Abstract
Regulatory T (Treg) cells respond to immune and inflammatory signals to mediate immunosuppression, but how functional integrity of Treg cells is maintained under activating environments remains elusive. Here we found that autophagy was active in Treg cells and supported their lineage stability and survival fitness. Treg cell-specific deletion of the essential autophagy gene Atg7 or Atg5 led to loss of Treg cells, increased tumor resistance, and development of inflammatory disorders. Atg7-deficient Treg cells had increased apoptosis and readily lost Foxp3 expression, especially after activation. Mechanistically, autophagy deficiency upregulated mTORC1 and c-Myc function and glycolytic metabolism that contributed to defective Treg function. Therefore, autophagy couples environmental signals and metabolic homeostasis to protect lineage and survival integrity of Treg cells in activating contexts.
Introduction
Regulatory T (Treg) cells play an indispensable role in preventing autoimmune disease and establishing self-tolerance1. The activation states and functional capacities of Treg cells are dynamically programmed by environmental signals2. Treg cells emerge from the thymus as quiescent central Treg cells (cTreg; CD44loCD62Lhi)3. In response to environmental cues in the periphery, a fraction of Treg cells are continuously activated and converted into effector Treg cells (eTreg; CD44hiCD62Llo) under steady state3,4. After an inflammatory challenge, Treg cells are further activated and potently upregulate their suppressive activity and contribute to the regulation of inflammatory responses induced by autoimmunity, tumor and other stimuli5. Thus, the activation states and functional capacities of Treg cells are dynamically programmed by environmental signals. As for cell-intrinsic pathways, continued expression of Foxp3 is required to reinforce Treg cell functional integrity1. While Foxp3 expression is stable in vivo6, Treg cells can lose Foxp3 expression and acquire effector function in certain inflammatory conditions7–10, suggesting that activating environments could destabilize Foxp3 expression. Aside from lineage stability, maintenance of the anti-apoptotic program also contributes to the functional integrity of Treg cells in maintaining immune tolerance11.
Macroautophagy (herein referred to as autophagy) is an evolutionarily conserved self-digestive process that targets intracellular substrates for lysosomal degradation and recycling in response to stress and other environmental signals12–14. Autophagy plays important and context-dependent roles in T cell-mediated immune responses. For example, autophagy is required for survival and TCR-induced proliferation of T cells15. In contrast, activated CD8+ cells deficient in autophagy exhibit normal proliferation and effector function, but with impaired memory cell formation16. Autophagy is induced after TCR and cytokine stimulation15,17,18, but virus-specific CD8+ T cells downregulate autophagy activity during clonal expansion, followed by induction of autophagy when they stop dividing16. These studies highlight dynamic and signal-dependent function and regulation of autophagy.
We report here that autophagy is actively regulated in Treg cells, and serves as a central signal-dependent controller of Treg cells by restraining excessive apoptotic and metabolic activities. We found that Treg cell-specific loss of the essential autophagy gene Atg7 or Atg5 was sufficient to break self-tolerance while facilitating tumor clearance. Atg7-deficient Treg cells exhibited impaired lineage stability and increased apoptosis, thereby compromising their functional integrity. Although autophagy is known to promote energy balance14,17,19, we found that Treg cells deficient in autophagy showed increased mTORC1 activity, c-Myc expression and glycolytic metabolism, characteristic of anabolic upregulation20. Inhibition of mTORC1 or c-Myc in Atg7-deficient Treg cells partly restored Treg cell stability and metabolic homeostasis. Collectively, our studies establish a crucial role of autophagy in establishing Treg cell-mediated immune tolerance by coordinating immune signals and metabolic homeostasis to protect the functional integrity of Treg cells.
RESULTS
Autophagy is functionally active in Treg cells
To investigate regulation of autophagy in Treg cells, we quantified autophagosomes in peripheral Treg cells and naïve CD4+ cells using transgenic mice expressing the green fluorescent protein (GFP) fused to LC3 (GFP-LC3), which labels autophagic membranes21. Treg cells had significantly more cells labeled with GFP-LC3+ puncta than did naïve CD4+ cells (Fig. 1a), suggesting increased autophagosomes in Treg cells. Lipidated LC3 (LC3-II) is another marker of autophagic membranes12–14; immunoblot analysis showed that Treg cells had higher amount of LC3-II than naïve CD4+ cells (Supplementary Fig. 1a). Treatment of cells with a lysosome inhibitor bafilomycin A1 (Baf1A), which blocks lysosome-mediated degradation of autophagosomes, increased the amount of LC3-II in both Treg cells and naïve CD4+ cells, but Treg cells still had higher amount of LC3-II than naïve CD4+ cells (Supplementary Fig. 1a). Therefore, Treg cells have higher autophagy activity than naïve CD4+ cells, indicating a possible role of autophagy in Treg cells.
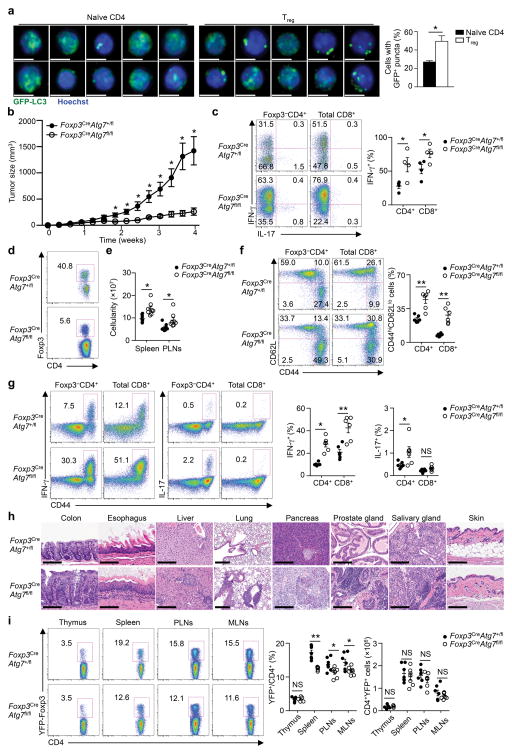
Figure 1. Treg cells have active autophagy and require Atg7 for mediating tumor immune tolerance and self-tolerance.
(a) Representative images (scale bars, 5 μm) (left) and quantification of percentages of cells with GFP-LC3+ puncta (right) in peripheral naïve CD4+ cells and Treg cells purified from GFP-LC3 mice (n=3 mice). (b–d) Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice (n=4 mice per genotype) were inoculated with MC38 colon adenocarcinoma cells, and tumor growth (b) was measured. Flow cytometry analyzing IFN-γ expression in Foxp3−CD4+ and CD8+ T cells (c, left), frequency of IFN-γ+ cells (c, right) and Foxp3 expression in CD4+ T cells (d) in tumor-infiltrating lymphocytes. Numbers in quadrants indicate percent cells in each throughout, and numbers adjacent to outlined areas indicate percent Foxp3+ cells (d). (e–g) Analysis of Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice (10–12 weeks old) under steady state. Total cellularity of spleen and PLNs (n=8 mice per genotype) (e). Flow cytometry analyzing the expression of CD62L and CD44 (f, left) and IFN-γ and IL-17 (g, left), and frequency of CD44hiCD62Llo cells (n=6 mice per genotype) (f, right) and IFN-γ+ or IL-17+ cells (n=6 mice per genotype) (g, right) in splenic Foxp3−CD4+ and CD8+ T cells. Numbers adjacent to outlined areas indicate percent IFN-γ+ or IL-17+ cells (g, left). (h) Hematoxylin and eosin staining of colon, esophagus, liver, lung, pancreas, prostate gland, salivary gland, and skin from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice (19–23 weeks old). Magnification and scale bars: ×40 and 100 μm (colon and skin), ×20 and 200 μm (esophagus, liver, pancreas, prostate gland and salivary gland) and ×10 and 200 μm (lung). (i) Flow cytometry analyzing YFP-Foxp3 expression in CD4+ T cells (left), and frequency and number of YFP-Foxp3+ cells (right) in the thymus, spleen, PLNs and mesenteric lymph nodes (MLNs) of Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice (10–12 weeks old) (n=7 mice per genotype). Numbers adjacent to outlined areas indicate percent YFP-Foxp3+ cells (g, left). NS, not significant (P > 0.05); * P < 0.05 and **P < 0.001 (two-tail unpaired Student’s t-test in a-c,e–g,i). Data are representative of two (a–d,h) experiments, or pooled from five out of six (e), four out of six (f,g) or three out of six (i) experiments (mean ± s.e.m in a–c,e–g,i).
To test this hypothesis, we crossed mice with loxP-flanked Atg7 alleles (Atg7fl/fl) with Foxp3YFP-Cre (Foxp3Cre) mice to delete the essential autophagy gene Atg7 in Treg cells (hereafter Foxp3CreAtg7fl/fl). Deletion of Atg7 abrogated autophagy in Treg cells, as indicated by the absence of LC3-II in immunoblot analysis (Supplementary Fig. 1a). To determine whether Treg cells require autophagy to suppress antitumor immune responses, we inoculated Foxp3CreAtg7fl/fl mice with MC38 colon adenocarcinoma cells. Tumor growth was severely inhibited in Foxp3CreAtg7fl/fl mice, suggesting that Atg7-deficient Treg cells failed to inhibit antitumor immune response (Fig. 1b). Consistent with this notion, Foxp3CreAtg7fl/fl mice had greatly increased percentage of tumor-infiltrating CD8+ cells (Supplementary Fig. 1b), and expression of interferon-γ (IFN-γ) in effector CD4+ and CD8+ T cells (Fig. 1c). However, Foxp3CreAtg7fl/fl mice had a profound loss of Treg cells in the tumor site (Fig. 1d). These results identify a crucial role of Atg7 in endowing Treg cells the ability to suppress antitumor immune responses.
Treg deletion of Atg7 or Atg5 alters immune homeostasis
The indispensable role of Atg7 in maintaining Treg cells in a pathological condition prompted us to determine the requirement of autophagy in Treg cells in maintaining self-tolerance under homeostatic conditions. Foxp3CreAtg7fl/fl mice at 10–12 weeks of age developed lymphoid hyperplasia with increased cellularity of the spleen and peripheral lymph nodes (PLNs) (Fig. 1e, Supplementary Fig. 1c). Foxp3CreAtg7fl/fl mice contained a higher proportion of the effector or memory population (CD44hiCD62Llo) in the CD4+ and CD8+ compartments (Fig. 1f). Moreover, CD44hi cells from Foxp3CreAtg7fl/fl mice showed increased expression of IFN-γ and interleukin 17 (IL-17) (Fig. 1g), but not IL-4 (Supplementary Fig. 1d). Therefore, T cells from Foxp3CreAtg7fl/fl mice were spontaneously activated in vivo. Moreover, severe systemic inflammatory disorders were observed in aged Foxp3CreAtg7fl/fl mice (19–23 weeks old) with infiltrations of lymphocytes and myeloid cells observed in various organs (Fig. 1h). Thus, Atg7 is essential for Treg cell-mediated immune homeostasis.
As autoimmune disease is frequently associated with loss of Treg cells, we examined Treg cells in the lymphoid organs of Foxp3CreAtg7fl/fl mice. Treg cell percentages were significantly reduced in spleen, PLNs and mesenteric lymph nodes (MLNs), but not the thymus, although Treg cell numbers remained largely unaltered due to the increase of total T cells (Fig. 1i). A more severe reduction of Treg cells was observed in colon lamina propria, a representative site of Treg activation, even in very young Foxp3CreAtg7fl/fl mice (Supplementary Fig. 1e). We next investigated whether Treg cell reduction in Foxp3CreAtg7fl/fl mice was a cell-autonomous defect. We generated mixed bone marrow (BM) chimeras by reconstituting Rag1−/− mice with BM cells from CD45.1+ mice mixed 1:1 with those from either Foxp3CreAtg7+/fl or Foxp3CreAtg7fl/fl CD45.2+ mice. Atg7-deficient Treg cells were underrepresented in the spleen, PLNs and MLNs, but not the thymus (Supplementary Fig. 1f), indicative of a cell-autonomous requirement of Atg7 in Treg cell maintenance.
To conclusively test the role of autophagy in Treg cells, we deleted another essential autophagy gene, Atg5, in Treg cells by crossing Atg5fl/fl mice with Foxp3YFP-Cre mice (Foxp3CreAtg5fl/fl). Foxp3CreAtg5fl/fl mice had disrupted immune homeostasis of CD4+ and CD8+ cells (Supplementary Fig. 1g), associated with increased IFN-γ expression (Supplementary Fig. 1h). Additionally, Foxp3CreAtg5fl/fl mice had reduced Treg cell percentage (Supplementary Fig. 1i). Therefore, these results establish autophagy as a central and intrinsic regulator of Treg cell maintenance and immune homeostasis.
Impaired survival and stability of Atg7-null Treg cells
To investigate the underlying basis for the reduced cellularity of Atg7-deficient Treg cells, we first examined Treg cell proliferation. Treg cells in Foxp3CreAtg7fl/fl mice actually contained a higher percentage of Ki67+ cells than those in Foxp3CreAtg7+/fl mice (Supplementary Fig. 2a), but Atg7-deficient Treg cells from the mixed BM chimeras had normal percentage of Ki67+ cells (Supplementary Fig. 2b). Moreover, Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl Treg cells showed comparable proliferation after in vitro stimulation or adoptive transfer into Rag1−/− mice (Supplementary Fig. 2c,d). Thus, Atg7 is dispensable for Treg cell proliferation, and the reduced Treg cellularity in the absence of Atg7 is unlikely to result from a proliferative defect.
Because peripheral Treg cell number is tightly regulated by apoptosis11, we next examined apoptosis of Treg cells. Treg cells in Foxp3CreAtg7fl/fl mice had greatly increased staining of active caspase-3 as compared to those in Foxp3CreAtg7+/fl mice (Fig. 2a), indicative of a higher rate of apoptosis. Additionally, upon in vitro stimulation, Atg7-deficient Treg cells were impaired in survival, as indicated by the increased staining with active caspase-3 and 7-AAD (Fig. 2b), and upregulation of Bim, which initiates Treg apoptosis11 (Fig. 2c). Atg7-deficient Treg cells from mixed BM chimeras also had increased active caspase-3 and Bim expression (Supplementary Fig. 2e,f), indicative of a cell-autonomous requirement of Atg7 in Treg cell survival.
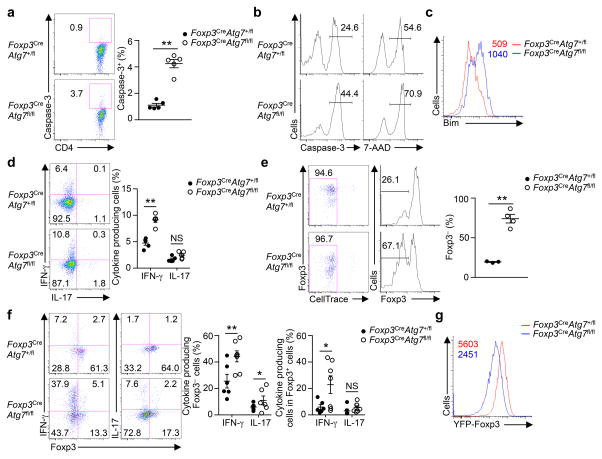
Figure 2. Atg7 contributes to Treg cell survival and lineage stability.
(a) Flow cytometry analyzing active caspase-3 expression (left), and frequency of caspase-3+ cells (right) in Treg cells from the spleen of Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice (n=5 mice per genotype). Numbers adjacent to outlined areas indicate percent caspase-3+ cells (left). (b,c) Flow cytometry analyzing active caspase-3 expression and 7-AAD staining (b) and Bim expression (c) in Treg cells (from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice) stimulated with anti-CD3, anti-CD28, and IL-2 for overnight. Numbers above bracketed lines indicate percent caspase-3+ or 7-AAD+ cells (b), and numbers above graph indicate mean fluorescence intensity (MFI) of Bim (c). (d) Flow cytometry analyzing the expression of IFN-γ and IL-17 (left), and frequency of IFN-γ+ cells and IL-17+ cells (right) in splenic Treg cells from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice (n=5 mice per genotype). Numbers in quadrants indicate percent cells in each throughout. (e,f) Treg cells (sorted from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice) were transferred into Rag1−/− mice. Flow cytometry analyzing CellTrace dilution (e, left) and the expression of Foxp3 (e, left) and IFN-γ and IL-17 (f, left), and frequency of Foxp3− cells (e, right) (n=3 mice for Atg7+/fl and n=4 mice for Atg7fl/fl) and IFN-γ+ cells and IL-17+ cells (f, right) (n=6 mice for Atg7+/fl and n=7 mice for Atg7fl/fl) in divided CellTrace-labeled donor cells (gated on CD4+TCRβ+). Numbers adjacent to outlined areas indicate percent CellTracelo cells (e, left), and numbers above bracketed lines indicate percent Foxp3− cells (e, left). (g) Flow cytometry analyzing YFP-Foxp3 expression in divided Treg cells (from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice) that were activated in vitro with anti-CD3, anti-CD28 and IL-2 for 96 h. Numbers above graphs indicate MFI of YFP-Foxp3. NS, not significant (P > 0.05); * P < 0.05 and ** P < 0.001 (two-tail unpaired Student’s t-test in a,d–f). Data are pooled from two out of five (a), four out of ten (d) or two out of two (f) experiments, or representative of three (b,c), ten (e) or five (g) experiments (mean ± s.e.m in a,d–f).
Aside from cell survival, lineage stability of Treg cells is crucial for their maintenance and function7–10. Although mean fluorescence intensity (MFI) of Foxp3 was comparable in Atg7-sufficient and deficient Treg cells (data not shown), Treg cells from Foxp3CreAtg7fl/fl mice had significantly elevated expression of IFN-γ under steady state (Fig. 2d), and upon tumor inoculation (Supplementary Fig. 2g). To directly examine the role of autophagy in maintaining Foxp3 expression in activated Treg cells in vivo, we transferred Atg7-sufficient and deficient Treg cells into Rag1−/− mice and assessed Foxp3 expression at 7–10 days after transfer. While only a small proportion of Atg7-sufficient Treg cells lost Foxp3 expression following homeostatic proliferation, the majority of Atg7-deficient Treg cells were unable to maintain Foxp3 (Fig. 2e). Loss of Foxp3 expression in Atg7-deficient Treg cells was associated with acquisition of production of IFN-γ, and to a lesser extent, IL-17 (Fig. 2f). IFN-γ expression was also elevated in the residual Foxp3+ Treg cells deficient in Atg7 (Fig. 2f). Moreover, in an in vitro system to measure stability of activated Treg cells22,23, Atg7-deficient Treg cells had greatly reduced Foxp3 (Fig. 2g) and elevated IFN-γ expression (Supplementary Fig. 2h). Collectively, Treg cells lacking Atg7 show impaired Foxp3 expression but aberrant acquisition of inflammatory cytokine expression in vivo and in vitro, indicating a central role of autophagy in maintaining the stability of Treg cells.
To explore the relationship between survival and stability defects of Atg7-deficient Treg cells, we crossed Foxp3CreAtg7fl/fl mice with mice expressing a Bcl2 transgene in lymphocytes (Bcl2-transgenic, Bcl2-TG)24. The excessive apoptosis of Foxp3CreAtg7fl/fl Treg cells was reduced in Foxp3CreAtg7fl/flBcl2-TG cells (Supplementary Fig. 2i). However, Foxp3CreAtg7fl/flBcl2-TG mice still had reduced Treg cell percentage and spontaneously activated conventional T cells (Supplementary Fig. 2j,k). Additionally, as compared with Treg cells from control Bcl2-TG mice, those from Foxp3CreAtg7fl/flBcl2-TG mice had elevated expression of IFN-γ (Supplementary Fig. 2l), and reduced Foxp3 expression after in vitro culture (Supplementary Fig. 2m). Therefore, survival and stability defects of Atg7-deficient Treg cells represent two discrete effects induced by loss of autophagy.
Atg7 restricts TCR-dependent mTORC1 signaling
To explore the biochemical basis for Atg7 functions, we performed functional genomics studies and found that phosphatidylinositol-3-OH kinase (PI(3)K) p110δ signaling, which was crucial for mTORC1 activation, was enhanced in Foxp3CreAtg7fl/fl Treg cells (data not shown). Further, Foxp3CreAtg7fl/fl Treg cells had increased cell size and CD71 and CD98 expression (Fig. 3a), all of which are dependent upon mTORC1 signaling25. Indeed, flow cytometry analysis showed that Treg cells from Foxp3CreAtg7fl/fl mice had increased S6 phosphorylation, indicative of mTORC1 activation (Fig. 3a). After anti-CD3 and anti-CD28 stimulation, Atg7-deficient cells exhibited a more pronounced upregulation of S6 and 4EBP1phosphorylation, as compared with Atg7-sufficient Treg cells (Fig. 3b). Furthermore, Atg5-deficient Treg cells had increased cell size, CD71 and CD98 expression, and S6 phosphorylation (Supplementary Fig. 3a). Therefore, autophagy is essential for restraining mTORC1 activity in Treg cells.
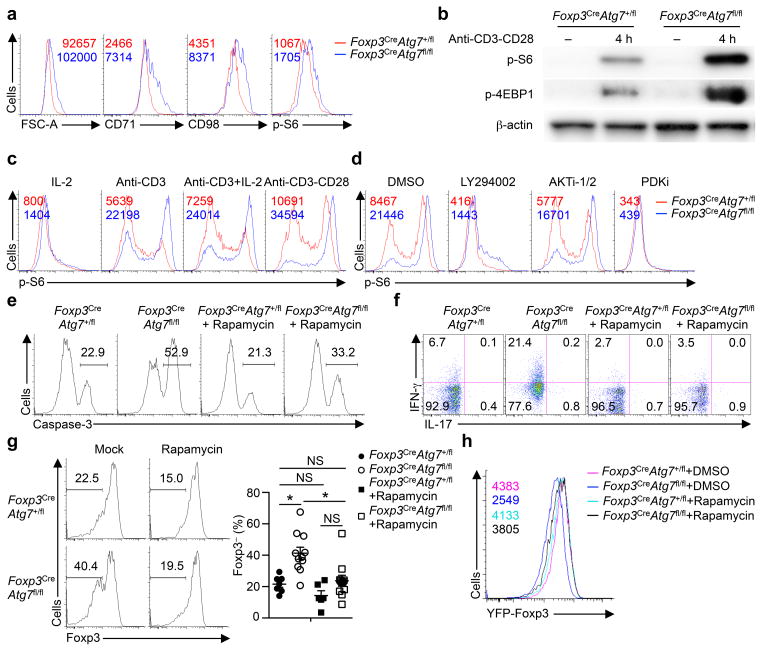
Figure 3. Atg7 restrains TCR-dependent mTORC1 activity in Treg cells.
(a) Flow cytometry analyzing cell size, and the expression of CD71, CD98 and p-S6 in Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl splenic Treg cells. Numbers above graphs indicate MFI of FSC-A, CD71, CD98 or p-S6. (b) Immunoblot analysis of p-S6 and p-4EBP1 in resting, and anti-CD3 and antiCD28-stimulated Treg cells from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice. (c) Flow cytometry analyzing p-S6 expression in Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl Treg cells stimulated with the indicated stimuli for 4 h. Numbers above graphs indicate MFI of p-S6. (d) Flow cytometry analyzing p-S6 expression in Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl Treg cells stimulated with anti-CD3 and anti-CD28 for 4 h in the presence of DMSO, LY294002 (10 μM), AKTi-1/2 (1 μM) or PDKi (10 μM). Numbers above graphs indicate MFI of p-S6. (e,f) Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice received mock or rapamycin treatment. Treg cells were isolated and stimulated overnight with anti-CD3, anti-CD28, and IL-2 for analysis of caspase-3 activity (e) or stimulated for 4 h for analysis of IFN-γ and IL-17 expression (f). Numbers above bracketed lines indicate percent caspase-3+ cells (e), and numbers in quadrants indicate percent cells in each (f). (g) Foxp3CreAtg7+/fl or Foxp3CreAtg7fl/fl Treg cells were transferred into Rag1−/− mice, followed by mock (n=7 mice for Atg7+/fl and n=11 mice for Atg7fl/fl) or rapamycin (n=6 mice for Atg7+/fl and n=11 mice for Atg7fl/fl) treatment. Flow cytometry analyzing Foxp3 expression (left), and frequency of Foxp3− cells (right) in divided donor cells (gated on CD4+TCRβ+) in the PLNs of recipients. Numbers above bracketed lines indicate percent Foxp3− cells. (h) Flow cytometry analyzing YFP-Foxp3 expression in divided Treg cells from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice, following DMSO or rapamycin treatment and activation with anti-CD3, anti-CD28, and IL-2 in vitro for 96 h. NS, not significant (P > 0.05); * P < 0.05 (one-way ANOVA in g). Data are representative of three (a,b,d–f,h) or two (c) experiments, or pooled from three out of three (g) experiments (mean ± s.e.m in g).
Signals from TCR, CD28 co-stimulation and IL-2 elicit mTORC1 activity26. To investigate the involvements of upstream inputs for mTORC1 regulation, we activated Treg cells with different stimuli and measured S6 phosphorylation. Anti-CD3 stimulation resulted in hyperactivation of S6 phosphorylation in Foxp3CreAtg7fl/fl Treg cells, and CD28 co-stimulation further boosted S6 phosphorylation in both Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl Treg cells (Fig. 3c). The kinases PI(3)K, Akt and PDK1 are known to contribute to mTORC1 activation in response to TCR-CD28 stimulation26. To determine the molecular basis for TCR-CD28-induced aberrant mTORC1 activation in the absence of Atg7, we treated cells with inhibitors for PI(3)K (LY294002), Akt (AKTi-1/2), and PDK1 (PDKi). Inhibition of PI(3)K and PDK1, but not Akt, blocked excessive S6 phosphorylation in Atg7-deficient Treg cells (Fig. 3d). Moreover, compared with control cells, Atg7-deficient Treg cells had moderately increased expression of the PI(3)K components p110δ and p85, and PDK1 (Supplementary Fig. 3b), while expression of Lck or phosphorylation of total tyrosine residues including Lck was unaltered (Supplementary Fig. 3b and data not shown). These results indicate that autophagy negatively regulates PI(3)K-PDK1 abundance and activation.
To determine the contribution of aberrant mTORC1 signaling to the defects in Atg7-deficient Treg cells, we treated Foxp3CreAtg7fl/fl mice with rapamycin in vivo. Rapamycin treatment moderately reduced active capase-3 staining (Fig. 3e), but more importantly, greatly diminished IFN-γ production in Treg cells in Foxp3CreAtg7fl/fl mice (Fig. 3f). Moreover, following adoptive transfer of Atg7-sufficient and deficient Treg cells into Rag1−/− mice, treatment of recipients with rapamycin largely restored Foxp3 expression in donor-derived Atg7-deficient Treg cells (Fig. 3g). Rapamycin also rectified Foxp3 expression in Atg7-deficient Treg cells in the in vitro Treg cell stability assay (Fig. 3h). Therefore, autophagy maintains Treg cell stability, at least in part, by restraining mTORC1 signaling.
Atg7-mediated transcriptional programs rely on mTORC1
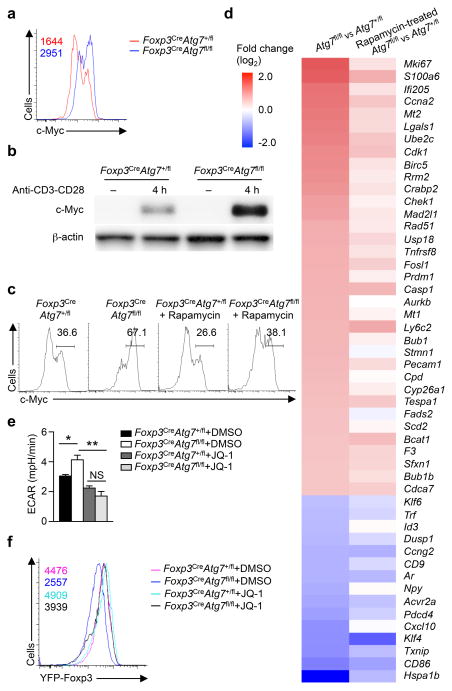
To explore autophagy-dependent transcriptional programs, we analyzed gene expression profiles of in vitro activated Treg cells. Compared to Foxp3CreAtg7+/fl cells, expression of 360 and 398 probes were respectively upregulated and downregulated (by greater than 0.5 log2 fold change) in Foxp3CreAtg7fl/fl Treg cells (Fig. 4a). To identify key networks regulated by autophagy in activated Treg cells, we did gene-set enrichment analysis (GSEA)27. Consistent with our finding that Atg7-deficient Treg cells had defective survival and stability, the caspase and cytokine pathways were enriched in these cells (Fig. 4b,c). Furthermore, T helper cell differentiation pathway was identified to be the most enriched canonical pathway in Atg7-deficient Treg cells by ingenuity pathway analysis (IPA) of the differentially expressed genes at the 0.5 log2 cut-offs (Supplementary Fig. 4a). Also, IPA of upstream regulation revealed the activation of PI(3)K in Atg7-deficient Treg cells, but suppression of Foxp3, Foxo3 and Bach2 – factors crucial for Treg cell generation and maintenance by repressing effector programs1,28,29 (Supplementary Fig. 4b). Therefore, these functional genomics analyses support a crucial role of Atg7 in restraining cytokine expression and effector programs in Treg cells.
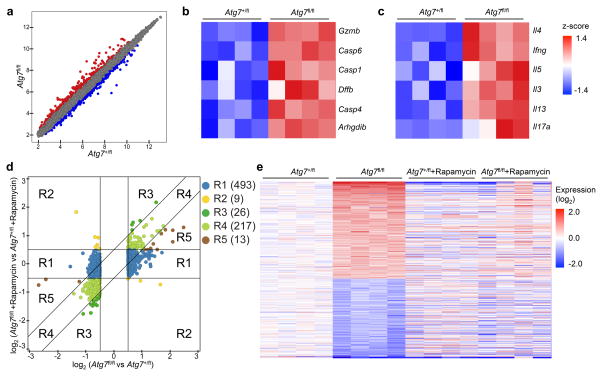
Figure 4. mTORC1 signaling is critical for Atg7-dependent transcriptional program.
Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice were treated with or without rapamycin (n=4 mice per group). Treg cells were sorted and activated with anti-CD3 and anti-CD28 for 4 h for gene expression profiling. (a) Scatterplot comparing global gene-expression profiles between Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl Treg cells. Transcripts with > 0.5 log2 fold increased (360 probes) or decreased (398 probes) expression in Foxp3CreAtg7fl/fl Treg cells are shown in red or blue respectively. (b,c) GSEA reveals that caspase (b) and cytokine pathways (c) are among the most extensively upregulated pathways in Foxp3CreAtg7fl/fl Treg cells as compared with Foxp3CreAtg7+/fl Treg cells. Heat maps of top hit genes in caspase (b) and cytokine (c) pathways. Differentially expressed genes were normalized by z-score. Expression levels are shown as green for low intensities, and red for high intensities. (d) Comparison of expression changes in rapamycin-treated Foxp3CreAtg7fl/fl versus Foxp3CreAtg7+/fl Treg cells with those in non-treated Foxp3CreAtg7fl/fl versus Foxp3CreAtg7+/fl Treg cells. The 758 Atg7 target genes (with > 0.5 log2 fold change) were partitioned into five main clusters, shown and colored by regions (R1–R5). Right, numbers indicate the number of probes within each region. (e) Heat maps of 515 rapamycin responsive genes that are differentially expressed in Foxp3CreAtg7fl/fl Treg cells (with > 0.5 log2 fold change) and have diminished response after rapamycin treatment. Red color denotes upregulated genes in Foxp3CreAtg7fl/fl Treg cells, and blue color denotes downregulated genes in Foxp3CreAtg7fl/fl Treg cells. Data are from one experiment (a–e).
To determine the contribution of aberrant mTORC1 to Atg7-mediated transcriptional programs, we compared gene expression profiles of in vitro activated Treg cells from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice with or without rapamycin treatment. Atg7-dependent targets (a total of 758 probes) were partitioned into five distinct clusters that differed in their responses to rapamycin (Fig. 4d). A salient feature was that the majority of Atg7 targets (493 out of 758 probes) fell into cluster 1, in which their expression was rectified by rapamycin. Moreover, cluster 5 contained 13 probes whose expression was partially rectified by rapamycin, and cluster 2 contained 9 probes that showed the opposite direction of change in expression. Thus, ~68% (clusters 1, 2 and 5) of all Atg7 targets were rapamycin responsive as they had a diminished response after rapamycin treatment (Fig. 4d,e). In contrast, only 217 probes in cluster 4 had equal magnitude of change (> 0.5 log2 fold change) in both rapamycin-treated and non-treated Foxp3CreAtg7fl/fl Treg cells, thus representing rapamycin non-responsive genes. In addition, cluster 3 contained 26 probes that were differentially expressed in both types of comparisons, but to a greater extent in rapamycin-treated Foxp3CreAtg7fl/fl Treg cells compared with non-treated Foxp3CreAtg7fl/fl cells. Furthermore, rapamycin considerably rectified expression of genes associated with gene sets altered by Atg7 deficiency described above, namely caspase and cytokine pathways (Supplementary Fig. 4c,d). These results identify a crucial contribution of mTORC1 to autophagy-dependent transcriptional programs.
Atg7 restraints glycolytic metabolism in Treg cells
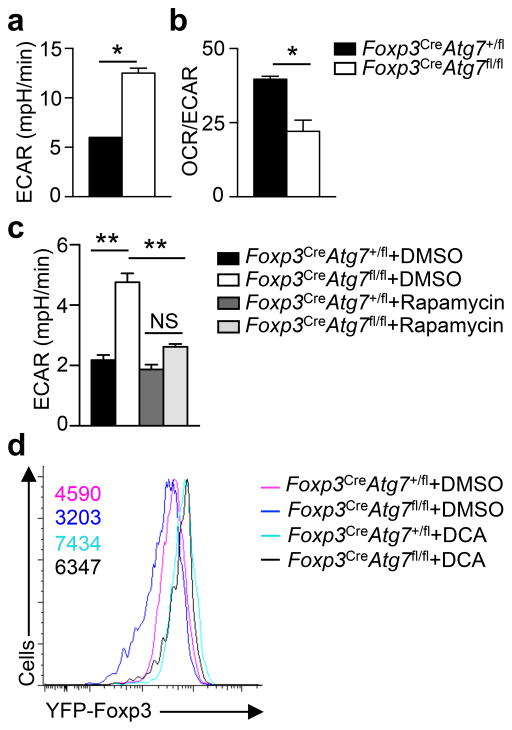
Differentiation of Treg cells is shaped by metabolic programs30–32, but how Treg stability is controlled by cellular metabolism remains unclear. The aberrant activation of mTORC1 in Atg7-deficient Treg cells prompted us to examine the involvement of metabolic programs. We measured mitochondrial oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), which denote mitochondrial OXPHOS and glycolytic activities, respectively. After TCR-CD28 stimulation, Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl Treg cells had comparable OCR (Supplementary Fig. 5a), but ECAR was significantly elevated in Foxp3CreAtg7fl/fl Treg cells (Fig. 5a). The reduced OCR/ECAR ratio in Atg7-deficient Treg cells indicated a preferential use of glycolysis over OXPHOS by these cells (Fig. 5b). Moreover, rapamycin treatment reduced ECAR in Atg7-deficient Treg cells (Fig. 5c), indicating a crucial role of mTORC1 in Atg7-dependent metabolic homeostasis.
Figure 5. Dysregulation of glycolytic metabolism in Atg7-deficient Treg cells contributes to impaired Treg cell stability.
(a) Measurement of ECAR in Treg cells (from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice) stimulated with anti-CD3 and anti-CD28 for 4 h. (b) Ratio of OCR to ECAR of Treg cells (from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice) stimulated with anti-CD3 and anti-CD28 for 4 h. (c) Measurement of ECAR in Treg cells (from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice) stimulated with anti-CD3 and anti-CD28 for 4 h in the presence of DMSO or rapamycin. (d) Flow cytometry analyzing YFP-Foxp3 expression in divided Treg cells (from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice) that were activated in vitro with anti-CD3, anti-CD28, and IL-2 for 96 h in the presence of DMSO or DCA. Numbers above graphs indicate MFI of YFP-Foxp3. NS, not significant (P > 0.05); * P < 0.05 and ** P < 0.001 (two-tail unpaired Student’s t-test in a,b and one-way ANOVA in c). Data are representative of two (a,b), three (c) or four (d) experiments (mean ± s.e.m in a–c).
To examine the functional importance of cellular metabolism, we treated Atg7-deficient cells with dichloroacetate (DCA) in the Treg stability assay; DCA shifts glycolysis towards OXPHOS by targeting pyruvate dehydrogenase kinase30. DCA treatment elevated Foxp3 expression in Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl Treg cells to a largely comparable level (Fig. 5d). Of note, methylation of Treg cell-specific demethylated region (TSDR, also known as CNS2) is associated with maintenance of Foxp3 expression and Treg cell stability23,33. TSDR methylation was comparable between Atg7-sufficient and deficient Treg cells, and DCA treatment had no effect (Supplementary Fig. 5b). Thus, autophagy-dependent metabolic regulation contributes to Foxp3 expression but in a process independent of TSDR methylation.
To determine molecular basis for the altered glycolysis, we examined expression of hexokinase 2 (HK2), a rate-limiting enzyme in glycolysis. HK2 expression was increased in Foxp3CreAtg7fl/fl Treg cells (Supplementary Fig. 5c), but was considerably reduced by rapamycin treatment (Supplementary Fig. 5d). In contrast, although the mTORC1-HIF1α pathway promotes glycolysis in TH17 cells and effector CD8+ T cells32,34, HIF1α expression was not altered in Atg7-deficient Treg cells (Supplementary Fig. 5e,f). Altogether, Atg7 negatively controls mTORC1-dependent glycolytic metabolism in Treg cells.
c-Myc links mTORC1 to glycolysis of Treg cells
Aside from HIF1α, c-Myc is another crucial regulator of T cell glycolysis35. c-Myc pathway was enriched in our IPA analysis of Atg7-deficient Treg cells (data not shown). Compared with Treg cells from Foxp3CreAtg7+/fl mice, those from Foxp3CreAtg7fl/fl Treg mice had increased c-Myc expression following anti-CD3-CD28 stimulation (Fig. 6a,b). To determine if upregulation of c-Myc in Atg7-deficient Treg cells depends upon mTORC1, we treated Foxp3CreAtg7fl/fl mice with rapamycin and examined c-Myc expression in Treg cells. Rapamycin treatment considerably reduced c-Myc expression in Atg7-deficient Treg cells (Fig. 6c). Moreover, dysregulated expression of c-Myc-associated genes in Atg7-deficient Treg cells was rectified upon rapamycin treatment (Fig. 6d). To further explore the role of mTORC1 in mediating c-Myc expression in Atg7-deficient Treg cells, we crossed Cd4CreAtg7fl/fl mice with Rptorfl/fl mice to abolish Raptor, the essential component of mTORC1, in Atg7-deficient T cells. Raptor deletion strongly reduced c-Myc expression in Atg7-deficient Treg cells (Supplementary Fig. 6a). These results indicate that Atg7 regulates c-Myc expression in Treg cells in an mTORC1-dependent manner.
Figure 6. Autophagy protects Treg cell stability by restraining mTORC1-dependent c-Myc expression and function.
(a) Flow cytometry analyzing c-Myc in Treg cells (from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice) activated with anti-CD3 and anti-CD28 for 4 h. Numbers above graphs indicate MFI of c-Myc. (b) Immunoblot analysis of c-Myc in resting, and anti-CD3 and anti-CD28 stimulated Treg cells from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice. (c,d) Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice received mock or rapamycin treatment. Treg cells were isolated and stimulated with anti-CD3 and anti-CD28 for 4 h for analysis of c-Myc expression (c). Numbers above bracketed lines indicate percent c-Myc+ cells (c). Heat maps of Myc target gene expression in non-treated Foxp3CreAtg7fl/fl versus Foxp3CreAtg7+/fl Treg cells, and rapamycin-treated Foxp3CreAtg7fl/fl versus Foxp3CreAtg7+/fl Treg cells (d). Red color denotes upregulated genes in Foxp3CreAtg7fl/fl Treg cells, and blue color denotes downregulated genes in Foxp3CreAtg7fl/fl Treg cells (d). (e) Measurement of ECAR in Treg cells (from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice) stimulated with anti-CD3 and anti-CD28 for 4 h in the presence of DMSO or JQ-1. (f) Flow cytometry analyzing YFP-Foxp3 expression in divided Treg cells (from Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice) that were activated in vitro with anti-CD3 and anti-CD28, and IL-2 for 96 h in the presence of DMSO or JQ-1. Numbers above graphs indicate MFI of YFP-Foxp3. NS, not significant (P > 0.05); * P < 0.05 and ** P < 0.001 (one-way ANOVA in e). Data are representative of four (a,f), three (b,c) or two (e) experiments, or from one (d) experiments (mean ± s.e.m in e).
The Myc locus is enriched with binding sites for bromodomain-containing proteins that recognize acetylated lysine residues of histones36, and two bromodomain inhibitors, JQ-1 and i-BET-762, effectively inhibit c-Myc expression and function36,37 (data not shown). To determine the contribution of aberrant c-Myc expression to increased glycolysis in Foxp3CreAtg7fl/fl Treg cells, we treated cells with JQ-1 and measured ECAR. JQ-1 treatment reduced ECAR in Atg7-deficient Treg cells (Fig. 6e). In the in vitro Treg stability assay, JQ-1 or i-BET-762 restored Foxp3 expression in Atg7-deficient Treg cells (Fig. 6f and Supplementary Fig. 6b). Therefore, Atg7 maintains Treg cell stability by targeting mTORC1-c-Myc pathway.
Activated Treg cells are sensitive to Atg7 deficiency
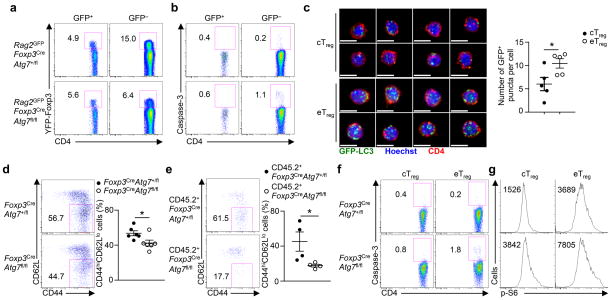
Under steady state, Treg cells are spontaneously and continuously activated in response to self-antigens and environmental cues3,4. To explore the role of autophagy in Treg cells with different activation states, we crossed Foxp3CreAtg7fl/fl mice with mice transgenic for GFP driven by the recombination-activating gene 2 (Rag2) promoter to label recent thymic emigrates (RTEs) with GFP38. In these mice, GFP+ peripheral T cells represent RTEs that have left the thymus within 2–3 weeks and show naïve phenotypes, while GFP− cells are mature peripheral T cells that have experienced peripheral environment cues3,38. Treg cells in Rag2GFPFoxp3CreAtg7fl/fl mice were diminished in the GFP− compartment, but not in the GFP+ compartment, as compared with the counterparts in Rag2GFPFoxp3CreAtg7+/fl mice (Fig. 7a). Additionally, the increased apoptosis of Atg7-deficient Treg cells, as determined by active caspase-3 staining, was observed only in the GFP− compartment (Fig. 7b). These results identify a preferential requirement of autophagy in maintaining the cellularity and survival of activated Treg cells.
Figure 7. Autophagy is preferentially required for activated Treg cell maintenance.
(a,b) Flow cytometry analyzing the expression of YFP-Foxp3 in GFP+ and GFP− CD4+ T cells (a) and active caspase-3 in GFP+ and GFP− Treg cells (b) in the spleen of Rag2GFPFoxp3CreAtg7+/fl and Rag2GFPFoxp3CreAtg7fl/fl mice. Numbers adjacent to outlined areas indicate percent YFP-Foxp3+ cells (a) and caspase-3+ cells (b). (c) Representative images (scale bars, 5 μm) (left) and quantification of the number of GFP-LC3+ puncta per cell (right) in cTreg and eTreg cells purified from the spleen of GFP-LC3 mice (n=5 mice). (d,e) Flow cytometry analyzing the proportion of CD44hiCD62Llo eTreg cells among total Treg cells in the spleen of Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice (n=6 mice per genotype) (d, left) and Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl chimeras (n=4 mice per group) (e, left), and frequency of CD44hiCD62Llo eTreg cells (d,e, right). Numbers adjacent to outlined areas indicate percent CD44hiCD62Llo eTreg cells (d,e, left). (f,g) Flow cytometry analyzing the expression of active caspase-3 (f) and p-S6 (g) in CD44loCD62Lhi cTreg cells and CD44hiCD62Llo eTreg cells in the spleen of Foxp3CreAtg7+/fl and Foxp3CreAtg7fl/fl mice. Numbers adjacent to outlined areas indicate percent caspase-3+ cells (f), and numbers above graphs indicate MFI of p-S6 (g). * P < 0.05 (two-tail unpaired Student’s t-test in c–e). Data are representative of five (a,f), three (b,g), or four (e) experiments, or pooled from two out of two (c) or five out of five (d) experiments (mean ± s.e.m in c–e).
Published reports highlight that the activated eTreg cells differentiate from the quiescent cTreg population in response to environmental cues3,4. Autophagy activity was upregulated in eTreg cells, as revealed by the significantly more GFP-LC3+ puncta in eTreg than cTreg cells (Fig. 7c). In Foxp3CreAtg7fl/fl mice, the eTreg population was modestly underrepresented in Treg cells (Fig. 7d), but such defect was more pronounced in a competitive environment created by the mixed BM chimeras (Fig. 7e), indicating a cell-autonomous requirement of Atg7 in this process. Moreover, eTreg cells in Foxp3CreAtg7fl/fl mice showed a more pronounced upregulation of apoptosis compared with cTreg cells (Fig. 7f). In line with this, eTreg cells had higher basal level of mTORC1 activity than cTreg cells as determined by S6 phosphorylation, and Atg7 deficiency augmented mTORC1 activity to a greater degree in eTreg cells (Fig. 7g). Thus, autophagy functions in both Treg subsets, but eTreg cells are more sensitive to autophagy deficiency, in agreement with their more activated state and elevated mTORC1 activity (Supplementary Fig. 7).
Discussion
A salient feature of the autoreactive Treg cells is the dynamic programming of their activation states in response to environmental and immune signals. How Treg cells maintain their survival fitness and lineage stability under the activating environments is poorly understood. Here we identify that autophagy is dynamically regulated in Treg cells, and deletion of Atg7 or Atg5 specifically in Treg cells results in increased apoptosis and impaired lineage stability. We further reveal an inhibitory effect of autophagy on mTORC1 that contributes to the survival and stability of Treg cells. Furthermore, aberrant mTORC1 elevates c-Myc expression and glycolytic metabolism in autophagy-deficient Treg cells, and pharmacological blocking of excessive mTORC1, c-Myc or glycolytic activities restores, at least in part, the impaired stability of autophagy-deficient Treg cells. Our studies therefore establish autophagy as a crucial regulator of Treg functional integrity, and identify a key role of autophagy in restraining mTORC1 and c-Myc function and glycolytic metabolism.
Homeostatic stimuli and environmental cues drive the continuous activation and progressive functional maturation of Treg cells2. However, after activation in vivo and in vitro, a proportion of Treg cells lose Foxp3 expression and lineage stability22,23. One unanswered question is therefore how Treg cells retain their survival fitness and functional integrity in activating contexts. Our studies identify an important role of autophagy in this process. Treg cells exhibit higher autophagy activity than naïve T cells, and they further upregulate autophagy in the activated eTreg subset. The exact stimuli that induce autophagy in Treg cells remain to be identified. Autophagy deficiency has a more pronounced effect on the maintenance of activated Treg cells than resting Treg cells in the periphery under steady state, and at sites of inflammation including tumor microenvironment and colon lamina propria. Moreover, Atg7-deficient Treg cells readily lose Foxp3 expression after extensive proliferation in Rag1−/− mice, or after TCR stimulation in vitro. These results identify a previously unappreciated mechanism that functions preferentially in activated Treg cells to protect their survival fitness and lineage stability.
mTORC1 signaling is widely recognized as a negative regulator of autophagy. Specifically, in a nutrient-replete environment, activation of mTORC1 inhibits autophagy; downregulation of mTORC1 activity under nutrient deprivation facilitates the induction of autophagy12–14. However, prolonged starvation can result in the reactivation of mTORC1 in an autophagy-dependent manner by degradation of autolysosomal products39. Unexpectedly, we found that autophagy plays an important role in restricting mTORC1 activation in Treg cells activated by TCR and other stimuli. Of note, mTORC1 is a crucial regulator in Treg cells, and either diminished or excessive mTORC1 disrupts Treg cell suppressive functions40,41. The in vivo rapamycin treatment experiment highlights the impacts of mTORC1 dysregulation on the stability and survival of Treg cells, and transcriptional programs controlled by Atg7. As for the biochemical mechanism by which autophagy regulates mTORC1 signaling, one possibility is that autophagy targets selective TCR signaling components or mTORC1 upstream regulators for degradation to modulate the strength of mTORC1 signaling. For example, a recent study indicates that autophagy shapes how TCR signals to NF-κB in effector T cells by selective degradation of Bcl-10, although the underlying mechanisms and functional outcomes are context-dependent42. In support of this notion, protein abundance of PI(3)K components and PDK1 are increased in the absence of autophagy, and pharmacological inhibition of PI(3)K or PDK1 blocks mTORC1 hyperactivation in Atg7-deficient Treg cells. Future studies are warranted to reveal the detailed biochemical pathway by which autophagy modulates the activity of PI(3)K and PDK1 signaling.
T cell survival, proliferation and function require dynamic reprogramming of cellular metabolism20, and glycolytic capacity and reserve are severely impaired in the in vitro generated Treg cells compared with TH1 and TH17 cells30. How glycolytic activity is restrained in Treg cells remains poorly defined, and our results identify a crucial inhibitory effect of autophagy on Treg cell glycolysis. Importantly, pharmacological blocking of glycolytic metabolism or c-Myc function partly restores the defective stability of Atg7-deficient Treg cells, thereby highlighting the functional contribution of dysregulated metabolism in this process. We and others have recently described a role of the phosphatase PTEN in the regulation of T cell glycolysis in Treg cells43,44. However, PTEN mainly restricts mTORC2 but not mTORC1 activity in Treg cells, and acts to control TH1 and TFH cell responses43,44. In contrast, Atg7-deficient Treg cells show dysregulated mTORC1-c-Myc signaling and are selectively defective in controlling TH1 cell response, without affecting TFH cell responses (our unpublished observation), suggesting that Treg cells employ discrete mechanisms to properly establish their metabolic programs. Interestingly, whereas our study indicates a negative role of glycolysis in the maintenance of Treg cell stability, a recent study identifies that induction of Treg cells from human conventional T cells is dependent on glycolysis45, thereby highlighting context-dependent functions of glycolysis in Treg cell biology.
In summary, our study has unveiled the interplay between autophagy and metabolic programming as a new mechanism to enforce Treg cell functional integrity in response to immune signals. We further establish that autophagy acts as a negative regulator of mTORC1 and c-Myc function and glycolytic metabolism to maintain metabolic balance in activated Treg cells. The identification of autophagy as a central signal-dependent quality control mechanism in Treg cells provides new opportunities for therapeutic intervention of autoimmune diseases and cancer. From this perspective, by strengthening tumor-associated immune responses, targeting Treg cell autophagy could act in synergy with strategies that block autophagy in tumor cells for added benefits in cancer therapy13.
Online methods
Mice
Rag1−/− and Rag2GFP mice38 were purchased from the Jackson Laboratory. GFP-LC3, Atg7fl/fl and Atg5fl/fl mice were as described21,46,47. Foxp3YFP-Cre mice were a gift from A. Rudensky48. Foxp3CreAtg7fl/fl mice were used at 7–16 weeks old unless otherwise noted, with the age and gender-matched Foxp3CreAtg7+/fl mice as controls. For treatment with rapamycin, mice were injected intraperitoneally with rapamycin (4 mg per kg body weight) daily, and then analyzed five days later. BM chimeras were generated by transferring 5 × 106 T cell-depleted BM cells into sublethally irradiated (5 Gy) Rag1−/− mice. All mice were kept in a specific pathogen-free facility in the Animal Resource Center at St. Jude Children’s Research Hospital. Animal protocols were approved by the Institutional Animal Care and Use Committee of St. Jude Children’s Research Hospital.
Flow cytometry
For analysis of surface markers, cells were stained in PBS containing 2% (wt/vol) BSA, with anti-CD4 (RM4-5), anti-CD8α (53-6.7), anti-TCRβ (H57-597), anti-CD44 (1M7), anti-CD62L (MEL-14), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD71 (R17217), and anti-CD98 (RL388; all from eBioscience). Intracellular Foxp3 (FJK-16s), Ki67 (SolA15), IFN-γ (XMG1.2), IL-4 (11B11), IL-17 (17B7; all from eBioscience), Bim (C34C5), c-Myc (D84C12), and p-S6 (D57.2.2E; all from Cell Signaling Technology) were analyzed by flow cytometry according to the manufacturer’s instructions. For intracellular cytokine staining, T cells were stimulated for 4 h with PMA plus ionomycin in the presence of monensin before intracellular staining according to the manufacturer’s instructions (eBioscience). Caspase-3 activity was measured using active caspase-3 apoptosis kit (BD Biosciences). To monitor cell division, lymphocytes were labeled with CellTrace™ violet (Life Technologies). Flow cytometry data were acquired on LSRII or LSR Fortessa (BD Biosciences) and analyzed using Flowjo software (Tree Star).
Imaging and histology
Purified GFP-LC3 naïve CD4+ cells and Treg cells were rested in complete medium for 1 h at 37°C. Cells were harvested and fixed by 4% (vol/vol) neutral buffered paraformaldehyde solution. Images were acquired using a Zeiss Axio ObserverZ.1 microscope equipped with a CSU-22 spinning disk (Yokagawa), Delta Evole EMCCD camera (Photometrics), 100× 1.45 NA oil objective and Slidebook imaging software (3i Intelligent Imaging Innovations). Images were subsequently processed using a Laplacian filter to detect GFP-LC3+ punctum objects having a minimum volume of 0.1 μm3. Frequency of cells with GFP-LC3+ puncta and number of GFP-LC3+ puncta per cell were determined for each sample. For histology analysis, tissues were fixed by 10% (vol/vol) neutral buffered formalin solution, embedded in paraffin, sectioned and stained with hematoxylin and eosin, and the clinical signs of autoimmune diseases were analyzed by an experienced pathologist (P. Vogel).
Tumor model
MC38 colon adenocarcinoma cells were maintained in our laboratory and cultured in DMEM medium supplemented with 10% (vol/vol) FBS and 1% (vol/vol) penicillin-streptomycin. Gender-matched Foxp3CreAtg7+/fl mice and Foxp3CreAtg7fl/fl mice were injected subcutaneously with 2 × 105 MC38 colon adenocarcinoma cells in the right flank. Tumors were measured regularly with digital calipers and tumor volumes were calculated by the formula: Length × Width × [(Length × Width) ^ 0.5] × π/6. To prepare tumor infiltrating lymphocytes (TILs), tumor was excised, minced and digested with 0.5 mg/ml Collagenase IV (Roche) + 200 U/ml DNase I (Sigma) for 1 h at 37°C. TILs were isolated by density-gradient centrifugation over Percoll (Life Technologies).
Cell purification and culture
Unless otherwise noted, lymphocytes were isolated from spleen and PLNs, that included inguinal, auxiliary and cervical lymph nodes, and naïve CD4+ cells (CD4+Foxp3-YFP−CD44loCD62Lhi), Treg cells (CD4+Foxp3-YFP+), cTreg cells (CD4+Foxp3-YFP+CD44loCD62Lhi) and eTreg cells (CD4+Foxp3-YFP+CD44hiCD62Llo) were sorted on a MoFlow (Beckman-Coulter) or Reflection (i-Cyt). Sorted Treg cells were cultured in plates coated with anti-CD3 (145-2C11) and anti-CD28 (37.51; both from eBioscience) for 4 days in Click’s medium supplemented with β-mercaptoethanl, 10% (vol/vol) FBS, 1% (vol/vol) penicillin-streptomycin and 200 U/ml IL-2. In some experiments, rapamycin (50 nM), JQ-1 (500 nM), i-BET-762 (500 nM) and DCA (10 mM) were added to the culture.
Adoptive transfer
For adoptive transfer, Treg cells from Foxp3CreAtg7+/fl mice and Foxp3CreAtg7fl/fl mice were transferred to the Rag1−/− mice. Seven to ten days after the transfer, recipients were euthanized for the analysis of Foxp3 and cytokine expression in transferred cells. For treatment with rapamycin, Rag1−/− mice were injected intraperitoneally with rapamycin (2 mg per kg body weight) every other day from day 1, and then analyzed at day 10.
RNA and Immunoblot analysis
Real-time PCR analysis was performed with primers and probe sets from Applied Biosystems, as described49. Immunoblots were performed as described previously24, using the following antibodies: Lck (2752), HK2 (C64G5), p85 (19H8), PDK1 (3062), p-S6 (2F9), p-4EBP1 (236B4), c-Myc (D84C12; all from Cell Signaling Technology), LC3 (NB100-2220; Novus), p110δ (EPR386; Abcam), HIF1α (10006421; Cayman) and β-actin (AC-15; Sigma).
Seahorse assays
Sorted Treg cells were stimulated with plate-bound anti-CD3 and anti-CD28 for 4 h in the presence of IL-2. In certain experiments, rapamycin (50 nM) and JQ-1 (500 nM) were added to the culture. After stimulation, cells were re-plated in XF media (non-buffered DMEM containing 5 mM glucose, 2 mM L-glutamine and 1 mM sodium pyruvate). XF-24 Extracellular Flux Analyzer (Seahorse Bioscience) was used to measure OCR and ECAR in response to 1 μM oligomycin, 2 μM fluoro-carbonyl cyanide phenylhydrazone (FCCP) and 1 μM Rotenone.
Methylation analysis of Treg cell-specific demethylated region
CellTrace-labeled Treg cells were cultured with anti-CD3, anti-CD28, and IL-2 for 4 days in the presence of DMSO or DCA. Divided cells were sorted, and genomic DNA was prepared by a DNeasy Blood & Tissue kit (Qiagen). Bisulfite conversion of DNA was conducted with an EZ DNA Methylation-Direct kit (Zymo Research). Intron 1 of Foxp3 (corresponding to CNS2) was amplified with a primer set as described (forward, 5′-TATTTTTTTGGGTTTTGGGATATTA-3′ and reverse, 5′-AACCAACCAACTTCCTACACTATCTAT-3′)44. PCR products were ligated into pGEM-T Easay vectors (Promega) and sequenced (more than 29 sequences per sample).
Gene expression profiling and bioinformatic analysis
Treg cells from Foxp3CreAtg7+/fl mice (n=4), Foxp3CreAtg7fl/fl mice (n=4), rapamycin-treated Foxp3CreAtg7+/fl mice (n=4) and rapamycin-treated Foxp3CreAtg7fl/fl mice (n=4) were stimulated with plate-bound anti-CD3 and anti-CD28 for 4 h. RNA samples from these cells were analyzed with the Mouse Gene 2.0 ST Signals array. Differentially expressed transcripts were identified by ANOVA (Partek Genomics Suite v6.5) and the Benjamini-Hochberg method was used to estimate the false discovery rate (FDR) as described previously25. Lists of differentially expressed genes at the 0.5 log2 cut-offs were used for IPA canonical pathway and upstream signaling analyses. GSEA was performed as described27. The microarray data have been deposited into the GEO series database (GSE75218).
Statistical analysis
Prism 5 software (GraphPad) was used to analyze data by performing two-tail unpaired Student’s t-test. When multiple groups were compared, one-way ANOVA with the Tukey test was performed. P value of less than 0.05 was considered significant. All error bars represent the s.e.m.
Supplementary Material
Acknowledgments
The authors acknowledge B. Rhode, C. Cloer and S. Rankin for animal colony management, X. Du for help with immunoblot analysis, N. Kalupahana for initial analysis of Atg7-deficient mice, A. Rudensky for Foxp3YFP-Cre mice, J.E. Bradner for providing the JQ-1 compound, St. Jude Immunology FACS core facility for cell sorting, and Y. Wang for editing. This work was supported by US National Institutes of Health (NIH AI105887, AI101407, CA176624 and NS064599), and Crohn’s and Colitis Foundation of America (to H.C.).
Footnotes
Accession codes
Gene Expression Omnibus: microarray data have been deposited under the accession code GSE75218.
Author contributions
J.W. designed and performed cellular, molecular, and biochemical experiments and wrote the manuscript; L.L. performed immunoblot analysis; K.Y. performed Seahorse assays and helped early development of the project; C.G. performed imaging assays; S.S., Z.C. and C.W. performed the TSDR methylation analysis; P.V. performed histopathology analysis; G.N. performed bioinformatic analysis; D.R.G. contributed genetic models; and H.C. designed experiments, wrote the manuscript, and provided overall direction.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liston A, Gray DHD. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 3.Smigiel KS, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisson S, et al. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvey A, et al. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014;15:580–587. doi: 10.1038/ni.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubtsov YP, et al. Stability of the Regulatory T Cell Lineage in Vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou XY, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey-Bucktrout SL, et al. Self-antigen-Driven Activation Induces Instability of Regulatory T Cells during an Inflammatory Autoimmune Response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komatsu N, et al. Pathogenic conversion of Foxp3(+) T cells into T(H)17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji M, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 11.Pierson W, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Overholtzer M, Thompson CB. Autophagy in cellular metabolism and cancer. J Clin Invest. 2015;125:47–54. doi: 10.1172/JCI73942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell. 2014;159:1263–1276. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, et al. Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nat Immunol. 2014;15:1152–1161. doi: 10.1038/ni.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard VM, et al. Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol. 2010;185:7349–7357. doi: 10.4049/jimmunol.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, et al. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. 2006;177:5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- 19.Pengo N, et al. Plasma cells require autophagy for sustainable immunoglobulin production. Nat Immunol. 2013;14:298–305. doi: 10.1038/ni.2524. [DOI] [PubMed] [Google Scholar]
- 20.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell. 2014;158:734–748. doi: 10.1016/j.cell.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Y, et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158:749–763. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang K, et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity. 2013;39:1043–1056. doi: 10.1016/j.immuni.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roychoudhuri R, et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature. 2013;498:506–510. doi: 10.1038/nature12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang W, et al. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 30.Gerriets VA, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polansky JK, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 34.Finlay DK, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delmore JE, et al. BET Bromodomain Inhibition as a Therapeutic Strategy to Target c-Myc. Cell. 2011;146:903–916. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandukwala HS, et al. Selective inhibition of CD4+ T-cell cytokine production and autoimmunity by BET protein and c-Myc inhibitors. Proc Natl Acad Sci USA. 2012;109:14532–14537. doi: 10.1073/pnas.1212264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng H, et al. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park Y, et al. TSC1 regulates the balance between effector and regulatory T cells. J Clin Invest. 2013;123:5165–5178. doi: 10.1172/JCI69751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul S, Kashyap AK, Jia W, He YW, Schaefer BC. Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-kappaB. Immunity. 2012;36:947–958. doi: 10.1016/j.immuni.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shrestha S, et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huynh A, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Rosa V, et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat Immunol. 2015;16:1174–1184. doi: 10.1038/ni.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuma A, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 47.Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 48.Rubtsov YP, et al. Reaulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, et al. Tuberous sclerosis 1 (Tsc1)-dependent metabolic checkpoint controls development of dendritic cells. Proc Natl Acad Sci USA. 2013;110:E4894–4903. doi: 10.1073/pnas.1308905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.