Abstract
The conventional method of estimating parasite densities employs an assumption of 8000 white blood cells (WBCs)/µl. However, due to leucopenia in malaria patients, this number appears to overestimate parasite densities. In this study, we assessed the accuracy of parasite density estimated using this assumed WBC count in eastern Myanmar, where Plasmodium vivax has become increasingly prevalent. From 256 patients with uncomplicated P. vivax malaria, we estimated parasite density and counted WBCs by using an automated blood cell counter. It was found that WBC counts were not significantly different between patients of different gender, axillary temperature, and body mass index levels, whereas they were significantly different between age groups of patients and the time points of measurement. The median parasite densities calculated with the actual WBC counts (1,903/µl) and the assumed WBC count of 8,000/µl (2,570/µl) were significantly different. We demonstrated that using the assumed WBC count of 8000 cells/µl to estimate parasite densities of P. vivax malaria patients in this area would lead to an overestimation. For P. vivax patients aged five years and older, an assumed WBC count of 5,500/µl best estimated parasite densities. This study provides more realistic assumed WBC counts for estimating parasite densities in P. vivax patients from low-endemicity areas of Southeast Asia.
Keywords: malaria parasite, white blood cell count, parasite density, parasite species, age
Graphical Abstract
Introduction
Malaria continues to be a leading cause of morbidity and mortality worldwide, with more than 200 million cases of disease annually. In 2014, it is estimated that malaria infection results in 438,000 deaths (WHO, 2015). In the Greater Mekong Subregion (GMS) of Southeast Asia, the tropical and subtropical climate is highly conducive to malaria transmission (Cui et al., 2012). Despite that several countries in this region are moving toward malaria elimination, malaria remains highly prevalent along international borders, where hill tribes and ethnic minorities reside. In the border regions, political, economic and geographic factors hinder efforts to eliminate malaria. Increased control efforts, better diagnosis and treatment, and efficient management of drug resistance are needed.
Estimating malaria parasite density (number of parasites per µl of blood) is necessary for disease management, clinical trials and drug efficacy studies (WHO, 2010). White blood cell (WBC) counts have been a useful surrogate for direct scoring of parasite load under field conditions. Complete blood counts, particularly WBC counts, can be performed with automated hematological analyzers, or manually using stains, a microscope and a cell counting chamber and counters. However, in most field settings, it is not practical to score WBC values of malaria patients by automated blood cell counters. To allow for estimation of parasite densities in areas lacking infrastructure required for scoring actual WBC counts, assumed WBC values have been used. The World Health Organization (WHO) recommended the use of an assumed WBC value of 8,000/µl (WHO, 2010), which was based on a study with a Nigerian population with a large proportion of children younger than five years (Dowling and Shute, 1966). Yet, recent studies found that estimating malaria parasite densities using this WBC value yielded results that were inconsistent with the actual observed malaria parasite density of patients in some areas (Alves-Junior et al., 2014; Jeremiah and Uko, 2007; McKenzie et al., 2005; Rishikesh et al., 2015). Furthermore, whereas most studies conducted so far focused on P. falciparum patients, studies conducted in areas co-endemic for P. vivax and P. falciparum revealed considerable differences in WBC counts between patients infected with these two parasite species (Alves-Junior et al., 2014; McKenzie et al., 2005). Since the abundance of WBCs is influenced by the host immune status (Berens-Riha et al., 2014), and may vary in people of different races, ages, nutritional conditions, and pathogen infections, the baseline values of leukocytes in patients may differ significantly between populations and geographical regions (Bain, 1996). Therefore, it has been suggested that the best solution for estimating parasite densities is to use different WBC counts for different geographical regions, age groups and sub-populations (e.g., pregnant women), and epidemiological settings (Adu-Gyasi et al., 2012; Alves-Junior et al., 2014; Haggaz et al., 2014).
Here we assessed the accuracy of WHO recommended WBC count for estimating malaria parasite density in an area of the GMS along the China-Myanmar border. Plasmodium vivax is the predominant parasite species and the study population consists primarily of peoples of the Kachin (Jingpo) ethnicity (Li et al., 2013). By quantifying WBCs using an automated hematological analyzer, we found that the assumed WBC count of 8,000 cells/µl significantly overestimated parasite densities, particularly in patients ≥5 years. We further established that a WBC count of 5,500 provides a significantly closer estimation of the parasite density in this region among patients of five years and older.
2. Materials and methods
2.1. Study site and participants
The study was carried out in two settlements for internally displaced people and four villages near Laiza city in northeastern Myanmar along the China-Myanmar border. A total of 256 patients of mostly Kachin (Jingpo) ethnicity, 1 to 59 years old, with uncomplicated P. vivax infections were recruited from April 2014 to October 2015. Pregnant women, those suffering severe malnutrition, and patients found to harbor other infectious diseases were excluded. Demographic information of patients was obtained using a questionnaire during admission of patients. Axillary temperature of the participants was measured at the time of admission. Their body weight and heights were measured. Body mass index (BMI) was calculated using the formula: [weight (kg)/height squared (m2)]. Informed consent/assent was obtained from enrolled patients/guardians and this study was approved by the Kachin bureau of public health and institutional review board of Kunming Medical University (#KMC2012-01).
2.2. Blood sample collection and analysis
Malaria diagnosis was performed by light microscopy of thin and thick Giemsa-stained blood smears from finger prick blood. A venous blood sample (approximately 0.5–2 ml) was drawn from each patient into an EDTA-filled tube for WBC counting by using an automated hematological analyzer (TEK-II Mini, China) on day 0 and day 7. Blood was analyzed within 30 min of collection. Daily internal quality controls and scheduled external quality assessments were performed following the instructions from the manufacturer. P. vivax malaria patients were treated with chloroquine and primaquine per local drug policy.
2.3. Estimation of parasite density
Plasmodium parasites were counted against 200 WBCs (or 500 WBCs when the initial number of parasites was less than 99) on thick blood films. Parasite densities were first recorded as the average value of parasites per 200 leukocytes counted by two trained microscopists. For numbers differing by >20% (and negative slides), a senior microscopist provided an additional evaluation to reconcile the divergent results. Parasite densities (parasite/µl of whole blood) were then calculated as: (Number of parasites counted/WBC counts) × WBC count/µl. Calculation of parasite densities was based on either assumed WBC counts (ranging from 4,000 to 11,000 WBCs/µl of blood) or actual WBC counts from the automated WBC counter.
2.4. Statistical analysis
Data was analyzed and visualized using Graphpad Prism (version 6.0). Since the WBC counts had a skewed distribution, we assessed parameters that could influence the WBC counts using several non-parametric tests. Kruskal-Wallis test was used for comparison among multiple age groups and BMI data, whereas Dunn's multiple comparison test was used for between-group comparisons among multiple age groups. Mann-Whitney U test was used for comparing two groups of gender, stratified BMI data, and two axillary temperature categories (≤38.0 vs >38.0 °C). The Wilcoxon matched-pairs signed rank test was used for comparison of WBC counts between the two detection time points, and between parasite densities calculated by actual WBC counts and estimated by assumed WBC counts. To evaluate the best assumed WBC for the estimating of parasite densities, Bland-Altman plot analysis was carried out after logarithmic transformation of parasite densities.
3. Results
We recruited a total of 256 P. vivax patients in northeast Myanmar to assess the validity and accuracy of determining parasite density using the assumed WBC count of 8,000/µl. Among these patients, fever, chills, headache and weakness were the most common symptoms. Automated blood count revealed that 51 (19.92%) and 12 (4.69%) patients had leucopenia (<4,000/µl) and leukocytosis (>10,000/µl), respectively.
Automated blood counts were stratified by patient age, gender, axillary temperature, and BMI (Table 1). WBC counts were not significantly different between patients of different genders, presence (>38.0°C) and absence of fever (≤38.0°C), and the two BMI groups (Table 1). In contrast, there was a significant difference in WBC counts between different age groups. Specifically, WBC counts from children younger than five years were significantly different from those of other groups (P = 0.001 and P = 0.0002 compared with the 5–14 and ≥15 years groups, respectively; Dunn's multiple comparison test). In addition, WBC counts were also significantly different between the two time points of measurement, with WBC counts performed on day 7 being significantly higher than those on day 0 (P =0.0163, Wilcoxon matched-pairs signed rank test). It is noteworthy that all P. vivax patients enrolled were parasite-negative on day 7 based on microscopic examinations of finger-prick blood smears.
Table 1.
Comparison of white blood cell (WBC) counts between different malaria patient groups
| Groups | N | WBC/µL Median |
WBC/µL Mean (SD) |
P | |
|---|---|---|---|---|---|
| Age (years) | <5 | 26 | 7,750 | 7,719 (2,536) | 0.0003a* |
| 5–14 | 129 | 5,800 | 5,868 (2,200) | 0.0010b(vs <5 group) | |
| ≥15 | 101 | 5,400 | 5,571 (2,025) | 0.0002b(vs <5 group) | |
| Gender | Female | 123 | 6,000 | 6,060 (2,349) | 0.4501c |
| Male | 133 | 5,800 | 5,828 (2,151) | ||
|
Axillary temperature (°C) |
≤38.0 | 96 | 5,600 | 5,800 (2,102) | 0.3223c |
| >38.0 | 160 | 6,050 | 6,023 (2,331) | ||
|
Measurement time |
Day 0 | 135 | 5,500 | 5,767 (2,076) | 0.0163d |
| Day 7 | 135 | 5,600 | 6,562 (3,365) | ||
| Body mass index | <18.5 | 152 | 6,000 | 6,109 (2,414) | 0.1255c |
| ≥18.5 | 95 | 5,400 | 5,537 (1,927) | ||
P value for the entire age groups;
Kruskal-Wallis test;
Dunn's multiple comparison test;
Mann Whitney test; and
Wilcoxon matched-pairs signed rank test. SD, standard deviation.
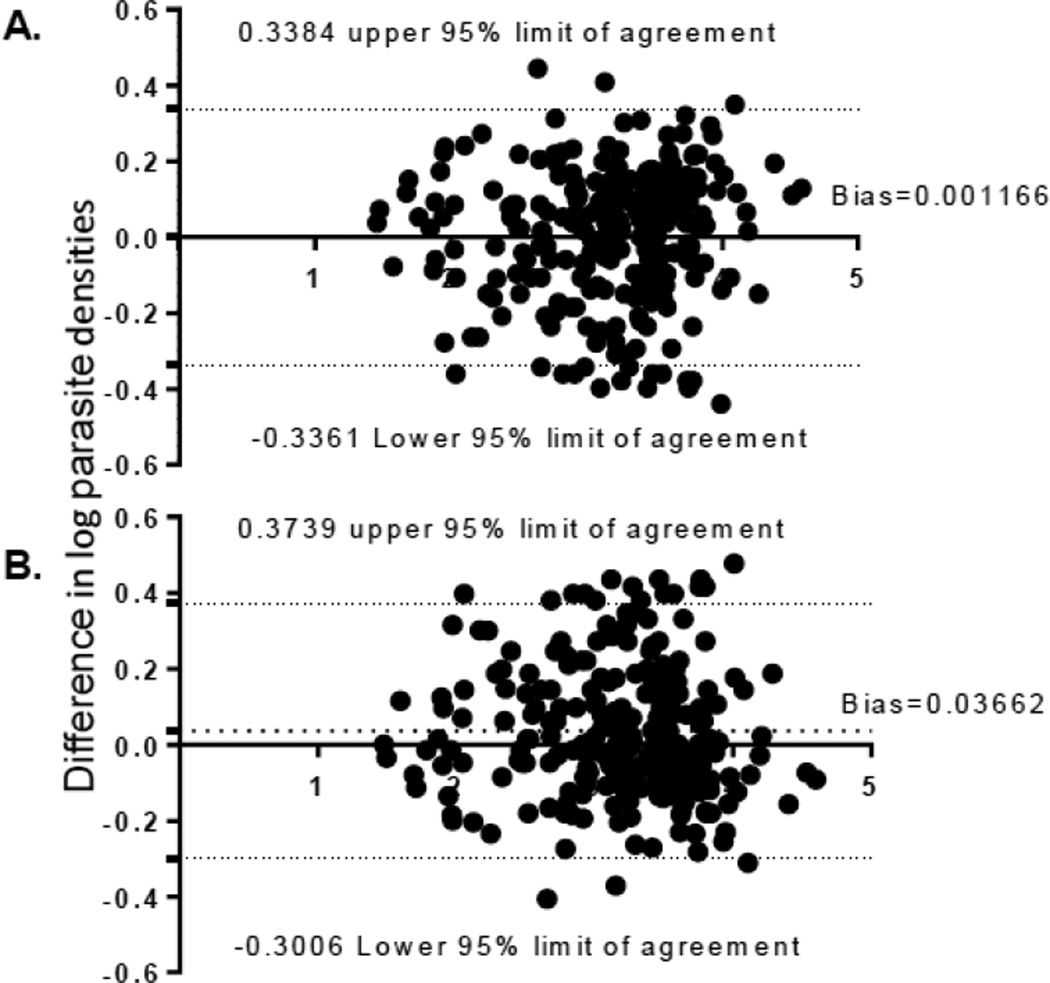
The median parasite density determined by automated WBC counting was 1,903/µl (interquartile range: 752–3,579/µl). To determine which assumed WBC count best estimates parasite density, we used assumed WBC counts of 8,000, 7,000, 6,000, 5,500, 5,000 and 4,000/µl, and predicted a median parasite density of 2,570, 2,249, 1,928, 1,767, 1,607 and 1,285/µl, respectively (Table 2). The parasite densities estimated by assumed WBC counts of 8,000 and 7,000 cells/µl were significantly higher than the densities calculated by the actual WBC counts, whereas assumed WBC counts of 5,000 and 4,000 cells/µl significantly underestimated parasite densities. The parasite density estimated using the assumed WBC counts of 5,500 (P = 0.2315) and 6,000 cells/µl (P = 0.0814) were not statistically significantly different from that calculated using the WBC counts determined by the automated counter (Table 2). Comparison using the Bland-Altman plot showed that parasite density estimated using the assumed WBC count of 5,500 cells/µl was most approximate to that calculated by using the actual WBC count (Figure 1).
Table 2.
Comparison between parasite densities (parasites/µl) calculated using actual WBC count and those estimated using assumed WBC counts
| WBC count/µl | Median | Range (min~max) |
IQR | Mean ± SD | P* |
|---|---|---|---|---|---|
| Actual count | 1,903 | 30~44,493 | 752~3,579 | 3,196±4,881 | -- |
| 8000 | 2,570 | 40~48,100 | 1,055~5,320 | 4,362±5,801 | <0.0001 |
| 7000 | 2,249 | 35~42,088 | 923~4,655 | 3,817±5,076 | <0.0001 |
| 6000 | 1,928 | 30~36,075 | 791~3,990 | 3,272±4,351 | 0.2315 |
| 5500 | 1,767 | 28~33,069 | 725~3,658 | 2,999±3,988 | 0.0814 |
| 5000 | 1,607 | 25~30,063 | 660~3,325 | 2,727±3,626 | <0.0001 |
| 4000 | 1,285 | 20~24,050 | 528~2,660 | 2,181±2,901 | <0.0001 |
P: Wilcoxon matched-pairs signed rank test between the median parasite density derived from an assumed WBC count (4,000–8,000) and that from an automated WBC counter. IQR, interquartile range; SD, standard deviation.
Fig. 1.
A comparison of different parasite densities of the entire study population estimated using assumed WBC counts (A. 5,500 cells/µl; B. 6,000 cells/µl) and automated WBC counts by Bland-Altman plot after logarithmic transformation of parasite densities.
The WBC count of 8,000/µl recommended by WHO was based on a study of a population in Africa, which consisted of almost 50% children under the age of five (Dowling and Shute, 1966). To determine whether this number is suitable for children of <5 years in our study group, we made similar comparison between parasite densities calculated using assumed and actual WBC counts. This analysis showed that parasite densities estimated using assumed WBC counts of 7,000 and 8,000 were not significantly different from that calculated from the actual WBC count (Table 3) (P > 0.05, Wilcoxon matched-pairs signed rank test). For patients five years and older, only parasite densities estimated from assumed WBC value of 5,500 (P = 0.4967) cells/µl was not significantly different from that calculated using the actual WBC counts (Table 3).
Table 3.
Comparison of different parasite densities between estimated by assumed and automated WBC counts in different age groups.
| <5 years | ≥5 years | |||
|---|---|---|---|---|
| Assumed WBC/µl |
Median Parasite density (/µl) |
P* | Median Parasite density (/µl) |
P* |
| 11,000 | 5,308 | < 0.0001 | 3,479 | < 0.0001 |
| 10,000 | 4,825 | 0.0001 | 3,163 | < 0.0001 |
| 9,000 | 4,343 | 0.0072 | 2,847 | < 0.0001 |
| 8,000 | 3,860 | 0.7454 | 2,530 | < 0.0001 |
| 7,000 | 3,378 | 0.1574 | 2,214 | < 0.0001 |
| 6,000 | 2,895 | 0.0010 | 1,898 | 0.0117 |
| 5,500 | 2,654 | 0.0003 | 1,740 | 0.7353 |
| 5,000 | 2,413 | 0.0001 | 1,582 | 0.0016 |
| 4,000 | 1,930 | < 0.0001 | 1,265 | < 0.0001 |
| Automated count | 3,630 | - | 1,760 | - |
P: Wilcoxon matched-pairs signed rank test between the median parasite density derived from an assumed WBC count (4,000–11,000) and that from an automated WBC counter. The most appropriate WBC counts for the two age groups were highlighted in bold.
4. Discussion
Estimation of blood stage malaria parasite densities by microscopy conventionally adopts an assumed constant WBC count of 8000 cells/µl in peripheral blood. In this study we further evaluated the suitability of this number for estimating malaria parasite density in an endemic area of Southeast Asia. After stratifying the WBC data by potential factors (age, gender, fever, and time of measurement), we identified that age and the time of measurement had a substantial influence on WBC counts.
One major finding of this study is that using the assumed WBC count of 8000 cells/µl for estimating parasite density significantly exaggerated the parasite density in this region. Our study further suggested that the proportions of different age groups in a study population are probably the most important factor influencing WBC counts and estimation of parasite densities. We found that an assumed WBC count of 5,500/µl best approximated the parasite density in our entire patient population, especially for patients five years of age and older. For children younger than five years, a WBC count of 8,000/µl was appropriate, though the small number of patients in this age group (26) prevented a more vigorous analysis. Nonetheless, this notion has been repeatedly observed in similar studies conducted in other malaria-endemic regions (Table 4). Of particular notice is that despite regional difference, the assumed WBC count of 5,500 cells/µl from this study coincided well with the proposed WBC count in a region of the Brazilian Amazon, where the majority (92.6%) of the study subjects was adults (Alves-Junior et al., 2014). An assumed WBC count of 5,000 cells/µl for patients five years and older matched well with a recent finding in south-western India, where all patients were adults and infected with P. vivax only (Rishikesh et al., 2015). In patient populations of exclusively or mostly children under five years, it has been documented that the recommended WBC count 8,000 cells/µl either was appropriate (Laman et al., 2014) or in a rare case underestimated parasite densities (Adu-Gyasi et al., 2012). In contrast, in populations consisting of mostly adults, this assumed WBC value would lead to significant overestimates of parasite densities (Alves-Junior et al., 2014; Haggaz et al., 2014; Jeremiah and Uko, 2007; McKenzie et al., 2005; Rodriguez-Morales et al., 2005; Tangpukdee et al., 2008). Taken together, the WHO recommend WBC value of 8000 cells/µl, based on a study cohort that was nearly 50% under the age of five, may have inflated the WBC counts in a population consisting of mostly adults (Dowling and Shute, 1966).
Table 4.
Summary of studies for comparison between parasite densities estimated using actual and assumed (8,000 cells/µl) WBC counts
| Study location | N | Age distribution (%) |
Parasite species | Parasite densities estimated using WBC count of 8,000/µl |
Recommended WBC count (cells/µl) |
Reference | ||
|---|---|---|---|---|---|---|---|---|
| <5 | 5–14 | ≥15 | ||||||
| Nigeria | 231 | 48.9 | 31.6 | 19.5 | P. falciparum | Recommended | 8,000 | (Dowling and Shute, 1966) |
| PNG | 168 | 100 | - | - | P. falciparum/P. vivax | Appropriate | 8,000 | (Laman et al., 2014) |
| Ghana | 5,902 | 100 | - | - | P. falciparum | Underestimated | 10,000 | (Adu-Gyasi et al., 2012) |
| Sudan | 98 | - | - | 100 | P. falciparum | Overestimated | - | (Haggaz et al., 2014) |
| Nigeria | 240 | 41.3 | 58.7 | - | P. falciparum | Overestimated | - | (Jeremiah and Uko, 2007) |
| Thailand, Peru | 4,697 | ? | ? | >69.0 | P. falciparum/P. vivax | Overestimated | - | (McKenzie et al., 2005) |
| Brazil | 403 | ? | ? | 92.6 | P. vivax/P. falciparum | Overestimated | 5,500 | (Alves-Junior et al., 2014) |
| India | 284 | - | - | 100 | P. vivax | Overestimated | 5,000–5,500 | (Rishikesh et al. 2015) |
| East Myanmar | 256 | 10.2 | 50.4 | 39.5 | P. vivax | Overestimated (for ≥5 years) | 5,500 | This study |
The time when WBCs are counted during the course of the disease or after treatment appears to also impact the outcome of the WBC counts. On day 7 after treatment, there was a recovery of WBC counts and this finding is largely consistent with those from other malarious regions. For example, in Papua New Guinea WBC counts in children younger than five years are dependent on the time of examination relative to the onset of illness (Laman et al., 2014). Similarly, WBC counts in Thai adults of both P. falciparum and P. vivax malaria quickly recovered from a significantly lower than normal level at the time of admission to the normal level within a week after treatment (Tangpukdee et al., 2008). The reduced WBC counts and in some cases leucopenia during an initial infection need to be taken into account when parasite densities are estimated based on assumed WBC numbers.
In areas of co-existence of different parasite species, WBC counts in patients of different malaria parasites have been compared. It has been documented in both Southeast Asia and South America that P. falciparum patients tended to have lower WBC counts than P. vivax patients (McKenzie et al., 2005; Tangpukdee et al., 2008). However, some studies did not find differences in WBC counts between patients of the two parasite species (Jadhav et al., 2003; Reiley and Barrett, 1971). In addition, the opposite was also noticed in a study conducted in South America, where lower WBC counts and leucopenia were more common among P. vivax patients than in P. falciparum patients (Rodriguez-Morales et al., 2005). P. vivax and P. falciparum are both endemic in our study region, where the former is the predominant species. Future studies should be conducted to make a comparison between the two species.
In conclusion, our results showed that in a malaria hypoendemic region of Southeast Asia a WBC count of 5,500 cells/µl is most appropriate for estimating malaria parasite densities of P. vivax malaria patients 5 years of age and older. These findings may have implications for other populations, as the influence of age on actual values may be greater than heretofore recognized. Though parasite density estimation is not needed for routine treatment of malaria cases diagnosed using either rapid diagnostic kits or microscopy, accurate parasite counts are more critical for clinical management of severe, hospitalized cases and for research purposes. Therefore, based on the consistent findings from multiple sites, WHO should consider modifying the current recommendation of using a WBC count of 8,000 cells/µl for estimating malaria parasite density.
Highlights.
We compare Plasmodium vivax parasite density using assumed and actual WBC counts.
The WHO recommended WBC count of 8000/µl blood overestimates parasite density.
A WBC count of 5500/µl most closely estimates parasite density in patients ≥5 years.
Acknowledgments
We thank the staff at the clinics and patients for participation in this study. This work was supported by National Natural Science Foundation of China (#U1202226 and #31260508), Special Research Funds from the Ministry of Education of China (#20125317110001), a talent introduction project of Yunnan province (# 2013HA026), and National Institutes of Health, USA (U19AI089672). HL is a recipient of the Academic Newcomer Scholarship from Yunnan province.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zhaoqing Yang, Email: Zhaoqingy92@hotmail.com.
Liwang Cui, Email: Luc2@psu.edu.
References
- Adu-Gyasi D, Adams M, Amoako S, Mahama E, Nsoh M, Amenga-Etego S, Baiden F, Asante KP, Newton S, Owusu-Agyei S. Estimating malaria parasite density: assumed white blood cell count of 10,000/mul of blood is appropriate measure in Central Ghana. Malaria Journal. 2012;11:238. doi: 10.1186/1475-2875-11-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Junior ER, Gomes LT, Ribatski-Silva D, Mendes CR, Leal-Santos FA, Simoes LR, Mello MB, Fontes CJ. Assumed white blood cell count of 8,000 cells/muL overestimates malaria parasite density in the Brazilian Amazon. PloS One. 2014;9:e94193. doi: 10.1371/journal.pone.0094193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. Journal of Clinical Pathology. 1996;49:664–666. doi: 10.1136/jcp.49.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens-Riha N, Kroidl I, Schunk M, Alberer M, Beissner M, Pritsch M, Kroidl A, Froschl G, Hanus I, Bretzel G, von Sonnenburg F, Nothdurft HD, Loscher T, Herbinger KH. Evidence for significant influence of host immunity on changes in differential blood count during malaria. Malaria Journal. 2014;13:155. doi: 10.1186/1475-2875-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, Fan Q, Fang Q, Jongwutiwes S, Parker D, Sirichaisinthop J, Kyaw MP, Su XZ, Yang H, Yang Z, Wang B, Xu J, Zheng B, Zhong D, Zhou G. Malaria in the Greater Mekong Subregion: Heterogeneity and complexity. Acta Tropica. 2012;121:227–239. doi: 10.1016/j.actatropica.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling MA, Shute GT. A comparative study of thick and thin blood films in the diagnosis of scanty malaria parasitaemia. Bulletin of the World Health Organization. 1966;34:249–267. [PMC free article] [PubMed] [Google Scholar]
- Haggaz AD, Elbashir LM, Adam GK, Rayis DA, Adam I. Estimating malaria parasite density among pregnant women at central Sudan using actual and assumed white blood cell count. Malaria Journal. 2014;13:6. doi: 10.1186/1475-2875-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav UM, Singhvi R, Shah R. Prognostic implications of white cell differential count and white cell morphology in Malaria. Journal of Postgraduate Medicine. 2003;49:218–220. discussion 221. [PubMed] [Google Scholar]
- Jeremiah ZA, Uko EK. Comparative analysis of malaria parasite density using actual and assumed white blood cell counts. Annals of Tropocal Paediatrics. 2007;27:75–79. doi: 10.1179/146532807X170547. [DOI] [PubMed] [Google Scholar]
- Laman M, Moore BR, Benjamin J, Padapu N, Tarongka N, Siba P, Betuela I, Mueller I, Robinson LJ, Davis TM. Comparison of an assumed versus measured leucocyte count in parasite density calculations in Papua New Guinean children with uncomplicated malaria. Malaria Journal. 2014;13:145. doi: 10.1186/1475-2875-13-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Parker DM, Yang Z, Fan Q, Zhou G, Ai G, Duan J, Lee MC, Yan G, Matthews SA, Cui L, Wang Y. Risk factors associated with slide positivity among febrile patients in a conflict zone of north-eastern Myanmar along the China-Myanmar border. Malaria Journal. 2013;12:361. doi: 10.1186/1475-2875-12-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Prudhomme WA, Magill AJ, Forney JR, Permpanich B, Lucas C, Gasser RA, Jr, Wongsrichanalai C. White blood cell counts and malaria. The Journal of Infectious Diseases. 2005;192:323–330. doi: 10.1086/431152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley CG, Barrett O., Jr Leukocyte response in acute malaria. Americam Journal of Medical Sciences. 1971;262:153–158. doi: 10.1097/00000441-197109000-00002. [DOI] [PubMed] [Google Scholar]
- Rishikesh K, Madivala SK, Prabhu P, Kamath A, Ashok H, Vidyasagar S, Shastry AB, Saravu K. Surmised total leucocyte counts miscalculate the parasite index of Plasmodium vivax malaria patients of tertiary and primary care settings in South-Western India. Malaria Journal. 2015;14:163. doi: 10.1186/s12936-015-0669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales AJ, Sanchez E, Arria M, Vargas M, Piccolo C, Colina R, Franco-Paredes C. White blood cell counts in Plasmodium vivax malaria. The Journal of Infectious Diseases. 2005;192:1675–1676. doi: 10.1086/496993. [DOI] [PubMed] [Google Scholar]
- Tangpukdee N, Yew HS, Krudsood S, Punyapradit N, Somwong W, Looareesuwan S, Kano S, Wilairatana P. Dynamic changes in white blood cell counts in uncomplicated Plasmodium falciparum and P. vivax malaria. Parasitology International. 2008;57:490–494. doi: 10.1016/j.parint.2008.06.005. [DOI] [PubMed] [Google Scholar]
- WHO. Basic Malaria Microscopy. Learner's guide. 80. 2010
- WHO. World Malaria Report 2015. 2015