Abstract
Background
HIV infection, depression, and cocaine use are independently associated with increased inflammatory signal production. There is increasing evidence about the role of inflammation in depression. In HIV disease, cocaine use may increase disease progression as well as alter T-cell functioning resulting in cytokine activation and thereby increasing susceptibility to depression.
Methods
We examined the association between cocaine use and depression among 447 African American persons infected with HIV who were frequent cocaine users or non-users, enrolled in an observational study in Baltimore, Maryland, between August 2003 and December 2012.
Results
The overall prevalence of depression was 40.9% (183 of 447) participants. Among persons who were depressed, the prevalence of cocaine use was 81.4% (149 of 183), compared to 69.3% among persons who were not depressed (183 of 264), P=0.004. Cocaine use was associated with nearly 2-fold increased odds of depression, unadjusted odds ratio (OR)=1.94, (95% CI: 1.23, 3.06); P=0.004, compared to never using cocaine, and OR=1.02, (95% CI: 1.10, 1.05); P=0.04 in adjusted analysis. A dose-response relationship between increasing duration of cocaine use and depression was observed.
Discussion
Frequency and duration of cocaine use may be associated with depression. We speculate that depression among cocaine users with HIV may involve an inflammatory component that needs further examination.
Keywords: HIV, Cocaine, Depression, Mental health
Introduction
Major depression is a common co-morbidity in persons with HIV and substance use disorders (1-3). This co-morbidity represents a multifactorial relationship. Whereas depression increases the risk of acquiring HIV and substance use disorders, both depression and substance use disorders are associated with increased progression and mortality in persons with HIV (3-7). Major depression, HIV infection, and cocaine use have all been independently associated with increased inflammation (8-12), and this convergence may result in increased medical comorbidity.
Depression is the most common psychiatric disorder associated with HIV/AIDS with a lifetime prevalence of 22-45% (13,14). Persons who are depressed are more likely to contract HIV through engaging in risky behavior than non-depressed individuals, including having sex for money or drugs, having sex when intoxicated by drugs or alcohol, having sex with intravenous drug users, and having a greater number of lifetime sexual partners (15).
Substance use and depression in persons infected with HIV are associated with increased likelihood of treatment non-adherence (16-21), which may result in antiviral drug resistance and poor HIV outcomes. In persons with comorbid HIV and depression, the use of stimulants such as cocaine may alter the biological pathways for both conditions (9,22-25).
Cocaine use is associated with increased T-cells susceptibility to HIV and increased rate of viral integration into host cells (26). A hypothesized pathway by which cocaine may affect T-cell functioning in the presence of HIV may be related to cytokine release from immune activation in response to cocaine use (23,26-28).
Cocaine use is associated with increased risk of HIV infection, decreased adherence to antiretroviral therapy (ART), lower CD4+ T-cell count, higher plasma HIV RNA and increased HIV disease progression compared with other substances of abuse such as marijuana and alcohol. This potentially bi-directional association between cocaine use and decreased ART adherence may increase HIV progression further worsening depression (29-32).
Recent findings have identified altered cytokine profiles, IL-4 and IL-10, to be positively associated with cocaine use in African American male cocaine users (33). Although Whites are more likely than African Americans to report a lifetime use of any cocaine use, African Americans are more likely than Whites to report lifetime use of crack cocaine (34), a more additive type of cocaine. The disparity of HIV infection continues to persist. The Centers for Disease Control and Prevention in 2010 reports a 7.9 fold higher rate of new HIV infections among African Americans compared with Whites (35). In 2010 although they made up only 12% of the United States population, African Americans experienced 44% of all new HIV infections compared with 31% new infections among White persons (36).
Because of increasing evidence supporting cytokine-induced depression (10,37-44), we examined the association between cocaine use and depression among African American persons infected with HIV who were on antiretroviral therapy (ART). An evaluation of the association between cocaine use and depression in persons with HIV would inform future research to identify inflammatory markers of depression in HIV. We hypothesized that cocaine use is associated with increased depression in persons with HIV. We also sought to evaluate how HIV treatment (duration of each ART drug use and duration of ART use for each ART class), viral control (CD4 cell count and HIV RNA quantification at study entry), cigarette smoking and alcohol use may modify the association between cocaine use and depression
Methods
Study Participants and Measures
Between August 2003 and December 2012, 447 African American persons infected with HIV who were frequent cocaine users or non-users, were enrolled in an observational study investigating the effects of cocaine use and antiretroviral regimens on subclinical atherosclerosis in Baltimore. The goal of the overall study was to investigate the association between cocaine abuse, antiretroviral therapy (ART), and other factors which might be associated with increased risk for subclinical atherosclerosis in African American men and women with HIV infection aged 25 years or older. All persons on ART were eligible to be included in these analyses. Exclusion criteria were any evidence of active clinical coronary artery disease at entry (CAD), any symptoms believed to be related to CAD at entry, glomerular filtration rate <60 mL/min/1.73 m2, known allergy to the contrast used for the computerized tomography scan, and pregnancy. The study was restricted to non-cocaine users and chronic cocaine users defined as cocaine use at least 4 times a month for at least 6 months, by any route. We excluded infrequent cocaine users, persons who use cocaine fewer than 4 times a month, or for less than six consecutive months.
Clinical information including the diagnosis of major depressive disorder (MDD) was obtained from a database of abstracted medical history in medical charts, and medication information was obtained from medical records. A diagnosis of MDD by Diagnostic and Statistical Manual of Mental Disorder, 4th Edition (DSM-IV) criteria within the last 6 months determined by Psychiatric providers who work closely with treating clinicians was recorded as depression. Persons with other mood disorders were not excluded. Persons identified with MDD included newly diagnosed and untreated MDD, and previously diagnosed but unresolved MDD regardless of treatment status. Interviews regarding sociodemographics and drug-use behaviors were conducted; clinical examinations, blood pressure (BP) measurement, echocardiography and 64-slice multidetector computed tomography coronary angiography (contrast-enhanced) were performed; and lipid profiles, vitamin D, and high sensitivity C-reactive protein (hsCRP) levels obtained. The Johns Hopkins Medicine Institutional Review Board approved the study protocol and all study participants provided written informed consent. All procedures used in this study were in accordance with Institutional guidelines.
Statistical analysis
Statistical analysis was performed using SAS (version 9.3, SAS Institute, Cary, NC). Continuous parameters were summarized by medians and interquartile ranges (IQRs), and categorical parameters were summarized as proportions. To compare between-group differences, the nonparametric Wilcoxon two-sample test was used for continuous variables and the Fisher's exact test was used for categorical variables.
We fit logistic regression models to assess the association between cocaine use and depression. Univariate logistic regression models were fit to evaluate the crude association between each covariate and depression. Potential confounders and effect modifiers of the association between cocaine use and depression that were assessed in univariate analysis include age, sex, cigarette smoking, alcohol use, baseline CD4 cell count, baseline HIV RNA quantification, duration of each ART drug use, duration of ART use for each ART class [nucleoside reverse-transcriptase inhibitors (NRTI), non-nucleoside reverse-transcriptase inhibitors (NNRTI), protease inhibitors (PI), fusion inhibitors (FI) or integrase inhibitors], duration of any ART use, and cocaine or other illicit drug use. We retained covariates that were significant at P≤0.20 level in univariate models for inclusion in multivariable logistic regression models. Covariates that ceased to make significant contributions to the model were eliminated in a stepwise backwards manner, yielding a final model. The P-values reported are two-sided and statistical significance was determined at P<0.05.
Results
A total of 447 participants were included in these analyses. Mean age was 46 years, 38.6% were female and prevalence of cocaine use was 74.3% (Table 1). The overall prevalence of depression was 40.9% (183 of 447) participants. Among persons who were depressed, the prevalence of cocaine use was 81.4% (149 of 183), compared to 69.3% among persons who were not depressed (183 of 264), P=0.004 (Table 1). Persons who were depressed had a longer duration of HIV infection, were more likely to be on protease inhibitor based ART regimen, and were more likely to have longer duration and higher frequency of cocaine use. There was no difference in hsCRP levels among cocaine users and non-users
Table 1.
Characteristicsa of Study Participants, by the Presence of Depression
| Characteristic | Total (N = 447) | Depression | P-value | |
|---|---|---|---|---|
| No (N = 264) | Yes (N = 183) | |||
| Age (year) | 46 (41-51) | 46 (41-52) | 46 (42-50) | 0.74 |
| Female (%) | 38.6 | 34.3 | 44.8 | 0.025 |
| Cocaine use (%) | 74.3 | 69.3 | 81.4 | 0.004 |
| Years of cocaine use | 8 (0-18) | 6(0-15) | 10 (3-19) | 0.001 |
| Times of cocaine use per day | 2 (0-4) | 2 (0-4) | 2 (1-4) | 0.03 |
| Cigarette smoking (%) | 82.6 | 82.3 | 83.1 | 0.83 |
| Years of cigarette smoking | 21 (7-30) | 21 (7-31) | 22 (9-30) | 0.83 |
| Alcohol use (%) | 87.5 | 87.9 | 86.9 | 0.74 |
| Years of alcohol use | 16 (5-27) | 15 (5-25) | 18 (4-28) | 0.49 |
| Heroin use (%) | 50.2 | 48.3 | 53.0 | 0.33 |
| Marijuana use (%) | 72.1 | 72.5 | 71.6 | 0.84 |
| Speedball (%) | 32.7 | 30.2 | 36.3 | 0.17 |
| Amphetamine (%) | 4.5 | 4.5 | 4.4 | 0.94 |
| Methadone (%) | 19.2 | 16.7 | 23.0 | 0.10 |
| hsCRP ≥2 mg/dL (%) | 49.2 | 47.7 | 51.4 | 0.45 |
| hsCRP (mg/dL) | 2.0 (0.6-5.0) | 1.9 (0.6-4.8) | 2.1 (0.6-5.1) | 0.94 |
| BMI (kg/m2) | 25.0 (22.1-29.3) | 25.0 (22.2-28.9) | 25.2 (22.0-29.9) | 0.95 |
| CD4 (cells/mm3) | 462(284-686) | 463 (274-702) | 461 (302-669) | 0.90 |
| HIV Viral load (c/mL) | 34(20-400) | 48(20-400) | 23 (20-114) | 0.12 |
| Years of HIV infection | 12.4 (6.6-17.6) | 10.4 (4.5-16.2) | 14.4 (10.0-18.7) | <0.0001 |
| NRTI use (%) | 72.5 | 67.9 | 79.2 | 0.008 |
| NNRTI use (%) | 34.6 | 35.5 | 33.3 | 0.64 |
| PI use (%) | 60.0 | 50.6 | 73.8 | <0.0001 |
| ART use (%) | 77.9 | 72.8 | 85.3 | 0.0018 |
Median (interquartile range) for continuous variables, proportion (%) for categorical variables.
Abbreviations: hsCRP: high-sensitivity C-reactive protein; BMI: body mass index; ART: antiretroviral therapy; NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: nonnucleoside reverse transcriptase inhibitor; PI: protease inhibitor.
In univariate analysis, any use of cocaine was associated with approximately 2-fold increase in odds of depression, compared to never using cocaine, OR=1.94, (95% CI: 1.23, 3.06); P=0.004), Table 2. The odds of depression was increased regardless of ART use, OR=2.16; (95% CI: 1.32, 3.52); P=0.002. Among persons on protease inhibitor-based ART the odds of depression was increased by 2.75, (95%CI: 1.87, 4.14); P<0.0001. Persons on NRTI based regimen also had an increased odds of depression, OR=1.80, (95% CI: 1.16, 2.80); P=0.009 (Table 2). Female sex was associated with a 1.5 fold increase in odds for depression. There was no association between alcohol use, cigarette smoking, use of other drugs and depression.
Table 2.
Unadjusted Odds Ratios for the Association between Demographic, Laboratory, and Clinical Factors and Clinical Depression by Logistic Regression Analysis
| Covariate | Crude OR (95% CI) | P-value |
|---|---|---|
| Age | 1.00 (0.98-1.02) | 0.87 |
| Sex | ||
| Male | 1.00 | |
| Female | 1.55 (1.06-2.28) | 0.03 |
| Cigarette smoking | ||
| Never | 1.00 | |
| Ever | 1.06 (0.64-1.74) | 0.83 |
| Alcohol use | ||
| No | 1.00 | |
| Yes | 0.91 (0.52-1.60) | 0.74 |
| Cocaine use | ||
| Never | 1.00 | |
| Ever | 1.94 (1.23-3.06) | 0.004 |
| Duration of cigarette smoking (year) | 1.00 (0.99-1.01) | 0.81 |
| Duration of alcohol use (year) | 1.01 (0.99-1.02) | 0.47 |
| Duration of cocaine use (year) | 1.03 (1.01-1.05) | 0.004 |
| Years of HIV infection | 1.06 (1.04-1.09) | <0.0001 |
| hsCRP>2 mg/dL | ||
| No | 1.00 | |
| Yes | 1.16 (0.79-1.69) | 0.45 |
| BMI (kg/m2) | 0.99 (0.97-1.03) | 0.71 |
| NRTI use | ||
| No | 1.00 | |
| Yes | 1.80 (1.16-2.80) | 0.009 |
| NNRTI use | ||
| No | 1.00 | |
| Yes | 0.91 (0.61-1.35) | 0.64 |
| PI use | ||
| No | 1.00 | |
| Yes | 2.75 (1.83-4.14) | <0.0001 |
| ART use | ||
| No | 1.00 | |
| Yes | 2.16 (1.32-3.52) | 0.002 |
OR: odds ratio; hsCRP: high-sensitivity C-reactive protein; BMI: body mass index (kg/m2); NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: nonnucleoside reverse transcriptase inhibitor; PI: protease inhibitor; ART: antiretroviral therapy.
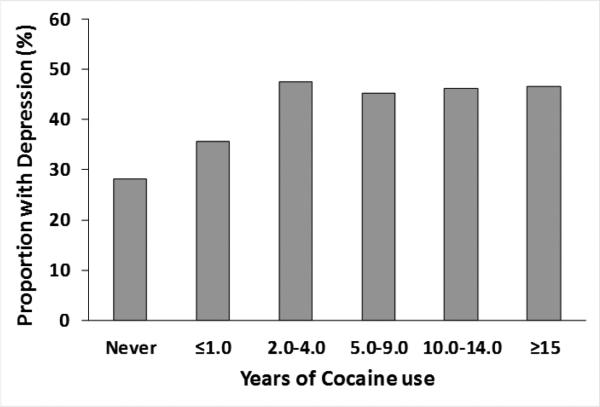
When adjusted for sex, duration of cocaine use, years of HIV infection and Protease inhibitor use, each year of cocaine use was associated with a 2% increased odds of depression, adjusted odds ratio =1.02, 95% CI: 1.01, 1.05); P=0.04 (Table 3). Each year of HIV infection was associated with a 5% increase in odds of depression and a protease inhibitor-based ART regimen was associated with a more than 2-fold increase in odds of depression. Figure 1 shows a dose-response relationship between increasing duration of cocaine use and increasing depression. After 5 years of cocaine use the prevalence of depression among persons with HIV was at least 45% compared to 28% among persons who had never used cocaine.
Table 3.
Adjusted Odds Ratios for the Association between Demographic, Laboratory, and Clinical Factors and Clinical Depression by Logistic Regression Analysis
| Covariate | Adjusted OR (95% CI) | P-value |
|---|---|---|
| Sex | ||
| Male | 1.00 | |
| Female | 1.60 (1.06-2.41) | 0.03 |
| Duration of cocaine use, years (continuous) | 1.02 (1.01-1.05) | 0.04 |
| Years of HIV infection | 1.05 (1.02-1.08) | 0.001 |
| Protease Inhibitor use | 2.22 (1.43-3.45) | 0.0004 |
OR: odds ratio; Adjusted for sex, duration of cocaine use, years of HIV infection and Protease inhibitor use.
Figure 1.
Prevalence of Depression by Duration of Cocaine Use Among Persons with HIV
Discussion
We determined that cocaine use, duration and frequency of use, were associated with increased odds for depression. On the other hand alcohol use, cigarette smoking and use of other drugs were not associated with increased depression in this study population. Our study population has been previously studied with findings to support that cocaine use is associated with the development of soft and hard plaque in cardiac blood vessels (45,46), suggesting an association between cocaine use and chronic inflammatory damage. Under the inflammatory model for depression, cocaine use may increase depression in persons with HIV by inducing chronic inflammatory changes rather than acute inflammation, suggested by normal hsCRP levels which showed no difference by depression status.
African Americans are disproportionately affected by HIV and substance use disorders, compared with Whites. Disparities concerning access to health care persist for African Americans (47), which places them a higher disadvantage of experiencing the untoward effects of HIV including comorbid HIV infection, substance use and depression. Because HIV increases the risk of MDD and substance use, our finding that frequent cocaine use may be associated with MDD in African Americans has implication for HIV care and psychiatric care.
Protease inhibitor use was associated with increased odds of depression. A number of explanations remain for this observation. The association between protease inhibitors and increased odds for depression may be a result of confounding by indication. Protease inhibitors by themselves may have good CNS penetration and therefore reduce the CNS HIV reservoirs (48). However because protease inhibitors have been associated with decreased depression scores (49), persons with depression or at increased risk for depression may be assigned treatment with protease inhibitors. As such, protease inhibitors may be used at higher rates with patients likely to exhibit higher symptoms of depression, representing confounding by indication. Also protease inhibitors have a higher threshold for resistance compared to (50), therefore persons more likely to be non-adherent may be assigned this class of drugs. Protease inhibitors have been associated with endoplasmic reticulum stress (51,52), which may lead to activation of proviral HIV particles and increased CNS inflammation, which may precipitate depression.
For each year a person is infected with HIV, the odds of depression increases. This may suggest an ongoing pathogenic role of the virus in developing depression. Our findings are consistent with prior reports of increased depression among females (53). We did not observe associations between alcohol and cigarettes use and depression. Previous reports support an association of increased depression among smokers and persons and alcohol use (54,55). Our observed lack of association could be due to the fact that cocaine use in this population may be a stronger predictor of depression than alcohol or cigarette use.
Because the current work evaluates depression diagnosis within the last 6 months including previously diagnosed but unresolved depression regardless of treatment status, our findings may represent an underestimation of the true association between cocaine use and depression. Furthermore among persons with HIV, depression may be under diagnosed and undocumented in as many as 45% or persons with HIV (56,57). This may additionally support an underestimated of the reported association between cocaine use and depression.
A limitation in these analyses is the cross-sectional study design. We are therefore unable to ascertain a causal association between cocaine use and depression. However our findings provide basis for this association to be further evaluated. By including persons who used cocaine for more than 6 months in these analyses, cocaine use was assessed to precede a diagnosis of depression among persons without a prior depression diagnosis. We did not account for depression treatment received which could affect the observed association. This may translate into our results being conservative estimates. We also did not account for hepatitis C viral (HCV) infections or treatment for HVC. HCV is a frequent comorbidity in persons with HIV and is associated with high depression rates (58-60). The dose-dependent association we observed between duration of cocaine use and depression strengthens our findings. Future studies need to evaluate a temporal association and the mechanisms by which cocaine use may increase depression in persons with HIV. Because infrequent cocaine users (persons who used cocaine fewer than 4 times a month or for less than six consecutive months) were excluded from these analyses, we reduced the possibility of misclassifying cocaine use. This approach may limit interpretation and comparison of our findings to prior research. By excluding infrequent users however, we increased the likelihood that persons captured in these analyses are cocaine abusers or dependent on cocaine. These findings may therefore not be generalizable to infrequent cocaine users. However our approach is relevant to frequent cocaine users and the observed dose-response association between frequent cocaine use and depression may suggest a potential association even among infrequent cocaine users, a hypothesis that needs further exploration. These findings are not intended to make an inference regarding all cocaine users but instead about frequent cocaine users.
Our findings among African American men may not be completely generalizable to the general population without understanding trends in depression among races, male and female sex, and substance differences between genders. Generally depression rates in females are higher compared with males (53), with Whites reporting higher depression rates than African Americans (61), and observed racial differences in substance abuse across populations (62,63). Race, sex and drug substance abuse rates may be effect modifiers of this association between cocaine use and depression which future research could help to understand better.
Conclusion
In conclusion, cocaine use, frequency and duration, may be associated with depression. Alcohol use, cigarette smoking and abuse of other substances were not associated with depression in our study population. We speculate that depression among cocaine users with HIV may involve an inflammatory component that needs further examination of CSF and plasma inflammatory signals.
References
- 1.Lopes M, Olfson M, Rabkin J, Hasin DS, Alegria AA, Lin KH, et al. Gender, HIV status, and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2012 Mar;73(3):384–391. doi: 10.4088/JCP.10m06304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001 Aug;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 3.DeLorenze GN, Weisner C, Tsai AL, Satre DD, Quesenberry CP., Jr. Excess mortality among HIV-infected patients diagnosed with substance use dependence or abuse receiving care in a fully integrated medical care program. Alcohol Clin Exp Res. 2011 Feb;35(2):203–210. doi: 10.1111/j.1530-0277.2010.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001 Mar 21;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 5.Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, et al. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med. 2002 Aug;32(6):1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 6.Whetten K, Reif S, Ostermann J, Pence BW, Swartz M, Whetten R, et al. Improving health outcomes among individuals with HIV, mental illness, and substance use disorders in the Southeast. AIDS Care. 2006;18(Suppl 1):S18–26. doi: 10.1080/09540120600839330. [DOI] [PubMed] [Google Scholar]
- 7.Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004 Jul;94(7):1133–1140. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009 Jan;71(1):57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin GC, Roth MD, Tashkin DP. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J Neuroimmunol. 1998 Mar 15;83(1-2):133–138. doi: 10.1016/s0165-5728(97)00229-4. [DOI] [PubMed] [Google Scholar]
- 10.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009 Feb 15;65(4):296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19851–19856. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boasso A, Hardy AW, Anderson SA, Dolan MJ, Shearer GM. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS One. 2008 Aug 13;3(8):e2961. doi: 10.1371/journal.pone.0002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008 Nov;5(4):163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan KR, Delong M, Kraemer H, Carney R, Spiegel D, Gordon C, et al. Comorbidity of depression with other medical diseases in the elderly. Biol Psychiatry. 2002 Sep 15;52(6):559–588. doi: 10.1016/s0006-3223(02)01472-5. [DOI] [PubMed] [Google Scholar]
- 15.Hutton HE, Lyketsos CG, Zenilman JM, Thompson RE, Erbelding EJ. Depression and HIV risk behaviors among patients in a sexually transmitted disease clinic. Am J Psychiatry. 2004 May;161(5):912–914. doi: 10.1176/appi.ajp.161.5.912. [DOI] [PubMed] [Google Scholar]
- 16.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000 Jul 24;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 17.Yun LW, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005 Apr 1;38(4):432–438. doi: 10.1097/01.qai.0000147524.19122.fd. [DOI] [PubMed] [Google Scholar]
- 18.Weber R, Huber M, Rickenbach M, Furrer H, Elzi L, Hirschel B, et al. Uptake of and virological response to antiretroviral therapy among HIV-infected former and current injecting drug users and persons in an opiate substitution treatment programme: the Swiss HIV Cohort Study. HIV Med. 2009 Aug;10(7):407–416. doi: 10.1111/j.1468-1293.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 19.Glass TR, Battegay M, Cavassini M, De Geest S, Furrer H, Vernazza PL, et al. Longitudinal analysis of patterns and predictors of changes in self-reported adherence to antiretroviral therapy: Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2010 Jun;54(2):197–203. doi: 10.1097/QAI.0b013e3181ca48bf. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez A, Barinas J, O'Cleirigh C. Substance use: impact on adherence and HIV medical treatment. Curr HIV/AIDS Rep. 2011 Dec;8(4):223–234. doi: 10.1007/s11904-011-0093-5. [DOI] [PubMed] [Google Scholar]
- 21.Stein MD, Rich JD, Maksad J, Chen MH, Hu P, Sobota M, et al. Adherence to antiretroviral therapy among HIV-infected methadone patients: effect of ongoing illicit drug use. Am J Drug Alcohol Abuse. 2000 May;26(2):195–205. doi: 10.1081/ada-100100600. [DOI] [PubMed] [Google Scholar]
- 22.Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007 Mar;11(2):185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gekker G, Hu S, Sheng WS, Rock RB, Lokensgard JR, Peterson PK. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. Int Immunopharmacol. 2006 Jun;6(6):1029–1033. doi: 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Mao JT, Huang M, Wang J, Sharma S, Tashkin DP, Dubinett SM. Cocaine down-regulates IL-2-induced peripheral blood lymphocyte IL-8 and IFN-gamma production. Cell Immunol. 1996 Sep 15;172(2):217–223. doi: 10.1006/cimm.1996.0235. [DOI] [PubMed] [Google Scholar]
- 25.Nair MP, Mahajan SD, Schwartz SA, Reynolds J, Whitney R, Bernstein Z, et al. Cocaine modulates dendritic cell-specific C type intercellular adhesion molecule-3-grabbing nonintegrin expression by dendritic cells in HIV-1 patients. J Immunol. 2005 Jun 1;174(11):6617–6626. doi: 10.4049/jimmunol.174.11.6617. [DOI] [PubMed] [Google Scholar]
- 26.Kim SG, Jung JB, Dixit D, Rovner R, Jr, Zack JA, Baldwin GC, et al. Cocaine exposure enhances permissiveness of quiescent T cells to HIV infection. J Leukoc Biol. 2013 Oct;94(4):835–843. doi: 10.1189/jlb.1112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhillon NK, Williams R, Peng F, Tsai YJ, Dhillon S, Nicolay B, et al. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007 Dec;13(6):483–495. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- 28.Nair MP, Chadha KC, Hewitt RG, Mahajan S, Sweet A, Schwartz SA. Cocaine differentially modulates chemokine production by mononuclear cells from normal donors and human immunodeficiency virus type 1-infected patients. Clin Diagn Lab Immunol. 2000 Jan;7(1):96–100. doi: 10.1128/cdli.7.1.96-100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009 Jan 1;50(1):93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 30.Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. J Community Health. 2004 Apr;29(2):117–127. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- 31.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002 Mar 29;16(5):767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 32.Khan MR, Berger A, Hemberg J, O'Neill A, Dyer TP, Smyrk K. Non-injection and injection drug use and STI/HIV risk in the United States: the degree to which sexual risk behaviors versus sex with an STI-infected partner account for infection transmission among drug users. AIDS Behav. 2013 Mar;17(3):1185–1194. doi: 10.1007/s10461-012-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parikh N, Dampier W, Feng R, Passic SR, Zhong W, Frantz B, et al. Cocaine alters cytokine profiles in HIV-1-infected African American individuals in the DrexelMed HIV/AIDS genetic analysis cohort. J Acquir Immune Defic Syndr. 2014 Jul 1;66(3):256–264. doi: 10.1097/QAI.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palamar JJ, Davies S, Ompad DC, Cleland CM, Weitzman M. Powder cocaine and crack use in the United States: an examination of risk for arrest and socioeconomic disparities in use. Drug Alcohol Depend. 2015 Apr 1;149:108–116. doi: 10.1016/j.drugalcdep.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, Sexual Transmitted Diseases and Tuberculosis Prevention [July 16, 2015];HIV Incidence. http://www.cdc.gov/hiv/statistics/surveillance/incidence.html. Page last reviewed and updated: May 20, 2015.
- 36.Centers for Disease Control and Prevention Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, Sexual Transmitted Diseases and Tuberculosis Prevention. [July 16, 2015];Basic Statistics HIV. http://www.cdc.gov/hiv/statistics/basics.html. Page last reviewed and updaed: May 20, 2015.
- 37.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009 May 1;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, et al. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007 Dec 1;62(11):1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007 Aug;21(6):727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 40.D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009 Feb 18;29(7):2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003 Jul;160(7):1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 42.Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008 May;85(1):1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008 Jan;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weidenfeld J, Yirmiya R. Effects of bacterial endotoxin on the glucocorticoid feedback regulation of adrenocortical response to stress. Neuroimmunomodulation. 1996 Nov-Dec;3(6):352–357. doi: 10.1159/000097295. [DOI] [PubMed] [Google Scholar]
- 45.Du J, Wasserman BA, Tong W, Chen S, Lai S, Malhotra S, et al. Cholesterol is associated with the presence of a lipid core in carotid plaque of asymptomatic, young-to-middle-aged African Americans with and without HIV infection and cocaine use residing in inner-city Baltimore, Md., USA. Cerebrovasc Dis. 2012;33(3):295–301. doi: 10.1159/000334661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai H, Fishman EK, Gerstenblith G, Moore R, Brinker JA, Keruly JC, et al. Vitamin D deficiency is associated with development of subclinical coronary artery disease in HIV-infected African American cocaine users with low Framingham-defined cardiovascular risk. Vasc Health Risk Manag. 2013;9:729–737. doi: 10.2147/VHRM.S50537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lesko CR, Cole SR, Miller WC, Westreich D, Eron JJ, Adimora AA, et al. Ten-year Survival by Race/Ethnicity and Sex Among Treated, HIV-infected Adults in the United States. Clin Infect Dis. 2015 Jun 1;60(11):1700–1707. doi: 10.1093/cid/civ183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010 Apr-May;18(2):45–55. [PMC free article] [PubMed] [Google Scholar]
- 49.Low-Beer S, Chan K, Yip B, Wood E, Montaner JS, O'Shaughnessy MV, et al. Depressive symptoms decline among persons on HIV protease inhibitors. J Acquir Immune Defic Syndr. 2000 Apr 1;23(4):295–301. doi: 10.1097/00126334-200004010-00003. [DOI] [PubMed] [Google Scholar]
- 50.Hsieh SM, Pan SC, Chang SY, Hung CC, Sheng WH, Chen MY, et al. Differential impact of resistance-associated mutations to PIs and NNRTIs on HIV-1 replication capacity. AIDS Res Hum Retroviruses. 2013 Apr 17; doi: 10.1089/aid.2013.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou H, Pandak WM, Jr, Lyall V, Natarajan R, Hylemon PB. HIV protease inhibitors activate the unfolded protein response in macrophages: implication for atherosclerosis and cardiovascular disease. Mol Pharmacol. 2005 Sep;68(3):690–700. doi: 10.1124/mol.105.012898. [DOI] [PubMed] [Google Scholar]
- 52.Chen L, Jarujaron S, Wu X, Sun L, Zha W, Liang G, et al. HIV protease inhibitor lopinavir-induced TNF-alpha and IL-6 expression is coupled to the unfolded protein response and ERK signaling pathways in macrophages. Biochem Pharmacol. 2009 Jul 1;78(1):70–77. doi: 10.1016/j.bcp.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. Br J Psychiatry. 2000 Dec;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- 54.Baek JH, Eisner LR, Nierenberg AA. Smoking and suicidality in subjects with major depressive disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J Affect Disord. 2013 Sep 25;150(3):1158–1166. doi: 10.1016/j.jad.2013.05.082. [DOI] [PubMed] [Google Scholar]
- 55.Chou SP, Huang B, Goldstein R, Grant BF. Temporal associations between physical illnesses and mental disorders--results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Compr Psychiatry. 2013 Aug;54(6):627–638. doi: 10.1016/j.comppsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Israelski DM, Prentiss DE, Lubega S, Balmas G, Garcia P, Muhammad M, et al. Psychiatric co-morbidity in vulnerable populations receiving primary care for HIV/AIDS. AIDS Care. 2007 Feb;19(2):220–225. doi: 10.1080/09540120600774230. [DOI] [PubMed] [Google Scholar]
- 57.Asch SM, Kilbourne AM, Gifford AL, Burnam MA, Turner B, Shapiro MF, et al. Underdiagnosis of depression in HIV: who are we missing? J Gen Intern Med. 2003 Jun;18(6):450–460. doi: 10.1046/j.1525-1497.2003.20938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011 Sep;31(8):1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 59.Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005 Oct;19(Suppl 3):S99–105. doi: 10.1097/01.aids.0000192077.11067.e5. [DOI] [PubMed] [Google Scholar]
- 60.Roux P, Lions C, Cohen J, Winnock M, Salmon-Ceron D, Bani-Sadr F, et al. Impact of HCV treatment and depressive symptoms on adherence to HAART among coinfected HIV-HCV patients: results from the ANRS-CO13-HEPAVIH cohort. Antivir Ther. 2013 Oct 28; doi: 10.3851/IMP2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnes DM, Keyes KM, Bates LM. Racial differences in depression in the United States: how do subgroup analyses inform a paradox? Soc Psychiatry Psychiatr Epidemiol. 2013 Dec;48(12):1941–1949. doi: 10.1007/s00127-013-0718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alvanzo AA, Storr CL, La Flair L, Green KM, Wagner FA, Crum RM. Race/ethnicity and sex differences in progression from drinking initiation to the development of alcohol dependence. Drug Alcohol Depend. 2011 Nov 1;118(2-3):375–382. doi: 10.1016/j.drugalcdep.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend. 1997 Jan 10;44(1):11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]