Abstract
Background
Drugs acting on μ-opioid receptors (MOR) are widely used as analgesics, but present side-effects including life-threatening respiratory depression. MOR are G-protein-coupled receptors inhibiting neuronal activity through calcium channels, adenylyl cyclase, and/or G-protein–gated inwardly-rectifying potassium (GIRK) channels. The pathways underlying MOR-dependent inhibition of rhythmic breathing are unknown.
Methods
Using a combination of genetic, pharmacological and physiological tools in rodents in vivo, we aimed to identify the role of GIRK channels in MOR-mediated inhibition of respiratory circuits.
Results
GIRK channels were expressed in the ventrolateral medulla, a neuronal population regulating rhythmic breathing, and GIRK channel activation with flupirtine reduced respiratory rate in rats (percentage of baseline rate in mean±SD: 79.4±7.4%, n=7), wild-type mice (82.6±3.8%, n=3), but not in mice lacking the GIRK2 subunit, an integral subunit of neuronal GIRK channels (GIRK2−/−, 101.0±1.9%, n=3). Application of the MOR agonist DAMGO to the ventrolateral medulla depressed respiratory rate, an effect partially reversed by the GIRK channel blocker Tertiapin Q (baseline: 42.1±7.4 breath/min, DAMGO: 26.1±13.4 breath/min, TertiapinQ+DAMGO: 33.9±9.8 breath/min, n=4). Importantly, DAMGO applied to the ventrolateral medulla failed to reduce rhythmic breathing in GIRK2−/− mice (percentage of baseline rate: 103.2±12.1%, n=4), whereas it considerably reduced rate in wild-type mice (62.5±17.7% of baseline, n=4). Respiratory rate depression by systemic injection of the opioid analgesic fentanyl was markedly reduced in GIRK2−/− (percentage of baseline: 12.8±15.8%, n=5) compared to wild-type mice (72.9±27.3%).
Conclusion
Overall, these results identify that GIRK channels contribute to respiratory inhibition by MOR, an essential step toward understanding respiratory depression by opioids.
Introduction
Drugs acting on μ-opioid receptors (MOR) are widely used in pain management or as drug of abuse, but present unwanted side-effects including addiction and life-threatening respiratory depression1. Respiratory depression and related overdoses kill over 15,000 prescription opioid users each year in the United States2 and is therefore a major health issue3-5. Respiratory depression is characterized by hypoventilation with reduction of respiratory rate and airflow, and fatal apnea can occur with opioid overdose5. Depression in respiratory rate is mediated by the action of MOR drugs on discrete groups of brainstem neurons6-9. For instance, the preBötzinger Complex (preBötC), a cluster of cells essential for the generation of rhythmic breathing in mammals10,11, is highly sensitive to opioids and mediates an important component of respiratory depression by opioids6,12,13. MOR are G-protein-coupled receptors modulating neuronal activity by interacting with adenylyl cyclase, calcium channels and/or GIRK channels14. Inhibition of adenylyl cyclase may play a substantial role in MOR-dependent inhibition15, but there is conflicting evidence also suggesting that potassium channels may be involved in preBötC neuronal inhibition by MOR13.
Many neurotransmitters, therapeutic agents and drugs of abuse activate G-protein-coupled receptors, modulate neuronal excitability and synaptic transmission, at least in part, by opening or closing G-protein–gated inwardly-rectifying potassium (GIRK or Kir3) channels16 (Fig. 1a). MOR can exert their effects through coupling to GIRK channels17,18. The physiological activation of GIRK channels can therefore shape the behaviour of various neural circuits regulating functions such as nociception16,19 or addiction20. In the brainstem respiratory network, however, GIRK channels have not been described and their contribution to MOR-induced respiratory depression is unknown.
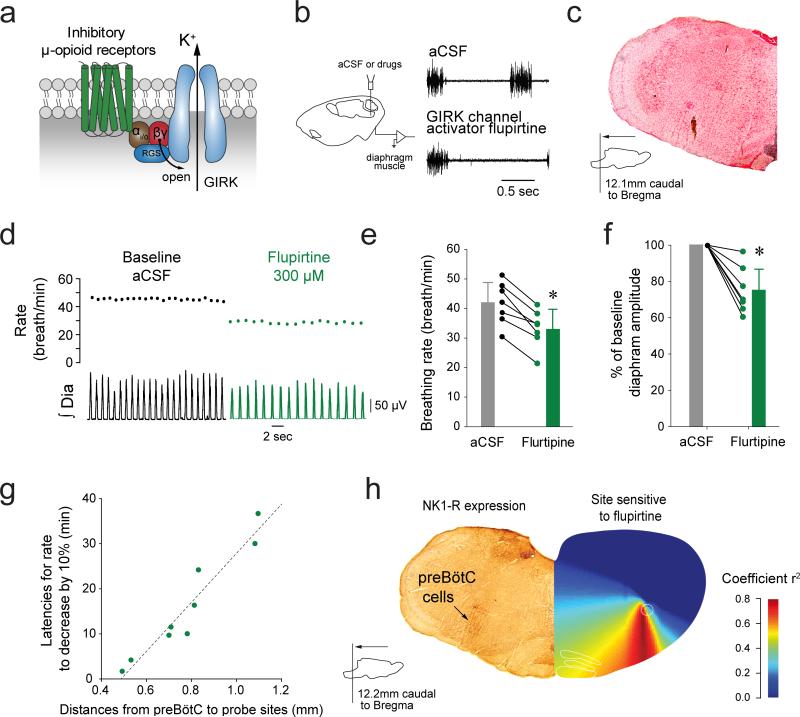
Figure 1. GIRK channels in the brainstem respiratory network modulate breathing in vivo.
(a) Schematic representation of the putative signaling pathways mediating MOR inhibition. Activation of GIRK channels by microperfusion of flupirtine (300 μM) into the preBötC reduced rhythmic breathing in anesthetized rats (b-e, n=7, P<0.001) and also significantly affected diaphragm amplitude (f, n=7, P=0.002). The latency for rate to decrease by 10% following flupirtine was correlated to the distance from preBötC to the probe sites (g, n=9, R=0.957, P<0.001). (h) The site of action of flupirtine identified by correlation maps12,22 overlapped the region where preBötC neurons expressed NK1-Rs (data representative of n=2 rats). Values are presented as mean ± SD. Data points with distances from perfusion sites to preBötC greater than 1 mm were not used in e because drugs have little effect on rhythmic breathing beyond this distance. * indicate significantly different mean values using Holm-Sidak post-hoc tests with P<0.05. AC, adenylyl cyclase, RGS, regulator of G-protein signalling, cAMP, cyclic AMP. aCSF, artificial cerebro-spinal fluid. NK-1R, neurokinin-1 receptors. GIRK, G-protein-gated inwardly rectifying potassium channels. Dia, Diaphragm muscle activity. preBötC, preBötzinger Complex.
Here, we hypothesized that GIRK channels contribute to inhibition of rhythmic breathing by MOR and opioid analgesics. We first identified that GIRK channels modulated rhythmic breathing and the inhibitory action of MOR in vivo. We showed that GIRK channels were expressed in the ventrolateral medulla, and that they constitute a signaling pathway for the inhibition of rhythmic breathing by MOR. By identifying the mechanisms of opioid-induced respiratory depression operating at the central circuits where breathing is generated, this new knowledge may lead to the development of new strategies to prevent this depression and reactivate breathing when it is depressed or failing in response to opioid drugs.
Methods
Experimental animals
All procedures were performed in accordance with the recommendations of the Canadian Council on Animal Care, and were approved by the University of Toronto Animal Care Committee. Twenty-two adult male Wistar rats (250-350g, Charles River, Saint-Constant, Quebec, Canada) were used for respiratory recordings and immunohistochemistry. Twenty-one wild-type mice and nineteen mice lacking the GIRK2 subunit (GIRK2−/−) were used for physiological recordings and immunohistochemistry (body weight: 40-50g). We used both males and females. The GIRK2 subunit is an integral subunit of GIRK channels and its absence eliminates a vast majority of functional GIRK channels in the brain21. The generation of GIRK2−/− mice was described previously21. Mice and rats were housed with free access to food and water under a 12-hour light 12-hour dark cycle (lights on at 7am).
Immunohistochemistry
Adult wild-type and GIRK2−/− mice were given a euthanizing dose of ketamine/xylazine (100 mg/kg and 10 mg/kg, respectively) and perfused transcardially with Ca2+-free Tyrode's solution followed by 4% paraformaldehyde containing 14% picric acid. Brains were post-fixed overnight in Sorenson's Buffer containing 10% sucrose at 4°C. Tissue was sectioned coronally at 12 μm by cryostat and mounted to slides. Sections containing the preBötzinger Complex were washed in Tris-buffered saline (TBS) for 10 min then placed in boiling citric acid (10mM; 30 min). Slides were then washed in TBS (3 × 5 min), incubated for 1 hr in TBS blocking solution containing casein, Tween20 and Triton X-100 (all 0.2%). Sections were exposed to rabbit anti-GIRK2 (1:1000; Alomone labs, Jerusalem, Israel) diluted in TBS solution containing casein and Tween20 (both 0.2%) overnight at RT in a humidity chamber. The following day, sections were washed in TBS (3 × 20 min) and then incubated for 2 hrs in donkey anti-rabbit IgG Cy3 (1:500; Jackson Immunoresearch, West Grove, Pennsylvania, USA) secondary antibody diluted in TBS containing casein and Tween20 (both 0.2%). Sections were washed in TBS (3 × 20 min), then dehydrated through a series of graded alcohols, cleared in xylene, and cover-slipped with DPX (Fisher Scientific, Waltham, Massachusetts, USA). Fluorescent immunoreactivity was visualized at 10X with an Olympus IX81 confocal microscope and imaged with MetaMorph Advanced software (Molecular Devices Inc., Sunnyvale, California, USA). Digital images were colorized and only brightness enhanced in ImageJ 64 (National Institute of Health). Exposure time, brightness and contrast intensity were identical across genotypes. Images were prepared for photographic presentation in Adobe Illustrator 6 and Photoshop 6 (Adobe Systems Inc., San Jose, California, USA). Immunohistochemistry for neurokinin-1 receptors (NK-1R) was performed in two adult rats as previously described12.
Microperfusion and recordings in anesthetized adult rodents
In anesthetized adult male rats, we used reverse-microdialysis to unilaterally microperfuse selected agents into the preBötC. The experimental procedures were as described previously12,22. Briefly, we recorded diaphragm muscle activity in isoflurane-anesthetized (2–2.5%), tracheotomised and spontaneously breathing (50% oxygen gas mixture, balance nitrogen) adult rats (average body weight: 305g). Diaphragm muscle activity was recorded using stainless steel bipolar electrodes positioned and sutured on the right side of the crural diaphragm. Genioglossus muscle activity was monitored during experiment. Electromyography signals were amplified (CWE Inc, Ardmore, Pennsylvania, USA), band-pass filtered (100-1000Hz), integrated and digitized at a sampling rate of 1000 Hz using CED acquisition system and Spike v6 software (Cambridge Electronic Design Limited, Cambridge, England). Rats were kept warm with a heating pad during all experiments. Using a dorsal approach, a microdialysis probe (CX-I-12-01) of 200 μm diameter, length of diffusing membrane 1 mm (Eicom, Kyoto, Japan) was inserted into the preBötC using a stereotaxic frame and micromanipulator (ASI Instruments, Warren, Michigan, USA). The probe was placed 12.2 mm posterior, 2 mm lateral, and 10.5 mm ventral to bregma. To accurately target the preBötC, we used several criteria to better position and to confirm the probe location as described previously12,22. (i) When the probe was inserted in the brain, genioglossus muscle activity showed a reduction of about 30% as it reached the vicinity of the preBötC. (ii) Post-mortem histology was used to confirm the probe location in the preBötC using standard anatomical markers such as the nucleus ambiguus, the caudal part of the facial nucleus, and the inferior olive, and immunohistochemistry of NK-1R. (iii) We created correlation maps associating the latency for breathing to respond to drug perfusion and the distances from the preBötC to probe locations, therefore identifying the region of the medulla highly sensitive to the drug perfused. We used these three anatomical and functional criteria and experience from our previous studies, to confirm that the probes were positioned in the region of the preBötC. On rare occasions (1/20 experiments), the probes damaged the preBötC and respiratory rhythm was irregular and unstable. In such an event, we did not continue the experiment. We perfused the probe with artificial cerebrospinal fluid (aCSF) and pH was adjusted to 7.4 by bubbling carbon dioxide in aCSF. Baseline levels of the physiological variables were recorded for at least 30 min while perfusing aCSF into the preBötC. Following this control period, the μ-opioid receptor agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO, 5 μM) or the GIRK channel activator flupirtine (300 μM) were added to the aCSF perfusing the preBötC. The responses to DAMGO or flupirtine were recorded for the next 30 min. For the DAMGO experiments in rats, a solution of DAMGO and the potassium blocker barium chloride, or GIRK channel blocker Tertiapin Q (TQ), were added to the solution for another 30 min. All drugs were obtained from Tocris (Minneapolis, Minnesota, USA).
In anesthetized (isoflurane, 1.5-2%), spontaneously breathing (50% oxygen, balance nitrogen) adult mice, we used reverse microdialysis to perfuse agents into the preBötC of wild-type and GIRK2−/− animals, while recording diaphragm muscle activity using a similar approach to the rats. The mice were also kept warm with a heating pad. We inserted the microdialysis probe into the brainstem 6.7 mm posterior, 1.2 mm lateral, and 5.7 mm ventral to bregma. For wild-type or GIRK2−/− mice, baseline levels were recorded for at least 30 min followed by DAMGO (5 μM), the GABAA receptor agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol THIP (50 μM), or flupirtine (300 μM) for at least 30 min. The anatomical and functional criteria defined in rats were also used for experiments in mice. In addition, we used he GABAA receptor agonist 4,5,6,7-Tetrahydroisoxazolo[5,4-c]pyridin-3-ol hydrochloride (THIP) in experiments where DAMGO was not expected to reduce respiratory rate (in GIRK2−/− mice) to ensure that drugs were functional modulating respiratory rhythm. Because of the random production of GIRK2−/− knockout animals, we did not randomize wild-type and GIRK2−/− mice. However, as standard practice in the lab, we alternated recordings from wild-type and knockout mice to avoid order effects or experimental conditions that may affect one group temporarily. To avoid experimenter bias, similar standard procedures, timelines for dosing, and automated analyses were used for both animal groups.
We also performed a separate set of experiments with systemic injection of the opioid analgesic fentanyl while recording diaphragm activity in wild-type and GIRK2−/− mice. Data in mice were normalized as percentage of baseline respiratory rate and diaphragm amplitude to remove potential variability between mice.
Anatomy and correlation maps
The locations of the intervention (perfusion) sites and correlation maps were performed as previously described22 using MATLAB 12 software (Mathworks, Natick, Massachusetts, USA).
Statistical Analysis
All data are presented as mean ± standard deviation (SD) with n values also indicated. For studies in rats, we used 1-way ANOVAs with the repeated factor being treatments with aCSF or drugs. For studies in mice, we tested for group differences using 2-way ANOVA tests, with a factor being genotype (i.e. wild-type or GIRK2−/−) and the repeated factor being drug treatments (aCSF or drugs of choice). If the ANOVA was statistically significant, Holm-Sidak post-hoc tests were then used to determine significant differences between conditions. No formal a priori power calculation was conducted. Sample sizes were based on previous studies. In response to reviewer concerns, sample sizes were increased in the experiments combining DAMGO, Tertiapin Q, and BaCl2. No attempts were made to adjust the p-values for this additional analysis. Data points of each animal were presented to appreciate data distribution. Where appropriate, all hypothesis tests are two-tailed with the level of significance set at P<0.05. All tests were performed with SigmaPlot version 11 (Systat Software Inc, San Jose, California, USA).
Results
We first investigated the role of GIRK channels in mediating rhythmic breathing generated by the preBötC in vivo. When flupirtine, a GIRK channel opener23, was applied to the preBötC (Fig. 1b), the frequency of diaphragm rhythmic activity was reduced (Fig. 1c, d). Flupirtine (300 μM) reduced respiratory rate by 22.2% (aCSF: 41.7±7.2 breath/min, flupirtine: 33.2±6.4 breath/min, n=7, Fig. 1e) and diaphragm amplitude (Fig. 1f, n=7, P=0.002). We then plotted the relationship between the latency for rate to decrease by 10% following flupirtine and the distance from preBötC to the probe sites (Fig. 1g). There was a significant positive correlation between latencies and distances, suggesting that it took less time for flupirtine to diffuse through tissue and depress respiratory rate when it was perfused close to the preBötC. Using this relationship, we identified highly responsive regions12,22 and created correlation maps (Fig. 1h). Using these maps, we localized the region of the medulla where flupirtine was most effective in suppressing respiratory rate, and this site corresponded to NK1-R-expressing preBötC neurons (Fig.1h), which are known to be markers of preBötC neurons24.
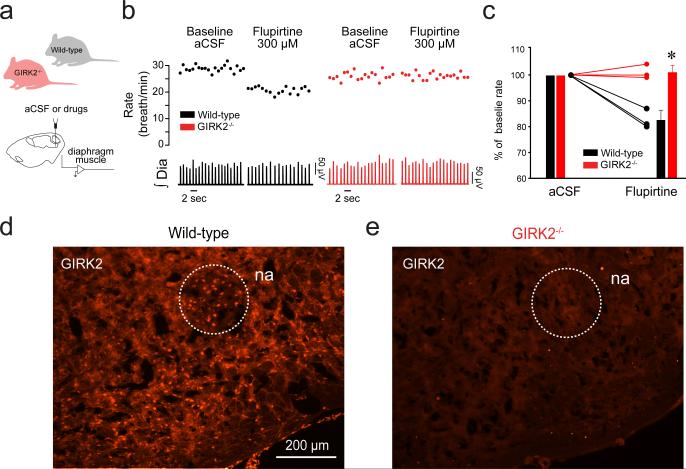
Flupirtine also indirectly antagonizes NMDA receptors23, activates GABAA receptors25 and activates other inward rectifier potassium channels25, which can modulate preBötC activity26. To determine whether flupirtine depresses rhythmic breathing by selectively acting on GIRK channels, we applied it to the preBötC (Fig. 2a) of GIRK2−/− adult mice21. In baseline conditions (aCSF), respiratory rates were not significantly different between wild-type and GIRK2−/− groups (wild-type: 22.9±2.3 breath/min, GIRK2−/: 20.9±1.7 breath/min, P=0.395). In wild-type mice, flupirtine (300 μM) significantly decreased respiratory rate (percentage of baseline: 82.6±2.7%, n=3, Fig. 2b, c), without affecting the amplitude of the diaphragm activation, whereas in GIRK2−/− mice the same concentration of flupirtine had no effect on respiratory rate (percentage of baseline: 101.0±1.9%, n=3). This result identifies that flupirtine activates GIRK channels to inhibit rhythmic breathing. We then identified the presence of GIRK2 subunits in the preBötC of wild-type (Fig. 2d) mice, but not in GIRK2−/− mice (Fig. 2e). In summary, these data demonstrate that GIRK channels are expressed in the region of the preBötC, and that activation of GIRK channels has the capacity to inhibit respiratory rate.
Figure 2. GIRK channels modulate breathing in vivo and are expressed in the ventrolateral medulla.
Activation of GIRK channels by flupirtine (300 μM) at the preBötC (a) decreased rhythmic breathing in wild-type (black), but not in GIRK2−/− mice (red, b, c, 2-way ANOVA, F(0,1)=46.64, P=0.002, n=3 for each group). Immunolabelling for GIRK2 subunits (d) in wild-type and GIRK2−/− mice (e) showing no GIRK2 subunits in GIRK2−/− mice. Representative of 4 wild-type and 3 GIRK2−/− mice. Data are presented as mean ± SD and as individual data. Data were normalized as percent of aCSF value to better represent responses to flupirtine in wild-type and GIRK2−/− mice. * indicate significant Holm-Sidak post-hoc tests for different mean values with P<0.05 between wild-type and GIRK2−/− mice in flupirtine condition. na, nucleus ambiguus. aCSF, artificial cerebro-spinal fluid. GIRK, G-protein-gated inwardly rectifying potassium channels. Dia, Diaphragm muscle activity. preBötC, preBötzinger Complex.
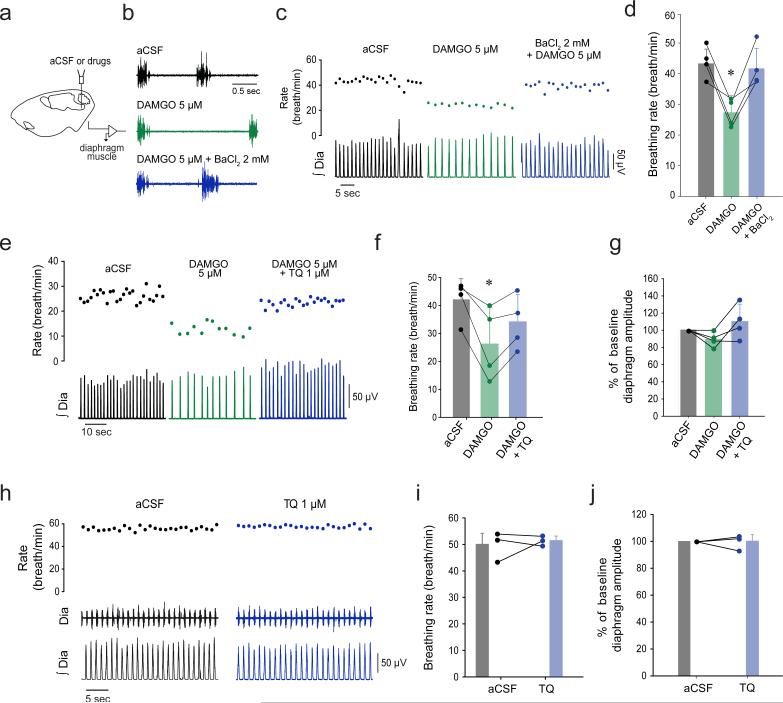
MORs inhibit rhythmic breathing when applied to the preBötC in various experimental conditions7,12. To test the hypothesis that GIRK channels play a significant role in the MOR-induced inhibition of rhythmic breathing, we focally applied potassium channel blockers to the preBötC of adult rats. Application of DAMGO (5 μM) to the preBötC region (Fig. 3a) of anesthetized adult rats significantly decreased respiratory rate (aCSF: 44.0±5.1 breath/min, DAMGO: 27.7±4.5 breath/min, n=4, Fig. 3b-d)12, and this reduction was reversed by co-application of the broad spectrum potassium blocker barium chloride (Ba+DAMGO: 42.3±6.6 breath/min, Fig. 3b-d). The GIRK channel blocker TQ 1 μM partially reversed DAMGO-related inhibition (aCSF: 42.1±7.4 breath/min, DAMGO: 26.1±13.4 breath/min, TQ+DAMGO: 33.9±9.8 breath/min, n=4, Fig. 3e-g). To determine whether blocking GIRK channels increases respiratory rate rather that blocking MOR activation, we applied TQ alone at the preBötC. TQ 1 μM did not significantly change respiratory rate or diaphragm amplitude (Fig. 3h-j), suggesting that at this concentration TQ blocks MOR inhibition (n=3). Additional power analysis showed that 30 animals would be needed to detect a difference of 5% between aCSF and TQ conditions (power: 80%). Overall, these data show that pharmacological blockade of GIRK channels antagonizes MOR-mediated inhibition of respiratory rate in vivo, with minimal and no physiologically relevant effects on diaphragm amplitude.
Figure 3. Blocking potassium or GIRK channels reverses respiratory rate inhibition by MORs.
In adult anesthetized rats, application of the potassium channel blocker barium chloride (2 mM) to the preBötC (a) reversed inhibition of respiratory rate by the MOR agonist DAMGO (b-d, F(2,1) = 18.14, P=0.003, n=4). Additional post-hoc analysis showed that the DAMGO and DAMGO+BaCl2 conditions were significantly different (P=0.005). DAMGO applied to the preBötC decreased rhythmic breathing, and this depression was partially reversed by the specific GIRK channel blocker Tertiapin Q (e-f, 1 μM, F(2,1) = 11.58, P=0.009, n=4). Additional post-hoc analysis showed that the DAMGO and DAMGO+TQ conditions were significantly different (P=0.048). No effect was observed on diaphragm muscle amplitude (g, P=0.125). Blocking alone GIRK channels with Tertiapin Q at the preBötC did not significantly change respiratory rate (h-j) (P=0.680, n=3) or diaphragm amplitude (P=0.951, n=3). BaCl2, barium chloride. TQ, Tertiapin Q. aCSF, artificial cerebro-spinal fluid. GIRK, G-protein-gated inwardly rectifying potassium channels. Dia, Diaphragm muscle activity. preBötC, preBötzinger Complex. Values are presented as means ± SD and as individual data. * indicate means significantly different from aCSF with Holm-Sidak post-hoc test with P<0.05.
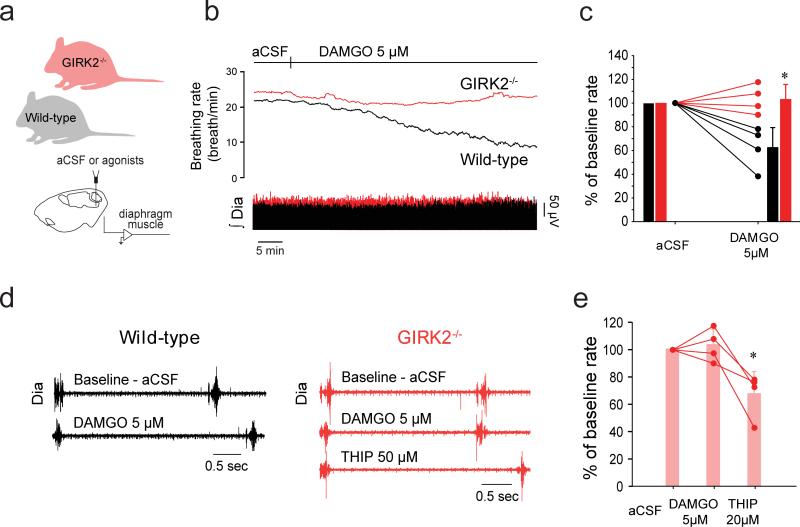
To avoid the limitations of pharmacological blockade of GIRK channels with TQ, we applied the MOR agonist DAMGO to the preBötC of wild-type and GIRK2−/− adult mice while recording rhythmic diaphragm activity (Fig. 4a). In baseline conditions (aCSF), respiratory rates were not significantly different between wild-type and GIRK2−/− groups (wild-type: 24.8±3.8 breath/min, GIRK2−/: 31.8±5.0 breath/min, P=0.084). Although DAMGO (5 μM) induced a significant reduction of respiratory rate in wild-type mice (percentage of aCSF mean: 62.5±17.7%), it had no effect in GIRK2−/− mice (percentage of aCSF: 103.2±12.1%, n=4, Fig. 4b, c). To address the possibility that the absence of a respiratory-inhibitory effect of DAMGO in GIRK2−/− mice was due to preBötC neurons being insensitive to modulation of neurotransmitters per se, we administered the GABAA receptor agonist THIP which is known to depress respiratory rate by inhibiting respiratory rhythm generating preBötC cells13. THIP (20 μM) significantly reduced respiratory rate in GIRK2−/− mice (67.4±16.6%, n=4, Fig. 4d, e), in accordance with effects previously identified in vitro13. These data suggest that ionotropic GABAA receptor function was not affected by the absence of GIRK channels, and that MOR inhibition of rhythmic breathing was absent when GIRK channels were not present in rodents in vivo.
Figure 4. GIRK channels contribute to inhibition by MOR.
(a) In anesthetized mice, DAMGO (5 μM) applied into the preBötC substantially slowed rhythmic breathing (b) in wild-type (black), but not in GIRK2−/− animals (c, repeated-measures 2-way ANOVA, F(0,3)=14.43, P=0.009 with Holm-Sidak post-hoc test, n=4 for each group). (d, e) GIRK2−/− mice did not respond to microperfusion of DAMGO into the preBötC, but responded to the GABAA receptor agonist THIP (20 μM, 1-way ANOVA, F(2,1)=12.66, P=0.007, n=4). aCSF, artificial cerebrospinal fluid. GIRK, G-protein-gated inwardly rectifying potassium channels. Dia, Diaphragm muscle activity. preBötC, preBötzinger Complex. Values are presented as means ± SD (bars) and as individual values. * indicate significantly different mean values with Holm-Sidak post-hoc tests with P<0.05.
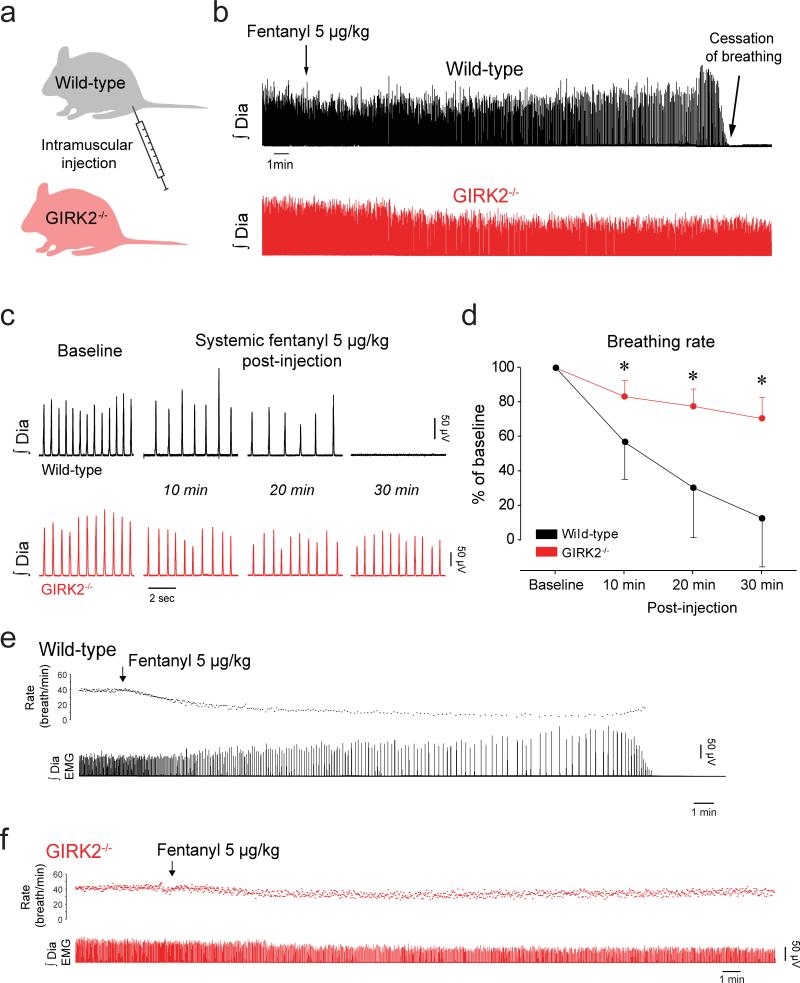
Drugs acting on MORs are widely used as analgesics or as drugs of abuse, but can induce life-threatening respiratory depression. To establish whether GIRK channels contribute to respiratory depression following systemic administration of the opioid analgesic fentanyl, an opioid used routinely in clinical practice, we performed additional studies in anesthetised GIRK2−/− mice. Under baseline conditions, there were no differences in respiratory rate between wild-type and GIRK2−/− mice (P=0.67). In wild-type mice, a single intra-muscular injection of fentanyl (5 μg/kg, Fig. 5a) elicited a potent depression of respiratory rate (Fig. 5b, c), whereas an identical intervention in GIRK2−/− mice only moderately decreased respiratory rate. Although all wild-type mice showed a substantial respiratory depression in response to fentanyl, this dose of fentanyl was enough to abolish breathing in 4/6 wild-type mice evaluated after 30 min (Fig. 5d). Importantly, such cessations of breathing were not observed in GIRK2−/− mice (Fig. 5e, f). These persistent apneas in wild-type mice were fatal unless there was intervention with mechanical ventilation. These experiments were performed in spontaneously breathing animals where carbon dioxide level was not controlled. It is likely that partial pressure of arterial carbon dioxide increased with respiratory rate depression in wild-type mice which would explain the increase in diaphragm muscle activity observed before breathing ceased in these animals (Fig. 5b, e).
Figure 5. GIRK channels contribute to respiratory depression induced by a systemically-applied opioid drug.
Intramuscular administration of the MOR analgesic fentanyl at a dose of 5μg/kg (a) caused a substantial reduction in respiratory rate in wild-type mice (n=6, b) whereas it produced only a mild respiratory depression in GIRK2−/− mice (n=5, c, d, repeated-measures 2-way ANOVA, F(2,2)=5.6, P=0.006). Representative tracings showing diaphragm activities in a wild-type (e) and a GIRK2−/− mice (f). GIRK, G-protein-gated inwardly rectifying potassium channels. Dia, Diaphragm muscle activity. preBötC, preBötzinger Complex. * indicate Holm-Sidak post-hoc tests with significantly different mean values with P<0.05. Values are presented as means ± SD.
Discussion
Using a combination of genetic, molecular, and physiological approaches, these data collectively identify that GIRK channels modulate rhythmic breathing, contribute to respiratory rate inhibition by MOR, and contribute to respiratory depression by the MOR drug fentanyl. Here we show that GIRK2 subunits are expressed in the preBötC region, a population of cells essential for generating rhythmic breathing in mammals11,13. The presence of inhibitory GIRK channels suggests that GIRK channels regulate preBötC neuronal excitability and contribute to MOR modulation. These data identify that activation of GIRK channels inhibits rhythmic breathing, and that the region most sensitive to GIRK channel activation corresponds to high expression of NK1-R which identifies the preBötC region. This result is consistent with the high sensitivity of NK1-R-expressing neurons to MOR agonists12. Importantly, we found that GIRK channels contribute to the inhibition of a population of neurons in the preBötC region by MOR agonists. Using GIRK2 subunit knockout mice, we showed that GIRK channels contribute to a substantial part of the respiratory rate depression following systemic administration of the MOR analgesic fentanyl. Overall, our results identify for the first time that GIRK channels modulate rhythmic breathing and contribute to respiratory rate depression by opioid drugs.
Here, we first determined that activation of GIRK channels by flupirtine decreased respiratory rate in rats. Flupirtine perfused in the preBötC region significantly depressed respiratory rate in anesthetized rats. Using correlation maps12, we identified the region of the medulla most sensitive to flupirtine and found that this region corresponds to high expression of NK-1R. Although correlation maps cannot exclude that flupirtine may diffuse beyond the preBötC and may activate GIRK channels in other populations of neurons, it indicated that there was a region of the brainstem with a higher sensitivity to flupirtine that also expresses NK-1R. Flupirtine also indirectly antagonizes NMDA receptors23, and activates GABAA receptors25 and other inward rectifier potassium channels25, which may explain why it decreased diaphragm muscle amplitude. To determine whether the effects of flupirtine were due to its action on GIRK channels only, we used mice lacking functional GIRK channels. GIRK1, GIRK2 and GIRK3 subunits form tetrameric channels that are postsynaptic effectors for G-protein-coupled receptor signalling16, with the GIRK2 subunit present in 3 out of 4 possible GIRK channel conformations. The absence of GIRK2 subunits therefore eliminates a vast majority of functional GIRK channels in the brain21. Accordingly, we used GIRK2 subunit knockout mice to identify whether flupirtine acts on GIRK channels. We showed that flupirtine at the concentration used in this study depressed respiratory rate in wild-type but not in GIRK2−/− mice. These results suggest that, in our study, flupirtine acts on GIRK channels and that GIRK channels in the region of the preBötC have the capacity to modulate rhythmic breathing. Because GIRK channels are widely expressed in the brain27 and in the vicinity of the preBötC (Fig. 2), it cannot be excluded that flupirtine diffused beyond the preBötC and activated GIRK channels in other parts of the respiratory network.
GIRK channels mediate MOR inhibition of various neural circuits16 and are involved in the effects of MOR drugs on functions such as addiction16,17 and nociception19. Here, the contribution of GIRK channels to MOR inhibition in the respiratory network is identified using pharmacological blockade and mice lacking GIRK2 subunits. Without GIRK2-containing channels, MOR activation in the preBötC region failed to decrease rhythmic breathing, and respiratory depression by systemic MOR drugs was significantly reduced, suggesting that GIRK channels play a crucial role in MOR inhibition of the respiratory network. The mechanisms underlying MOR inhibition of the respiratory network have been investigated15. It has been proposed that MORs inhibit neuronal activity by interacting with adenylyl cyclase15, but there is conflicting evidence also suggesting that potassium channels may be involved in MOR neuronal inhibition of the preBötC13. The adenylyl cyclase and MOR/GIRK pathways are tightly associated since both pathways involve Gαi/o. It is therefore not surprising that modulation of the adenylyl cyclase / cAMP / protein kinase A pathway has the capacity to increase respiratory rate and reverse the MOR-induced suppression of respiratory rate28. Those previous data, however, do not exclude the essential role of GIRK channels that involve a parallel, and partially overlapping, pathway to inhibit preBötC neurons. Our study clearly identifies GIRK channels as critical components mediating respiratory inhibition by MOR receptors.
MOR drugs are widely used as analgesics, although they can present the severe and sometimes fatal side-effect of respiratory depression. Despite the broad action of the MOR analgesic fentanyl on the entire central nervous system and the periphery, we found that the respiratory depression by fentanyl was significantly and markedly reduced in the absence of functional GIRK channels, and importantly no respiratory arrest was observed in GIRK2−/− mice. There was nonetheless a moderate decrease in breathing after fentanyl in GIRK2−/− mice probably due to the action of opioid drugs on other components of the respiratory network that do not involve GIRK channels such as the adenylyl cyclase pathway15 or pre-synaptic inhibition of voltage-dependent calcium channels29. Activation of GIRK channels that do not contain the GIRK2 subunit, i.e. GIRK1/3 channels may also be underlying the moderate depression by fentanyl observed in GIRK2−/− mice. This depression was not due to degradation of breathing over time due to anesthesia since the effects of systemic fentanyl disappeared after about an hour and respiratory rate returned to its baseline value. Overall, these results showed that GIRK channels containing the GIRK2 subunit underlie a major component of respiratory rate depression by MOR analgesics. Since we did not measure GIRK currents in the medulla, it cannot be excluded that fentanyl may modulate other neurotransmitters that also work through GIRK channels.
The development of new therapies to reverse respiratory depression by opioids has been of great interest over the last 10 years1,15,30-32. However, the mechanism of action of opioids on respiratory circuits was not known until we identified GIRK channels as an important mechanism contributing to MOR-mediated respiratory rate inhibition. Importantly, our data suggest that pharmacological strategies targeting the GIRK pathway may lead to new therapies to reverse or prevent respiratory depression induced by MOR drugs, although the development of new drugs acting on GIRK channels has been hindered by the wide distribution of GIRK channels and their contribution to cardiac and endocrine functions16. Since channel function and trafficking are highly dependent on the channel subunit composition, selectively blocking GIRK channels with specific subunit compositions33 may lead to new strategies to prevent respiratory depression19. The development of subunit-selective GIRK modulators34 or targeting the other components of the GIRK channel pathway such as regulators of G-protein signalling35 or βγ G-protein dimers36 (Fig. 1a) that are specific to respiratory inhibition by MORs, but not analgesia, may be a viable strategy to prevent respiratory depression by MOR drugs.
Acknowledgements
We would like to thank Laura Vechio, MSc, Associate Professor Ali Salahpour, PhD (Department of Pharmacology, University of Toronto), and Professor Beverly Orser, MD, PhD for technical support with genotyping (Department of Physiology, University of Toronto). G.M. was supported by a Parker B. Francis Fellowship. R.L.H was supported by a Tier 1 Canada Research Chair. N.C.V. was supported by a National Institute on Drug Abuse Training Grant (T32 DA07234). This research was supported by the Canadian Institutes for Health Research (separate grants to R.L.H. and J.J.G.), the National Sanitarium Association Innovative Research Program (R.L.H.) and the National Institutes for Health (MH061933 and DA034696; K.W.).
Footnotes
Authors’ contributions
GM, RLH; conception, design, analysis, interpretation, drafting manuscript. JR, NCV, HL, KW, JJG; analysis, interpretation, and drafting.
Conflicts of interest disclosure
The authors declare no competing interests.
References
- 1.Dahan A, Aarts L, Smith TW. Incidence, Reversal, and Prevention of Opioid-induced Respiratory Depression. Anesthesiology. 2010;112:226–38. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid- overdose epidemic. N Engl J Med. 2014;370:2063–6. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- 3.Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, Crosby AE, Paulozzi LJ. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–20. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 4.Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146–55. doi: 10.1056/NEJMra1202561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahan A. Respiratory depression with opioids. J Pain Palliat. Care Pharmacother. 2007;21:63–66. [PubMed] [Google Scholar]
- 6.Montandon G, Horner R. CrossTalk proposal: The preBotzinger complex is essential for the respiratory depression following systemic administration of opioid analgesics. J Physiol. 2014;592:1159–62. doi: 10.1113/jphysiol.2013.261974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips RS, Cleary DR, Nalwalk JW, Arttamangkul S, Hough LB, Heinricher MM. Pain-facilitating medullary neurons contribute to opioid-induced respiratory depression. J Neurophysiol. 2012;108:2393–404. doi: 10.1152/jn.00563.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EA, Hopp FA, Dean C, Zuperku EJ. Pontine mu-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. J Neurophysiol. 2012;108:2430–41. doi: 10.1152/jn.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31:1292–301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu YJ, Arttamangkul S, Evans CJ, Williams JT, von Zastrow M. Neurokinin 1 receptors regulate morphine-induced endocytosis and desensitization of mu-opioid receptors in CNS neurons. J Neurosci. 2009;29:222–33. doi: 10.1523/JNEUROSCI.4315-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- 16.Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–15. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci. 2002;22:4328–34. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajic D, Koike M, Albsoul-Younes AM, Nakajima S, Nakajima Y. Two different inward rectifier K+ channels are effectors for transmitter-induced slow excitation in brain neurons. Proc Natl Acad Sci U S A. 2002;99:14494–9. doi: 10.1073/pnas.222379999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marker CL, Stoffel M, Wickman K. Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J Neurosci. 2004;24:2806–12. doi: 10.1523/JNEUROSCI.5251-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labouebe G, Lomazzi M, Cruz HG, Creton C, Lujan R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Luscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–68. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- 21.Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci U S A. 1997;94:923–7. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montandon G, Horner RL. State-dependent contribution of the hyperpolarization-activated Na+/K+ and persistent Na+ currents to respiratory rhythmogenesis in vivo. J Neurosci. 2013;33:8716–28. doi: 10.1523/JNEUROSCI.5066-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raffa RB, Pergolizzi JV., Jr The evolving understanding of the analgesic mechanism of action of flupirtine. J Clin Pharm Ther. 2012;37:4–6. doi: 10.1111/j.1365-2710.2010.01233.x. [DOI] [PubMed] [Google Scholar]
- 24.McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat. Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinger F, Geier P, Dorostkar MM, Chandaka GK, Yousuf A, Salzer I, Kubista H, Boehm S. Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine. Br J Pharmacol. 2012;166:1631–42. doi: 10.1111/j.1476-5381.2011.01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgado-Valle C, Feldman JL. NMDA receptors in preBotzinger complex neurons can drive respiratory rhythm independent of AMPA receptors. J Physiol. 2007;582:359–68. doi: 10.1113/jphysiol.2007.130617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saenz del Burgo L, Cortes R, Mengod G, Zarate J, Echevarria E, Salles J. Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. J Comp Neurol. 2008;510:581–606. doi: 10.1002/cne.21810. [DOI] [PubMed] [Google Scholar]
- 28.Ballanyi K, Lalley PM, Hoch B, Richter DW. cAMP-dependent reversal of opioid- and prostaglandin-mediated depression of the isolated respiratory network in newborn rats. J Physiol. 1997;504(Pt 1):127–34. doi: 10.1111/j.1469-7793.1997.127bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altier C, Zamponi GW. Signaling complexes of voltage-gated calcium channels and G protein- coupled receptors. J Recept Signal Transduct Res. 2008;28:71–81. doi: 10.1080/10799890801941947. [DOI] [PubMed] [Google Scholar]
- 30.Lalley PM. D1-dopamine receptor agonists prevent and reverse opiate depression of breathing but not antinociception in the cat. Am J Physiol Regul Integr Comp Physiol. 2005;289:R45–R51. doi: 10.1152/ajpregu.00868.2004. [DOI] [PubMed] [Google Scholar]
- 31.Roozekrans M, Olofsen E, van der Schrier R, van Gerven J, Peng S, McLeod J, Dahan A. Reversal of opioid-induced respiratory depression by BK-channel blocker GAL021: A pharmacokinetic pharmacodynamic modeling study in healthy volunteers. Clin Pharmacol Ther. 2015;97:641–649. doi: 10.1002/cpt.99. [DOI] [PubMed] [Google Scholar]
- 32.Ren J, Poon BY, Tang Y, Funk GD, Greer JJ. Ampakines alleviate respiratory depression in rats. Am J Respir Crit Care Med. 2006;174:1384–1391. doi: 10.1164/rccm.200606-778OC. [DOI] [PubMed] [Google Scholar]
- 33.Walsh KB. Targeting GIRK Channels for the Development of New Therapeutic Agents. Front Pharmacol. 2011;2:64. doi: 10.3389/fphar.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lujan R, Marron Fernandez de Velasco E, Aguado C, Wickman K. New insights into the therapeutic potential of Girk channels. Trends Neurosci. 2014;37:20–9. doi: 10.1016/j.tins.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou H, Chisari M, Raehal KM, Kaltenbronn KM, Bohn LM, Mennerick SJ, Blumer KJ. GIRK channel modulation by assembly with allosterically regulated RGS proteins. Proc Natl Acad Sci U S A. 2012;109:19977–82. doi: 10.1073/pnas.1214337109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei Q, Jones MB, Talley EM, Garrison JC, Bayliss DA. Molecular mechanisms mediating inhibition of G protein-coupled inwardly-rectifying K+ channels. Mol Cells. 2003;15:1–9. [PubMed] [Google Scholar]