Abstract
Background
Distinguishing alveolar rhabdomyosarcoma (ARMS) from embryonal rhabdomyosarcoma (ERMS) is of prognostic and therapeutic importance. Criteria for classifying these entities evolved significantly from 1995 to 2013. ARMS is associated with inferior outcome; therefore patients with alveolar histology have generally been excluded from low-risk therapy. However, patients with ARMS and low-risk Stage and Group (Stage 1 Group I/II/orbit III or Stage 2/3 Group I/II) were eligible for the Children's Oncology Group (COG) low-risk rhabdomyosarcoma (RMS) study D9602 from 1997 to 1999. The characteristics and outcomes of these patients have not been previously reported, and the histology of these cases has not been reviewed using current criteria.
Procedure
We re-reviewed cases that were classified as ARMS on D9602 using current histologic criteria, determined PAX3/PAX7-FOXO1 fusion status, and compared these data with outcome for this unique group of patients.
Results
Thirty-eight patients with ARMS were enrolled onto D9602. Only one-third of cases with slides available for re-review (11/33) remained classified as ARMS by current histologic criteria. Most were reclassified as ERMS (17/33, 51.5%). Cases that remained classified as ARMS were typically fusion-positive (8/11, 73%), therefore current classification results in a similar rate of fusion-positive ARMS for all clinical risk groups. In conjunction with data from COG intermediate-risk treatment protocol D9803, our data demonstrates excellent outcomes for fusion-negative ARMS with otherwise low-risk clinical features.
Conclusions
Patients with fusion-positive RMS with low-risk clinical features should be classified and treated as intermediate-risk, while patients with fusion-negative ARMS could be appropriately treated with reduced intensity therapy.
Keywords: Rhabdomyosarcoma, Alveolar Rhabdomyosarcoma, Embryonal Rhabdomyosarcoma, Low-Risk, Outcomes, Histology, Fusion Status, D9602, D9803
Introduction
Due to inferior outcomes associated with ARMS, the histologic classification of rhabdomyosarcoma (RMS) as alveolar RMS (ARMS) or embryonal RMS (ERMS) has prognostic and therapeutic importance, yet the criteria for making this diagnosis have changed over time. The International Classification of RMS (ICR) published in 1995 provided the first prognostically relevant classification system for RMS.[1] The ICR identified prognostic groups as follows: superior prognosis (botryoid and spindle cell RMS), intermediate prognosis (conventional ERMS), and poor prognosis (ARMS). Additionally, the ICR modified the previous diagnostic criteria for ARMS to include a solid variant and tumors with even focal alveolar histology. This represented a significant departure from the previous definition of ARMS as tumors with >50% alveolar histology. This change resulted in an increase in the frequency of ARMS and doubled the proportion of FOXO1 fusion-negative ARMS cases.[2, 3]
To better understand the outcome for patients enrolled in protocols using the ICR criteria in the context of current histologic criteria, we recently re-reviewed the histology of patients enrolled in Children's Oncology Group (COG) intermediate-risk RMS study D9803 from 1999 to 2005. Re-review of pathology materials from this study using current stricter histologic and cytologic criteria showed that only two-thirds remained classified as ARMS.[4] In many cases, a dense pattern of ERMS closely resembled the solid variant of ARMS. When classified by current criteria, the percentage of fusion-negative ARMS cases decreased from 37% to 18% of the total ARMS diagnoses[4], a percentage more closely approaching earlier reports.
During a time period overlapping with D9803, COG low-risk RMS study D9602 enrolled patients with low-risk RMS from 1997 to 2004 with the goal of reducing toxicity for these patients.[5] From 1997 to 1999, prior to the opening of enrollment for intermediate-risk RMS study D9803, patients with ARMS histology and low-risk Stage and Group criteria (either Stage 1 (favorable site), Group I/II (resected), or orbital Group III (unresected), or Stage 2/3 (unfavorable site), Group I/II (resected)) were enrolled in D9602. However, the original outcome analysis of D9602 was limited to patients with ERMS, so that outcome for patients with ARMS has not been reported.[5] After September 1, 1999, patients with a histologic diagnosis of ARMS and low-risk Stage and Group were excluded from D9602 and enrolled onto D9803.[6]
A recent study confirmed that fusion status impacts outcome for children with RMS, suggesting that fusion-negative ARMS in patients with low-risk clinical features have favorable outcome.[7] However, this analysis of low-risk fusion-negative ARMS did not reach statistical significance, likely due to the small sample size. Given that patients treated on the low-risk RMS protocol D9602 were not included in the study group, we expanded this analysis by performing the first detailed correlation of current histologic classification, fusion status, and outcome for clinically low-risk patients enrolled in D9602 as ARMS. When combined with patients with low-risk clinical features enrolled in D9803 as ARMS, our analysis of these re-classified cases represents the largest number of patients with low-risk Stage and Group ARMS to date. Our data demonstrates that the rate of fusion negativity in low risk ARMS is similar to all other clinical risk strata. Further, our combined data show significantly superior event-free survival (EFS) for patients with clinically low-risk fusion-negative ARMS compared with clinically low-risk fusion-positive ARMS, suggesting that reduced intensity therapy may be appropriate for patients with clinically low-risk fusion-negative ARMS.
Materials and Methods
Histology review
Thirty-eight patients with ARMS and low-risk clinical features were enrolled in COG study D9602. H&E slides for histology re-review and classification were available for 33 of these patients. Two pathologists (ERR and DMP) conducted an initial re-review, and four pathologists (ERR, DMP, MAA and LAT) participated in the final re-review. Reviewers were not always initially in agreement but reached a consensus diagnosis for all cases by examining nuclear features; round, monotonous nuclei favored the diagnosis of ARMS. In cases difficult to classify, diffuse myogenin reactivity favored the diagnosis of ARMS over ERMS. A Kappa statistic for interobserver agreement was calculated. Cases were reclassified as ARMS (predominant solid and/or alveolar histology), ERMS (predominant embryonal histology), mixed RMS (characterized by separate, distinct areas of ARMS and ERMS histology) or sclerosing/spindle cell RMS (SSCRMS) as previously described.[4, 8] A diagnosis of RMS not otherwise specified (NOS) was made if the available material was too small, crushed, or necrotic for definitive classification. Immunohistochemical stains were available for some cases, including myogenin for 26 of 33 cases. Nuclear myogenin expression was scored from 0 to 4+ based on the following percentages of tumor cells: 0 (absent expression), 1+ (<10%), 2+ (10-50%), 3+ (>50-90%), and 4+ (>90%). The histologic type was determined prior to FOXO1 fusion status testing.
PAX3/PAX7-FOXO1 Fusion status
Sufficient material for FOXO1 fusion status testing and identification of the PAX3 or PAX7 fusion partner was available for 29 the 33 cases that underwent histologic re-review. Four cases reclassified as ERMS had no material available for fusion status determination. One additional case did not have material available for histologic re-review but had material available for fusion status determination. Of the 30 cases with available material, fusion status was determined for 19 cases by fluorescence in situ hybridization (FISH) to detect rearrangements of the FOXO1 (13q14), PAX3 (2q35) and PAX7 (1p36) loci using unstained formalin-fixed, paraffin embedded tissue sections, as previously described.[4, 9] In the remaining 11 cases, fusion status was determined by quantitative reverse transcription-polymerase chain reaction (RT-PCR) to detect expression of a PAX3-FOXO1 or PAX7-FOXO1 fusion transcript.[2, 4]
Patient Outcome and Statistical Analyses
EFS was defined as the time from study entry to the first occurrence of disease progression, disease relapse, or death. For those not experiencing one of these events, EFS was censored at last contact. Estimates of overall survival (OS) and EFS as time-to-event distributions were calculated using the Kaplan-Meier method, and distributions were compared using log-rank tests.
Results
Histology of Low-Risk Patients Enrolled in D9602 as ARMS
COG low-risk RMS study D9602 enrolled 38 patients as ARMS with low-risk clinical features. Of these, material from 34 was available for histologic re-review and/or PAX3/PAX7-FOXO1 fusion status determination. A total of thirty-three cases with material available for histologic re-review were examined by current histologic criteria, and only 11 (33%) remained classified as ARMS. Most cases that remained classified as ARMS had a classic pattern (7/11, 64%) (Figure 1, panel A). Two otherwise classic ARMS cases had prominent areas of sclerosis (Figure 1, panel B), but 4+ myogenin expression helped to confirm the diagnosis of ARMS and exclude SSCRMS. The remaining 22 cases were reclassified by current histologic criteria as ERMS (17/22, 52%), mixed RMS (3/22, 9%), or RMS NOS (2/22, 6%).
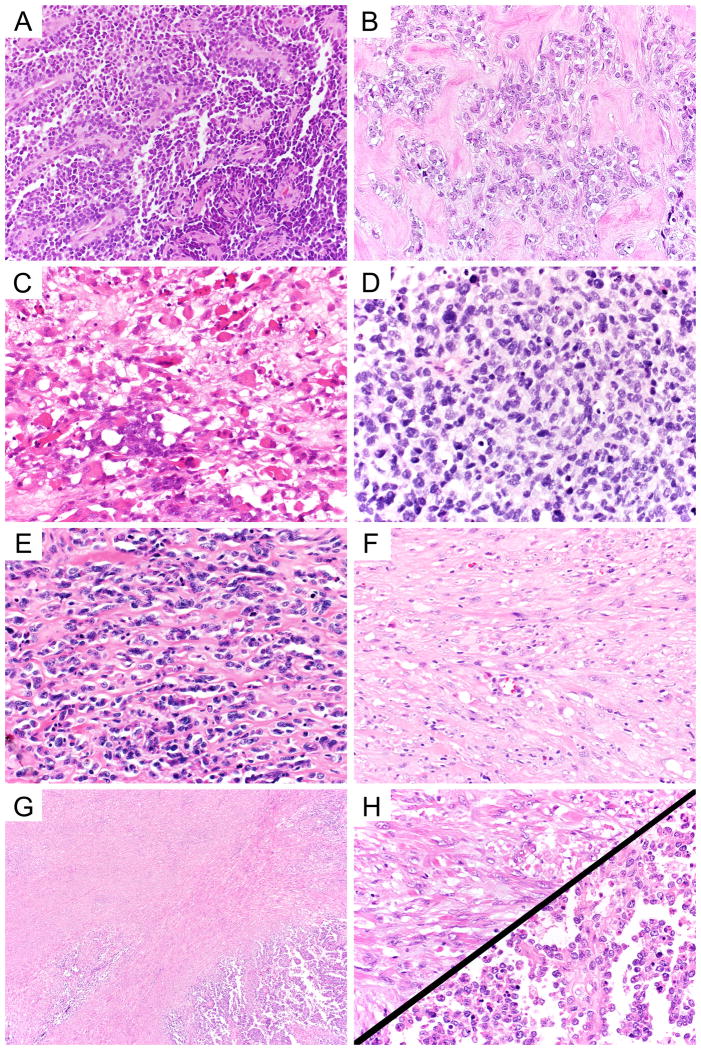
Figure 1.
Histologic heterogeneity among D9602 cases previously classified as ARMS. Cases enrolled in D9602 included classic ARMS (A, 40× objective magnification) and ARMS with regions of dense sclerosis (B, 40× objective magnification). Variants of ERMS included typical (C, 40× objective magnification), dense (D, 40× objective magnification), sclerosing (E, 40× objective magnification) and spindled patterns (F, 40× objective magnification). Mixed RMS typically featured distinct areas of ERMS and ARMS (G, 4× objective magnification; H upper left ERMS, 40× objective magnification; H lower right ARMS, 40× objective magnification).
Cases reclassified as ERMS or mixed RMS represented a spectrum of ERMS histology, with typical ERMS (Figure 1, panel C), dense ERMS (Figure 1, panel D), sclerosing (Figure 1, panel E), and spindled patterns (Figure 1, panel F). Multiple patterns were often seen in the same tumor. The presence of at least focal dense, alveolar, or sclerosing patterns were seen in nearly all reclassified cases and accounted for the original diagnosis of ARMS by previous histologic criteria. The nuclei of ERMS were variably sized and were often ovoid, angulated or spindled. In contrast, nuclei of ARMS were monotonous and round (compare Figures 1, panel A and 1, panel D). The consensus diagnosis of the reviewing pathologists was based on a combination of cytologic features and the distribution of the components. Composite ERMS showed intermingled patterns while mixed RMS was segregated into distinct areas of ARMS and ERMS histology (Figures 1, panel G and 1, panel H). Interobserver agreement between initial re-review and final consensus re-review was good (Kappa=0.704). Although only a small group of patients were enrolled in low-risk study D9602 with a diagnosis of ARMS, the frequency of histologic reclassification as ERMS in these cases was higher than the rate for all patients enrolled as ARMS in D9803 (52% vs. 33%, p=0.051).[4] Patients enrolled in D9803 with a histologic diagnosis of ARMS and low-risk clinical features were also reclassified as ERMS at a significantly higher rate compared with all patients with ARMS enrolled in D9803 (47% vs. 33%, p=0.024).
Fusion Status and Clinical Features of Low-Risk Patients Enrolled in D9602 as ARMS
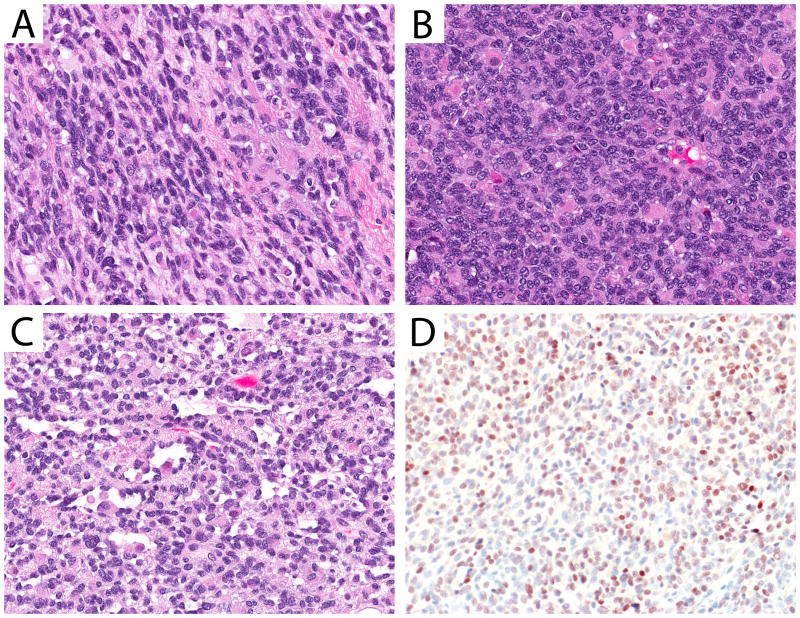
Following histologic classification, PAX3/PAX7-FOXO1 gene fusion status was determined for 29 of the 33 re-reviewed cases, and one additional case with material available only for molecular testing. Four cases reclassified as ERMS had no material available for additional testing. Of the 29 cases with histology re-review and fusion status determination, a PAX3/PAX7-FOXO1 fusion gene was detected in 13 (46.4% fusion-positive). PAX3 or PAX7 fusion partners were seen at comparable frequencies (Table I). Amongst the cases that remained classified as ARMS, 8 of 11 (73%) were fusion-positive. Other fusion-positive tumors included 1 of 3 mixed RMS, both RMS NOS, and the 1 case that was not available for histology review. Additionally, one case reclassified as ERMS was found to have a PAX3-FOXO1 fusion. This tumor, which arose in the extremity, showed features of typical ERMS (Figure 2, panel A) with ovoid to spindled nuclei in the majority of the specimen; supporting histologic classification as ERMS based on current criteria. This tumor also contained admixed densely cellular areas (Figure 2, panel B) showing rounder nuclei, and foci demonstrating a micro-alveolar pattern (Figure 2, panel C). Myogenin immunohistochemistry (IHC) performed in this case demonstrated moderate but inconclusive (3+) reactivity (Figure 2, panel D). All 9 fusion-positive cases with slides available to review showed 3+ or 4+ myogenin reactivity. By comparison, in 17 fusion-negative cases with available myogenin immunohistochemistry 3+ or 4+ myogenin reactivity was seen in only 5 cases (29.4%; 3+ n=5 and 4+ n=2). Patients with fusion-positive RMS were also more likely to be female (p=0.02), have Stage 2 rather than Stage 1 disease (p=0.06), and to have smaller-sized tumors (<5cm, p=0.06) compared with fusion-negative RMS. In the cases we examined, no fusion-positive RMS occurred in a low risk genitourinary primary site (0 of 8); instead, most fusion-positive RMS with low-risk clinical features occurred at head and neck primary sites, including the orbit (Table II).
TABLE I. Fusion Status in Original ARMS Cases by Re-review Diagnosis.
| Total Cases | Re-review Diagnosis, n (%) | |||||
|---|---|---|---|---|---|---|
| ARMS | ERMS | Mixed RMS | RMS, NOS | Not reviewed | ||
| PAX3-FOXO1 | 7 | 5 (45%) | 1 (6%) | 0 | 1 (50%) | 0 |
| PAX7-FOXO1 | 6 | 3 (27%) | 0 | 1 (33%) | 1 (50%) | 1 |
| Fusion-negative | 17 | 3 (27%) | 12 (70%) | 2 (67%) | 0 | 0 |
| Unknown | 4 | 0 | 4 (24%) | 0 | 0 | 0 |
| Total | 34 | 11 | 17 | 3 | 2 | 1 |
Figure 2.
ERMS with a PAX3-FOXO1 fusion. The histologic features of this case were variable, including a predominant spindle cell pattern (A, 40× objective magnification) with areas of dense ERMS (B, 40× objective magnification). Focal areas showed microalveolar architecture (C, 40× objective magnification). Myogenin IHC performed with this case showed strong (3+) nuclear reactivity (D, 40× objective magnification).
TABLE II. Comparison of FOXO1 Fusion Status with Patient Characteristics for D9602 Patients.
| Characteristic | FOXO1 Fusion Positive | FOXO1 Fusion Negative | p-value Fisher's exact test |
|---|---|---|---|
| (N=13) | (N=21) | ||
| Age | |||
| <1 | 2 | 1 | |
| 1-9 | 10 | 15 | |
| ≥10 | 1 | 5 | 0.31 |
| Sex | |||
| Male | 6 | 18 | |
| Female | 7 | 3 | 0.02 |
| Primary site | |||
| Orbit | 5 | 6 | |
| Other head and neck (not parameningeal) | 5 | 6 | |
| GU-non bladder/prostate | 0 | 8 | |
| Extremity | 2 | 1 | |
| Trunk | 1 | 0 | 0.04 |
| Stage | |||
| 1 | 9 (69%) | 20 (95%) | |
| 2 | 4 | 1 | 0.06 |
| Group | |||
| I | 4 | 11 | |
| IIa | 4 | 5 | |
| III | 5 | 5 | 0.45 |
| Size | |||
| ≤ 5 cm | 13 (100%) | 15 (71%) | |
| > 5 cm | 0 | 6 | 0.06 |
| Regional lymph nodes | |||
| N-0 | 13 | 21 |
Outcome of Patients Enrolled in D9602as ARMS
Although D9602 included a small number of patients enrolled as ARMS, there was a trend toward superior outcome for patients with fusion-negative RMS versus fusion-positive RMS. Patients with fusion-negative confirmed ARMS (n=3) had a 5-year EFS of 100%, and patients reclassified as ERMS (presumed or proven fusion-negative, n=18) had a 5-year EFS of 81% (CI: 51%, 93%). Patients with fusion-positive RMS did poorly; patients with PAX3-FOXO1 fusion-positive RMS (n=7) had a 43% 5-year EFS (CI: 10%, 73%) and patients with PAX7-FOXO1 fusion-positive RMS (n=6) had a 67% 5-year EFS (CI: 19%, 90%) (Supplemental Figure 1). Regrouping these patients by fusion status alone (fusion-positive RMS, n=13 versus fusion-negative RMS, n=21) suggested a trend but did not reach statistical significance, with 5-year EFS estimates of 54% for fusion-positive RMS (CI: 25%, 76%) compared with 83% for fusion-negative RMS (CI: 56%, 94%) (p=0.15) (Supplemental Figure 2). 5-year OS for fusion-positive RMS was 85% (CI: 51%, 96%) versus 100% for fusion-negative RMS (p=0.27) (Supplemental Figure 3).
Outcome of ARMS with Low-risk Clinical Features Enrolled in D9602 and D9803
Our review identified 16 clinically low-risk patients enrolled in D9602 with ARMS confirmed by current histologic criteria, or fusion-positive RMS of other histologic types (mixed RMS, ERMS or RMS NOS). The previous histologic re-classification of patients enrolled in D9803 identified fifty patients with ARMS confirmed by current histologic criteria and fusion-positive RMS.[4] Thus, outcome data are available for 66 patients with either fusion-negative ARMS or fusion-positive RMS with low-risk clinical features. The characteristics of this group are shown in Supplemental Table I. Within this group, 53 (80%) were fusion-positive, with 35 (53%) PAX3-FOXO1 and 18 (27%) PAX7-FOXO1.
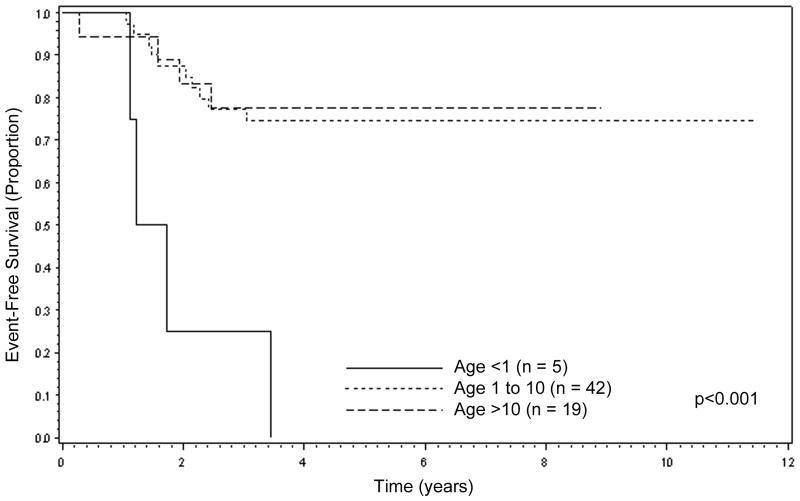
On examining patient characteristics, including age, sex, Group, tumor size, lymph node status, and FOXO1 fusion status, only age <1 year was significantly associated with inferior EFS. The 5-year EFS for patients <1 year of age was 0% (n=5), compared to 75% for patients aged 1-9 (n=42, CI: 58%, 86%) and 78% for patients ≥10 years of age (n=19, CI: 51%, 91%) (p=0.0001, Figure 3). However, despite the differences in 5-year EFS, 5-year OS was similar for each age group: <1 year old, 75% (CI: 13%, 96%); 1-9 years old, 85% (CI: 70%, 93%); and ≥10 years old, 78% (CI; 51%, 91%, p=0.69).
Figure 3.
Event-Free Survival for ARMS with low-risk clinical features by patient age, combined D9602 and D9803 cases (n=66).
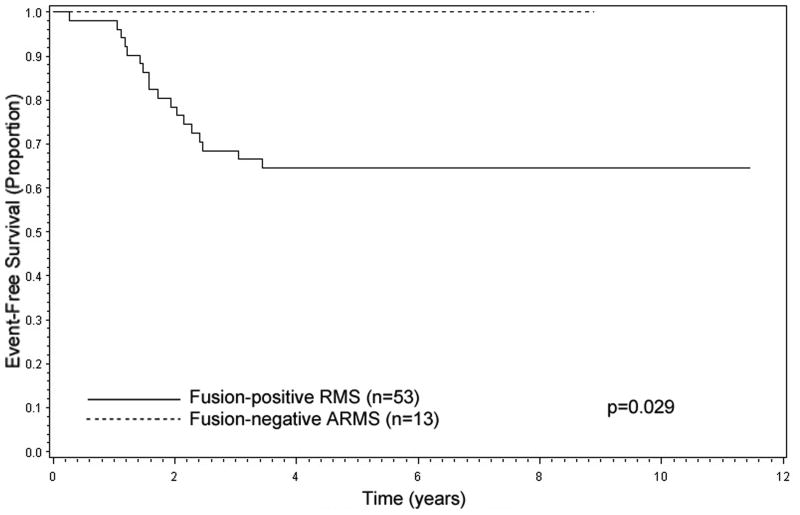
Among patients with confirmed ARMS and low-risk clinical features, fusion status predicted outcome. Patients with fusion-positive RMS had inferior 5-year EFS compared to fusion-negative ARMS: fusion-positive RMS, 64% (n=53, CI: 50%, 76%); fusion-negative ARMS, 100% (n=13, p=0.029) (Figure 4). 5-year OS also tended to show inferior OS for fusion-positive RMS; fusion-negative ARMS, 100%; fusion-positive RMS, 79% (p=0.11) (data not shown).
Figure 4.
Event-Free Survival for ARMS with low-risk clinical features by FOXO1 fusion status, combined D9602 and D9803 cases (n=66).
Discussion
Since 1999, patients with ARMS have been excluded from low-risk studies due to their inferior prognosis. However, we and others recently confirmed that fusion status is a key determinant of outcome for children with RMS.[7] To better understand the histologic, biologic, and prognostic features of patients with ARMS and low-risk clinical features, we analyzed the group of patients treated on low-risk COG RMS study D9602. Our results highlight the complex and often challenging histologic features of this group, in which PAX7-FOXO1 fusion events comprised a relatively high proportion of cases. When combined with the previous results of D9803, our data demonstrate that fusion status significantly predicts outcome for patients with ARMS with low-risk clinical features. The excellent outcome for patients with low-risk fusion-negative ARMS suggests that these patients may be appropriately treated with reduced intensity therapy.
The histologic shift that accompanied the ICR increased the rate of ARMS diagnoses and it provided a unique opportunity to examine a subset of cases with particularly challenging histology. Re-review of patients enrolled in intermediate-risk study D9803 with an enrollment diagnosis of ARMS resulted in reclassification of 33% of cases as ERMS by current criteria.[4] Surprisingly, the histology of RMS with low-risk clinical features is more dramatically shifted by current histologic classification than the histology of intermediate-risk RMS patients enrolled as ARMS in D9803 (low-risk 52% vs. intermediate risk 33%, p=0.051).
Since FOXO1 fusion status is prognostically important for low and intermediate-risk RMS, the relevance of histologic classification could be questioned. Indeed, the varied morphologic patterns in ERMS are particularly important and challenging to recognize. We currently recommend fusion testing for all cases of RMS. Reducing costs is highly valued in the current era of medicine. Since 80% of all RMS cases are ERMS, virtually all of which are fusion-negative, fusion testing may be unnecessary in cases of classic ERMS and significant cost savings could be realized. Future cost analysis may demonstrate the benefits of limiting testing. Myogenin IHC and other surrogate markers[10] may also be useful in triaging molecular tests, as fusion-positive tumors essentially always show abundant myogenin expression (3+ or 4+). As we learn more about the biology and histology of fusion-negative RMS, recognition of specific histologic patterns may help triage these cases to alternative molecular tests, as demonstrated by the MYOD1 mutations and NCOA2 fusions seen in SSCRMS.[11-13] Additionally, in both intermediate-risk and low-risk RMS, reexamination of histology identified morphologic patterns that mimicked ARMS, particularly foci of dense or sclerosing RMS. This area of histologic overlap is especially important to identify, given the recent WHO classification of SSCRMS as a distinct entity.[14] Sclerosing patterns were also frequently admixed with typical or dense embryonal patterns. The occasional observation of sclerosis in classic ARMS adds to the diagnostic challenges in classifying RMS, although the presence of diffuse myogenin reactivity (4+) should favor the diagnosis of ARMS over SSCRMS. Interestingly, all of the ARMS cases in low-risk study D9602 with areas of sclerosis harbored a PAX7-FOXO1 fusion. Here and in previous reviews, [4, 15] we have noted that ARMS cases with PAX7-FOXO1 often have more varied histologic patterns. The over-representation of tumors with PAX7-FOXO1 in low-risk study D9602 may contribute to the varied histology of ARMS in this cohort. We found a higher rate of PAX7-FOXO1 fusions in histologically confirmed ARMS in D9602 (27%), compared with 16% of confirmed ARMS in D9803 and in 22% of ARMS in IRS IV.[3, 4] Notably, D9803 patients with confirmed ARMS and low-risk clinical features showed a 24% rate of PAX7-FOXO1.[7]
Diagnostic challenges are not limited to tumors with PAX7-FOXO1, as evidenced by the single case of ERMS with a PAX3-FOXO1 fusion. Overall, the presence of a PAX3/PAX7-FOXO1 fusion remains highly specific for ARMS.[3] In our review, the fusion-positive reclassified ERMS tumor showed predominantly typical ERMS morphology with ovoid to spindled nuclei admixed with areas of more dense cellularity. The nuclear features in these dense areas, as well as in scattered micro-alveolar foci, are arguably those of ARMS; however these admixed areas represented a minority of the tumor compared with the majority of areas that showed typical ERMS histology. The location of this tumor in the extremity is somewhat unusual for ERMS.
As in intermediate-risk RMS, FOXO1 fusion status for patients with ARMS with low-risk clinical features is a prognostic marker. All patients with ARMS enrolled in D9602 received VAC chemotherapy regimen similar to the D9803 intermediate-risk RMS study, including a total cumulative cyclophosphamide dose of 28.6 g/m2. This combined cohort represents the largest study of fusion status and outcome in ARMS with low-risk clinical features. The outcome for patients with fusion-positive RMS with low-risk clinical features (5-year EFS 54%, 5-year OS 85%) appears inferior to patients with low-risk ERMS treated with VAC chemotherapy in Subgroup B of D9602 (5-year EFS 85%, 5-year OS 95%); outcome for fusion-positive RMS with low-risk clinical features is similar to outcome for patients with intermediate-risk Stage 2/3, Group III ARMS enrolled in D9803 (5-year EFS 55%, 5-year OS 68%)[4]. We also show that outcome for histologically confirmed yet fusion-negative ARMS is excellent; in D9602 and D9803 these patients had a 5-year EFS of 100%. These findings support treating patients with fusion-positive RMS with low-risk clinical features on intermediate-risk protocols and treating fusion-negative alveolar rhabdomyosarcoma with low-risk clinical features with reduced intensity therapy.
Supplementary Material
Supplemental Figure 1: Event-Free Survival by FOXO1 fusion status and fusion partner, D9602 low-risk cases (n=34).
Supplemental Figure 2: Event-Free Survival for RMS with low-risk clinical features by FOXO1 fusion status, D9602 low-risk cases (n=34).
Supplemental Figure 3: Overall Survival for RMS with low-risk clinical features by FOXO1 fusion status, D9602 low-risk cases (n=34).
Supplemental Table I: Patient Characteristics of Confirmed ARMS and Fusion-positive RMS with Low-risk Clinical Features Enrolled on D9602 or D9803.
Acknowledgments
This study was supported by St. Baldrick's Foundation Consortium Research Grant (244660), and National Cancer Institute grants U10CA180886, U10CA180899, U10CA098543, and U10CA098413. FGB was supported by the Intramural Research Program of the National Cancer Institute. All studies were conducted in accordance with institutional review board approved protocols.
Abbreviations
- ARMS
alveolar rhabdomyosarcoma
- COG
Children's Oncology Group
- EFS
event-free survival
- ERMS
embryonal rhabdomyosarcoma
- ICR
International Classification of Rhabdomyosarcoma
- OS
overall survival
- RMS
rhabdomyosarcoma
- SSCRMS
sclerosing/spindle cell RMS
- VAC
Vincristine, dactinomycin and cyclophosphamide
- VTC
Vincristine, topotecan, and cyclophosphamide
Footnotes
Disclosures: All studies were conducted in accordance with institutional review board approved protocols.
Conflict of Interest Statement: The authors have no conflicts of interest.
References
- 1.Newton WA, Jr, Gehan EA, Webber BL, Marsden HB, van Unnik AJ, Hamoudi AB, Tsokos MG, Shimada H, Harms D, Schmidt D, Ninfo V, Cavazzana AO, Gonzalez-Crussi F, Parham DM, Reiman HM, Asmar L, Beltangady MS, Sachs NE, Triche TJ, Maurer HM. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer. 1995;76(6):1073–1085. doi: 10.1002/1097-0142(19950915)76:6<1073::aid-cncr2820760624>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Barr FG, Smith LM, Lynch JC, Strzelecki D, Parham DM, Qualman SJ, Breitfeld PP. Examination of gene fusion status in archival samples of alveolar rhabdomyosarcoma entered on the Intergroup Rhabdomyosarcoma Study-III trial: a report from the Children's Oncology Group. J Mol Diagn. 2006;8(2):202–208. doi: 10.2353/jmoldx.2006.050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorensen PH, Lynch JC, Qualman SJ, Tirabosco R, Lim JF, Maurer HM, Bridge JA, Crist WM, Triche TJ, Barr FG. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the children's oncology group. J Clin Oncol. 2002;20(11):2672–2679. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- 4.Rudzinski ER, Teot LA, Anderson JR, Moore J, Bridge JA, Barr FG, Gastier-Foster JM, Skapek SX, Hawkins DS, Parham DM. Dense pattern of embryonal rhabdomyosarcoma, a lesion easily confused with alveolar rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Am J Clin Pathol. 2013;140(1):82–90. doi: 10.1309/AJCPA1WN7ARPCMKQ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raney RB, Walterhouse DO, Meza JL, Andrassy RJ, Breneman JC, Crist WM, Maurer HM, Meyer WH, Parham DM, Anderson JR. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2011;29(10):1312–1318. doi: 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arndt CA, Stoner JA, Hawkins DS, Rodeberg DA, Hayes-Jordan AA, Paidas CN, Parham DM, Teot LA, Wharam MD, Breneman JC, Donaldson SS, Anderson JR, Meyer WH. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children's oncology group study D9803. J Clin Oncol. 2009;27(31):5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skapek SX, Anderson J, Barr FG, Bridge JA, Gastier-Foster JM, Parham DM, Rudzinski ER, Triche T, Hawkins DS. PAX-FOXO1 fusion status drives unfavorable outcome for children with rhabdomyosarcoma: a children's oncology group report. Pediatr Blood Cancer. 2013;60(9):1411–1417. doi: 10.1002/pbc.24532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudzinski ER, Anderson JR, Hawkins DS, Skapek SX, Parham DM, Teot LA. The World Health Organization Classification of Skeletal Muscle Tumors in Pediatric Rhabdomyosarcoma: A Report From the Children's Oncology Group. Arch Pathol Lab Med. 2015;139:1281–1287. doi: 10.5858/arpa.2014-0475-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishio J, Althof PA, Bailey JM, Zhou M, Neff JR, Barr FG, Parham DM, Teot L, Qualman SJ, Bridge JA. Use of a novel FISH assay on paraffin-embedded tissues as an adjunct to diagnosis of alveolar rhabdomyosarcoma. Lab Invest. 2006;86(6):547–556. doi: 10.1038/labinvest.3700416. [DOI] [PubMed] [Google Scholar]
- 10.Rudzinski ER, Anderson JR, Lyden ER, Bridge JA, Barr FG, Gastier-Foster JM, Bachmeyer K, Skapek SX, Hawkins DS, Teot LA, Parham DM. Myogenin, AP2beta, NOS-1, and HMGA2 Are Surrogate Markers of Fusion Status in Rhabdomyosarcoma: A Report From the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Am J Surg Pathol. 2014;38(5):654–659. doi: 10.1097/PAS.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agaram NP, Chen CL, Zhang L, LaQuaglia MP, Wexler L, Antonescu CR. Recurrent MYOD1 mutations in pediatric and adult sclerosing and spindle cell rhabdomyosarcomas: evidence for a common pathogenesis. Genes Chromosomes Cancer. 2014;53(9):779–787. doi: 10.1002/gcc.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohsaka S, Shukla N, Ameur N, Ito T, Ng CK, Wang L, Lim D, Marchetti A, Viale A, Pirun M, Socci ND, Qin LX, Sciot R, Bridge J, Singer S, Meyers P, Wexler LH, Barr FG, Dogan S, Fletcher JA, Reis-Filho JS, Ladanyi M. A recurrent neomorphic mutation in MYOD1 defines a clinically aggressive subset of embryonal rhabdomyosarcoma associated with PI3K-AKT pathway mutations. Nat Genet. 2014;46(6):595–600. doi: 10.1038/ng.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szuhai K, de Jong D, Leung WY, Fletcher CD, Hogendoorn PC. Transactivating mutation of the MYOD1 gene is a frequent event in adult spindle cell rhabdomyosarcoma. J Pathol. 2014;232(3):300–307. doi: 10.1002/path.4307. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher CDM, World Health Organization. International Agency for Research on Cancer . WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013. p. 468. [Google Scholar]
- 15.Parham DM, Barr FG. Classification of rhabdomyosarcoma and its molecular basis. Adv Anat Pathol. 2013;20(6):387–397. doi: 10.1097/PAP.0b013e3182a92d0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Event-Free Survival by FOXO1 fusion status and fusion partner, D9602 low-risk cases (n=34).
Supplemental Figure 2: Event-Free Survival for RMS with low-risk clinical features by FOXO1 fusion status, D9602 low-risk cases (n=34).
Supplemental Figure 3: Overall Survival for RMS with low-risk clinical features by FOXO1 fusion status, D9602 low-risk cases (n=34).
Supplemental Table I: Patient Characteristics of Confirmed ARMS and Fusion-positive RMS with Low-risk Clinical Features Enrolled on D9602 or D9803.