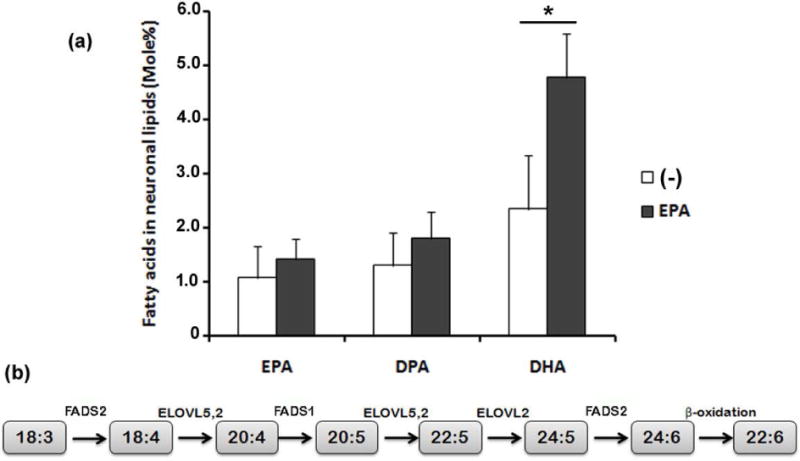
Retina neurons were supplemented with vehicle (−) or with 3 μM EPA added at day 1 in vitro and the fatty acid composition of neuronal phospholipids was determined by GLC at day 4. Bars (a) depict mean ± SD of the mole % content of EPA, docosapentaenoic acid (DPA) and DHA in neuronal phospholipids. Three different experiments with two dishes for each condition were analyzed. * p≤0.05, statistically significant differences, compared to the respective controls, determined by a Student’s t-test. Sequence of metabolic reactions (b) leading to 22:6 n-3 (DHA) synthesis from 18:3 n-3 that involves its desaturation by FADS2, the further elongation and desaturation catalyzed by ELOVL5,2 and FADS1 to synthesize 20:5 n-3 (EPA), which is successively elongated by ELOVL5,2 and ELOVL2 to 24:5 n-3, which is then desaturated by FADS2 to 24:6 n-3, which after a (peroxisomal) β-oxidation gives rise to DHA.
Neurons and glial cells express FADS2
The ability to synthesize DHA using EPA as a precursor requires the presence of FADS2 to introduce the last double bond. Immunocytochemical analysis of neuronal cultures revealed that opsin-positive cells (arrows in Fig. 6a, d) expressed FADS2 (Fig. 6b, d). This expression colocalized with TOPRO labeling (Figs. 6c, d), implying this enzyme was present in photoreceptors and had a nuclear localization. In neuro-glial cultures, FADS2 expression was observed both in vimentin-labeled glial cells (Fig. 6e, g, arrowheads) and in neurons (Fig. 6e, g, arrows), and it mostly colocalized with TOPRO labeling (Figs. 6f, g) suggesting it localized in nuclei in both cell types. FADS2 expression was also observed in ARPE-19 cells, and though colocalization with DAPI labeling was observed, it evidenced a predominantly cytoplasmic localization (Fig. 6h–j).
Figure 6. Retina neurons expressed FADS2.
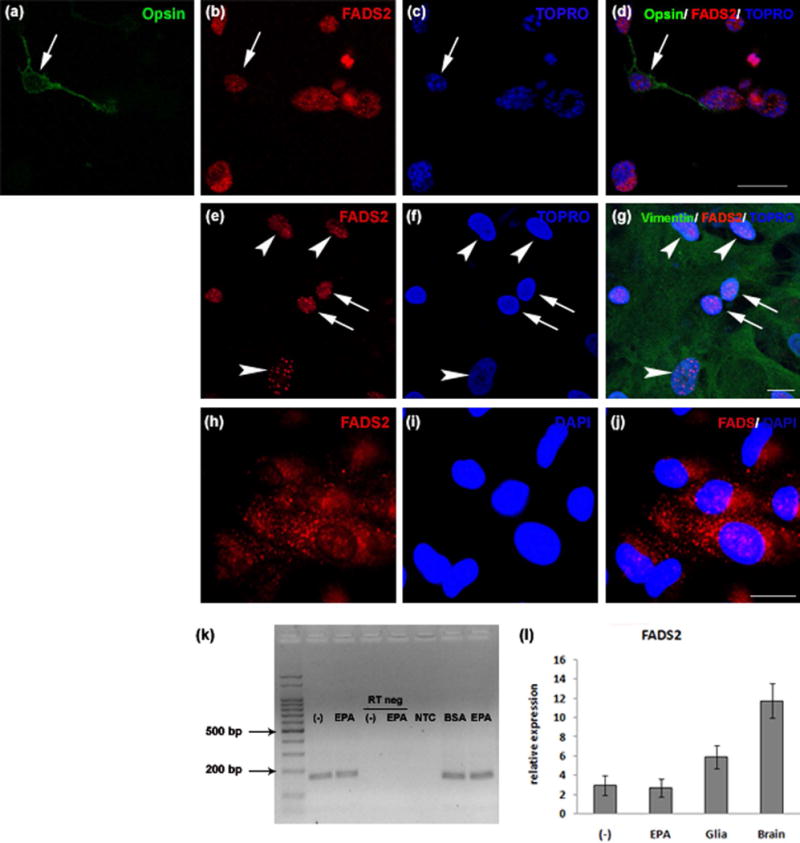
Fluorescence confocal photomicrographs (a–j) of 6 day neuronal cultures (a–d), and neuroglial mixed cultures (e–g) and epifluorescence photomicrographs of ARPE-19 cell cultures (h–j) showing expression of opsin (green in a, d), FADS2 (red in b, d, e, g, h, j), vimentin (green in g) and nuclei labeled with TOPRO (c, d, f, g) or DAPI (i, j). Note that glial cell nuclei (arrowheads in f) were much larger than those of neurons (arrows in f). FADS2 expression was observed in nuclei in opsin-labeled photoreceptors (arrows in a–d), neurons (arrows in e, g) and vimentin-labeled glial cells (arrowheads in e, g). In contrast, in ARPE-19 cells expression of FADS2 was mainly observed in the cytoplasm (h, j). Bars, 20 μm. Electrophoresis of FADS2 mRNAs after RT-PCR (k) in 4 day neuronal cultures supplemented with vehicle (−) or 3 μM EPA; a specific 167 bp band was observed in samples from neuronal cultures, which was absent in Reverse Transcriptase negative control (RT negative) and No Template Control (NTC) conditions. Bars (l) depict relative quantification of FADS2 mRNA levels by RT-PCR in lysates prepared from 4 day neuronal cultures (n=3) supplemented with vehicle (−) or 3 μM EPA, from 13 day pure glial cultures (n=3) from rat retina (glia) and from PN10 rat brain (n=1).
Analysis by RT-PCR showed that retinal neurons in control conditions expressed FADS2 and this expression was the same when neuronal cultures were supplemented with EPA (Fig. 6k, l). Pure glial cultures had high levels of FADS2 mRNA, which was also present in the rat brain lysates used as positive controls (Fig. 6l).
Synthesis of DHA is required for EPA effects on photoreceptors
Our results demonstrated that the effects of EPA addition were similar to those of DHA, protecting photoreceptors from apoptosis induced by oxidative stress and advancing photoreceptor differentiation. The data also showed that EPA supplementation led to DHA accretion in cultured neurons. To discern whether EPA exerted its effects by itself or through its further elongation and desaturation to DHA, we inhibited DHA synthesis by pre-treating neuronal cultures on day 1 with CP, an inhibitor of Δ5/Δ6 desaturases, before EPA supplementation. As expected, analysis of phospholipid fatty acid composition revealed that pre-treatment with CP completely prevented the increase in the (mole) percent of DHA induced by EPA supplementation, leading to a small, though non-significant, increase in n-3 DPA percentage in EPA-supplemented neurons (Fig. 7a).
Figure 7. Inhibition of DHA synthesis blocked EPA effects on photoreceptor apoptosis and differentiation.
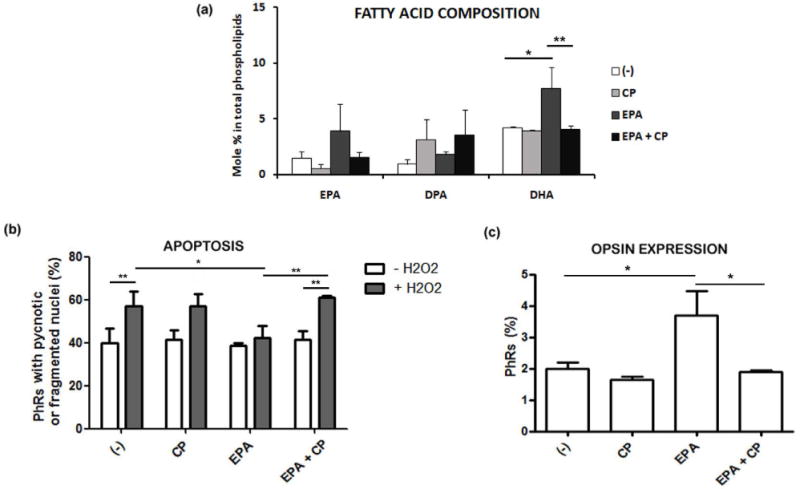
Neuronal cultures were treated with or without CP, a FADS1/FADS2 inhibitor, before supplementation with vehicle or with 3 μM EPA at day 1. Bars (a) depict mean ± SD of EPA, DPA and DHA mole % content in total phospholipids extracted from 4 day neuronal cultures supplemented with vehicle (−), CP, EPA and EPA+CP. Three separate experiments with two dishes for each condition were analyzed. Bars (b) depict mean ± SD of the percentage of photoreceptors (PhRs) showing pyknotic or fragmented (apoptotic) nuclei in neuronal cultures treated without (white bars) or with H2O2 (grey bars) at day 3. Bars (c) depict the mean ± SD of the percentage of photoreceptors expressing opsin after 6 days in culture in each experimental condition. Three separate experiments with three dishes for each condition were analyzed and 10 fields per sample were counted blindly in (b) and (c). *p≤0.05, **p≤0.01, statistically significant differences determined by a one way-ANOVA test followed by a Tukey’s test.
We then evaluated the effect of inhibiting DHA synthesis on EPA protection from oxidative damage. While the apoptosis of H2O2-treated photoreceptors in EPA-supplemented cultures remained at the same levels as found in controls, pre-treatment with CP completely blocked EPA protection, increasing the percentage of apoptotic photoreceptors to the values observed in H2O2-treated cultures lacking EPA (Fig. 7b).
Finally, we analyzed whether inhibiting DHA synthesis affected EPA enhancement of photoreceptor differentiation. While EPA increased the number of opsin-positive photoreceptors compared with controls (Fig. 7c), pre-treatment with CP completely blocked this increase; the percentage of photoreceptors expressing opsin was similar to that observed in controls.
Discussion
Though several studies have shown that DHA is synthesized in the eye, the ability of retinal neurons to perform this synthesis had not been established. Here, we demonstrate that isolated retinal neurons in culture can synthesize DHA, using EPA as a precursor. Our results show that adding EPA to retinal neuron cultures leads to increased DHA levels in neuronal phospholipids, prevents oxidative stress-induced photoreceptor apoptosis, and enhances photoreceptor differentiation. The data also demonstrate that FADS2, which catalyzes the desaturation reaction required for the synthesis of DHA from EPA, is present in photoreceptors, and its inhibition blocks the effects of EPA on these cells. This finding suggests that isolated retinal neurons in culture can elongate and desaturate EPA to synthesize DHA, and that this synthesis is required for EPA protection and enhancement of differentiation in photoreceptors.
EPA and DHA are known for their beneficial effects on brain and retinal function (Calder 2007; Deckelbaum and Torrejon 2012). DHA promotes cell survival in ischemic stroke and prevents apoptosis of a neuron-like cell line (Eady et al. 2012; Kim et al. 2000). DHA also protects retinal ganglion cells from oxidative stress (Shimazawa et al. 2009), attenuates retinal degeneration in a mouse model of inherited retinal degeneration (Ebert et al. 2009), and prevents the apoptosis of rat retina photoreceptors during development in vitro and when subjected to oxidative stress (Rotstein et al. 1996a; Rotstein et al. 2003). Mounting evidence supports the protective effect of EPA in the eye. An EPA-enriched diet reduces choroidal neovascularization (Koto et al. 2007) and ocular inflammation in experimentally induced uveitis (Suzuki et al. 2010). Although higher plasma levels of EPA have been associated with higher macular pigment density (Delyfer et al. 2012) and a decreased risk of progression of AMD (SanGiovanni et al. 2008), the beneficial effects of EPA on AMD are still under debate (2013; Merle et al. 2014; 2015). Here, we demonstrate that supplementation with EPA efficiently promoted the survival of photoreceptors in culture and prevented their apoptosis triggered by the oxidants PQ and H2O2. EPA supplementation preserved mitochondrial membrane potential in photoreceptors, which is consistent with the involvement of the mitochondrial pathway in triggering the apoptosis of these cells (Rotstein et al. 2003; German et al. 2006). Non n-3 fatty acids, such as PAM, OLA, and ARA, have beneficial functions in other systems (Juman et al. 2013; Murakami et al. 2003; Sales-Campos et al. 2013; Lopez-Huertas 2010; Shrestha et al. 2013), but did not prevent photoreceptor apoptosis upon oxidative stress, suggesting that only supplementation with EPA and DHA protected these cells from apoptosis.
When cultured in the absence of their trophic factors, photoreceptors show little differentiation, remaining as round cells with small cell bodies and short cilia (Rotstein et al. 1996a). DHA advances the differentiation of cultured photoreceptors (Rotstein et al. 1996a; Rotstein et al. 1998; Garelli et al. 2006). Supplementation with EPA also advanced the differentiation of photoreceptors, increasing opsin expression. Hence, the addition of EPA turned on the survival and differentiation pathways in photoreceptors.
Since EPA is a metabolic precursor of DHA, the similarity between its effects and those of DHA led us to reason that EPA might exert these effects through its successive elongation and desaturation to DHA. This fatty acid is synthesized in the eye in dogs and rats. Pigment epithelium and microvascular endothelial cells can actively synthesize DHA (Wang and Anderson 1993; Delton-Vandenbroucke et al. 1997). Intravitreal injection of radioactive precursors of DHA leads to the rapid synthesis and acylation of DHA in retinal lipids (Bazan et al. 1982; Wetzel et al. 1991; Stinson et al. 1991). The retina has high levels of elongase and low levels of desaturase expression (Tikhonenko et al. 2010). Isolated retinas incubated with DHA precursors, such as [I4C]- ALA or DPA n-3 acids, synthesize modest amounts of radioactive DHA (Wang and Anderson 1993), can elongate [14C]DPA n-3 to [14C]24:5 n-3, and form labeled 24:6 n-3 and DHA (Rotstein et al. 1996b). Since both neurons and glial cells are present in the whole retinas used in these studies, it was essential that we establish whether neurons or glial cells were responsible for the synthesis of DHA. Our results show that FADS2, the enzyme involved in the last desaturation step in this synthesis (Voss et al. 1991), is present in isolated retina neurons, and is expressed in photoreceptors. EPA is elongated by either ELOVL5 or ELOVL2 to DPA n-3 (Gregory et al. 2013) (Fig. 5b), which is further elongated to 24:5 n-3; this fatty acid is then desaturated by FADS2 to 24:6 n-3, which gives rise to DHA after its peroxisomal β-oxidation. Supplementation of isolated retinal neurons with EPA increased DHA content in neuronal lipids without EPA accretion. Inhibiting FADS2 activity in these neurons abolished the increase in DHA content and prompted an accumulation of DPA n-3 in neuronal lipids. Hence, our data support that isolated retinal neurons can synthesize DHA from EPA. Our results offer evidence of the presence of active FADS2 in retinal neurons in culture, and suggest that the enzymes required for EPA elongation (i.e., ELOVL2 and/or 5) are also present and active in these neurons.
Elongase and desaturase activities are generally lower in the brain than in the liver (Igarashi et al. 2007). The liver has a greater capacity for DHA synthesis from circulating ALA than the brain and it is the primary source of brain and retinal DHA (Rapoport et al. 2007; Igarashi et al., 2007; Scott and Bazan 1989). The contribution and the physiological relevance of neuronal enzymatic activities to the high levels of DHA present in the retina has yet to be established. Although it is highly unlikely for neuronal enzymatic activities to be responsible for these levels, they might participate in keeping DHA levels constant in photoreceptors upon transient decreases in the plasma levels of this fatty acid. In rats fed with [3H]ALA, [3H]EPA appears more rapidly and at higher levels in plasma than [3H]DHA (Domenichiello et al. 2014). Since at least in brain, the rates of uptake of plasma EPA and DHA have been shown to be similar (reviewed in Chen et al. 2015), EPA might be a readily available precursor for DHA synthesis in neuronal tissues in an ALA-enriched, DHA-deficient, diet. These enzymes might also have a role in the event of minor local changes in DHA levels in photoreceptor phospholipids. Since DHA is released from these lipids and subsequently activates RXR and the ERK/MAPK pathway to achieve photoreceptor protection (German et al. 2006; German et al. 2013), neuronal fatty acid elongase and desaturase enzymes might be activated to synthesize and replenish DHA in phospholipids, once it is released. Interestingly, large scale human genotyping projects have associated different forms of AMD with variants in genes encoding FADS2 and proteins involved in DHA signaling pathway, such as a co-activator of the n-3 long chain PUFA sensing peroxisome proliferator activated receptor (PPAR)-RXR transcription complex and constituents of the stress-activated MAPK pathway (Edwards et al. 2008; SanGiovanni et al. 2013; SanGiovanni and Lee 2013; Meyers et al. 2014). Further work is required to establish the function of these proteins in retina neurons, both in vitro and in vivo.
FADS2 is also present in pigment epithelium and glial cells. Pigment epithelium cells have been proposed as relevant sources of retinal DHA (Wang and Anderson 1993). Brain glial cells provide neurons with DHA (Moore et al. 1991). Retinal glial cells can take up DHA and deliver it to photoreceptors in co-culture (Politi et al. 2001b). Hence, retinal pigment epithelium and glial cells might also contribute to maintain DHA levels in photoreceptors, through its synthesis and further delivery to neurons.
Adding DHA to neuronal cultures leads to a 5-fold increase in its content in neuronal lipids, amounting to about 25% of the total fatty acids (Rotstein et al. 1996b), a level similar to that found in the mature rat retina (Fliesler and Anderson 1983; Rotstein et al. 1996b). Interestingly, though EPA supplementation led to a much lower (2-fold) accumulation of DHA in neuronal lipids, this slight increase in DHA levels was sufficient to promote photoreceptor survival. The high content of DHA in retina photoreceptors is undoubtedly relevant to modulate conformational changes in rhodopsin upon light exposure and appropriate function of retina enzymes (Grossfield et al. 2006; Mitchell et al. 2001; Gawrisch et al. 2008). However, as occurs with other signaling molecules, the function of DHA as a signal might only require a minute accrual of DHA to reach a threshold level that allows the release of DHA and subsequent RXR activation. In addition, once released, DHA might serve as a precursor for several DHA-derived bioactive metabolites, such as Neuroprotectin D1, which has a protective effect in retinal pigment epithelia and in a cone photoreceptor cell line (Bazan et al. 2010; Halapin and Bazan 2010; Bazan et al. 2011; Kanan et al. 2014) and reduces pathological retinal neovascularization (Connor et al. 2007).
EPA is known to be a precursor of potent lipid mediators, such as resolvins, which are responsible for several of its beneficial effects (Serhan et al. 2000; Dona et al. 2008). However, our data show that the protective effects of supplementation with EPA require its elongation and desaturation to DHA in neuronal cultures. Inhibition of FADS2 and, consequently, of DHA synthesis completely blocked EPA’s prevention of oxidative stress-induced apoptosis and prevented the increase in opsin levels in photoreceptors. This implies that, at least in isolated retinal neurons, the effects of EPA on survival and differentiation of photoreceptors are derived from its metabolism to DHA, and not to EPA itself or EPA-derived metabolites.
In conclusion, this work shows that EPA supplementation can protect photoreceptors from oxidative stress-induced apoptosis, and this protection derives from EPA’s elongation and desaturation to DHA. We also provide the first evidence that retinal neurons have the enzymatic machinery for synthesizing DHA, which is crucial for photoreceptor function.
Acknowledgments
We are grateful to Elisa Beatriz de los Santos and Edgardo Buzzi for their technical assistance.
This work was supported by grants from FONCyT, the Argentinean National Research Council (CONICET), and the Universidad Nacional del Sur, Bahia Blanca, Argentina to NPR; and NIH grants EY00871, EY04149, and EY021725 to REA. NPR was supported by a Research Sabbatical Award from Research to Prevent Blindness.
Abbreviations
- ARA
arachidonic acid
- BSA
bovine serum albumin
- CP
CP-24879 hydrochloride
- DHA
docosahexaenoic acid
- DMEM
Dulbecco’s modified Eagle medium
- DPA
docosapentaenoic acid
- FADS2
fatty acyl Δ6 desaturase
- FCS
fetal calf serum
- OLA
oleic acid
- PAM
palmitic acid
- PBS
phosphate buffer saline
- PF
paraformaldehyde
- PI
propidium iodide
- PQ
paraquat dichloride
- PUFA
polyunsaturated fatty acids
- RXR
retinoid X receptor
- TdT
terminal deoxy-nucleotidyl transferase
- TUNEL
Terminal Deoxynucleotide Transferase dUTP Nick End Labeling assay
Footnotes
ARRIVE guidelines have been followed:
-
=>if No, skip complete sentence
-
=>if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.”
-
=>if ‘none’, insert “The authors have no conflict of interest to declare.”
-
=>otherwise insert info unless it is already included
References
- Abrahan CE, Insua MF, Politi LE, German OL, Rotstein NP. Oxidative stress promotes proliferation and dedifferentiation of retina glial cells in vitro. J Neurosci Res. 2009;87:964–77. doi: 10.1002/jnr.21903. [DOI] [PubMed] [Google Scholar]
- Adler R. Regulation of neurite growth in purified retina neuronal cultures: effects of PNPF, a substratum-bound, neurite-promoting factor. J Neurosci Res. 1982;8:165–77. doi: 10.1002/jnr.490080207. [DOI] [PubMed] [Google Scholar]
- Alvarez RA, Aguirre GD, Acland GM, Anderson RE. Docosapentaenoic acid is converted to docosahexaenoic acid in the retinas of normal and prcd-affected miniature poodle dogs. Invest Ophthalmol Vis Sci. 1994;35:402–8. [PubMed] [Google Scholar]
- Anderson RE, Maude MB. Lipids of ocular tissues. 8. The effects of essential fatty acid deficiency on the phospholipids of the photoreceptor membranes of rat retina. Arch Biochem Biophys. 1972;151:270–6. doi: 10.1016/0003-9861(72)90497-3. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ. Monoclonal antibodies which recognize different cell types in the rat retina. Nature. 1980;286:231–5. doi: 10.1038/286231a0. [DOI] [PubMed] [Google Scholar]
- Bazan HE, Careaga MM, Sprecher H, Bazan NG. Chain elongation and desaturation of eicosapentaenoate to docosahexaenoate and phospholipid labeling in the rat retina in vivo. Biochim Biophys Acta. 1982;712:123–8. doi: 10.1016/0005-2760(82)90093-5. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–31. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31:321–51. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty S, Boulton M, Henson D, Koh HH, Murray IJ. Macular pigment and age related macular degeneration. Br J Ophthalmol. 1999;83:867–77. doi: 10.1136/bjo.83.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fat Acids. 2007;77:327–335. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–88. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- Carmody RJ, McGowan AJ, Cotter TG. Reactive oxygen species as mediators of photoreceptor apoptosis in vitro. Exp Cell Res. 1999;248:520–30. doi: 10.1006/excr.1998.4421. [DOI] [PubMed] [Google Scholar]
- Chen CT, Kitson AP, Hopperton KE, Domenichiello AF, Trépanier MO, Lin LE, Ermini L, Post M, Thies F, Bazinet RP. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci Rep. 2015;5:15791. doi: 10.1038/srep15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chucair AJ, Rotstein NP, Sangiovanni JP, During A, Chew EY, Politi LE. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2007;48:5168–77. doi: 10.1167/iovs.07-0037. [DOI] [PubMed] [Google Scholar]
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crupi R, Marino A, Cuzzocrea S. n-3 fatty acids: role in neurogenesis and neuroplasticity. Curr Med Chem. 2013;20:2953–63. doi: 10.2174/09298673113209990140. [DOI] [PubMed] [Google Scholar]
- Deckelbaum RJ, Torrejon C. The omega-3 fatty acid nutritional landscape: health benefits and sources. J Nutr. 2012;142:587S–591S. doi: 10.3945/jn.111.148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delton-Vandenbroucke I, Grammas P, Anderson RE. Polyunsaturated fatty acid metabolism in retinal and cerebral microvascular endothelial cells. J Lipid Res. 1997;38:147–59. [PubMed] [Google Scholar]
- Delyfer MN, Buaud B, Korobelnik JF, Rougier MB, Schalch W, Etheve S, Vaysse C, et al. Association of macular pigment density with plasma n-3 fatty acids: the PIMAVOSA study. Invest Ophthalmol Vis Sci. 2012;53:1204–10. doi: 10.1167/iovs.11-8721. [DOI] [PubMed] [Google Scholar]
- Domenichiello AF, Chen CT, Trepanier MO, Stavro PM, Bazinet RP. Whole body synthesis rates of DHA from α-linolenic acid are greater than brain DHA accretion and uptake rates in adult rats. J Lipid Res. 2014;55:62–74. doi: 10.1194/jlr.M042275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, Andrian UH, von, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–55. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall SC, Michael-Titus AT. Neurological benefits of omega-3 fatty acids. Neuromolecular Med. 2008;10:219–35. doi: 10.1007/s12017-008-8036-z. [DOI] [PubMed] [Google Scholar]
- Eady TN, Belayev L, Khoutorova L, Atkins KD, Zhang C, Bazan NG. Docosahexaenoic acid signaling modulates cell survival in experimental ischemic stroke penumbra and initiates long-term repair in young and aged rats. PLoS One. 2012;7:e46151. doi: 10.1371/journal.pone.0046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert S, Weigelt K, Walczak Y, Drobnik W, Mauerer R, Hume DA, Weber BHF, Langmann T. Docosahexaenoic acid attenuates microglial activation and delays early retinal degeneration. J Neurochem. 2009;110:1863–75. doi: 10.1111/j.1471-4159.2009.06286.x. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Fridley BL, James KM, Sharma AK, Sharma AS, Cunningham JM, Tosakulwong N. Evaluation of clustering and genotype distribution for replication in genome wide association studies: the age-related eye disease study. PLoS One. 2008;3:e3813. doi: 10.1371/journal.pone.0003813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fu Z, Lofqvist CA, Shao Z, Sun Y, Joyal JS, Hurst CG, Cui RZ, et al. Dietary ω-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. Am J Clin Nutr. 2015;101:879–88. doi: 10.3945/ajcn.114.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelli A, Rotstein NP, Politi LE. Docosahexaenoic acid promotes photoreceptor differentiation without altering Crx expression. Investig Ophthalmol Vis Sci. 2006;47:3017–3027. doi: 10.1167/iovs.05-1659. [DOI] [PubMed] [Google Scholar]
- Gawrisch K, Soubias O, Mihailescu M. Insights from biophysical studies on the role of polyunsaturated fatty acids for function of G-protein coupled membrane receptors. Prostaglandins Leukot Essent Fatty Acids. 2008;79:131–4. doi: 10.1016/j.plefa.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German OL, Buzzi E, Rotstein NP, Rodríguez-Boulan E, Politi LE. Retinal pigment epithelial cells promote spatial reorganization and differentiation of retina photoreceptors. J Neurosci Res. 2008;86:3503–14. doi: 10.1002/jnr.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German OL, Insua MF, Gentili C, Rotstein NP, Politi LE. Docosahexaenoic acid prevents apoptosis of retina photoreceptors by activating the ERK/MAPK pathway. J Neurochem. 2006;98:1507–1520. doi: 10.1111/j.1471-4159.2006.04061.x. [DOI] [PubMed] [Google Scholar]
- German OL, Monaco S, Agnolazza DL, Rotstein NP, Politi LE. Retinoid X receptor activation is essential for docosahexaenoic acid protection of retina photoreceptors. J Lipid Res. 2013;54:2236–2246. doi: 10.1194/jlr.M039040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MK, Geier MS, Gibson RA, James MJ. Functional characterization of the chicken fatty acid elongases. J Nutr. 2013;143:12–6. doi: 10.3945/jn.112.170290. [DOI] [PubMed] [Google Scholar]
- Grossfield A, Feller SE, Pitman MC. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc Natl Acad Sci U S A. 2006;103:4888–93. doi: 10.1073/pnas.0508352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halapin NA, Bazan NG. NPD1 induction of retinal pigment epithelial cell survival involves PI3K/Akt phosphorylation signaling. Neurochem Res. 2010;35:1944–7. doi: 10.1007/s11064-010-0351-8. [DOI] [PubMed] [Google Scholar]
- Hicks D, Barnstable CJ. Different rhodopsin monoclonal antibodies reveal different binding patterns on developing and adult rat retina. J Histochem Cytochem. 1987;35:1317–28. doi: 10.1177/35.11.3655327. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–8. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt KR, Gallagher AJ, Hastings TG, Reynolds IJ. Characterization of hydrogen peroxide toxicity in cultured rat forebrain neurons. Neurochem Res. 1997;22:333–40. doi: 10.1023/a:1022403224901. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–70. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- Jeffrey BG, Weisinger HS, Neuringer M, Mitchell DC. The role of docosahexaenoic acid in retinal function. Lipids. 2001;36:859–71. doi: 10.1007/s11745-001-0796-3. [DOI] [PubMed] [Google Scholar]
- Jordán J, Galindo MF, Prehn JH, Weichselbaum RR, Beckett M, Ghadge GD, Roos RP, Leiden JM, Miller RJ. p53 expression induces apoptosis in hippocampal pyramidal neuron cultures. J Neurosci. 1997;17:1397–405. doi: 10.1523/JNEUROSCI.17-04-01397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juman S, Hashimoto M, Katakura M, Inoue T, Tanabe Y, Arita M, Miki T, Shido O. Effects of long-term oral administration of arachidonic acid and docosahexaenoic acid on the immune functions of young rats. Nutrients. 2013;5:1949–61. doi: 10.3390/nu5061949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanan Y, Gordon WC, Mukherjee PK, Bazan NG, Al-Ubaidi MR. Neuroprotectin D1 is Synthesized in the Cone Photoreceptor Cell Line 661W and Elicits Protection Against Light-Induced Stress. Cell Mol Neurobiol. 2014 doi: 10.1007/s10571-014-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima A, Harada T, Kami H, Yano T, Imada K, Mizuguchi K. Effects of eicosapentaenoic acid on synaptic plasticity, fatty acid profile and phosphoinositide 3-kinase signaling in rat hippocampus and differentiated PC12 cells. J Nutr Biochem. 2010;21:268–77. doi: 10.1016/j.jnutbio.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J Biol Chem. 2000;275:35215–23. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- Kljavin IJ, Lagenaur C, Bixby JL, Reh TA. Cell adhesion molecules regulating neurite growth from amacrine and rod photoreceptor cells. J Neurosci. 1994;14:5035–49. doi: 10.1523/JNEUROSCI.14-08-05035.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koto T, Nagai N, Mochimaru H, Kurihara T, Izumi-Nagai K, Satofuka S, Shinoda H, et al. Eicosapentaenoic acid is anti-inflammatory in preventing choroidal neovascularization in mice. Invest Ophthalmol Vis Sci. 2007;48:4328–34. doi: 10.1167/iovs.06-1148. [DOI] [PubMed] [Google Scholar]
- Krauss-Etschmann S, Shadid R, Campoy C, Hoster E, Demmelmair H, Jiménez M, Gil A, et al. Effects of fish-oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter trial. Am J Clin Nutr. 2007;85:1392–400. doi: 10.1093/ajcn/85.5.1392. [DOI] [PubMed] [Google Scholar]
- Labrousse VF, Nadjar A, Joffre C, Costes L, Aubert A, Grégoire S, Bretillon L, Layé S. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS One. 2012;7:e36861. doi: 10.1371/journal.pone.0036861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin G, Duffin KL, Obukowicz MG, Hummert SL, Fujiwara H, Needleman P, Raz A. Differential metabolism of dihomo-gamma-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem J. 2002;365:489–96. doi: 10.1042/BJ20011798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman BJ, Niu SL, Polozova A, Mitchell DC. The role of docosahexaenoic acid containing phospholipids in modulating G protein-coupled signaling pathways: visual transduction. J Mol Neurosci. 2001;16:237–42. doi: 10.1385/JMN:16:2-3:237. discussion 279–84. [DOI] [PubMed] [Google Scholar]
- Lohr HR, Kuntchithapautham K, Sharma AK, Rohrer B. Multiple, parallel cellular suicide mechanisms participate in photoreceptor cell death. Exp Eye Res. 2006;83:380–9. doi: 10.1016/j.exer.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Lopez-Huertas E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol Res. 2010;61:200–7. doi: 10.1016/j.phrs.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Lu DY, Tsao YY, Leung YM, Su KP. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for ω-3 fatty acids. Neuropsychopharmacology. 2010;35:2238–48. doi: 10.1038/npp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hackett SF, Mincey A, Lai H, Campochiaro PA. Effects of different types of oxidative stress in RPE cells. J Cell Physiol. 2006;206:119–25. doi: 10.1002/jcp.20439. [DOI] [PubMed] [Google Scholar]
- Luchtman DW, Meng Q, Wang X, Shao D, Song C. ω-3 fatty acid eicosapentaenoic acid attenuates MPP+-induced neurodegeneration in fully differentiated human SH-SY5Y and primary mesencephalic cells. J Neurochem. 2013;124:855–68. doi: 10.1111/jnc.12068. [DOI] [PubMed] [Google Scholar]
- Merle BM, Benlian P, Puche N, Bassols A, Delcourt C, Souied EH. Circulating omega-3 Fatty acids and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:2010–9. doi: 10.1167/iovs.14-13916. [DOI] [PubMed] [Google Scholar]
- Merle BM, Richard F, Benlian P, Puche N, Delcourt C, Souied EH. CFH Y402H and ARMS2 A69S Polymorphisms and Oral Supplementation with Docosahexaenoic Acid in Neovascular Age-Related Macular Degeneration Patients: The NAT2 Study. PLoS One. 2015;10:e0130816. doi: 10.1371/journal.pone.0130816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers KJ, Mares JA, Igo RP, Truitt B, Liu Z, Millen AE, Klein M, et al. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS) Invest Ophthalmol Vis Sci. 2014;55:587–99. doi: 10.1167/iovs.13-13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DC, Gawrisch K, Litman BJ, Salem N. Why is docosahexaenoic acid essential for nervous system function? Biochem Soc Trans. 1998;26:365–70. doi: 10.1042/bst0260365. [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Niu SL, Litman BJ. Optimization of receptor-G protein coupling by bilayer lipid composition I: kinetics of rhodopsin-transducin binding. J Biol Chem. 2001;276:42801–6. doi: 10.1074/jbc.M105772200. [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Niu SL, Litman BJ. Quantifying the differential effects of DHA and DPA on the early events in visual signal transduction. Chem Phys Lipids. 2012;165:393–400. doi: 10.1016/j.chemphyslip.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Moore SA, Yoder E, Murphy S, Dutton GR, Spector AA. Astrocytes, not neurons, produce docosahexaenoic acid (22:6 omega-3) and arachidonic acid (20:4 omega-6) J Neurochem. 1991;56:518–24. doi: 10.1111/j.1471-4159.1991.tb08180.x. [DOI] [PubMed] [Google Scholar]
- Moriguchi K, Yuri T, Yoshizawa K, Kiuchi K, Takada H, Inoue Y, Hada T, Matsumura M, Tsubura A. Dietary docosahexaenoic acid protects against N-methyl-N-nitrosourea-induced retinal degeneration in rats. Exp Eye Res. 2003;77:167–73. doi: 10.1016/s0014-4835(03)00114-3. [DOI] [PubMed] [Google Scholar]
- Murakami M, Masuda S, Kudo I. Arachidonate release and prostaglandin production by group IVC phospholipase A2 (cytosolic phospholipase A2gamma) Biochem J. 2003;372:695–702. doi: 10.1042/BJ20030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuringer M, Connor WE, Petten C, Van, Barstad L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J Clin Invest. 1984;73:272–6. doi: 10.1172/JCI111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obukowicz MG, Raz A, Pyla PD, Rico JG, Wendling JM, Needleman P. Identification and characterization of a novel delta6/delta5 fatty acid desaturase inhibitor as a potential anti-inflammatory agent. Biochem Pharmacol. 1998;55:1045–58. doi: 10.1016/s0006-2952(97)00665-5. [DOI] [PubMed] [Google Scholar]
- Okabe N, Nakamura T, Toyoshima T, Miyamoto O, Lu F, Itano T. Eicosapentaenoic acid prevents memory impairment after ischemia by inhibiting inflammatory response and oxidative damage. J Stroke Cerebrovasc Dis. 2011;20:188–95. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Patten AR, Sickmann HM, Dyer RA, Innis SM, Christie BR. Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposure. Neurosci Lett. 2013;551:7–11. doi: 10.1016/j.neulet.2013.05.051. [DOI] [PubMed] [Google Scholar]
- Politi LE, Bouzat C, los Santos EB, de, Barrantes FJ. Heterologous retinal cultured neurons and cell adhesion molecules induce clustering of acetylcholine receptors and polynucleation in mouse muscle BC3H-1 clonal cell line. J Neurosci Res. 1996;43:639–51. doi: 10.1002/(SICI)1097-4547(19960315)43:6<639::AID-JNR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Politi LE, Rotstein NP, Salvador G, Giusto NM, Insua MF. Insulin-like growth factor-I is a potential trophic factor for amacrine cells. J Neurochem. 2001a;76:1199–211. doi: 10.1046/j.1471-4159.2001.00128.x. [DOI] [PubMed] [Google Scholar]
- Politi L, Rotstein N, Carri N. Effects of docosahexaenoic acid on retinal development: cellular and molecular aspects. Lipids. 2001b;36:927–935. doi: 10.1007/s11745-001-0803-8. [DOI] [PubMed] [Google Scholar]
- Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fat Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R, Rosner B, Seddon JM. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology. 2013;120:1020–8. doi: 10.1016/j.ophtha.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein NP, Aveldaño MI, Barrantes FJ, Politi LE. Docosahexaenoic acid is required for the survival of rat retinal photoreceptors in vitro. J Neurochem. 1996a;66:1851–1859. doi: 10.1046/j.1471-4159.1996.66051851.x. [DOI] [PubMed] [Google Scholar]
- Rotstein NP, Aveldaño MI, Barrantes FJ, Roccamo AM, Politi LE. Apoptosis of retinal photoreceptors during development in vitro: protective effect of docosahexaenoic acid. J Neurochem. 1997;69:504–513. doi: 10.1046/j.1471-4159.1997.69020504.x. [DOI] [PubMed] [Google Scholar]
- Rotstein NP, Pennacchiotti GL, Sprecher H, Aveldaño MI. Active synthesis of C24:5, n-3 fatty acid in retina. Biochem J. 1996b;316(Pt 3):859–64. doi: 10.1042/bj3160859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein NP, Politi LE, Aveldaño MI. Docosahexaenoic acid promotes differentiation of developing photoreceptors in culture. Invest Ophthalmol Vis Sci. 1998;39:2750–8. [PubMed] [Google Scholar]
- Rotstein NP, Politi LE, German OL, Girotti R. Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors. Investig Ophthalmol Vis Sci. 2003;44:2252–2259. doi: 10.1167/iovs.02-0901. [DOI] [PubMed] [Google Scholar]
- Sales-Campos H, Souza PR de, Peghini BC, Silva JS da, Cardoso CR. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev Med Chem. 2013;13:201–10. [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY, Agrón E, Clemons TE, Ferris FL, Gensler G, Lindblad AS, et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126:1274–9. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanGiovanni JP, Chen J, Sapieha P, Aderman CM, Stahl A, Clemons TE, Chew EY, Smith LEH. DNA sequence variants in PPARGC1A, a gene encoding a coactivator of the ω-3 LCPUFA sensing PPAR-RXR transcription complex, are associated with NV AMD and AMD-associated loci in genes of complement and VEGF signaling pathways. PLoS One. 2013;8:e53155. doi: 10.1371/journal.pone.0053155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanGiovanni JP, Lee PH. AMD-associated genes encoding stress-activated MAPK pathway constituents are identified by interval-based enrichment analysis. PLoS One. 2013;8:e71239. doi: 10.1371/journal.pone.0071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3:69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86:2903–7. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazawa M, Nakajima Y, Mashima Y, Hara H. Docosahexaenoic acid (DHA) has neuroprotective effects against oxidative stress in retinal ganglion cells. Brain Res. 2009;1251:269–75. doi: 10.1016/j.brainres.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Shrestha C, Ito T, Kawahara K, Shrestha B, Yamakuchi M, Hashiguchi T, Maruyama I. Saturated fatty acid palmitate induces extracellular release of histone H3: a possible mechanistic basis for high-fat diet-induced inflammation and thrombosis. Biochem Biophys Res Commun. 2013;437:573–8. doi: 10.1016/j.bbrc.2013.06.117. [DOI] [PubMed] [Google Scholar]
- Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, Howe PRC. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr. 2012;107:1682–93. doi: 10.1017/S0007114511004788. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Babyak MA, Georgiades A, Sherwood A, Sketch MH, Watkins LL. Association between n-3 fatty acid consumption and ventricular ectopy after myocardial infarction. Am J Clin Nutr. 2009;89:1315–20. doi: 10.3945/ajcn.2008.26829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson AM, Wiegand RD, Anderson RE. Recycling of docosahexaenoic acid in rat retinas during n-3 fatty acid deficiency. J Lipid Res. 1991;32:2009–17. [PubMed] [Google Scholar]
- Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, Pariante CM. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:644–51. doi: 10.4088/jcp.v69n0418. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Noda K, Kubota S, Hirasawa M, Ozawa Y, Tsubota K, Mizuki N, Ishida S. Eicosapentaenoic acid suppresses ocular inflammation in endotoxin-induced uveitis. Mol Vis. 2010;16:1382–8. [PMC free article] [PubMed] [Google Scholar]
- Taepavarapruk P, Song C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: effects of omega-3 fatty acid EPA treatment. J Neurochem. 2010;112:1054–64. doi: 10.1111/j.1471-4159.2009.06524.x. [DOI] [PubMed] [Google Scholar]
- Tanito M, Brush RS, Elliott MH, Wicker LD, Henry KR, Anderson RE. High levels of retinal membrane docosahexaenoic acid increase susceptibility to stress-induced degeneration. J Lipid Res. 2009;50:807–19. doi: 10.1194/jlr.M800170-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanito M, Elliott MH, Kotake Y, Anderson RE. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest Ophthalmol Vis Sci. 2005;46:3859–68. doi: 10.1167/iovs.05-0672. [DOI] [PubMed] [Google Scholar]
- Tikhonenko M, Lydic TA, Wang Y, Chen W, Opreanu M, Sochacki A, McSorley KM, et al. Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes. 2010;59:219–27. doi: 10.2337/db09-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco J. Dietary requirements and functions of alpha-linolenic acid in animals. Prog Lipid Res. 1982;21:1–45. doi: 10.1016/0163-7827(82)90015-7. [DOI] [PubMed] [Google Scholar]
- Trépanier MO, Hopperton KE, Orr SK, Bazinet RP. N-3 polyunsaturated fatty acids in animal models with neuroinflammation: An update. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.05.045. [DOI] [PubMed] [Google Scholar]
- Tuo J, Ross RJ, Herzlich AA, Shen D, Ding X, Zhou M, Coon SL, Hussein N, Salem N, Chan CC. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am J Pathol. 2009;175:799–807. doi: 10.2353/ajpath.2009.090089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy RD, Birch DG, Birch EE, Tyson JE, Hoffman DR. Effect of dietary omega-3 fatty acids on retinal function of very-low-birth-weight neonates. Pediatr Res. 1990;28:485–92. doi: 10.1203/00006450-199011000-00014. [DOI] [PubMed] [Google Scholar]
- Unoda K, Doi Y, Nakajima H, Yamane K, Hosokawa T, Ishida S, Kimura F, Hanafusa T. Eicosapentaenoic acid (EPA) induces peroxisome proliferator-activated receptors and ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2013;256:7–12. doi: 10.1016/j.jneuroim.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Voss A, Reinhart M, Sankarappa S, Sprecher H. The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a 4-desaturase. J Biol Chem. 1991;266:19995–20000. [PubMed] [Google Scholar]
- Wang N, Anderson RE. Synthesis of docosahexaenoic acid by retina and retinal pigment epithelium. Biochemistry. 1993;32:13703–9. doi: 10.1021/bi00212a040. [DOI] [PubMed] [Google Scholar]
- Wetzel MG, Li J, Alvarez RA, Anderson RE, O’Brien PJ. Metabolism of linolenic acid and docosahexaenoic acid in rat retinas and rod outer segments. Exp Eye Res. 1991;53:437–46. doi: 10.1016/0014-4835(91)90161-7. [DOI] [PubMed] [Google Scholar]
- Wheeler TG, Benolken RM, Anderson RE. Visual membranes: specificity of fatty acid precursors for the electrical response to illumination. Science. 1975;188:1312–4. doi: 10.1126/science.1145197. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Horie K, Yamamoto T, Nagano T, Hirano T. Light-induced retinal damage in mice. Hydrogen peroxide production and superoxide dismutase activity in retina. Retina. 1992;12:59–66. [PubMed] [Google Scholar]
- Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–15. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]


