Abstract
Mucopolysaccharidosis IV A (MPS IV A), Morquio A, is caused by deficiency in lysosomal enzyme N-acetylgalactosamine-6-sulfate sulfatase (GALNS), which is responsible for the catabolism of the glycosaminoglycans (GAGs) keratan sulfate (KS) and chondroitin 6-sulfate (C6S). Accumulation of GAGs results in disrupted cartilage formation and skeletal dysplasia. In this prospective cross-sectional study, bone mineral density (BMD) of the whole body (WB), lumbar spine (LS), and lateral distal femur (LDF) was acquired by dual-energy X-ray absorptiometry (DXA) on patients with MPS IV A. Functional abilities, medical history, Tanner score, and laboratory results were reviewed. Age and sex-matched norms were used to calculate Z-scores. Participants included 18 patients (13 females; 16 were unrelated) with a mean age of 21.4 years (3.3 to 40.8 years). While every patient was able to bear weight, 9 were full-time ambulators. Whole-body DXA could be obtained on only 6 patients (5 full-time ambulators) because of respiratory compromise caused by the position, presence of hardware, or positioning difficulties. Mean WB Z-score was −2.0 (range − 0.3 to −4.1). Technical issues invalidating LS DXA in 8 patients included kyphosis at the thoracolumbar junction resulting in overlap of vertebrae in the posterior-anterior view. Mean LS BMD Z-score in full-time ambulators was −3.4 (range − 1.6 to −5.0) and in the non-/partial ambulator was −4.0 (−3.7 to −4.2). Lateral distal femur BMD was acquired on every patient, and average Z-scores were −2 or less at all sites; full-time ambulators exhibited higher BMD. In conclusion, the LDF proved to be the most feasible site to measure in patients with MPS IV A. The higher LDF values in ambulators suggest this should be a consideration in promoting bone health for this group.
Keywords: Bone mineral density, Dual-energy X-ray absorptiometry, DXA, Lateral distal femur, Lumbar spine, Morquio
1. Introduction
Morquio syndrome type A (mucopolysaccharidosis type IV A, MPS IV A) is an autosomal recessive lysosomal storage disorder caused by deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS) [1–3]. This deficiency leads to accumulation of excessive glycosaminoglycans (GAGs) keratan sulfate (KS) and chondroitin-6-sulfate (C6S) primarily in bone, cartilage, ligaments, and the extracellular matrix (ECM) [3].
In patients with MPS IV A, even at birth, cartilage formation is disrupted, resulting in poor bone mineralization [4]. Systemic skeletal symptoms are caused by excessive KS storage and result in spondyloepiphyseal dysplasia, striking short trunk stature, cervical spinal cord compression, pectus carinatum, kyphoscoliosis, knock-knee, hypermobile joints, and an abnormal gait with an increased tendency to fall. Many patients become wheelchair dependent in their second decade and undergo multiple surgeries to alleviate serious medical complications [5–6].
Three studies report bone mineral density (BMD) in MPS IV A, all with limited sample size (n = 2,9,2, respectively) [7–9]. Dual energy X-ray absorptiometry (DXA) was used to assess BMD in the studies. Both Rigante et al. and Koura et al. reported low BMD at several body sites measured. Lin et al. reported normal BMD results after applying the height-adjusted Z-score (HAZ) method [10] used to correct for the height deficits. Subsequently, the use of the HAZ method for correction of BMD has been brought into question in the presence of skeletal dysplasia [11].
Bone properties including shape, structure, and strength of bone are related to load and forces on the bone. The loss of BMD in medical conditions affecting the ability to bear weight such as cerebral palsy (CP), spina bifida, and muscular dystrophy has been described. Low BMD of the lower extremities as measured by the lateral distal femur (LDF) DXA is directly associated with lack of ambulation or weight bearing [12–16]. Henderson and colleagues described a strong association between low BMD at the LDF and fracture history [15].
In this report, we describe BMD, fracture history, and ambulation in 16 unrelated (18 total) MPS IV A patients to understand the natural course of BMD. We hypothesized a higher prevalence of low BMD in these patients compared with normal subjects. Because of the limitations of whole body (WB) and lumbar spine (LS) measurements in this patient population, we also evaluated BMD by using another site, the LDF. This alternative measurement site applied to conditions affecting ambulation and spine abnormality is directly applicable to patients with MPS IV A.
2. Material and methods
2.1. Subjects
In this prospective, cross-sectional study, we evaluated 18 patients (16 unrelated) with Morquio syndrome type A (13 females) ranging in age from 3.3 to 40.8 years (mean age 21.4 years) at our hospital. All patients were diagnosed by enzymatic assay. Current medical status (functional abilities, anthropometrics, Tanner stage, medications) and medical history (fracture, physical development, birth events, diagnosis, medications, surgery) were ascertained through questionnaire and review of available medical records. Ambulatory status was categorized as either full-time (able to ambulate without the use of an assistive device [cane, crutches, or walker]) or partial/non-ambulator (use of assistive device [walker or wheelchair] at school only or at the mall, or minimally able/unable to bear weight). Prior radiologic images, if available, were provided by subjects. Available laboratory results were reviewed. Height and weight measures were obtained at DXA scan acquisition, and height Z-scores were calculated using National Health and Nutrition Survey (NHANES) LMMS tables (accessed 9/5/15) [17]. The maximum age available (19.9 years) was used for patients over this age. The study was approved by the Nemours Institutional Review Board, and informed consent was obtained from all participants.
2.2. Bone mineral density evaluation
Bone mineral density was assessed by DXA using a Hologic (Bedford, MA, USA) Discovery A model machine located in the Medical Imaging Department. The following body sites were scanned: WB, LS, and LDF. In addition, on adult subjects, the proximal femur (hip) was also attempted. The LDF scans were analyzed for three distinct regions of interest previously described [18–19] to assess bone density in different types of bone. Region 1 (R1) is predominantly trabecular bone, region 2 (R2) is mixed trabecular and cortical bone, region 3 (R3) is primarily cortical bone. The LDF BMD was assessed bilaterally if possible; left and right femur BMD values were averaged, and Z-scores were calculated. Radiologic images of the lateral spine, including radiographs and inter-vertebral assessment by DXA, were used to aid in correct region of interest placement on the LS DXA.
Bone mineral density results were compared with age and sex-matched norms to calculate Z-scores using manufacturer-provided normative values for the WB and LS and published norms for the LDF [20]. T-scores, used in adult BMD assessment, are not reported with our MPS IV A adult BMD results because no subject is post-menopausal or over age 50 [21]. For subjects older than 18 years of age, LDF Z-score was calculated using the oldest normative values available for sex (18 years). The LDF values for patients younger than the lower age limit of the normative data available for the LDF were excluded from analysis. Data are evaluated based on age (adult over 18 years vs. children). The DXA values of BMD more than 2 standard deviations (SD) below the normal mean, expressed as Z-score ≤ −2, were considered abnormal. No height adjustment of BMD results was used because of the abnormal bone morphology seen in patients with MPS IV A. Since weight bearing is known to affect BMD, patients were grouped into two categories by ambulatory status: 1) full-time (F) ambulators and 2) part-time or non-ambulators (P/N). The BMD results are examined for the entire group and relative to the amount of ambulation.
3. Results
Eighteen patients (16 unrelated) with MPS IV A (13 females) were evaluated; average age was 21.4 years (3.3 to 40.8 years). Phenotype analyses based on height [5] were performed on all subjects. For the 6 patients with available genotype analysis, phenotype/genotype correlations were performed (Table 1).
Table 1.
Clinical data for mucopolysaccharidosis IV A patients.
| ID | Height (cm) | Height Z-score | Weight (kg) | Age (yrs) | Sex | ERT | Genotype | Ambulatory status |
|---|---|---|---|---|---|---|---|---|
| 1 | 88.9 | –2.2 | 12.7 | 3.3 | M | N | n/a | F |
| 2 | 97 | –2.2 | 16.2 | 4.9 | F | N | n/a | F |
| 3 | 99.1 | –3.5 | 15.9 | 6.1 | F | Y | p.G301C/G301C | F |
| 4 | 86.4 | –8.7 | 13.2 | 9.1 | M | Y | n/a | P/N |
| 5 | 106.7 | –8.7 | 37.6 | 15.9 | F | N | n/a | P/N |
| 6 | 91.44 | –8.7 | 28.6 | 16.3 | F | N | P.ivs8 + 1G > c/ivs8 + 1G > c | P/N |
| 7 | 114.3 | –7.7 | 35.5 | 18 | F | N | p.S287L/L352P | P/N |
| 8 | 116.8 | –8.1a | 33.2 | 18.9 | M | Y | n/a | P/N |
| 9 | 113.8 | –8.5a | 34.7 | 21.0 | M | Y | n/a | F |
| 10 | 96.5 | –10.1a | 25.4 | 21.1 | F | N | n/a | P/N |
| 11 | 118 | –8.0a | 34.5 | 22.4 | M | N | p.G42E/P125L | F |
| 12 | 126 | –5.7a | 34.5 | 23.0 | F | N | n/a | P/N |
| 13 | 129.5 | –5.2a | 36.4 | 25.6 | F | N | p.S287L/L352P | F |
| 14 | 91.4 | –10.8a | 22.7 | 30.6 | F | N | p.M41K/M41K | P/N |
| 15 | 91.4 | –10.8a | 25.5 | 34.2 | F | N | n/a | F |
| 16 | 122 | –6.3a | 34.4 | 36.5 | F | N | n/a | F |
| 17 | 106.7 | –8.6a | 36.4 | 40.8 | F | N | n/a | P/N |
| 18 | 96.5 | –10.1a | 24 | 38.4 | F | N | n/a | F |
ERT = enzyme replacement therapy; F = full-time ambulator; P/N = partial or non-ambulator.
Based on age 20 years.
Four patients were undergoing enzyme replacement therapy (ERT). While every subject had the ability to bear weight, 9 were full-time ambulators. Of the 9 P/N ambulators, 7 were able to walk/bear weight but chose to use a power wheelchair full time for speed and convenience. Only 2 patients (both adults) were not able to ambulate without assistance and were unable to walk for over 50 yards with a walker. Only two fractures were reported and were due to trauma (hand slammed in door; femur from tripping while running with crutches) (Table 1).
3.1. DXA utility by body site
The recommended body sites to measure by DXA differ between adults and children [22]. In children, WB and LS are the preferred sites along with LDF in certain conditions. In adults, the LS and proximal femur are recommended. Use of LDF in adults has been described by Henderson and colleagues; however, normative data are currently unavailable [23].
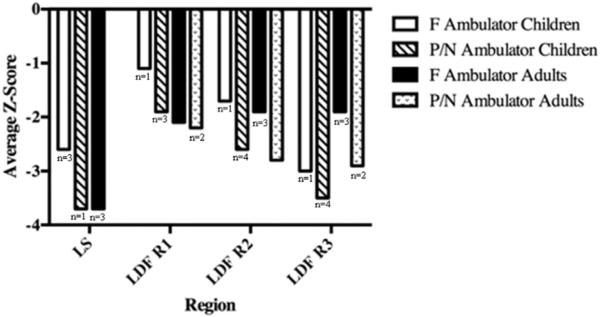
In the present study, we assessed the utility of DXA sites in patients with MPS IV A. Results for WB could be obtained on only 6 subjects (2 children, 4 adults) because of respiratory compromise caused by the position, the presence of hardware, or positioning difficulties. The WB was not well tolerated because of discomfort experienced by subjects when lying flat on their back; in a few cases, subjects were unable to breathe. The proximal femur was successfully acquired on only 1 of the 11 adults, but accurate analysis of the scan was impossible because of abnormal anatomy. The LS scan was successfully acquired in all patients, but, because of the abnormal shape and position of vertebrae seen in Morquio syndrome type A, the technical validity of the LS DXA was compromised and limited to 10 (55.6%) of our subjects (Fig 1).
Fig. 1.
Lumbar spine and lateral distal femur bone mineral density Z-scores in children and adults by ambulatory status. F = full-time ambulator; P/N = partial or non-ambulator; LS = lumbar spine; LDF R1 = lateral distal femur region 1; LDF R2 = lateral distal femur region 2; LDF R3 = lateral distal femur region 3.
The LDF was the only site at which all patients could be scanned, showing technically valid results (Table 2).
Table 2.
Utility of dual-energy X-ray absorptiometry in children and adults with mucopolysaccharidosis IV A: acquisition versus valid results.
| Body site scanned | n | Number of scans acquired | Number of technically valid scans |
|---|---|---|---|
| WB | 18 | 7 (38.9%) | 6 (33.3%) |
| Proximal femur (hip) | 11 adults | 1 (9.1%) | 0 |
| LS | 18 | 18 (100%) | 10 (55.6%) |
| LDF | 18 | 18 (100%) | 18 (100%) |
WB = whole body; LS = lumbar spine; LDF = lateral distal femur.
3.2. DXA results
3.2.1. Overall
We were able to obtain WB DXA results on 6 subjects (5 full-time ambulators). Average WB Z-score for the children was −1.6 (range −0.6 to −2.5) and for the adults was −2.2 (range −0.3 to −4.1). Average LS Z-score for the children was −2.9 (range −1.6 to −4.4) and for the adults was −4.0 (range −2.6 to −5.0). For the children, LDF Z-score results were as follows by region of interest: R1 = −2.1 (range −0.6 to −3.3); R2 = −2.6 (range −1.4 to −4.0); R3 = −3.1 (range −1.4 to −5.2). For the adults, LDF Z-score results were as follows by region of interest: R1 = −2.4 (range −1.3 to −4.3); R2 = −2.3 (range −0.8 to −3.2); R3 = −2.4 (range −1.3 to −3.7).
3.2.2. DXA by ambulatory status
Of the 18 patients, nine were F ambulators. Age did not predict full-time ambulatory status (Table 1). Of the 10 patients with valid LS BMD results, 8 were F ambulators. The LS BMD was low for both children and adults, regardless of ambulatory status. At the LDF, BMD Z-scores were consistently lower in the P/N ambulation group at all regions and in children and adults (Fig. 1).
3.2.3. DXA by genotype and ambulation status
For patients with available genotype analysis (Table 1), 2 patients had the same genotype (p. S287L/L352P). Both were untreated females, aged 18 years (partial/non-ambulator) and 26 years (full-time ambulator). The partial/non-ambulator had consistently lower BMD across all body sites measured than the full-time ambulator (Fig. 2).
Fig. 2.
Lumbar spine and lateral distal femur bone mineral density Z-scores by ambulatory status in two unrelated, untreated adult females sharing the same genotype (p.S287L/L352P).
F = full-time ambulator; P/N = partial or non-ambulator; DXA = dual energy X-ray absorptiometry; LS = lumbar spine; LDF R1 = lateral distal femur region 1; LDF R2 = lateral distal femur region 2; LDF R3 = lateral distal femur region 3.
4. Discussion
In this study of patients with MPS IV A, we have described the technical considerations of measuring BMD (Table 2), reported BMD results (Fig. 1), and evaluated the influence of ambulation (Fig. 2).
Undegraded C6S and KS accumulation leads to alterations in the connective tissue and cartilage ground substance, distorting bone mass acquisition and perturbing the regular microarchitecture of bone tissue [24]. Histopathological studies have indicated that patients with MPS IV A present impaired bone quality because of distortion of geometric shape, collagen disposition in ECM, and remodeling, resulting in poor bone mineralization [25–26]. As patients with MPS IV A age, they frequently decide to use wheelchairs full time for convenience and comfort because of bone deformity and chronic pain.
While we expected to see older patients using wheelchairs because of progression of the disease, in the present study, only our oldest patient required the use of a wheelchair full time. The elementary school-age patients used assistive devices at school but still ambulated elsewhere. The high school-aged and older patients who were P/N ambulators opted to use the power chair for convenience (rather than need), foregoing weight bearing.
Impairment or lack of ambulation corresponds with low bone BMD of the LDF in children with a variety of physical disabilities including CP, Duchenne muscular dystrophy (DMD), and spina bifida [12–16]. In this group of patients with MPS IV A, a trend of lower BMD in the P/N ambulators was observed in both adult and children and at all body sites measured (Fig. 1). The full-time ambulators had higher BMD at all ages. Overall, BMD values of the patients investigated in this study were below normal at most sites measured, but there was wide variation in BMD results in both age groups. Younger-aged patients had some normal BMD Z-scores regardless of ambulatory status. The P/N adults showed no normal BMD Z-scores.
There are more than 300 mutations already described in the GALNS gene illustrating the heterogeneity seen in this pathology [27]. In the present study, genotype analyses were available for 6 patients (Table 1). Within this subset of unrelated patients, two female adult patients shared the same genotype (p.S287L/L352P). In these two patients, one was a FT ambulator, and the other was a P/N ambulator. The BMD Z-scores were consistently higher at all body sites measured in the patient who walked full time, despite the fact that she was older (26 years), than the P/N ambulator (18 years) (Fig. 2). This finding suggests that ambulation provides a protective effect on BMD.
Despite the low BMD values and commensurate Z-scores, just two fractures from trauma were reported. Similarly, in two studies of MPS patients (I, II, and VI; II, VI), only traumatic fractures were reported in 1 of 8 and 2 of 40 patients, respectively [28,11]. In typical healthy adults, the correlation of low BMD DXA and fracture has been established. Only one study exists describing the relationship between LDF BMD and fracture. In 2010, Henderson and colleagues described a strong association between low BMD at the LDF and fracture history in children with CP and DMD [15]. The relationship of low LDF BMD and fracture was not observed in our patients with MPS IV A.
In this study, height adjustments for DXA were not used for BMD because of the large height deficits seen in our patients with MPS IV A (Table 1).When using height adjustments, BMD results are frequently normalized. This normalization of BMD results was reported by Lin and colleagues after using the HAZ method in 9 children with MPS IV A [8]. Fung et al. also used the HAZ method in a group of children and adults with MPS II and VI and showed the same results—overall normalization of WB and LS BMD after using the HAZ method [28]. It is important to recognize that the HAZ method was developed for children aged 6 to 18 years with height deficits, under the condition of normal bone morphometry. Zemel et al. describe that the shortest height used is −2.6 SD below that of the age-matched controls [10]. Polgreen and colleagues [11] describe that the HAZ method is valid at the LS in patients with height deficits as low as −6 SD, whereas WB BMD results are overestimated in the same population. The maximum height deficit acceptable for the HAZ method is unknown.
Polgreen et al. [11] demonstrated that HAZ overestimated the WB BMD results, likely because of overall atypical bone geometry seen in MPS. For the LS, they determined that the HAZ method yielded valid results. However, this is likely due to fewer spinal skeletal deformities and milder height deficits seen in MPS I and II. While MPS VI patients have skeletal spinal abnormalities and height deficits more similar to those of patients with MPS IV A, it is important to note the average height Z-score in the MPS VI subjects was −4.3 (±0.6), whereas the average height Z-score in our study was −7.4 (±2.8).
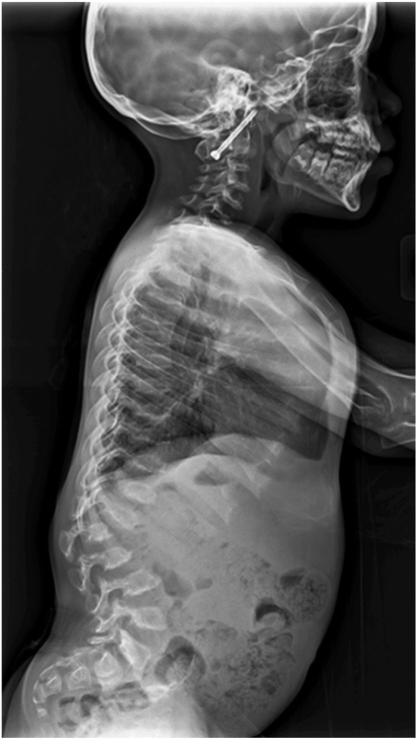
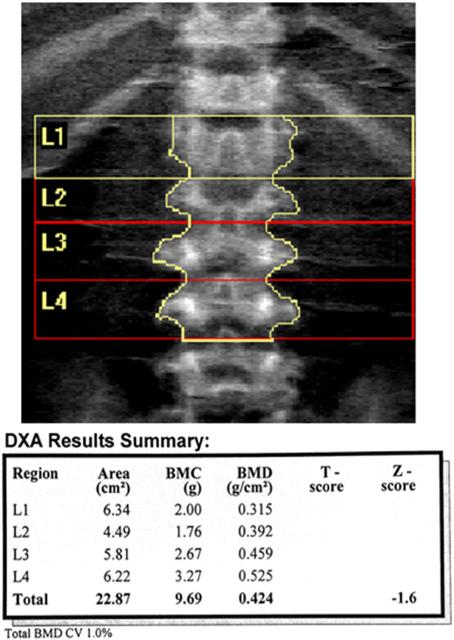
The skeletal abnormalities seen in our patients were significant, particularly at the LS. While we were able to acquire LS DXA scans, the atypical morphometry observed in patients with MPS IV A precluded valid use of the LS DXA in nearly half of our patients, and correct analysis required review of radiographic images (Figs. 3 & 4).
Fig. 3.
Morphologic changes in the spine in mucopolysaccharidosis IV A influence dual energy X-ray absorptiometry (DXA) assessment. (A) The lateral spine radiograph shows sharp angle kyphosis centered on L2 with a hypoplastic vertebral body. Anterior wedging and beaking are noted at multiple levels. There has been cervical fusion with fixation.
Fig. 4.
Lumbar spine DXA requires careful delineation of vertebral body levels and margins. The DXA results summary notes bone mineral content is low in L1 and L2 with differences in area resulting in bone mineral density (BMD) variation. Total BMD Z-score (−1.6) is in the normal range for age and sex.
The WB DXA scan was not well tolerated, as described earlier (Table 2). Only the LDF scan was feasible and well tolerated in all patients investigated in our study. The LDF analysis takes into consideration size of bone and adjusts the three regions of interest to the bone size. The HAZ method has been validated for the WB, LS, forearm, and proximal femur, but not the LDF.
This study has several limitations including a limited sample size with wide range of ages (3.1–40.8 years). By splitting the groups by age and then by ambulation, the results are limited by even smaller sample sizes within the categories. While this was a prospective study, we were limited by a convenience sample of patients seen at our hospital who were willing to enroll in the study—even being an autosomal recessive condition, our group was composed of 77% females. Genotype information was not available on every patient, limiting our ability to perform genotype/phenotype correlations or evaluating BMD relative to genotype. Our evaluation of bone was limited to DXA, which is an areal (2-dimensional) measurement that has limitations when assessing a 3-dimensional object (bone). Using DXA to assess bone with atypical bone morphometry presents technical problems that must be considered when interpreting results. Use of other volumetric assessment techniques would add value to the study of BMD in MPS IV A. While normative values are available for WB and LS within the age range in this study group, we were limited to the age ranges of 6 to 18 years that are currently available for the LDF. The age of peak bone mass for lower extremities is unknown and may exceed 18 years of age.
Despite limitations, this study has value in that we present BMD results for both adults and children in the largest cohort of MPS IV A patients to date. Furthermore, we report BMD DXA results at a novel body site for patients with MPS —the lateral distal femur. All images were evaluated and interpreted by a pediatric radiologist ensuring technical validity of our findings.
5. Conclusion
Measuring BMD by DXA in patients with MPS IV A presents unique and significant challenges when using standard body sites. The WB DXA was not well tolerated or feasible. Anatomical abnormalities of the spine and technical limitations of DXA made use of the LS challenging. The application of height adjustment Z-score correction to BMD results in patients with significant height deficits and atypical morphology and should be performed with caution. The Z-score LDF DXA was the most feasible BMD DXA measurement in patients with MPS IV A. On average, BMD was low at all body sites measured in children and adults, although there was broad variation. Full-time ambulation consistently was associated with higher BMD values at all ages. Fracture did not appear to be a major concern in this group of patients with MPS IV A.
Acknowledgments
This work was supported by grants from the Austrian MPS Society grant number 3212482005, The Bennett Foundation grant number 4399, and the International Morquio Organization (Carol Ann Foundation) grant number 3212482001. S.T. was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant numbers P20GM103464 and P30GM114736. F. K. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico from Brazil (CNPq).The content of the article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors. Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/Alfred I. duPont Hospital for Children.
Abbreviations
- BMD
bone mineral density
- CP
cerebral palsy
- C6S
chondroitin-6-sulfate
- DMD
Duchenne muscular dystrophy
- DXA
dual energy X-ray absorptiometry
- ECM
extracellular matrix
- ERT
enzyme replacement therapy
- F
full-time ambulator
- GAGs
glycosaminoglycans
- GALNS
N-acetylgalactosamine-6-sulfate sulfatase
- HAZ
height-adjusted Z-score
- HGMD
The Human Gene Mutation Database
- KS
keratan sulfate
- LDF
lateral distal femur
- LS
lumbar spine
- MPS IVA
mucopolysaccharidosis type IV A
- NHANES
National Health and Nutrition Survey
- P/N
part-time or non-ambulators
- WB
whole body
Footnotes
Contributions to the project
Heidi Kecskemethy was responsible for the planning, conduct, data acquisition and analysis, and reporting of the work described in this article.
Francyne Kubaski was responsible for the data analysis and reporting of the work described in this article.
Shunji Tomatsu was involved with the planning, conduct, and reporting of the work described in this article.
H. Theodore Harcke was involved with planning, conduct, data acquisition and analysis, and reporting of work described in this article.
References
- 1.Brailsford JF. Chondro-osteo-dystrophy, roentgenopgraphic & clinical features of a child with dislocation of vertebrae. Am. J. Surg. 1929;7:404–410. [PubMed] [Google Scholar]
- 2.Morquio L. Sur une forme de dystrophie osseuse familial. Arch. Méd. Enfants Paris. 1929;32:129–135. [Google Scholar]
- 3.Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. eighth ed. McGraw-Hill; New York: 2001. pp. 3421–3452. [Google Scholar]
- 4.Tomatsu S, Almeciga-Diaz CJ, Montano AM, Yabe H, Tanaka A, Dung VC, Giugliani R, Kubaski F, Mason RW, Yasuda E, Sawamoto K, Mackenzie W, Suzuki Y, Orii KE, Barrera LA, Sly WS, Orii T. Therapies for the bone in mucopolysaccharidoses. Mol. Genet. Metab. 2015;114:94–109. doi: 10.1016/j.ymgme.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J. Inherit. Metab. Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 6.Tomatsu S, Mackenzie WG, Theroux MC, Mason RW, Thacker MM, Shaffer TH, Montano AM, Rowan D, Sly W, Alméciga-Diaz CJ, Barrera LA, Chinen Y, Yasuda E, Ruhnke K, Suzuki Y, Orii T. Current and emerging treatments and surgical interventions for Morquio A syndrome: a review. Res. Rep. Endocr. Disord. 2012;65–77 doi: 10.2147/RRED.S37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koura HM, El-Katoury A, Abdallah NI, El-Bassyouni HT, Ayoub DF, Bassiouni RI. Bone mineral density in Egyptian children with mucopolysaccharidoses. J. Res. Med. Sci. 2009;4:100–106. [Google Scholar]
- 8.Lin HY, Shih SC, Chuang CK, Chen MR, Niu DM, Lin SP. Assessment of bone mineral density by dual energy X-ray absorptiometry in patients with mucopolysaccharidoses. Orphanet. J. Rare. Dis. 2013;8:1–7. doi: 10.1186/1750-1172-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigante D, Buonuomo PS, Caradonna P. Early-onset osteoporosis with high bone turnover in children with Morquio–Brailsford syndrome. Rheumatol. Int. 2006;26:1163–1164. doi: 10.1007/s00296-006-0150-3. [DOI] [PubMed] [Google Scholar]
- 10.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kallwarf HJ. Height adjustment in assessing dual energy X-ray absorptiometry measurements of bone mass and density in children. J. Clin. Endocrinol. Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polgreen LE, Thomas W, Fung E, Viskochil D, Stevenson DA, Steinberger J, Orchand P, Whitley CB, Ensurd KE. Low bone mineral content and challenges in interpretation of dual-energy X-ray absorptiometry in children with mucopolysaccharidosis types I, II, and VI. J. Clin. Densitom. 2014;17:200–206. doi: 10.1016/j.jocd.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas RE, Kecskemethy HH, Lopiccolo MA, Hossain J, Dy RT, Bachrach SJ. Lower extremity bone mineral density in children with congenital spinal dysfunction. Dev. Med. Child Neurol. 2012;54:1133–1137. doi: 10.1111/j.1469-8749.2012.04420.x. [DOI] [PubMed] [Google Scholar]
- 13.Harcke HT, Kecskemethy HH, Conklin D, Scavina M, Mackenzie WG, McKay CP. Assessment of bone mineral density in Duchenne muscular dystrophy using the lateral distal femur. J. Clin. Neuromuscul. Dis. 2006;8:1–6. [Google Scholar]
- 14.Henderson RC, Lark RK, Gurka MJ, Worley G, Fung EB, Conaway M, Stallings VA, Stevenson RD. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110(1Pt 1):e5. doi: 10.1542/peds.110.1.e5. [DOI] [PubMed] [Google Scholar]
- 15.Henderson RC, Berglund LM, May R, Zemel BS, Grossberg RI, Johnson J, Plotkin H, Stevenson RD, Szalay E, Wong B, Kecskemethy HH, Harcke HT. The relationship between fractures and DXA measures of BMD in the distal femur of children and adolescents with cerebral palsy or muscular dystrophy. J. Bone Miner. Res. 2010;25:520–526. doi: 10.1359/jbmr.091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szalay EA, Cheema A. Children with spina bifida are at risk for low bone density. Clin. Orthop. Relat. Res. 2011;469:2153–2157. doi: 10.1007/s11999-010-1634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention, National Center for Health Statistics LMS Parameters for Girls & Boys: Height for Age. [9/5/15];National Health and Nutrition Survey (NHANES) 2000 Retrieved from: http://www.cdc.gov/growthcharts/zscore.htm.
- 18.Harcke HT, Taylor A, Bachrach S, Henderson RC. Lateral femoral scan: an alternative method for assessing bone mineral density in children with cerebral palsy. Pediatr. Radiol. 1998;28:241–246. doi: 10.1007/s002470050341. [DOI] [PubMed] [Google Scholar]
- 19.Henderson RC, Lark RK, Newman JE, Kecskemthy H, Fung EB, Renner JB, Harcke HT. Pediatric reference data for dual X-ray absorptiometric measures of normal bone density in the distal femur. AJR Am. J. Roentgenol. 2002;178:439–443. doi: 10.2214/ajr.178.2.1780439. [DOI] [PubMed] [Google Scholar]
- 20.Zemel BS, Stallings VA, Leonard MB, Paulhamus DR, Kecskemethy HH, Henderson RC. Revised pediatric reference data for the lateral distal femur measured by hologic discovery/Delphi dual-energy X-ray absorptiometry. J. Clin. Densitom. 2009;12:207–218. doi: 10.1016/j.jocd.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive summary of the 2013 international society for clinical densitometry position development conference on bone densitometry. J. Clin. Densitom. 2013;16:455–466. doi: 10.1016/j.jocd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Crabtree NJ, Arabi A, Bachrach LK, Fewtrll M, El-Hajj Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD pediatric official positions. J. Clin. Densitom. 2014;17:225–242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Henderson RC, Henderson BA, Kecskemethy HH, Hidalgo ST, Nikolova BA, Sheridan K, Harcke HT, Thorpe DE. Adaptation of the lateral distal femur DXA scan technique to adults with disabilities. J. Clin. Densitom. 2015;18:102–108. doi: 10.1016/j.jocd.2014.04.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wraith JE. The mucopolysaccharidoses: a clinical review and guide to management. Arch. Dis. Child. 1995;72:263–267. doi: 10.1136/adc.72.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClure J, Smith PS, Sorby-Adams G, Hopwood J. The histological and ultrastructural features of the epiphyseal plate in Morquio type A syndrome (mucopolysaccharidosis type IVA) Pathology. 1986;18:217–221. doi: 10.3109/00313028609059462. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda E, Fushimi K, Suzuki Y, Takami T, Zustin J, Patel P, Ruhnke K, Shimada T, Boyce B, Kokas T, Barone C, Theroux M, Mackenzie W, Nagel B, Ryerse JS, Orii KE, Iida H, Orii T, Tomatsu S. Pathogenesis of Morquio A syndrome: an autopsied case reveals systemic storage disorder. Mol. Genet. Metab. 2013;109:301–311. doi: 10.1016/j.ymgme.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 27.The Human Gene Mutation Database (HGMD) [May 2015];2015 Available at: http://www.hgmd.cf.ac.uk/ac/gene.php?gene=GALNS.
- 28.Fung EB, Johnson JA, Madden J, Kim T, Harmatz P. Bone density assessment in patients with mucopolysaccharidosis: a preliminary report from patients with MPS II and VI. J. Pediatr. Rehabil. Med. 2010;3:13–23. [PMC free article] [PubMed] [Google Scholar]