Abstract
ROCK activity increases due to ECM rigidity in the tumor microenvironment and promotes a malignant phenotype via actomyosin contractility. Invasive migration is facilitated by actin-rich adhesive protrusions known as invadopodia that degrade the ECM. Invadopodia activity is dependent on matrix rigidity and contractile forces suggesting that mechanical factors may regulate these subcellular structures through ROCK-dependent actomyosin contractility. However, emerging evidence indicates that the ROCK1 and ROCK2 isoforms perform different functions in cells suggesting that alternative mechanisms may potentially regulate rigidity-dependent invadopodia activity. In this study, we found that matrix rigidity drives ROCK signaling in cancer cells but that ROCK1 and ROCK2 differentially regulate invadopodia activity through separate signaling pathways via contractile (NM II) and non-contractile (LIMK) mechanisms. These data suggest that the mechanical rigidity of the tumor microenvironment may drive ROCK signaling through distinct pathways to enhance the invasive migration required for cancer progression and metastasis.
1. Introduction
While clinical strategies have improved for treating primary tumors, 90% of patients will die due to the metastatic spread of cancer cells [1]. For metastasis to occur, malignant cells must migrate away from the primary tumor by invading neighboring tissues [2]. The rigidity of the tumor-associated ECM has been identified as a significant factor in driving malignant behavior by increasing actomyosin contractility through phosphorylation of the MLC of NM II by Rho-activated ROCK [3–6]. Matrix rigidity is known to augment the invasive properties of several types of cancer cells in vitro and enhance invasion and metastasis in vivo [7–14]. Therefore, ROCK has become a potential therapeutic candidate due to constitutively active mutations and/or overexpression in many cancers [15, 16]. However, current studies conflict as to whether the inhibition of ROCK increases or decreases migration and/or invasion [3, 17–20] which may be a result of the differences in the functions of the two ROCK isoforms that were originally thought to be similar [21]. Recent work has shown that ROCK1 and ROCK2 participate in non-redundant signaling pathways to regulate distinct cellular functions that often result in opposing or dissimilar effects in a variety of normal cell types [21–32]. In addition, ROCK has several other downstream substrates [33] which may also play a role in invasive behavior. Of these, LIMK has been shown to regulate proteolytic path generation by leading tumor cells during collective invasion [34].
To penetrate cross-linked tissues during invasive migration, cancer cells utilize actin-rich adhesive protrusions known as invadopodia [35]. Invadopodia formation is driven by signaling and actin assembly resulting in mature structures that utilize MMPs to focally degrade the ECM [36, 37]. Invadopodia are considered a hallmark of invasive cells and have been implicated in tumor cell invasion and metastasis [38, 39]. We recently showed that ECM degradation is dependent on cellular traction forces generated in response to matrix rigidity indicating that invadopodia activity is regulated by rigidity signals transmitted through cellular contractility [40]. Previous work has implicated both ROCK and MLCK, one of the other main effectors of NM II, in regulating ECM degradation by invadopodia [41]. However, inhibition of NM II and MLCK led to divergent effects on invadopodia expression [41], and MLCK inhibition does not affect cellular force generation in some cell types [14, 42]. Furthermore, ROCK inhibition has led to increases in invasive and migratory properties of cancer cells [19, 20]. Therefore, the underlying mechanism by which rigidity signaling drives invadopodia activity in cancer cells remains unknown. Using our recently modified PAA rigidity system to evaluate invasive and contractile properties of cancer cells [40, 43], the goal of this study was to test the hypothesis that matrix rigidity differentially regulates invadopodia activity through the ROCK isoforms.
2. Materials and Methods
2.1 Cell culture, siRNA, and inhibitors
SCC-61 cells, an invasive head and neck squamous cell carcinoma cell line model for invadopodia [40, 44–49], were cultured as previously described [40]. MDA-MB-231 cells, an invasive breast adenocarcinoma cell line model for invadopodia [50–55], were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; both from Life Technologies). For experiments, cells were incubated in invadopodia medium containing DMEM:RPMI 1640 supplemented with 5% NuSerum, 10% FBS, and 20 ng/ml epidermal growth factor (all from Life Technologies) [40, 43]. siGENOME SMARTpool siRNA against ROCK1, ROCK2, and MLCK (ThermoScientific) were used to maximize KD and minimize the risk of off-target effects [56] as well as potential compensatory effects between the ROCK isoforms [15, 27]. They were transfected with lipofectamine RNAiMAX (Life Technologies) per the manufacturer’s protocol. Non-targeting siRNA (siGENOME or ON-TARGETplus) was used as a control (ThermoScientific). Blebbistatin, a NM II inhibitor, and GM-6001, a broad-spectrum MMP inhibitor, were used at 25 and 15 μ;M, respectively, to inhibit invadopodia activity [41, 44]. Currently, pharmacological inhibitors do not exist that can differentiate between the ROCK isoforms due to their highly homologous catalytic kinase domains [15, 57, 58].
2.2 Western blotting
Whole cell lysates were scraped in SDS lysis buffer and combined with 5× SDS sample buffer and 2-mercaptoethanol and incubated at 95°C for 5 minutes. Relative protein concentrations were determined using the Protein Assay Reagent (BioRad Laboratories) as per the manufacturer’s instructions. Equal amounts of protein were loaded onto a 7% SDS-PAGE gel, separated by electrophoresis, and transferred to an Immobilon-P filter. The non-specific binding sites were blocked with 5% non-fat dried milk in Tris buffered saline. The blots were incubated with a ROCK1 mouse monoclonal antibody (Abcam), a ROCK2 rabbit polyclonal antibody (Abcam), a MLCK mouse monoclonal antibody (Santa Cruz), or a β-actin polyclonal antibody (Cell Signaling) for one hour at room temperature with gentle agitation. The blots were detected with a horse radish peroxidase-conjugated secondary antibody. Signal was detected by chemiluminescence using ECL Western Blotting Substrate reagent (Pierce) and quantitated with densitometry.
2.3 PAA substrates
PAAs were synthesized for the invadopodia and traction force assays as previously described [40, 43]. Briefly, soft, hard, and rigid PAAs were composed of varying ratios of acrylamide/BIS-acrylamide (8%/0.05%, 8%/0.35%, and 12%/0.6%, respectively), 0.1% N-hydroxysuccinimide ester, and fibronectin (200 μg/ml, 215 μg/ml, and 230 μg/ml, respectively). These formulations yielded substrates with elastic moduli as previously measured by rheometry assuming E=3G′ of 1,023, 7,307, 22,692 Pa, respectively, and the same relative concentrations of conjugated fibronectin at their surfaces [40, 43]. These values span the range of elastic moduli previously reported for normal and cancerous tissues [3, 59].
2.4 Traction force assays
As previously described [40, 43], PAAs containing 200 nm fluorescent beads (Life Technologies) at a final ratio of 1:125 were cast on activated glass coverslips of 35 mm MatTek dishes (MatTek). Beads were previously found not to alter the PAA mechanical properties [40]. 15,000 cells were incubated overnight (~18 hours) in invadopodia medium.
2.5 Invadopodia assays
PAAs were cast on activated glass coverslips of 35 mm MatTek dishes and overlaid with cross-linked (0.5% glutaraldehyde) 1% gelatin and FITC-labeled fibronectin as previously described [40, 43] or a cross-linked (0.05% glutaraldehyde) 0.2% FITC-labeled gelatin mixture (1:5 ratio of labeled to unlabeled gelatin) [53, 60, 61] to evaluate ECM degradation. 25,000 cells were incubated overnight (~18 hours) in invadopodia medium. The use of cross-linked fluorescent ECM for assessing invadopodia numbers and ECM degradation is the standard method in the field used as an indicator of cancer cell invasiveness [60, 62]. The utilization of PAAs underneath this ECM is for modulating matrix rigidity experienced by cancer cells [40, 41, 43, 63, 64].
2.6 Immunofluorescence
ROCK1 and ROCK2 were identified with mouse monoclonal antibodies (Abcam). pMLC (Cell Signaling) and pLIMK (Santa Cruz) were identified with rabbit polyclonal antibodies. For the invadopodia assays, the colocalization of actin and cortactin were used as a marker for invadopodia [40, 41, 63, 64]. F-actin and cortactin were identified with Alexa Fluor 546 phalloidin (Life Technologies) and a mouse monoclonal antibody (EMD Millipore), respectively. All proteins were visualized with appropriate Alexa Fluor secondary antibodies (Life Technologies). Fluorescent images were captured on a Nikon Eclipse TE2000-E inverted microscope with either a 40× Plan Fluor or a 60× Plan Apochromat oil immersion lens. Metamorph software (Molecular Devices) was used for image analyses and/or quantitation of invadopodia, ECM degradation, pMLC, and pLIMK as previously described [40, 41, 63, 64]. Invadopodia were further classified as either actively degrading or non-degrading depending on whether they were colocalized with ECM degradation or not (which are sometimes denoted as mature invadopodia or invadopodia precursors, respectively [65]). Integrated and pixel intensity levels were quantitated based on the summation or average of pixel intensity values in outlined areas of each cell, respectively, and maximum intensity levels at invadopodia were quantitated based on the average of the maximum intensity pixel value from a manual line scan at each structure.
2.7 Traction force microscopy and calculations
As previously described [40], cells were incubated in L-15 medium with the same supplements as the invadopodia medium for one hour in an environmentally controlled microscope chamber prior to microsphere and cell imaging with a 40× Plan Fluor objective to determine substrate displacements for traction force calculations based on the optical flow method for microsphere tracking, the PAA mechanical properties, and the maximum likelihood method for estimating cellular forces from elasticity theory [66] using the computer program LIBTRC from Micah Dembo of Boston University. The overall collective traction force exerted by a cell on a substrate is reported as the integral of the traction field over the cell area [67].
2.8 Transwell migration and invasion assays
Standard Transwell migration and invasion assays were performed using 0.5 and 0.8 μm pore 24-well Transwell plates (BD Biosciences), respectively [68]. For the invasion assay, plates were pre-coated with Matrigel, a commonly used tumor-derived ECM that requires proteolytic invasion [69, 70]. 75,000 cells were seeded in each Transwell and incubated overnight (~18 hours) with invadopodia medium serving as the attractant in the bottom of each well. After removing cells from the top side of the Transwell permeable supports with a cotton swab, samples were fixed with 4% paraformaldehyde for 20 minutes and then permeabilized with 0.1% Triton-X for 10 minutes. Cell nuclei were then stained with 1:1,000 Hoechst (Life Technologies) for 5 minutes. The number of cells that migrated or invaded to the other side of the Transwell permeable supports were fluorescently imaged with a 10× Plan Fluor objective and counted over four random areas for each sample. Matrigel is typically used for in vitro invasion assays since the presence of invadopodia in cancer cells is highly correlated with in vitro (as well as in vivo) invasive behavior [71–78].
2.9 Statistics
As previously described using SPSS Statistics (IBM) [40, 63], data were evaluated for normality with the Shapiro-Wilk or Kolmogorov-Smirnov test. Normal data were analyzed with a one-way ANOVA, while non-parametric data were analyzed with a Kruskal-Wallis test. Significance in groups was determined by a Student’s t-test or a Mann-Whitney test, either with a Bonferroni correction, or a Tamhane post hoc test. A p-value less than 0.05 was considered statistically significant. Given that the majority of results were not normal, the data were depicted using box-and-whisker plots for appropriate representation with only median values presented to simplify the plots.
3. Results
3.1 ROCK1 regulates traction forces
We previously demonstrated that traction forces generated by SCC-61 cells are regulated by matrix rigidity and predictive of ECM degradation by invadopodia [40]. While cellular force generation is driven by actomyosin-generated contractile forces, regulation of NM II activity by ROCK and MLCK can serve different functions in non-muscle cells [79, 80]. Therefore, we first tested the specific roles of ROCK1, ROCK2, and MLCK in regulating cellular contractility. SCC-61 cells were transfected with siGENOME SMARTpool siRNA against ROCK1, ROCK2, and MLCK, and KDs were confirmed with Western blots (Figures 1A–B). Traction force microscopy was performed on NTC and KD cells cultured overnight on soft PAAs (Figure 1C) to measure actomyosin contractility [40]. Soft PAAs were utilized since bead displacements used to calculate traction forces are at their largest given the relatively low rigidity of these substrates thus providing the maximum sensitivity to detect differences in contractility. Interestingly, we found that only ROCK1 KD led to a decrease in traction forces (Figure 1D), while KDs of ROCK2 (Figure 1E) and MLCK (Figure 1F) had no effect on cellular force generation. We also tested MDA-MB-231 cells, which we first confirmed also exhibited rigidity-dependent traction forces (Supplementary Figure 1A) like SCC-61 cells [40], and found a similar decrease in traction forces generated by ROCK1 KD cells but not ROCK2 KD cells when compared to NTC cells (Supplementary Figures 1B–C). To rule out the possibility of compensation between the ROCK isoforms, we also performed a double KD and found that the decrease in traction forces generated by ROCK1/ROCK2 KD cells were statistically similar to ROCK1 KD cells (p=0.57) but also significantly different from NTC cells (Supplementary Figure 2). These data indicate that only ROCK1 may regulate actomyosin contractility.
Figure 1.
ROCK1 regulates force production. (A) Representative Western blots and (B) total quantitation of non-targeting control (NTC) and knockdowns (KDs) of ROCK1, ROCK2, and MLCK with siRNAs in SCC-61 cells. Note the specificity of the siRNAs for the ROCK isoforms. (C) Representative traction maps with colors representing local traction stress levels (traction magnitude over the area) and quantitation of the average traction magnitudes per cell of NTC versus (D) ROCK1 KD, (E) ROCK2 KD, and (F) MLCK KD in the traction force assays on soft polyacrylamide gels. Data are presented as mean ± standard error, and * indicates p<0.05 for (B) while data in (D–F) are presented as box and whisker plots with the black lines indicating the medians, the whiskers representing the 10th and 90th percentiles, and * indicating p<0.05 for n=70–78, 67–86, and 79–86 cells, respectively, all from 3–5 independent experiments.
3.2 Both ROCK isoforms regulate ECM degradation
Since rigidity-dependent ROCK signaling promotes malignant behavior [3–5], we performed invadopodia assays on rigid PAAs or glass with the ROCK1 and ROCK2 KD cells to determine their roles in regulating invadopodia activity (Figure 2 and Supplementary Figure 3). Stiffer substrates were used since they induce more ECM degradation [40, 41, 63] thus providing the maximum sensitivity to detect differences in invadopodia activity. Interestingly, both KDs decreased ECM degradation by SCC-61 cells (Figures 2A,B,E) as well as the number of invadopodia actively degrading ECM (yellow arrows and outlined insets in Figure 2A; Figures 2C,F). While ROCK1 KD decreased the total number of invadopodia (actively degrading and non-degrading denoted by white arrows and outlined insets in Figure 2A; Figure 2D), ROCK2 KD had no effect on the total number of invadopodia in SCC-61 cells (Figure 2G). We also confirmed the roles of both ROCK isoforms in regulating ECM degradation by invadopodia in MDA-MB-231 cells (Supplementary Figure 3). These data indicate that ROCK1 and ROCK2 may regulate invadopodia and thus ECM degradation through different signaling pathways.
Figure 2.
ROCK1 and ROCK2 regulate ECM degradation. (A) Representative wide-field fluorescence images of non-targeting control (NTC), ROCK1 knockdown (KD), and ROCK2 KD SCC-61 cells in the invadopodia assays on rigid polyacrylamide gels. Invadopodia puncta were identified by colocalization of actin (red) and cortactin (blue). Active invadopodia (i.e., actively degrading; yellow arrows and yellow outlined insets) were identified by colocalization with black degraded areas of the ECM lacking FITC signal (green). Total invadopodia included active and non-degrading (white arrows and white outlined insets). Quantitation of the (B,E) degradation area per cell, (C,F) number of actively degrading invadopodia per cell, and (D,G) number of total invadopodia per cell for NTC versus ROCK1 KD or ROCK2 KD, respectively. Data are presented as box and whisker plots with the black lines indicating the medians, the whiskers representing the 10th and 90th percentiles, and * indicating p<0.05 for n=125–209 and 109–142 cells for ROCK1 KD and ROCK2 KD experiments, respectively, from 3–4 independent experiments. Scale bar represents 10 μm.
3.3 Matrix rigidity regulates ROCK signaling through NM II and LIMK
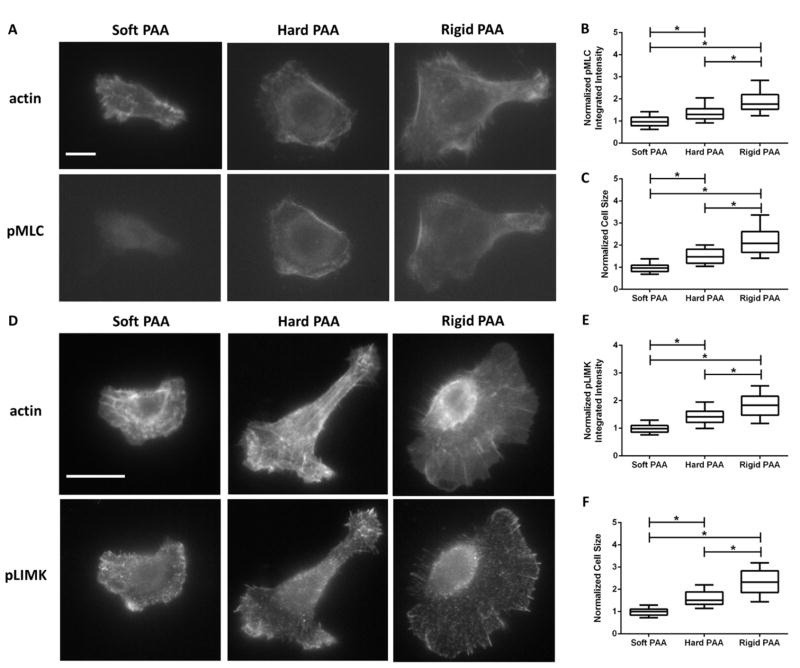
Of the other downstream targets of ROCK, LIMK is of particular interest since it is known to affect actin dynamics by inactivating cofilin to hinder barbed end formation and promote actin filament stabilization [34, 81]. To first verify whether matrix rigidity could drive ROCK signaling through both NM II and LIMK, we determined the overall cellular levels of pMLC and pLIMK in response to ECM rigidity using quantitative immunofluorescence (Figures 3A,D). In SCC-61 cells incubated overnight on the PAAs used in the traction force assays, we found that the integrated pixel intensity levels of overall cellular pMLC (Figure 3B) and pLIMK (Figure 3E) were highly dependent on ECM rigidity as was cell size (Figures 3C,F). These data suggest that ECM rigidity may promote ROCK signaling through the NM II and LIMK pathways.
Figure 3.
Overall cellular pMLC and pLIMK levels are dependent on matrix rigidity. Representative wide-field fluorescence images of SCC-61 cells immunostained for actin and (A) pMLC or (D) pLIMK on soft, hard, and rigid polyacrylamide gels used in the traction force assays. Quantitation of (B) pMLC and (E) pLIMK normalized integrated intensities and cell sizes from (C) pMLC and (F) pLIMK immunostaining. Data are presented as box and whisker plots with the black lines indicating the medians, the whiskers representing the 10th and 90th percentiles, and * indicating p<0.05 for n=84–87 and 83–89 cells for pMLC and pLIMK experiments, respectively, from 4 independent experiments. Scale bars represent 10 μm.
3.4 NM II and LIMK are differentially regulated by the ROCK isoforms
To test whether ROCK1 and ROCK2 specifically target NM II and LIMK to regulate invasiveness, we performed invadopodia assays on glass for optimal imaging and quantitated pMLC and pLIMK expression with ROCK1 KD and ROCK2 KD SCC-61 cells. In these cases, we quantitated average pixel intensities which are proportional to protein levels but independent of cell size. pMLC average intensity levels were significantly decreased with ROCK1 but not ROCK2 KD in SCC-61 cells (Figures 4A–B). While significant localization of pMLC has not been found at or around invadopodia [41] which we confirmed as well (Figure 4A), we verified the presence of pLIMK at invadopodia (Figure 4C). In contrast to pMLC, pLIMK average intensity levels were significantly decreased with ROCK2 but not ROCK1 KD in SCC-61 cells (Figure 4D). Furthermore, using line scans at invadopodia (vertical red lines in Figure 4C), pLIMK maximum intensity values at non-degrading invadopodia (white arrows and outlined insets in Figure 4C) were found to be reduced by ROCK2 but not ROCK1 KD (Figure 4E). However, pLIMK maximum intensity values at actively degrading invadopodia (yellow arrows and outlined insets in Figure 4C) were similarly decreased for both ROCK1 and ROCK2 KDs (Figure 4F). In addition, F-actin maximum intensity values at non-degrading invadopodia were also reduced with ROCK2 KD (Figure 4G) and similarly decreased for both ROCK1 and ROCK2 KDs at actively degrading invadopodia as well (Figure 4H). Interestingly, F-actin maximum intensity values were also decreased with ROCK1 KD but not to the extent as with ROCK2 KD at non-degrading invadopodia (Figure 4G). These data support the notion that invadopodia activity is differentially regulated by the ROCK isoforms through the NM II and LIMK pathways which may affect the formation and/or maturation of invadopodia.
Figure 4.
pMLC and pLIMK are dependent on specific ROCK isoforms. Representative wide-field fluorescence images of non-targeting control (NTC), ROCK1 knockdown (KD), and ROCK2 KD SCC-61 cells immunostained for (A) pMLC and (C) pLIMK in the invadopodia assays on glass. Quantitation of (B) pMLC and (D) pLIMK normalized average intensities per pixel. Quantitation of the pLIMK maximum intensities from maximum values taken from line scans (vertical red lines in (C)) at (E) non-degrading (white arrows and outlined insets in (C)) and (F) actively degrading invadopodia (yellow arrows and outlined insets in (C)). Quantitation of the F-actin maximum intensities from maximum values taken from line scans (vertical red lines in (C)) at (G) non-degrading (white arrows and outlined insets in (C)) and (H) actively degrading invadopodia (yellow arrows and outlined insets in (C)). Non-degrading and actively degrading invadopodia were identified by colocalization of actin (red) without and with black degraded areas of the ECM lacking FITC signal (green), respectively. Data are presented as box and whisker plots with the black lines indicating the medians, the whiskers representing the 10th and 90th percentiles, and * indicating p<0.05 for n=31–50 and 46–56 cells for pMLC and pLIMK experiments, respectively, and n=116–128 and 96–212 non-degrading and active invadopodia, respectively, from the cells in the pLIMK experiments, all from 3 independent experiments. Scale bars represent 10 μm.
3.5 ROCK1 regulates migration while both isoforms regulate invasion
Since both ROCK isoforms appear to regulate ECM degradation (Figure 2) but not traction forces (Figure 1), we wanted to test their roles in invasion and migration given previous conflicting studies regarding ROCK in these processes [3, 17–20]. We performed Transwell migration and invasion assays with ROCK1 KD and ROCK2 KD SCC-61 cells as well as NTC cells treated with blebbistatin or GM-6001 and quantitated the number of cells that traversed the Transwell permeable supports using nuclear fluorescence staining. For the migration assay in which the cells can freely migrate through the pores in the permeable supports (Figure 5A), ROCK1 but not ROCK2 KD significantly decreased Transwell migration (Figure 5B). These data support the role of ROCK1 in regulating actomyosin contractility which we confirmed with NM II inhibition with blebbistatin which mimicked ROCK1 KD (Figures 5A–B). For the invasion assay in which the cells must first degrade a layer of Matrigel (Figure 5C), Transwell invasion was significantly inhibited with both ROCK1 and ROCK2 KDs. These data provide further support regarding the roles of the ROCK isoforms in regulating proteolytic activity which we confirmed with the broad-spectrum MMP inhibitor GM-6001 which mimicked both KDs (Figures 5C–D). We found similar results for ROCK1 and ROCK2 KDs in MDA-MB-231 cells using the same migration and invasion assays (Supplementary Figure 4). These data confirm our findings regarding ROCK isoform regulation of actomyosin contractility and invadopodia activity.
Figure 5.
ROCK1 regulates migration while ROCK1 and ROCK2 regulate invasion. Representative wide-field fluorescence images of DMSO-treated non-targeting control (NTC), 25 μM blebbistatin-treated NTC, ROCK1 knockdown (KD), 15 μM GM-6001-treated NTC, and ROCK2 KD SCC-61 cells incubated overnight and then stained with Hoechst in Transwell (A) migration and (C) Matrigel-coated invasion assays with quantitation of the normalized number of (B) migrated and (D) invaded cells. Cells were plated in Transwells using serum-free invadopodia medium while complete invadopodia medium served as the attractant in the bottom well. Data are presented as a box and whisker plots with the black lines indicating the medians, the whiskers representing the 10th and 90th percentiles, and * indicating p<0.05 for n=24 areas for each condition from 3 independent experiments. Scale bars represent 20 μm.
4. Discussion
Overall, our results suggest that matrix rigidity may drive invasive migration through differential ROCK signaling to regulate invadopodia activity. Cell migration by invading cells is driven by actomyosin-generated contractile forces [82] that are transmitted to the ECM and can be measured as traction forces [83]. In general, actomyosin contractility is thought to promote cell migration through phosphorylation of MLC of NM II by MLCK [84] and ROCK [6]. Interestingly, we found that siRNA against MLCK had no effect on traction force levels (Figure 1F). MLCK has been shown to assemble cortical actin bundles responsible for only transient contractile activity in fibroblasts [79, 80]. In contrast, ROCK is involved in actin stress fiber assembly inside fibroblasts resulting in sustained contractile forces [79, 80]. These differences may explain why the ROCK inhibitor Y-27632 inhibits traction force generation in fibroblasts while inhibition of MLCK with ML-7 and a specific peptide inhibitor has no effect [42]. Collagen gel contraction by epithelial cells has also been shown to be inhibited by Y-27632 but not ML-7 [14]. Additionally, ML-7 has known off-target effects that include phosphoinositide 3-kinase [42] which is a key regulator of invadopodia formation [47]. These studies support our finding and suggest that MLCK may regulate invadopodia activity [41] through an alternative mechanism; therefore, we continued with a focus on the role of ROCK in invasiveness since matrix rigidity is known to drive a ROCK-dependent malignant phenotype [3].
Several studies have shown that inhibition of ROCK, usually with Y-27632, leads to decreases in ECM degradation, force generation, and invasion [3, 17, 18]. Therefore, we wanted to determine the precise role of the ROCK isoforms in regulating invadopodia activity since we previously found that ECM degradation was dependent on traction force levels [40]. Intriguingly, we found that only ROCK1 was involved in regulating traction force levels (Figure 1D and Supplementary Figure 1C) suggesting that this isoform is mainly responsible for rigidity-dependent invadopodia activity through actomyosin contractility [40, 41]. We confirmed the role of ROCK1 in regulating invadopodia numbers and/or ECM degradation (Figures 2B–D and Supplementary Figure 3) as well as migration and invasion (Figure 5 and Supplementary Figure 4). However, we also found that ROCK2 regulated invadopodia activity (Figures 2E–F) despite having no effect on cellular force generation (Figure 1E and Supplementary Figure 1C) indicating an alternative mechanism. While the ROCK isoforms have often been considered functionally similar [21], recent work in a variety of cell types has shown that ROCK1 and ROCK2 participate in diverse signaling pathways to differentially regulate cell behavior [21–32]. In particular, ROCK 1 KD in fibroblasts results in a loss of stress fibers, while ROCK2 KD enhances or stabilizes the cytoskeleton [21–23] which would support our traction force results for each isoform. Since Y-27632 inhibits ROCK1 and ROCK2 with equal potency [28], potential functional differences between ROCK1 and ROCK2 could be masked. Our data corroborates these findings and indicate that the ROCK isoforms regulate invadopodia activity through different pathways despite reports that both can phosphorylate NM II [21, 28].
One potential alternative pathway was through LIMK which has been shown to stabilize actin filaments at invadopodia and promote invasion [34]. Therefore, we wanted to first determine whether matrix rigidity could drive ROCK signaling through the NM II and LIMK pathways. We confirmed that both pMLC and pLIMK levels increased with matrix rigidity (Figures 3B,E) which was accompanied by an increase in cell size (Figures 3C,F). To determine the roles of these pathways on invasiveness independent of cell size, we knocked down ROCK1 and ROCK2 and quantitated the average pixel intensities of pMLC and pLIMK as a downstream indicator of ROCK activity. Previous work has shown strong NM II but a lack of pMLC localization in rings around invadopodia [41]. Therefore, we chose to look at pMLC average pixel intensity throughout the cell and found it to be dependent on ROCK1 but not ROCK2 (Figure 4B). In contrast, we found that pLIMK average pixel intensity throughout the cell was dependent on ROCK2 but not ROCK1 (Figure 4D) even though both isoforms have been reported to phosphorylate LIMK [15]. While cofilin activity has been shown near invadopodia [74, 85], we verified the presence of pLIMK at these invasive structures (Figure 4C). Overall, these data are supported by previous studies showing that LIMK and ROCK inhibition have similar effects on barbed end formation at invadopodia [85] and proteolytic invasion by collectively migrating cancer cells but not phosphorylation of MLC [34].
Given the presence of pLIMK at invadopodia and overall cellular intensity differences, we wanted to determine whether LIMK was regulated by ROCK2 at these invasive structures. We found that pLIMK maximum intensity values were dependent only on ROCK2 at non-degrading invadopodia (Figure 4E) which coincided with a decrease in F-actin maximum intensity values (Figure 4G). These results support a role for LIMK in invadopodia maturation [34, 85] but not formation since the total number of invadopodia were similar to NTC with ROCK2 KD (Figure 2G) but decreased for the number of actively degrading invadopodia (Figure 2F). Furthermore, we found that only ROCK2 KD inhibited Transwell invasion but not migration which was mimicked with GM-6001 (Figure 5) confirming a non-contractile regulatory role for ROCK2. Our findings are consistent with a previous report showing that ROCK2 is involved in invadopodia maturation by regulating MMP activity at these structures [86]. In contrast, ROCK1-driven actomyosin contractility may aid in anchoring invadopodia through local force generation to promote adhesion-based signaling and thus maturation [49, 87] similar to other adhesive structures like focal adhesions and podosomes [49, 88, 89]. A role for contractile forces in regulating invadopodia in this manner would be consistent with the decreased numbers of actively degrading and total invadopodia that were found with ROCK1 KD (Figure 2C–D) as well as the decrease in F-actin maximum intensity values at non-degrading invadopodia (Figure 4G) despite pLIMK levels being similar to NTC (Figure 4E). Since we did not find evidence of compensation between the ROCK isoforms (Supplementary Figure 2), diminished stability of invadopodia due to decreased cellular contractility with ROCK1 KD could account for the reduction in pLIMK maximum intensity values at actively degrading invadopodia (Figure 4F). Furthermore, decreased contractility and/or reduced pLIMK may explain the differences in F-actin maximum intensity values at actively degrading invadopodia (Figure 4H). While our data suggest that ROCK activity is driven by matrix rigidity to regulate invadopodia through actin filament dynamics and the contractile machinery, further studies are required to precisely determine how the ROCK isoforms affect invadopodia lifetime and turnover.
ROCK signaling and actomyosin contractility have also been implicated in regulating podosomes which are actin-rich protrusions similar to invadopodia used by non-cancer cells to degrade the ECM during invasive migration [90–92]. Podosomes have been shown to generate protrusive and traction forces that are dependent on matrix rigidity [89, 93]. However, inhibition of myosin activity with blebbistatin and/or Y-27632 has led to opposing effects on podosome formation in difference cell types [89, 94–96] which may be due to different levels and/or localization of upstream Rho GTPases [97]. In addition, actin polymerization is also a critical component of podosome dynamics which is not dependent on ROCK-mediated contractility [98]; however, the interplay of these processes regulates oscillations and mechanosensing at podosomes [99, 100]. Although cofilin has been shown to regulate actin polymerization at invadopodia through ROCK and LIMK [85], this pathway does not appear to be involved in cofilin activation that is required for podosome organization in osteoclasts [101]. While further studies are necessary to determine the precise functions of the ROCK isoforms in regulating podosomes, our data suggest unique roles for ROCK1 and ROCK2 in different signaling pathways that converge to regulate invadopodia dynamics. These differences may also be mediated by variations in upstream regulators of ROCK such as podoplanin and the different Rho GTPases as well as other pathways downstream of ROCK that include ezrin and moesin that also regulate invadopodia [53, 85, 102–104]. In particular, RhoA is known to regulate force sensing pathways including actomyosin contractility [105–107] as well as invasive migration and/or metastasis by cancer cells [108–110], while RhoC has been found to promote invadopodia activity locally through the LIMK-cofilin pathway [17, 34, 85]. Furthermore, podoplanin mediates ECM degradation by invadopodia through a ROCK-LIMK-cofilin pathway that is regulated by RhoC [104]. Therefore, matrix rigidity may further regulate ROCK signaling through differential Rho pathways dependent on cellular localization.
Contractile activity has also been demonstrated to be important in a non-proteolytic mode of cancer cell invasion known as amoeboid migration. In this mode of migration, cancer cells are characterized by a rounded morphology and generate ROCK-dependent propulsive forces through cortical actomyosin contractility to physically reorient porous ECM [2]. While both ROCK isoforms have been implicated in this phenotype [111–113], cells exhibiting amoeboid behavior do not have strong adhesions and thus exert very weak traction forces [114]. But, ROCK is also critical for driving malignant behavior through increased cellular contractility and focal adhesion formation consistent with a mesenchymal phenotype [3]. Furthermore, the formation of invadopodia is thought to be part of the conversion to a mesenchymal phenotype [115, 116] for migration through cross-linked ECM as it is proteolyzed [117, 118]. However, the ROCK1 isoform has been shown to interact with LIMK to promote a mesenchymal and not an amoeboid phenotype [119]. While our study cannot resolve differences in these modes of migration based on ROCK activity, our data do indicate that both ROCK isoforms differentially regulate invadopodia activity in a non-porous environment requiring ECM degradation.
5. Conclusion
Currently, tumor rigidity is known to increase ROCK signaling to promote malignant behavior by driving actomyosin contractility in cancer cells; however, this transformation is typically associated with the acquisition of a motile and proliferative phenotype [3–5]. In this study, our results suggest that rigidity-dependent ROCK signaling also regulates proteolytic ECM degradation by invadopodia through two distinct pathways. While matrix rigidity and density have been shown to drive malignant behavior primarily in breast cancer [3, 12, 13] which has been highly associated with ROCK expression and/or activity [120, 121], we and others have shown that these mechanical factors also alter the invasive properties of many cancer cell types [7–10, 40, 41, 63]. In particular, invadopodia activity has been shown to be dependent on rigidity in three unrelated cancer cell lines that contain different mutations [40, 41, 63] suggesting the potential for common mechanical signaling pathways regulated by the ROCK isoforms. Since the factors that contribute to tumor density are ubiquitous features of many cancers [122–124], this growing evidence suggests that rigidity signaling through ROCK may play an important role in driving invasive migration requiring ECM proteolysis in other cancers. Current models describing invadopodia stages do not address the roles of ROCK isoforms, actomyosin contractility, or matrix rigidity which could represent novel steps in invadopodia formation and/or maturation [36, 37]. In addition, differences in ROCK isoform signaling could also impact different modes of tumor cell dissemination, i.e., proteolytic versus non-proteolytic [82], with implications for specific anti-invasive therapies.
Supplementary Material
Acknowledgments
We would like to thank Micah Dembo of Boston University for licensing of the LIBTRC software. Research reported in this publication was supported by the National Institutes of Health grant K25CA143412 from the National Cancer Institute and supported in part by the Research Scholar Grant RSG-15-226-01-CSM from the American Cancer Society, both to A.P. Additional support was provided by the Vanderbilt Clinical and Translational Science Award UL1TR000445 from the National Center for Advancing Translational Sciences and resources from the Vanderbilt Advanced Computing Center for Research and Education. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, American Cancer Society, or National Center for Advancing Translational Sciences. We would also like to thank Alissa Weaver for her critical reading of the manuscript. Additional funding was provided by the Department of Otolaryngology.
Abbreviations
- ROCK
rho-associated kinase
- ECM
extracellular matrix
- NM II
non-muscle myosin II
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- LIMK
LIM kinase
- NTC
non-targeting control
- KD
knockdown
- PAA
polyacrylamide gel
- MMP
matrix metalloproteinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science (New York, NY. 2011;331:1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nature reviews Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 3.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nature reviews. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. Journal of mammary gland biology and neoplasia. 2004;9:325–42. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 6.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) The Journal of biological chemistry. 1996;271:20246–9. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 7.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10889–94. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraning-Rush CM, Califano JP, Reinhart-King CA. Cellular traction stresses increase with increasing metastatic potential. PLoS ONE. 2012;7:e32572. doi: 10.1371/journal.pone.0032572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haage A, Nam DH, Ge X, Schneider IC. Matrix metalloproteinase-14 is a mechanically regulated activator of secreted MMPs and invasion. Biochemical and biophysical research communications. 2014 doi: 10.1016/j.bbrc.2014.05.086. [DOI] [PubMed] [Google Scholar]
- 10.Haage A, Schneider IC. Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. FASEB J. 2014 doi: 10.1096/fj.13-245613. [DOI] [PubMed] [Google Scholar]
- 11.Mierke CT. The fundamental role of mechanical properties in the progression of cancer disease and inflammation. Reports on progress in physics Physical Society. 2014;77:076602. doi: 10.1088/0034-4885/77/7/076602. [DOI] [PubMed] [Google Scholar]
- 12.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–43. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC medicine. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. The Journal of cell biology. 2003;163:583–95. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO reports. 2012;13:900–8. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan-Fisher M, Wewer UM, Yoneda A. Regulation of ROCK activity in cancer. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2013;61:185–98. doi: 10.1369/0022155412470834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE. Hic-5 promotes invadopodia formation and invasion during TGF-beta-induced epithelial-mesenchymal transition. The Journal of cell biology. 2012;197:421–37. doi: 10.1083/jcb.201108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nature medicine. 1999;5:221–5. doi: 10.1038/5587. [DOI] [PubMed] [Google Scholar]
- 19.Harma V, Knuuttila M, Virtanen J, Mirtti T, Kohonen P, Kovanen P, et al. Lysophosphatidic acid and sphingosine-1-phosphate promote morphogenesis and block invasion of prostate cancer cells in three-dimensional organotypic models. Oncogene. 2012;31:2075–89. doi: 10.1038/onc.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vishnubhotla R, Bharadwaj S, Sun S, Metlushko V, Glover SC. Treatment with Y-27632, a ROCK Inhibitor, Increases the Proinvasive Nature of SW620 Cells on 3D Collagen Type 1 Matrix. International journal of cell biology. 2012;2012:259142. doi: 10.1155/2012/259142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. The Journal of cell biology. 2005;170:443–53. doi: 10.1083/jcb.200412043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoneda A, Ushakov D, Multhaupt HA, Couchman JR. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Molecular biology of the cell. 2007;18:66–75. doi: 10.1091/mbc.E06-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darenfed H, Dayanandan B, Zhang T, Hsieh SH, Fournier AE, Mandato CA. Molecular characterization of the effects of Y-27632. Cell motility and the cytoskeleton. 2007;64:97–109. doi: 10.1002/cm.20168. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, et al. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circulation research. 2009;104:531–40. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lock FE, Hotchin NA. Distinct roles for ROCK1 and ROCK2 in the regulation of keratinocyte differentiation. PLoS ONE. 2009;4:e8190. doi: 10.1371/journal.pone.0008190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lock FE, Ryan KR, Poulter NS, Parsons M, Hotchin NA. Differential regulation of adhesion complex turnover by ROCK1 and ROCK2. PLoS ONE. 2012;7:e31423. doi: 10.1371/journal.pone.0031423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, Surma M, Zhang L, Wei L. Dissecting the roles of ROCK isoforms in stress-induced cell detachment. Cell cycle. 2013;12:1492–500. doi: 10.4161/cc.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi J, Wu X, Surma M, Vemula S, Zhang L, Yang Y, et al. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell death & disease. 2013;4:e483. doi: 10.1038/cddis.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertsch S, Thanos S. Opposing Signaling of ROCK1 and ROCK2 Determines the Switching of Substrate Specificity and the Mode of Migration of Glioblastoma Cells. Molecular neurobiology. 2013 doi: 10.1007/s12035-013-8568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. The Journal of cell biology. 2011;193:655–65. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vemula S, Shi J, Hanneman P, Wei L, Kapur R. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood. 2010;115:1785–96. doi: 10.1182/blood-2009-08-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newell-Litwa KA, Badoual M, Asmussen H, Patel H, Whitmore L, Horwitz AR. ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. The Journal of cell biology. 2015;210:225–42. doi: 10.1083/jcb.201504046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surma M, Wei L, Shi J. Rho kinase as a therapeutic target in cardiovascular disease. Future cardiology. 2011;7:657–71. doi: 10.2217/fca.11.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott RW, Hooper S, Crighton D, Li A, Konig I, Munro J, et al. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. The Journal of cell biology. 2010;191:169–85. doi: 10.1083/jcb.201002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clinical & experimental metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 36.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nature reviews. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beaty BT, Condeelis J. Digging a little deeper: The stages of invadopodium formation and maturation. European journal of cell biology. 2014 doi: 10.1016/j.ejcb.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weaver AM. Invadopodia. Curr Biol. 2008;18:R362–4. doi: 10.1016/j.cub.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 39.Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Current opinion in cell biology. 2012;24:277–83. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jerrell RJ, Parekh A. Cellular traction stresses mediate extracellular matrix degradation by invadopodia. Acta biomaterialia. 2014;10:1886–96. doi: 10.1016/j.actbio.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, et al. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–9. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beningo KA, Hamao K, Dembo M, Wang YL, Hosoya H. Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Archives of biochemistry and biophysics. 2006;456:224–31. doi: 10.1016/j.abb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jerrell RJ, Parekh A. Polyacrylamide gels for invadopodia and traction force assays on cancer cells. J Vis Exp. 2015:52343. doi: 10.3791/52343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer research. 2007;67:4227–35. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 45.Clark ES, Weaver AM. A new role for cortactin in invadopodia: regulation of protease secretion. European journal of cell biology. 2008;87:581–90. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark ES, Brown B, Whigham AS, Kochaishvili A, Yarbrough WG, Weaver AM. Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene. 2009;28:431–44. doi: 10.1038/onc.2008.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoshino D, Jourquin J, Emmons SW, Miller T, Goldgof M, Costello K, et al. Network analysis of the focal adhesion to invadopodia transition identifies a PI3K-PKCalpha invasive signaling axis. Science signaling. 2012;5:ra66. doi: 10.1126/scisignal.2002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell reports. 2013;5:1159–68. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Branch KM, Hoshino D, Weaver AM. Adhesion rings surround invadopodia and promote maturation. Biol Open. 2012;1:711–22. doi: 10.1242/bio.20121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowden ET, Onikoyi E, Slack R, Myoui A, Yoneda T, Yamada KM, et al. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Experimental cell research. 2006;312:1240–53. doi: 10.1016/j.yexcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Monteiro P, Rosse C, Castro-Castro A, Irondelle M, Lagoutte E, Paul-Gilloteaux P, et al. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. The Journal of cell biology. 2013;203:1063–79. doi: 10.1083/jcb.201306162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayes KE, Walk EL, Ammer AG, Kelley LC, Martin KH, Weed SA. Ableson kinases negatively regulate invadopodia function and invasion in head and neck squamous cell carcinoma by inhibiting an HB-EGF autocrine loop. Oncogene. 2013;32:4766–77. doi: 10.1038/onc.2012.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaty BT, Wang Y, Bravo-Cordero JJ, Sharma VP, Miskolci V, Hodgson L, et al. Talin regulates moesin-NHE-1 recruitment to invadopodia and promotes mammary tumor metastasis. The Journal of cell biology. 2014;205:737–51. doi: 10.1083/jcb.201312046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valenzuela-Iglesias A, Sharma VP, Beaty BT, Ding Z, Gutierrez-Millan LE, Roy P, et al. Profilin1 regulates invadopodium maturation in human breast cancer cells. European journal of cell biology. 2015;94:78–89. doi: 10.1016/j.ejcb.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Artym VV, Swatkoski S, Matsumoto K, Campbell CB, Petrie RJ, Dimitriadis EK, et al. Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. The Journal of cell biology. 2015;208:331–50. doi: 10.1083/jcb.201405099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parsons BD, Schindler A, Evans DH, Foley E. A direct phenotypic comparison of siRNA pools and multiple individual duplexes in a functional assay. PLoS ONE. 2009;4:e8471. doi: 10.1371/journal.pone.0008471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nature reviews. 2003;4:446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 58.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–54. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samani A, Zubovits J, Plewes D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Physics in medicine and biology. 2007;52:1565–76. doi: 10.1088/0031-9155/52/6/002. [DOI] [PubMed] [Google Scholar]
- 60.Artym VV, Yamada KM, Mueller SC. ECM degradation assays for analyzing local cell invasion. Methods in molecular biology (Clifton, NJ. 2009;522:211–9. doi: 10.1007/978-1-59745-413-1_15. [DOI] [PubMed] [Google Scholar]
- 61.Beaty BT, Sharma VP, Bravo-Cordero JJ, Simpson MA, Eddy RJ, Koleske AJ, et al. beta1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Molecular biology of the cell. 2013;24:1661–75. S1–11. doi: 10.1091/mbc.E12-12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bowden ET, Coopman PJ, Mueller SC. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods in cell biology. 2001;63:613–27. doi: 10.1016/s0091-679x(01)63033-4. [DOI] [PubMed] [Google Scholar]
- 63.Parekh A, Ruppender NS, Branch KM, Sewell-Loftin MK, Lin J, Boyer PD, et al. Sensing and modulation of invadopodia across a wide range of rigidities. Biophysical journal. 2011;100:573–82. doi: 10.1016/j.bpj.2010.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weaver AM, Page JM, Guelcher SA, Parekh A. Synthetic and tissue-derived models for studying rigidity effects on invadopodia activity. Methods in molecular biology (Clifton, NJ. 2013;1046:171–89. doi: 10.1007/978-1-62703-538-5_10. [DOI] [PubMed] [Google Scholar]
- 65.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer research. 2006;66:3034–43. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 66.Munevar S, Wang Y, Dembo M. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophysical journal. 2001;80:1744–57. doi: 10.1016/s0006-3495(01)76145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reinhart-King CA, Dembo M, Hammer DA. The dynamics and mechanics of endothelial cell spreading. Biophysical journal. 2005;89:676–89. doi: 10.1529/biophysj.104.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kramer N, Walzl A, Unger C, Rosner M, Krupitza G, Hengstschlager M, et al. In vitro cell migration and invasion assays. Mutation research. 2013;752:10–24. doi: 10.1016/j.mrrev.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Marshall J. Transwell((R)) invasion assays. Methods in molecular biology (Clifton, NJ. 2011;769:97–110. doi: 10.1007/978-1-61779-207-6_8. [DOI] [PubMed] [Google Scholar]
- 70.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Seminars in cancer biology. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Thompson EW, Paik S, Brunner N, Sommers CL, Zugmaier G, Clarke R, et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. Journal of cellular physiology. 1992;150:534–44. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 72.Coopman PJ, Do MT, Thompson EW, Mueller SC. Phagocytosis of cross-linked gelatin matrix by human breast carcinoma cells correlates with their invasive capacity. Clin Cancer Res. 1998;4:507–15. [PubMed] [Google Scholar]
- 73.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–9. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. The Journal of cell biology. 2005;168:441–52. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. The Journal of cell biology. 2010;189:541–56. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takkunen M, Hukkanen M, Liljestrom M, Grenman R, Virtanen I. Podosome-like structures of non-invasive carcinoma cells are replaced in epithelial-mesenchymal transition by actin comet-embedded invadopodia. Journal of cellular and molecular medicine. 2010;14:1569–93. doi: 10.1111/j.1582-4934.2009.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamaguchi H, Yoshida S, Muroi E, Yoshida N, Kawamura M, Kouchi Z, et al. Phosphoinositide 3-kinase signaling pathway mediated by p110alpha regulates invadopodia formation. The Journal of cell biology. 2011;193:1275–88. doi: 10.1083/jcb.201009126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamamoto H, Sutoh M, Hatakeyama S, Hashimoto Y, Yoneyama T, Koie T, et al. Requirement for FBP17 in invadopodia formation by invasive bladder tumor cells. The Journal of urology. 2011;185:1930–8. doi: 10.1016/j.juro.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 79.Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. The Journal of cell biology. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katoh K, Kano Y, Noda Y. Rho-associated kinase-dependent contraction of stress fibres and the organization of focal adhesions. Journal of the Royal Society, Interface/the Royal Society. 2011;8:305–11. doi: 10.1098/rsif.2010.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, Yip SC, et al. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. The Journal of cell biology. 2004;166:697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nature cell biology. 2012;14:777–83. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 83.Beningo KA, Wang YL. Flexible substrata for the detection of cellular traction forces. Trends in cell biology. 2002;12:79–84. doi: 10.1016/s0962-8924(01)02205-x. [DOI] [PubMed] [Google Scholar]
- 84.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiological reviews. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 85.Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–44. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vishnubhotla R, Sun S, Huq J, Bulic M, Ramesh A, Guzman G, et al. ROCK-II mediates colon cancer invasion via regulation of MMP-2 and MMP-13 at the site of invadopodia as revealed by multiphoton imaging. Laboratory investigation; a journal of technical methods and pathology. 2007;87:1149–58. doi: 10.1038/labinvest.3700674. [DOI] [PubMed] [Google Scholar]
- 87.Parekh A, Weaver AM. Regulation of invadopodia by mechanical signaling. Experimental cell research. 2015 doi: 10.1016/j.yexcr.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roca-Cusachs P, Iskratsch T, Sheetz MP. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. Journal of cell science. 2012;125:3025–38. doi: 10.1242/jcs.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, et al. Self-organized podosomes are dynamic mechanosensors. Curr Biol. 2008;18:1288–94. doi: 10.1016/j.cub.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. Journal of cell science. 2009;122:3037–49. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schachtner H, Calaminus SD, Thomas SG, Machesky LM. Podosomes in adhesion, migration, mechanosensing and matrix remodeling. Cytoskeleton. 2013;70:572–89. doi: 10.1002/cm.21119. [DOI] [PubMed] [Google Scholar]
- 92.Hoshino D, Branch KM, Weaver AM. Signaling inputs to invadopodia and podosomes. Journal of cell science. 2013;126:2979–89. doi: 10.1242/jcs.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Labernadie A, Bouissou A, Delobelle P, Balor S, Voituriez R, Proag A, et al. Protrusion force microscopy reveals oscillatory force generation and mechanosensing activity of human macrophage podosomes. Nature communications. 2014;5:5343. doi: 10.1038/ncomms6343. [DOI] [PubMed] [Google Scholar]
- 94.Pan YR, Chen CL, Chen HC. FAK is required for the assembly of podosome rosettes. The Journal of cell biology. 2011;195:113–29. doi: 10.1083/jcb.201103016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Juin A, Planus E, Guillemot F, Horakova P, Albiges-Rizo C, Genot E, et al. Extracellular matrix rigidity controls podosome induction in microvascular endothelial cells. Biology of the cell/under the auspices of the European Cell Biology Organization. 2013;105:46–57. doi: 10.1111/boc.201200037. [DOI] [PubMed] [Google Scholar]
- 96.Yu CH, Rafiq NB, Krishnasamy A, Hartman KL, Jones GE, Bershadsky AD, et al. Integrin-Matrix Clusters Form Podosome-like Adhesions in the Absence of Traction Forces. Cell reports. 2013;5:1456–68. doi: 10.1016/j.celrep.2013.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Helden SF, Hordijk PL. Podosome regulation by Rho GTPases in myeloid cells. European journal of cell biology. 2011;90:189–97. doi: 10.1016/j.ejcb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 98.van den Dries K, Meddens MB, de Keijzer S, Shekhar S, Subramaniam V, Figdor CG, et al. Interplay between myosin IIA-mediated contractility and actin network integrity orchestrates podosome composition and oscillations. Nature communications. 2013;4:1412. doi: 10.1038/ncomms2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Labernadie A, Thibault C, Vieu C, Maridonneau-Parini I, Charriere GM. Dynamics of podosome stiffness revealed by atomic force microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21016–21. doi: 10.1073/pnas.1007835107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van den Dries K, Schwartz SL, Byars J, Meddens MB, Bolomini-Vittori M, Lidke DS, et al. Dual-color superresolution microscopy reveals nanoscale organization of mechanosensory podosomes. Molecular biology of the cell. 2013;24:2112–23. doi: 10.1091/mbc.E12-12-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blangy A, Touaitahuata H, Cres G, Pawlak G. Cofilin activation during podosome belt formation in osteoclasts. PLoS ONE. 2012;7:e45909. doi: 10.1371/journal.pone.0045909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, et al. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. The Journal of cell biology. 2008;181:985–98. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Antelmi E, Cardone RA, Greco MR, Rubino R, Di Sole F, Martino NA, et al. ss1 integrin binding phosphorylates ezrin at T567 to activate a lipid raft signalsome driving invadopodia activity and invasion. PLoS ONE. 2013;8:e75113. doi: 10.1371/journal.pone.0075113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martin-Villar E, Borda-d’Agua B, Carrasco-Ramirez P, Renart J, Parsons M, Quintanilla M, et al. Podoplanin mediates ECM degradation by squamous carcinoma cells through control of invadopodia stability. Oncogene. 2015;34:4531–44. doi: 10.1038/onc.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends in cell biology. 2007;17:178–86. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 106.Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. Journal of cell science. 2009;122:1059–69. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 107.Marjoram RJ, Lessey EC, Burridge K. Regulation of RhoA activity by adhesion molecules and mechanotransduction. Current molecular medicine. 2014;14:199–208. doi: 10.2174/1566524014666140128104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Costa P, Scales TM, Ivaska J, Parsons M. Integrin-specific control of focal adhesion kinase and RhoA regulates membrane protrusion and invasion. PLoS ONE. 2013;8:e74659. doi: 10.1371/journal.pone.0074659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin L, Yang XM, Li J, Zhang YL, Qin W, Zhang ZG. Microfilament regulatory protein MENA increases activity of RhoA and promotes metastasis of hepatocellular carcinoma. Experimental cell research. 2014;327:113–22. doi: 10.1016/j.yexcr.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 110.Paul NR, Allen JL, Chapman A, Morlan-Mairal M, Zindy E, Jacquemet G, et al. alpha5beta1 integrin recycling promotes Arp2/3-independent cancer cell invasion via the formin FHOD3. The Journal of cell biology. 2015;210:1013–31. doi: 10.1083/jcb.201502040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16:1515–23. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 112.Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nature cell biology. 2008;10:127–37. doi: 10.1038/ncb1675. [DOI] [PubMed] [Google Scholar]
- 113.Oppel F, Muller N, Schackert G, Hendruschk S, Martin D, Geiger KD, et al. SOX2-RNAi attenuates S-phase entry and induces RhoA-dependent switch to protease-independent amoeboid migration in human glioma cells. Molecular cancer. 2011;10:137. doi: 10.1186/1476-4598-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Indra I, Undyala V, Kandow C, Thirumurthi U, Dembo M, Beningo KA. An in vitro correlation of mechanical forces and metastatic capacity. Phys Biol. 2011;8:015015. doi: 10.1088/1478-3975/8/1/015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer cell. 2011;19:372–86. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer metastasis reviews. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 117.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Current opinion in cell biology. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 118.Wolf K, Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clinical & experimental metastasis. 2009;26:289–98. doi: 10.1007/s10585-008-9190-2. [DOI] [PubMed] [Google Scholar]
- 119.Shea KF, Wells CM, Garner AP, Jones GE. ROCK1 and LIMK2 interact in spread but not blebbing cancer cells. PLoS ONE. 2008;3:e3398. doi: 10.1371/journal.pone.0003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lane J, Martin TA, Watkins G, Mansel RE, Jiang WG. The expression and prognostic value of ROCK I and ROCK II and their role in human breast cancer. International journal of oncology. 2008;33:585–93. [PubMed] [Google Scholar]
- 121.Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer research. 2009;69:8742–51. doi: 10.1158/0008-5472.CAN-09-1541. [DOI] [PubMed] [Google Scholar]
- 122.Ariztia EV, Lee CJ, Gogoi R, Fishman DA. The tumor microenvironment: key to early detection. Critical reviews in clinical laboratory sciences. 2006;43:393–425. doi: 10.1080/10408360600778836. [DOI] [PubMed] [Google Scholar]
- 123.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nature reviews Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.